Abstract
Background and Objectives
To determine the accuracy of fingerstick hemoglobin assessment in blood donors, the performance of a portable hemoglobinometer (HemoCue Hb 201+) was prospectively compared with that of an automated hematology analyzer (Cell-Dyn 4000). Hemoglobin values obtained by the latter were used as the “true” result.
Material and Methods
Capillary fingerstick samples were assayed by HemoCue in 150 donors. Fingerstick samples from two sites, one on each hand, were obtained from a subset of 50 subjects. Concurrent venous samples were tested using both HemoCue and Cell-Dyn devices.
Results
Capillary hemoglobin values (HemoCue) were significantly greater than venous hemoglobin values (HemoCue), which in turn were significantly greater than venous hemoglobin values by Cell-Dyn (mean ± SD: 14.05 ± 1.51, 13.89 ± 1.31, 13.62 ± 1.23, respectively; p<0.01 for all comparisons among groups). Nine donors (6%) passed hemoglobin screening criteria (≥12.5 g/dL) by capillary HemoCue, but were deferred by Cell-Dyn values (false-pass). Five donors (3%) were deferred by capillary sampling, but passed by Cell-Dyn (false-fail). Substantial variability in repeated fingerstick HemoCue results was seen (mean hemoglobin 13.72 vs. 13.70 g/dL, absolute mean difference between paired samples 0.76 g/dL). Hand dominance was not a factor.
Conclusions
Capillary samples assessed via a portable device yielded higher hemoglobin values than venous samples assessed on an automated analyzer. False-pass and false-fail rates were low and acceptable in the donor screening setting, with “true” values not differing by a clinically significant degree from threshold values used to assess acceptability for blood donation.
Keywords: capillary hemoglobin determination, venous hemoglobin determination, hemoglobin screening of blood donors, blood donor deferrals, handedness
Introduction
To qualify as an allogeneic blood donor, subjects must have a hemoglobin of at least 12.5 g/dL at the time of donation, as established by the Food and Drug Association (FDA) and the American Association of Blood Banks (AABB).(1,2) This standard is intended to protect both the donor and the recipient. It is used to assess the general health of a donor, to make sure the donor can tolerate the loss of a unit of blood without developing symptomatic anemia, and to ensure that the red cell content of a unit of blood meets a minimum standard.
Hemoglobin screening is currently performed using one of several available methods, including the copper sulfate density method, the spun microhematocrit determination, and the spectrophotometric determination of hemoglobin. The FDA and AABB do not mandate a standardized method for hemoglobin screening of donors. The gravimetric copper sulfate method has been used for many decades and is still used in certain regions of the United States. This method is inexpensive and easy to use but does not provide values that are as accurate as other hemoglobin screening methods. The copper sulfate density method does not give quantitative results and relies on a subjective endpoint.(3–5) Some institutions use the spun microhematocrit method for initial screening or subsequently as a second testing method if donors fail copper sulfate testing, however, this too is time consuming and not as accurate as other screening tools.(6,7)
For efficiency and convenience, many donor centers use a spectrophotometric measurement of hemoglobin acquired with a portable hemoglobinometer, such as the HemoCue Hb 201+ device (HemoCue AB, Angelholm, Sweden), to measure hemoglobin values on capillary samples obtained by a fingerstick lancing technique.(8) Donor capillary hemoglobin levels are determined within seconds using this device; however, the accuracy and reproducibility of capillary hemoglobin assessments using the HemoCue analyzer has been a topic of debate.
Previous studies in non-donor populations suggest that capillary HemoCue hemoglobin values are higher than venous HemoCue hemoglobin values, and that HemoCue hemoglobin values were reproducible using venous and arterial but not capillary samples.(9,10) These studies did not focus on healthy subjects in the donor room environment, where specifically trained staff repeatedly perform the same assessment using the same technique. Other studies have confirmed the manufacturer’s claims for the reproducibility and accuracy of the HemoCue hemoglobin, and its correlation with concomitant venous samples assayed using a conventional cyanmethemoglobin method (relative error <3.5%, coefficient of correlation 0.96), however, these studies were performed under controlled laboratory settings and not in the clinical blood bank environment.(11,12)
A recent study in Ireland of over 36,000 paired capillary and venous samples for hemoglobin determinations from blood donors not meeting hemoglobin donation criteria (≥ 12.5 g/dL in women; ≥ 13.5 g/dL in males) revealed that venous hemoglobin levels were consistently higher than capillary levels when the hemoglobin levels were in the lower part of the normal range.13 These findings permitted the collection of donor units from donors with fingerstick hemoglobin levels of 12.0–12.5 g/dL in women and 13.0–13.5 g/dL in men since these levels were found to be equivalent to venous hemoglobin levels that met donation criteria.. The French Regional Blood Establishment has also reported similar findings in over 70,000 blood donors.14
The aim of this study was to determine the accuracy and agreement of capillary and venous hemoglobin measurements performed by the HemoCue technique, and to compare these results with a venous hemoglobin determination performed by an automated hematology analyzer in a healthy donor population.
Materials and Methods
Studies were conducted in 150 healthy prospective blood donors undergoing whole blood donation, plateletpheresis, or leukapheresis donation procedures in the Department of Transfusion Medicine at the National Institutes of Health (NIH), Bethesda, during June 2006. Approximately 4,500 donors make 12,000 visits per year to this donor center, donating 7,000 units of whole blood, 3,500 plateletpheresis and 1,500 leukapheresis concentrates. All donors in this study underwent a routine health history screen and vital signs assessment prior to donation. Informed consent was obtained for donation of samples for research use. HemoCue Hb 201+ devices underwent daily quality control testing as recommended by the manufacturer.
Donors were seated in a screening booth for no longer than 10 minutes before acquisition of the capillary sample. The donor center is well air conditioned during the summer months. In the screening booth, the donor’s finger was cleaned with alcohol and pricked with a lancet (Microtainer, BD Diagnostics, Franklin Lakes, NJ), using the arm closest to the screening booth desk. The first 2–3 drops of blood were wiped away. A drop of blood was then drawn into a microcuvette by capillary action. The cuvette was placed in the HemoCue photometer. After a few seconds, the hemoglobin reading appeared on the screen of the HemoCue device. Eligibility for donation was based on this initial HemoCue fingerstick hemoglobin determination as per donor center’s Standard Operating Procedures (SOP). Both the machine number and hemoglobin reading were recorded.
Immediately following capillary sample testing, the donor was moved to a donor phlebotomy recliner chair, where a pre-donation venous blood sample was drawn directly into an EDTA vacuum collection tube. The EDTA blood sample was mixed well by inversion 8–10 times, and 100 uL of venous blood was pipetted from the collection tube onto plastic film under aseptic conditions. To evaluate the comparability of capillary versus venous sample acquisition methods, the pipetted drop of venous blood was immediately used to fill a HemoCue microcuvette. Hemoglobin determination was performed using the same HemoCue device used for the capillary fingerstick sample. To evaluate the accuracy of the capillary and venous sample HemoCue assessments, the EDTA blood sample tube was transported within 30 minutes to the Department of Laboratory Medicine (Clinical Center, NIH) for analysis with use of an automated hematology analyzer (Cell-Dyn 4000, Abbott Labs, Abbott Park, IL).
To evaluate the reproducibility of capillary fingerstick hemoglobin determinations, a subgroup of 50 volunteer healthy donors were recruited. Selection was based on the first 50 volunteer donors who gave informed consent for double fingersticks. In these donors, capillary samples were obtained by the same phlebotomist from fingers on both hands. Hand dominance of the donor, hand used for each lancet stick, and machine number were recorded. Venous HemoCue and Cell-Dyn 4000 analyses were performed and analyzed as described above.
Data are shown as mean and standard deviation, unless otherwise noted. Significance is assigned at the 0.05 level. Comparisons among groups of paired donor samples are made with the Student’s t-test.
Results
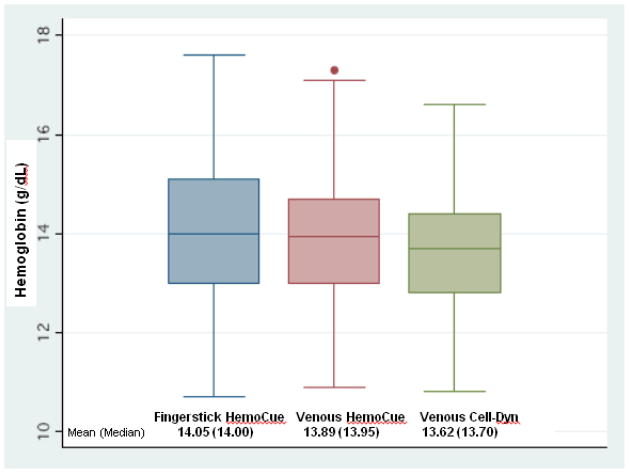
Sixty-five female and 85 male donors, mean age 50 (range 20 to 71) years were studied. Capillary fingerstick HemoCue hemoglobin values were significantly higher than venous HemoCue hemoglobin results, and both were significantly higher than venous Cell-Dyn results (mean hemoglobin values: 14.05 ± 1.51, 13.89 ± 1.31, 13.62 ± 1.23, respectively; p<0.01 for all comparisons among groups) (Table 1 and Figure 1). The dispersion of results around the mean was slightly greater for the capillary HemoCue method than the other two methods (coefficient of variation 10.6% for capillary HemoCue, 9.0% for venous HemoCue, and 8.6% for venous Cell-Dyn results).
Table 1.
Paired comparisons of hemoglobin determinations by three methods
| n | Hemoglobin g/dL (mean ± SD) | Mean Difference (g/dL)* | Relative Error (%)† | r (Pearson correlation coefficient) | p value | |
|---|---|---|---|---|---|---|
|
| ||||||
| Capillary HemoCue vs. |
150 | 14.05 ± 1.51 | 0.59 | 4.25 | 0.87 | 0.007 |
| Venous HemoCue | 150 | 13.89 ± 1.31 | ||||
|
| ||||||
| Capillary HemoCue vs. |
150 | 14.05 ± 1.51 | 0.69 | 5.08 | 0.88 | <0.001 |
| Venous Cell-Dyn 4000 | 150 | 13.62 ± 1.23 | ||||
|
| ||||||
| Venous HemoCue vs. |
150 | 13.89 ± 1.31 | 0.38 | 2.80 | 0.97 | <0.001 |
| Venous Cell-Dyn 4000 | 150 | 13.62 ± 1.23 | ||||
|
| ||||||
| Capillary HemoCue #1 vs. |
50 | 13.72 ± 1.64 | 0.76 | 5.57 | 0.84 | 0.92 |
| Capillary HemoCue #2 | 50 | 13.70 ± 1.73 | ||||
Differences expressed as absolute values
Relative error refers to the difference between the approximated value (capillary HemoCue hemoglobin) and the exact value (venous Cell-Dyn hemoglobin), divided by the exact value, and given as a percent. Venous HemoCue values were considered more exact than capillary values.
Figure 1.
Distribution of hemoglobin determinations obtained by fingerstick HemoCue, venous HemoCue, and venous Cell-Dyn methods
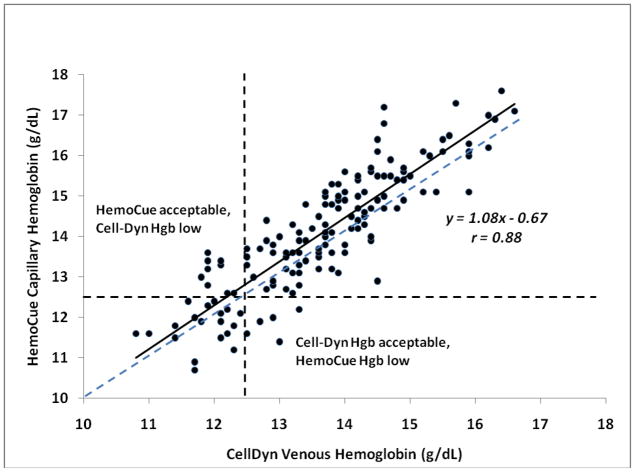
Nine donors (6%) passed hemoglobin screening criteria (hemoglobin ≥12.5 g/dL) by capillary HemoCue, but were deferred by Cell-Dyn values (these were termed false-pass HemoCue results) (Figure 2). “True” hemoglobin values (Cell-Dyn results) in these 9 subjects ranged from 11.8 to 12.3 g/dL. Five donors (3%) were deferred by capillary sampling, but passed by Cell-Dyn values (false-fail). “True” hemoglobin values in these subjects ranged from 12.5 to 13.3 g/dL. Differences between capillary hemoglobin values obtained with the HemoCue device and venous hemoglobins assayed on an automated analyzer (Cell-Dyn) were small, with a mean difference of 0.69 g/dL (relative error 5.08%). However, differences of greater than 10% were seen in 12% of cases.
Figure 2.
Accuracy of capillary HemoCue in comparison to venous Cell-Dyn hemoglobin values in healthy blood donors. Nine of 150 donors (6%) had “false-pass” HemoCue values, and five of 150 (3%) had “false-fail” HemoCue values when compared with Cell-Dyn reference values, using 12.5 g/dL as the criteria for deferral. Dashed line is the line of identity between HemoCue and Cell-Dyn values.
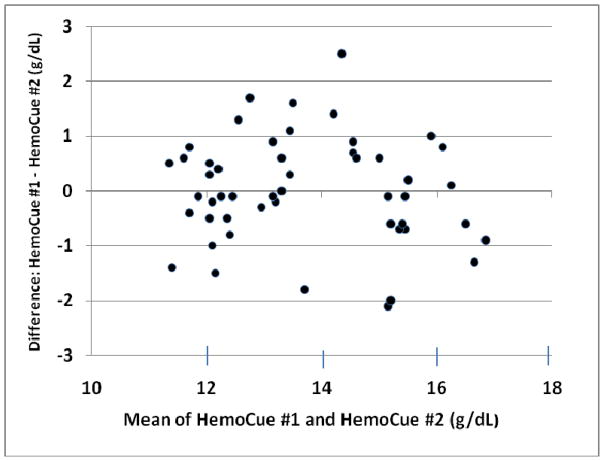
Repeat fingerstick determination in 50 donors (27 male, 23 female) revealed similar mean values but substantial inter-sample variability, with differences as great as 2.5 g/dL per donor (mean absolute difference 0.76 g/dL, relative error 5.57%) (Table 1 and Figure 3). Differences of greater than 10% in paired fingerstick hemoglobin values were seen in 16% of assessments. There was no correlation between hand dominance and higher or lower HemoCue hemoglobin values.
Figure 3.
Reproducibility of capillary HemoCue assessments. Capillary HemoCue values were obtained by the same operator on fingers of different hands. Aggregate mean hemoglobin was 13.71 g/dL, with a mean absolute difference in duplicate tests of 0.76 (relative error 5.6%).
Discussion
Rapid, accurate and reproducible assessment of donor hemoglobin is a critical feature of the blood donor screening process and has resulted in the availability of several point-of-care methods for minimally invasive hemoglobin determination. In this study of healthy blood donors, the accuracy of a portable hemoglobinometer (HemoCue) was evaluated by comparing capillary and venous hemoglobin values obtained with the hemoglobinometer to venous hemoglobin values obtained by an automated hematology analyzer (Cell-Dyn 4000). Capillary hemoglobin levels were found to be significantly higher than venous hemoglobin values obtained using the same hemoglobinometer, and both capillary and venous assessments using the hemoglobinometer were found to be significantly higher than “reference” hemoglobin values obtained using the automated analyzer. These findings support previous observations, wherein hemoglobin measurements obtained in capillary blood using the HemoCue device were uniformly higher than those obtained in venous blood, regardless of the device used for the venous assessment.(9,10,15)
The magnitude of the difference in hemoglobin values when the same venous sample was assessed by the HemoCue and the Cell-Dyn devices was small, with a mean difference of 0.38 g/dL (relative error 2.8%), and a strong correlation between the measurements (r = 0.97). Since the HemoCue device measures hemoglobin directly, whereas the automated cell counter requires high sample dilution, and since unlike automated counters, the HemoCue system is not affected by changes in sample turbidity, it has been suggested that the Hemocue may provide a more accurate assessment of hemoglobin than an automated cell counter.(12)
The composition of a drop of blood obtained from the capillaries by fingerstick technique is not the same as blood obtained from a vessel by venipuncture.(16–19) The fingerstick blood drop reflects the content of blood from various loop capillaries and small arterioles and venules in the finger. The fingerstick sample is also dependent on skin thickness, temperature of the skin, depth of penetration of the lancet, and potential “milking” of the finger by the phlebotomist. Venous sampling provides a more reliable assessment of hemoglobin as it reflects the blood coursing through the veins, heart, and arteries. Regulatory agencies do not specify the best technique for obtaining blood samples for hemoglobin testing to qualify individuals for blood donations. Each collection technique and testing methodology has its inherent variations and limitations, however, these differences should not be so significant as to compromise the donor’s health and safety.
The fingerstick HemoCue hemoglobin was significantly higher than both the venous HemoCue and venous Cell-Dyn hemoglobin measurements, but it is possible that non-analytical factors related to body position may have affected these values. The donors were seated for 10 minutes in a screening booth prior to fingerstick sample acquisition, but were then moved to a reclining chair, where they lay for several minutes before the venous samples were obtained. Upright body posture is known to cause mild hemoconcentration related to loss of plasma volume into the interstitial spaces of the dependent lower extremities. Conversely, assumption of the supine position is associated with as much as a 5–10% decrease in hemoglobin due to shifts of plasma volume from the extra- to the intravascular space.(20) Sample acquisition in the upright versus the supine position may thus have confounded the interpretation of the fingerstick versus the venous samples, independent of the instruments used to assess the samples.
Other factors that may affect hemoglobin determinations in addition to posture include season of the year and hemoglobin levels.(21,22) In the study by Tong and colleagues, the differences between paired capillary and venous samples were more pronounced in the winter months.(13) The cause of this observation is unclear. Also, venous hemoglobin levels were consistently at least 0.5 g/dL higher than capillary levels in female donors with capillary hemoglobin levels of 12.0 to 12.5 g/dL and in males with capillary hemoglobins of 13.0 to 13.5 g/dL. These findings permitted blood collection from donors who would have ordinarily been deferred, resulting in a 9.4% increase in annual blood donations in Ireland. Badevant et al. reported similar findings in France, supporting the observation that capillary hemoglobin values in the lower end of the normal range, just below the acceptable hemoglobin cutoffs for females and males, underestimated the venous levels in donors.(14) In our study, seasonal effects were not assessed since the study was conducted over a one month period in the summer. In donors deferred from donation due to capillary hemoglobin levels < 12.5 g/dL, the capillary hemoglobin results were still higher than the venous results in 55% of donors.
The precision or repeatability of capillary hemoglobin values using the HemoCue device has been found to be highly variable in prior studies, with nearly all studies showing lower reproducibility for capillary than for venous samples. Methodologies varied in these studies; in some cases, repeat capillary samples were obtained from the same finger by the same operator, in some cases from different fingers by different operators.(15) We found the reproducibility of repeated capillary measurements obtained by the same operator from fingers of different hands to be poor, with substantial inter-sample variability independent of operator training, specific HemoCue device, or donor handedness. Despite the concern that hand dominance may have had a role in the collection of a robust droplet of blood (larger dominant hand with increased vasculature, musculature, and degree of callused digits), hemoglobin measurements from the dominant hand were not predictably higher or lower than the non-dominant hand. The inter-sample variability was most likely due to inconsistencies inherent in the process of fingerstick sample acquisition, including the size and style of the lancet and the manner in which it is applied, and innate differences in individual blood droplets. Despite these variations, the mean absolute value of the differences between repeated fingerstick HemoCue measurements was small (0.76 g/dL, relative error 5.6%). The role of pre-analytical factors in determining capillary HemoCue values is acknowledged by the manufacturer, who recommends confirmation of an unexpected or unacceptable result with a second fingerstick performed by a different operator.(8)
The comparison of fingerstick HemoCue hemoglobin values with venous Cell-Dyn values yielded a 6% false-pass rate (hemoglobin ≥ 12.5 g/dL by capillary HemoCue testing but <12.5 g/dL by venous Cell-Dyn analysis). This cohort consisted entirely of donors with a reference hemoglobin value within 0.7 g/dL of the threshold value for donation. We found this 6% false-pass rate to be operationally acceptable and within the range reported by other investigators.(5) In addition, the 3% rate of falsely deferred donors (false-fail rate based on capillary HemoCue hemoglobin of < 12.5 g/dL with venous Cell-Dyn result of ≥ 12.5 g/dL) was small and operationally tolerable. Although occasional studies have found greater discrepancies between fingerstick and venous hemoglobin assessments, with one study predicting that 50% of donors with unacceptable venous hemoglobin levels would pass fingerstick HemoCue hemoglobin tests,(16) most studies have shown similarly low false-pass and false-fail results.(23–26)
Venous hemoglobin determination by an automated analyzer is viewed as the gold standard of hemoglobin testing; however, the collection and testing of venous blood as part of the routine blood donor screening process would be logistically difficult. It would require donors to spend more time in the screening process, necessitate an additional needlestick, and potentially compromise a phlebotomy site. An accepted approach to obtaining venous samples without an additional venipuncture is widely practiced in Europe and described by Lofti and colleagues.(27) Venous samples for automated analyzer assay are collected after the completion of the blood donation, and the hemoglobin values from the postdonation sample are used to qualify the donor for the next donation. Women with postdonation hemoglobin greater than or equal to 12.5 g/dL and men with postdonation hemoglobin greater than or equal to 13.5 g/dL were eligible to donate without having a predonation hemoglobin check prior to the next donation. The authors concluded that this was an acceptable alternative to performing predonation hemoglobin assays. However, this approach would not work for donors who do not return for donation within an acceptable time frame. Although eliminating blood sampling for predonation hemoglobin screening would certainly provide the greatest donor satisfaction, an editorial noted that donor satisfaction with fingerstick capillary sampling was 57%, only slightly higher than the 51% satisfaction obtained with needle insertion to perform to blood donation.(28)
The HemoCue hemoglobinometer has been used outside the donor room in critical care areas where accuracy is extremely important. Van de Louw et al. demonstrated that the HemoCue device could be used to quickly and accurately analyze hemoglobin levels in patients with active gastrointestinal bleeding.(29) In this study, the differences between capillary hemoglobin values obtained with the HemoCue device and venous sample assayed on an automated analyzer were in general small, with a mean difference of −0.06 g/dL. However, differences greater than 1% were seen in 21% of cases. Capillary hemoglobin values obtained using the HemoCue should be evaluated with caution when making therapeutic decisions.
In conclusion, we found that screening of fingerstick samples in blood donors using a HemoCue portable hemoglobinometer yields hemoglobin results that, while consistently slightly higher than values in concurrently drawn venous samples, were closely correlated with venous hemoglobin results from an automated analyzer. The false pass and false fail rates of the capillary HemoCue technique identified in this study were found to be low and operationally acceptable, with no evidence of compromise in donor health, although assessment of iron stores was not performed in these donors. Unfortunately, although the main purpose of the screening hemoglobin test is to ensure that donors are not ill or likely to be made ill by donating and to prevent the development or worsening of iron deficiency, screening hemoglobin levels in the range of 12.0 to 13.5 g/dL have recently been shown to be poor predictors of body iron stores.(30,31) It has been suggested that the screening hemoglobin assay is not a sufficient test to detect iron deficiency and that a more specific test, such as the serum ferritin, should be used when the hemoglobin is in the 12.0 to 13.5 g/dL range. Future studies assessing the accuracy and precision of fingerstick versus venous sampling techniques should ensure that changes in body position between samples do not confound the results.
New gender-based deferral criteria for donor hemoglobin values are being widely discussed in the U.S.(32) Attention to stringent training and periodic competency assessments of staff performing fingerstick hemoglobin assessments will be a critical part of this reevaluation of hemoglobin eligibility standards, with the intent of improving both the blood supply and the safety of blood donation.
Acknowledgments
Sources of Grant Support: None
We gratefully acknowledge the expertise of the Donor Room Staff of the NIH Department of Transfusion Medicine, and the generosity and commitment of our blood donors.
Footnotes
Authors AJP, RW, SJL, and BJB fulfill the following criteria: 1) substantial contributions to research design, or the acquisition, analysis, or interpretation of data; 2) drafting the paper or revising it critically, and 3) approval of the submitted and final versions.
Disclaimers: The authors have no conflict of interests to disclose.
References
- 1.United States Code of Federal Regulation, Title 21, CFR 640.3(b)(3) Washington, DC: US Government Printing Office; 2007. [Google Scholar]
- 2.Price TH, editor. Standards for blood banks and transfusion services. 25. Bethesda: AABB; 2008. p. 45. [Google Scholar]
- 3.Ross DG, Gilfillan AC, Houston DE, Heaton WAL. Evaluation of hemoglobin screening methods in prospective blood donors. Vox Sang. 1986;50:78–80. doi: 10.1111/j.1423-0410.1986.tb04850.x. [DOI] [PubMed] [Google Scholar]
- 4.Boulton FE, Nightingale MJ, Reynolds W. Improved strategy for screening prospective blood donors for anaemia. Transfusion Med. 1994;4:221–225. doi: 10.1111/j.1365-3148.1994.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 5.James V, Jones KF, Turner EM, Sokol RJ. Statistical analysis of inappropriate results from current hemoglobin screening methods for blood donors. Transfusion. 2003;43:400–404. doi: 10.1046/j.1537-2995.2003.00316.x. [DOI] [PubMed] [Google Scholar]
- 6.Avoy DR, Canuel ML, Otton BM, Mileski EB. Hemoglobin screening in prospective blood donors: a comparison of methods. Transfusion. 1977;17:261–264. doi: 10.1046/j.1537-2995.1977.17377196362.x. [DOI] [PubMed] [Google Scholar]
- 7.Cable RG. Hemoglobin determination in blood donors. Transfusion Med Rev. 1995;9:131–144. doi: 10.1016/s0887-7963(05)80052-5. [DOI] [PubMed] [Google Scholar]
- 8.HemoCue. HemoCueR Hb 201+ Operating Manual. HemoCue AB; Angelholm, Sweden: 2010. [Google Scholar]
- 9.de Paiva AA, Rondo PH, Silva SS, do Latorre MR. Comparison between the HemoCue and an automated counter for measuring hemoglobin. Rev Saude Publica. 2004;38:585–587. doi: 10.1590/s0034-89102004000400017. [DOI] [PubMed] [Google Scholar]
- 10.Chen PP, Short TG, Leung DHY, Oh TE. A clinical evaluation of the HemoCue haemoglobinometer using capillary, venous and arterial samples. Anaesth Intensive Care. 1992;20:497–503. doi: 10.1177/0310057X9202000419. [DOI] [PubMed] [Google Scholar]
- 11.Zwart A, Buursma A, Kwant G, Oeseburg B, Zijlstra WG. Determination of total haemoglobin in whole blood; further tests of the “Hemocue” method. Clin Chem. 1987;33:2307–2308. [PubMed] [Google Scholar]
- 12.Schenck von H, Falkensson M, Lundberg B. Evaluation of Hemocue, a device for determining haemoglobin. Clin Chem. 1986;32:526–529. [PubMed] [Google Scholar]
- 13.Tong E, Murphy WG, Kinsella A, Darragh E, Woods J, Murhpy C, McSweeney E. Capillary and venous haemoglobin levels in blood donors: a 42-month study of 36 258 paired samples. Vox Sang. 2010;98:547–553. doi: 10.1111/j.1423-0410.2009.01285.x. [DOI] [PubMed] [Google Scholar]
- 14.Badevant B, Porra V, Adjou C, Chuteau C, Chartois-Leaute AG, Bonaguidi M, Duvieu N, Prodhomme JY, Perdriau A, Schneider T, Follea G. Retrospective analysis of pre-donation hemoglobin screening implementation in a French regional blood establishment (EFS Pays De La Loire) Vox Sang. 2011;101(Suppl 1):P-144, 136. [Google Scholar]
- 15.Gomez-Simon A, Navarro-Nunez L, Perez-Ceballos E, et al. Evaluation of four rapid methods for hemoglobin screening of whole blood donors in mobile collection settings. Transf Apher Sci. 2007;36:235–242. doi: 10.1016/j.transci.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Radtke H, Polat G, Kalus U, Salama A, Kiesewetter H. Hemoglobin screening in prospective blood donors: Comparison of different blood samples and different quantitative methods. Transf Apher Sci. 2005;33:31–35. doi: 10.1016/j.transci.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Schalk E, Heim MU, Koenigsmann M, Jentsch-Ullrich K. Use of capillary blood count parameters in adults. Vox Sang. 2007;93:348–353. doi: 10.1111/j.1423-0410.2007.00978.x. [DOI] [PubMed] [Google Scholar]
- 18.Neufeld I, Garcia-Guerra A, Sanchez-Francia D, Newton-Sanchez O, Ramirez-Villalobos MD, Rivera-Dommarco J. Hemoglobin measured by HemoCue and a reference method in venous and capillary blood: a validation study. Salud Publica Mex. 2002;44:219–227. doi: 10.1590/s0036-36342002000300005. [DOI] [PubMed] [Google Scholar]
- 19.Daae LNW, Halvorsen S, Mathesin PM, Mironska K. A comparison between haematological parameters in capillary and venous blood from health adults. Scand J Clin Lab Invest. 1988;48:723–726. doi: 10.1080/00365518809085796. [DOI] [PubMed] [Google Scholar]
- 20.Hagan RD, Diaz FJ, Horvath SM. Plasma volume changes with movement to supine and standing positions. J Appl Physiol. 1978;45:414–417. doi: 10.1152/jappl.1978.45.3.414. [DOI] [PubMed] [Google Scholar]
- 21.Kristal-Boneh E, Froom P, Harari G, Shapiro Y, Green MS. Seasonal changes in red blood cell parameters. Br J Haematol. 1993;85:603–607. doi: 10.1111/j.1365-2141.1993.tb03354.x. [DOI] [PubMed] [Google Scholar]
- 22.Lau P, Hansen M, Sererat M. Influence of climate on donor deferrals. Transfusion. 1988;28:559–562. doi: 10.1046/j.1537-2995.1988.28689059031.x. [DOI] [PubMed] [Google Scholar]
- 23.Cable RG. Hemoglobin screening of blood donors: how close is close enough? Transfusion. 2003;43:306–308. doi: 10.1046/j.1537-2995.2003.00372.x. [DOI] [PubMed] [Google Scholar]
- 24.Munoz M, Romero A, Gomez JF, Manteca A, Naveira E, Ramirez G. Utility of point-of-care haemoglobin measurement in the HemoCue-B Haemoglobin for the initial diagnosis of anemia. Clin Lab Haematol. 2005;27:99–104. doi: 10.1111/j.1365-2257.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- 25.Mendrone A, Jr, Sabino EC, Sampaio L, et al. Anemia screening in potential female blood donors: comparison of two different quantitative methods. Transfusion. 2009;49:662–668. doi: 10.1111/j.1537-2995.2008.02023.x. [DOI] [PubMed] [Google Scholar]
- 26.Zoemann M, Lizardo B, Geusendam G, Schlenke P. Reliability of capillary hemoglobin screening under routine conditions. Transfusion. 2011;51:2714–2719. doi: 10.1111/j.1537-2995.2011.03183.x. [DOI] [PubMed] [Google Scholar]
- 27.Lofti R, Wernet D, Starke U, Northoff H, Cassens U. A noninvasive strategy for screening prospective blood donors for anemia. Transfusion. 2005;45:1585–1592. doi: 10.1111/j.1537-2995.2005.00574.x. [DOI] [PubMed] [Google Scholar]
- 28.Goldman MR. Another stab at donor hemoglobin screening. Transfusion. 2005;45:1552–1553. doi: 10.1111/j.1537-2995.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- 29.Van de Louw A, Lasserre N, Drouhin F, et al. Reliability of HemoCue in patients with gastrointestinal bleeding. Intensive Care Med. 2007;33:355–358. doi: 10.1007/s00134-006-0461-6. [DOI] [PubMed] [Google Scholar]
- 30.Bryant B, Yau YY, Arceo SM, Daniel-Johnson J, Hopkins JA, Leitman SF. Iron replacement therapy in the routine management of blood donors. Transfusion. 2012;52:1566–1575. doi: 10.1111/j.1537-2995.2011.03488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cable RG, Glynn SA, Kiss JA, Mast AE, Steele WR, Murphy EL, Wright DJ, Sacher RA, Gottschall JL, Vij V, Simon TL for the NHLBI Retrovirus Epidemiology Donor Study-II (REDS-II) Iron deficiency in blood donors: analysis of enrollment data from the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2011;51:511–522. doi: 10.1111/j.1537-2995.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman B. Improving the US blood supply and blood donation safety for both women and men. Transfusion. 2008;48:1032–1035. doi: 10.1111/j.1537-2995.2008.01664.x. [DOI] [PubMed] [Google Scholar]