Abstract
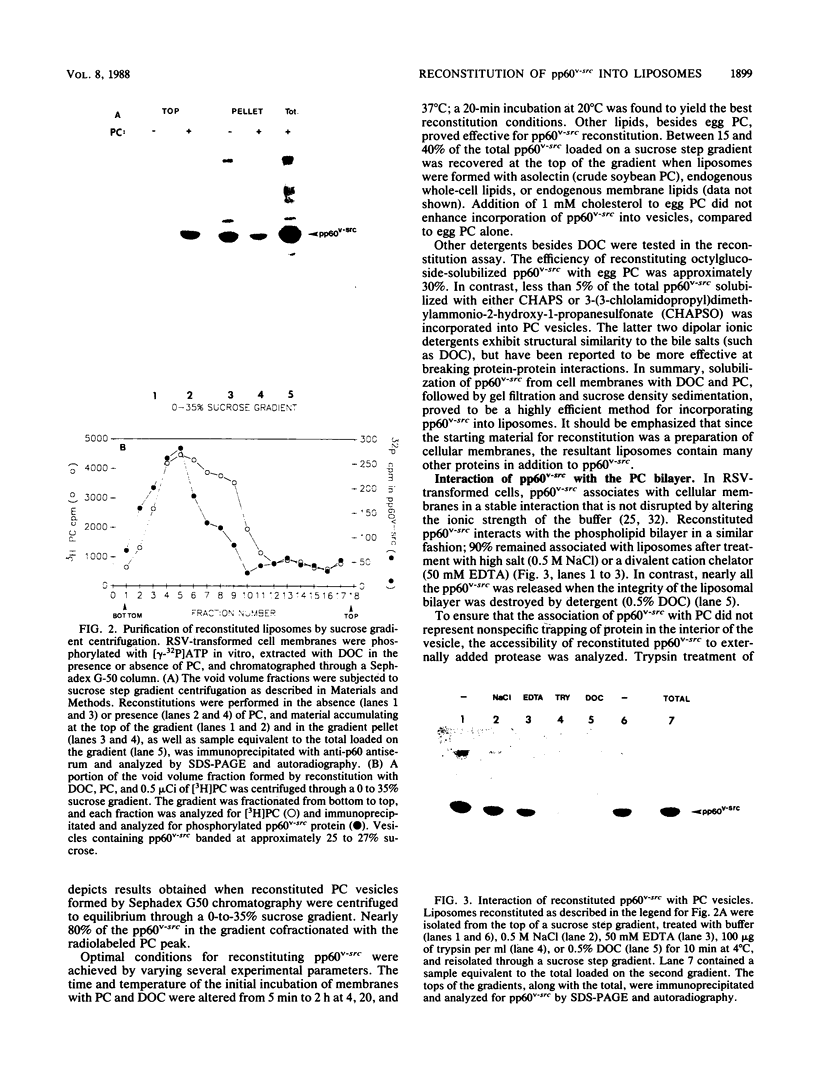
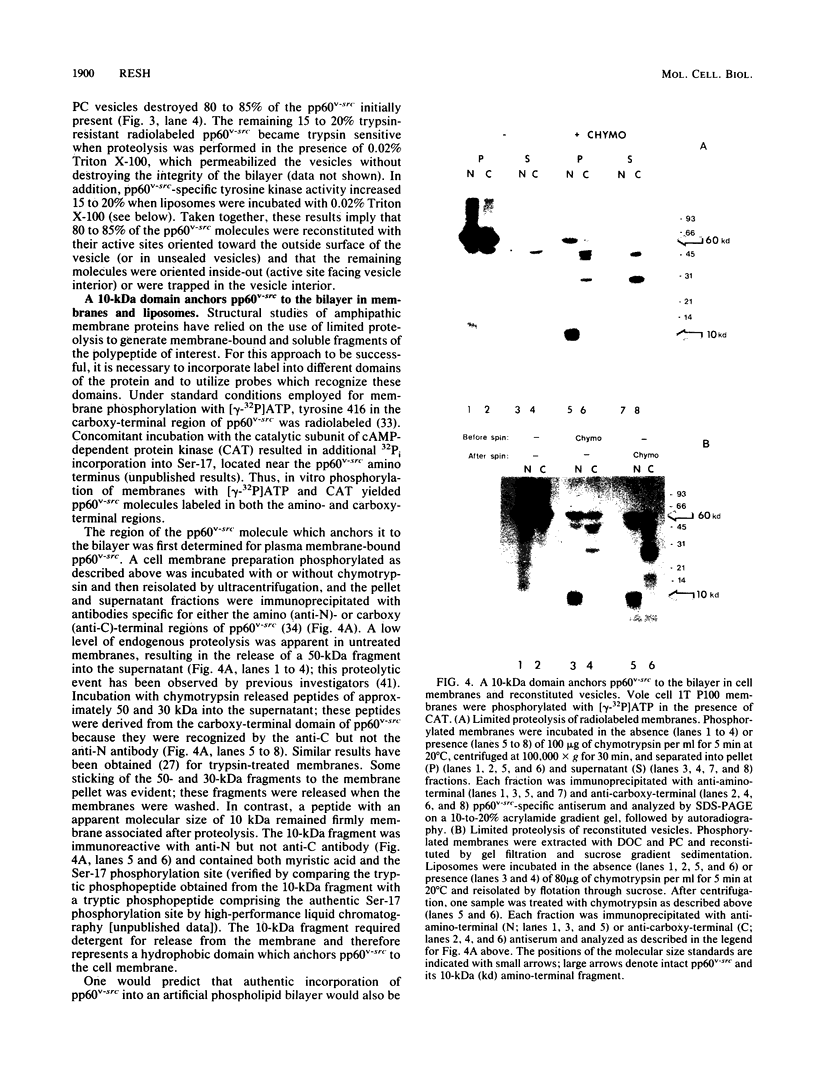
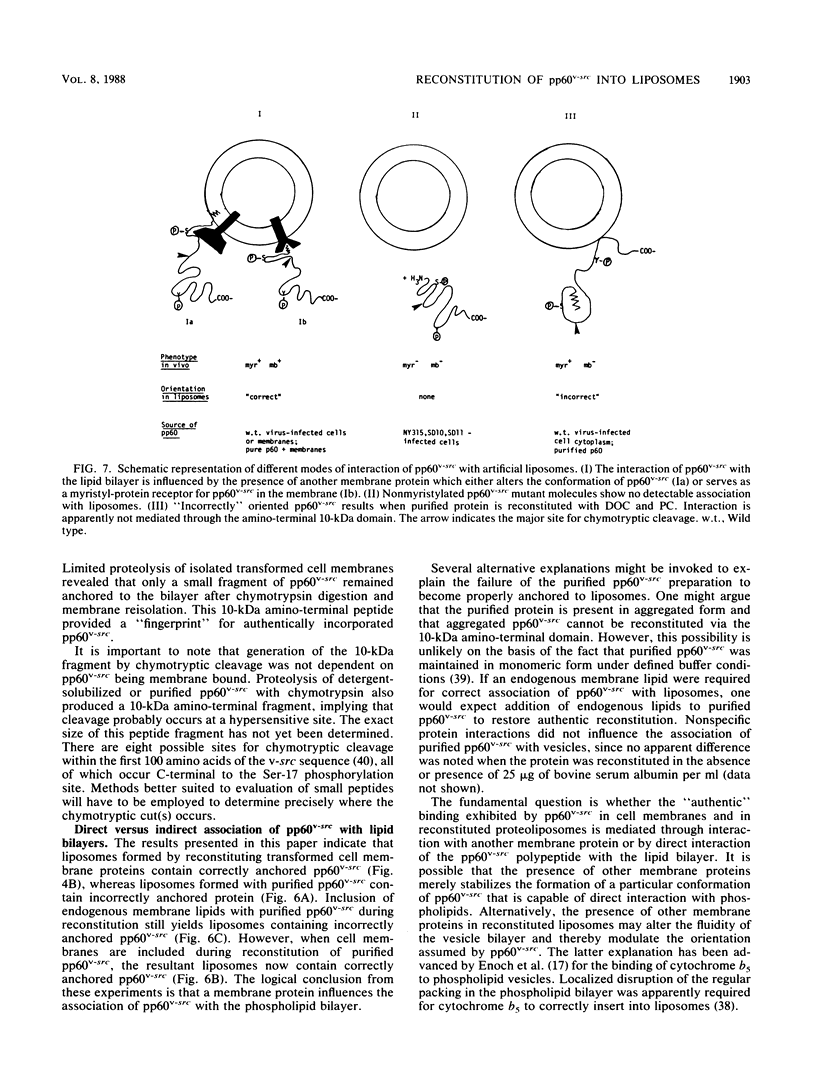
An artificial membrane system was developed to study the molecular basis for interaction of pp60v-src, the Rous sarcoma virus transforming protein, with lipid bilayers. pp60v-src was extracted from cell membranes by detergent solubilization and reincorporated into phospholipid vesicles. Reconstituted pp60v-src retained tyrosine kinase activity and was integrally associated with the liposome through a 10-kilodalton (kDa) amino-terminal domain. The same 10-kDa domain was shown to anchor pp60v-src to the plasma membrane of transformed cells. Reconstitution experiments performed with nonmyristylated pp60v-src proteins revealed that these polypeptides did not interact with phospholipid vesicles. In contrast, myristylated, soluble pp60v-src molecules (including a highly purified pp60v-src preparation) could be reconstituted into liposomes, but their interaction with the liposomal bilayer was not mediated by the 10-kDa amino-terminal domain. When membrane proteins were included during reconstitution of purified pp60v-src, binding through the 10-kDa anchor was restored. A model is presented to accommodate the different types of interactions of pp60v-src with liposomes; the model postulates the existence of an additional membrane component that anchors the pp60v-src polypeptide to the phospholipid bilayer.
Full text
PDF

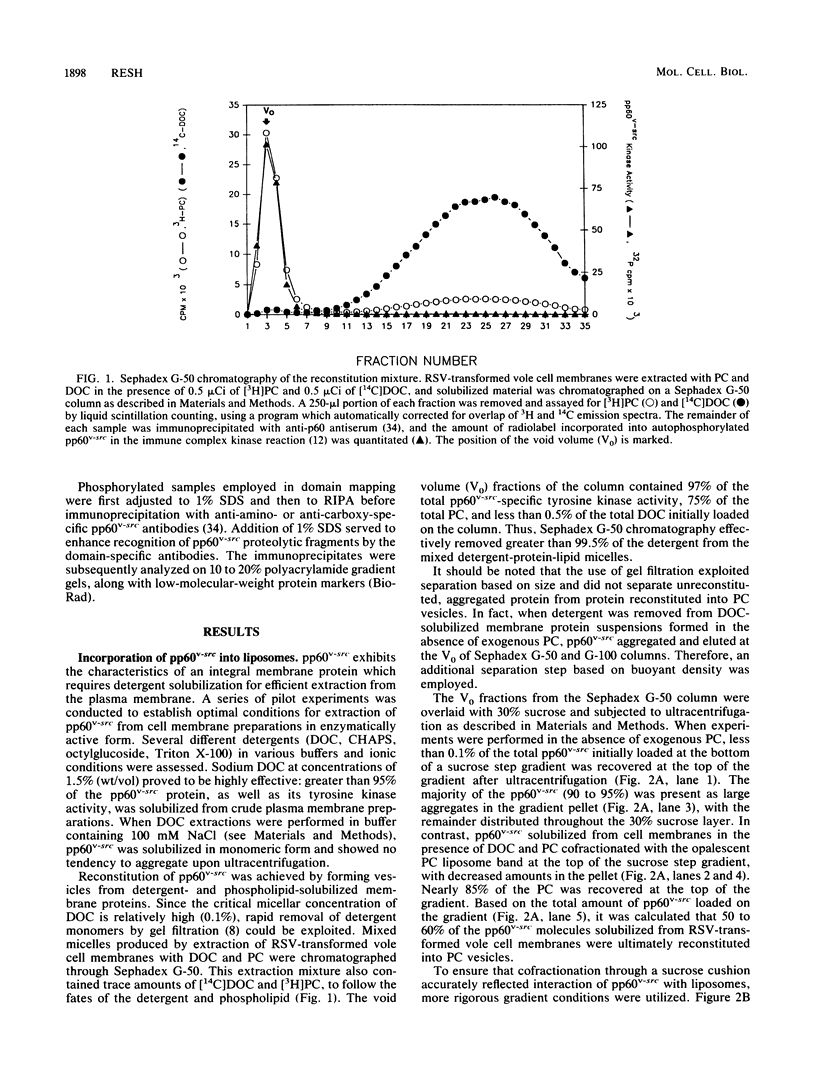




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A., Cohen P., Santikarn S., Williams D. H., Calder A. G., Smith A., Klee C. B. Identification of the NH2-terminal blocking group of calcineurin B as myristic acid. FEBS Lett. 1982 Dec 27;150(2):314–318. doi: 10.1016/0014-5793(82)80759-x. [DOI] [PubMed] [Google Scholar]
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Brugge J., Yonemoto W., Darrow D. Interaction between the Rous sarcoma virus transforming protein and two cellular phosphoproteins: analysis of the turnover and distribution of this complex. Mol Cell Biol. 1983 Jan;3(1):9–19. doi: 10.1128/mcb.3.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner J., Hauser H., Braun H., Wilson K. J., Wacker H., O'Neill B., Semenza G. The mode of association of the enzyme complex sucrase.isomaltase with the intestinal brush border membrane. J Biol Chem. 1979 Mar 25;254(6):1821–1828. [PubMed] [Google Scholar]
- Brunner J., Skrabal P., Hauser H. Single bilayer vesicles prepared without sonication. Physico-chemical properties. Biochim Biophys Acta. 1976 Dec 2;455(2):322–331. doi: 10.1016/0005-2736(76)90308-4. [DOI] [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Sefton B. M. Myristic acid is attached to the transforming protein of Rous sarcoma virus during or immediately after synthesis and is present in both soluble and membrane-bound forms of the protein. Mol Cell Biol. 1984 Dec;4(12):2697–2704. doi: 10.1128/mcb.4.12.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Sefton B. M. Myristic acid, a rare fatty acid, is the lipid attached to the transforming protein of Rous sarcoma virus and its cellular homolog. J Virol. 1985 Jan;53(1):7–12. doi: 10.1128/jvi.53.1.7-12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr S. A., Biemann K., Shoji S., Parmelee D. C., Titani K. n-Tetradecanoyl is the NH2-terminal blocking group of the catalytic subunit of cyclic AMP-dependent protein kinase from bovine cardiac muscle. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6128–6131. doi: 10.1073/pnas.79.20.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Bishop J. M. Transit of pp60v-src to the plasma membrane. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7117–7121. doi: 10.1073/pnas.79.23.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Levinson A. D., Bishop J. M. The protein encoded by the transforming gene of avian sarcoma virus (pp60src) and a homologous protein in normal cells (pp60proto-src) are associated with the plasma membrane. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3783–3787. doi: 10.1073/pnas.77.7.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Hanafusa H. N-terminal deletions in Rous sarcoma virus p60src: effects on tyrosine kinase and biological activities and on recombination in tissue culture with the cellular src gene. Mol Cell Biol. 1985 Oct;5(10):2789–2795. doi: 10.1128/mcb.5.10.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Pellman D., Hanafusa H. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984 Sep;4(9):1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch H. G., Fleming P. J., Strittmatter P. The binding of cytochrome b5 to phospholipid vesicles and biological membranes. Effect of orientation on intermembrane transfer and digestion by carboxypeptidase Y. J Biol Chem. 1979 Jul 25;254(14):6483–6488. [PubMed] [Google Scholar]
- Garber E. A., Cross F. R., Hanafusa H. Processing of p60v-src to its myristylated membrane-bound form. Mol Cell Biol. 1985 Oct;5(10):2781–2788. doi: 10.1128/mcb.5.10.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin S. M. Active transport of sodium and potassium ions by the sodium and potassium ion-activated adenosine triphosphatase from renal medulla. Reconstitution of the purified enzyme into a well defined in vitro transport system. J Biol Chem. 1977 Aug 25;252(16):5630–5642. [PubMed] [Google Scholar]
- Hamaguchi M., Hanafusa H. Association of p60src with Triton X-100-resistant cellular structure correlates with morphological transformation. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2312–2316. doi: 10.1073/pnas.84.8.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. G., Wang E., Goldberg A. R. Evidence that the src gene product of Rous sarcoma virus is membrane associated. Virology. 1980 Feb;101(1):25–40. doi: 10.1016/0042-6822(80)90480-8. [DOI] [PubMed] [Google Scholar]
- Krzyzek R. A., Mitchell R. L., Lau A. F., Faras A. J. Association of pp60src and src protein kinase activity with the plasma membrane of nonpermissive and permissive avian sarcoma virus-infected cells. J Virol. 1980 Dec;36(3):805–815. doi: 10.1128/jvi.36.3.805-815.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Varmus H. E., Bishop J. M. Virus-specific messenger RNAs in permissive cells infected by avian sarcoma virus. J Biol Chem. 1979 Aug 25;254(16):8015–8022. [PubMed] [Google Scholar]
- Levinson A. D., Courtneidge S. A., Bishop J. M. Structural and functional domains of the Rous sarcoma virus transforming protein (pp60src). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1624–1628. doi: 10.1073/pnas.78.3.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N., Towler D. A., Glaser L. Specificity of fatty acid acylation of cellular proteins. J Biol Chem. 1985 Mar 25;260(6):3784–3790. [PubMed] [Google Scholar]
- Ozols J., Carr S. A., Strittmatter P. Identification of the NH2-terminal blocking group of NADH-cytochrome b5 reductase as myristic acid and the complete amino acid sequence of the membrane-binding domain. J Biol Chem. 1984 Nov 10;259(21):13349–13354. [PubMed] [Google Scholar]
- Pellman D., Garber E. A., Cross F. R., Hanafusa H. Fine structural mapping of a critical NH2-terminal region of p60src. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1623–1627. doi: 10.1073/pnas.82.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M. D., Erikson R. L. Characterization of pp60src phosphorylation in vitro in Rous sarcoma virus-transformed cell membranes. Mol Cell Biol. 1985 May;5(5):916–922. doi: 10.1128/mcb.5.5.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M. D., Erikson R. L. Development and characterization of antisera specific for amino- and carboxy-terminal regions of pp60src. J Virol. 1985 Jul;55(1):242–245. doi: 10.1128/jvi.55.1.242-245.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M. D., Erikson R. L. Highly specific antibody to Rous sarcoma virus src gene product recognizes a novel population of pp60v-src and pp60c-src molecules. J Cell Biol. 1985 Feb;100(2):409–417. doi: 10.1083/jcb.100.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S., Garber E. A., Hanafusa H. Amino terminal myristylation of the protein kinase p60src, a retroviral transforming protein. Science. 1985 Jan 25;227(4685):427–429. doi: 10.1126/science.3917576. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Beemon K., Hunter T. Comparison of the expression of the src gene of Rous sarcoma virus in vitro and in vivo. J Virol. 1978 Dec;28(3):957–971. doi: 10.1128/jvi.28.3.957-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternweis P. C. The purified alpha subunits of Go and Gi from bovine brain require beta gamma for association with phospholipid vesicles. J Biol Chem. 1986 Jan 15;261(2):631–637. [PubMed] [Google Scholar]
- Sugimoto Y., Erikson E., Graziani Y., Erikson R. L. Inter- and intramolecular interactions of highly purified Rous sarcoma virus-transforming protein, pp60v-src. J Biol Chem. 1985 Nov 5;260(25):13838–13843. [PubMed] [Google Scholar]
- Takeya T., Feldman R. A., Hanafusa H. DNA sequence of the viral and cellular src gene of chickens. 1. Complete nucleotide sequence of an EcoRI fragment of recovered avian sarcoma virus which codes for gp37 and pp60src. J Virol. 1982 Oct;44(1):1–11. doi: 10.1128/jvi.44.1.1-11.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells S. K., Collett M. S. Specific proteolytic fragmentation of p60v-src in transformed cell lysates. J Virol. 1983 Jul;47(1):253–258. doi: 10.1128/jvi.47.1.253-258.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]