Abstract
Hypertension affects many older adults and is associated with impaired neural and cognitive functioning. We investigated whether a history of hypertension was associated with impairments to prospective memory, which refers to the ability to remember to perform delayed intentions such as remembering to take medication. Thirty-two cognitively-normal older adult participants with or without a history of hypertension (self-reported) performed two laboratory prospective memory tasks, one that relies more strongly on executive control (nonfocal prospective memory) and one that relies more strongly on spontaneous memory retrieval processes (focal prospective memory). We observed hypertension-related impairments for nonfocal, but not focal, prospective memory. To complement our behavioral approach, we conducted a retrospective analysis of available structural magnetic resonance imaging data. Lower white matter volume estimates in the anterior prefrontal cortex (aPFC) were associated with lower nonfocal prospective memory and with a history of hypertension. A history of hypertension may be associated with worsened executive control and lower prefrontal white matter volume. The translational implication is that individuals who must remember to take antihypertensive medications and to monitor their blood pressure at home may be impaired in the executive control process that helps to support these prospective memory behaviors.
Hypertension, or sustained high blood pressure, is a serious pathological disease that affects more than 50% of adults over the age of 60 (Kearney, Whelton, Reynolds, Muntner, Whelton, & He, 2005). Hypertension may be asymptomatic but still increase risk for stroke, and therefore, it is sometimes referred to as the “silent killer” (e.g., Cooper, 1973). Prior to stroke, hypertension impairs the cerebrovascular system’s ability to distribute blood to regions of need, such as areas with high neuronal activity (Novak & Hajjar, 2010). In older adults, hypertension is also associated with increases in white matter lesions (de Leeuw et al., 2002; Goldstein, Bartzokis, Hance, & Shapiro, 1998; Raz, Rodrigue, Kennedy, & Acker, 2007), impaired white matter microstructure (Kennedy & Raz, 2009), and decreased grey and white matter volume (Raz, Rodrigue, & Acker, 2003). These negative influences on the brain are often concomitant with behavioral deficits: an array of epidemiological and clinical studies has found that hypertension is associated with worse performance on cognitive tests as well as increased risk for dementia (e.g., Unverzagt et al., 2011; for reviews, see Elias, Goodell, & Dore, 2012; Igase, Kohara, & Miki, 2012; Novak & Hajjar, 2010; Qiu, Winblad, & Fratiglioni, 2005; for an example of a discrepant finding, see Jacobs et al., 2011). In the present study, we extended this literature by investigating hypertension-related impairments in prospective memory tasks, which we considered might have theoretical and translational implications.
Prospective Memory
Prospective memory refers to memory for performing an intended action in the future, such as remembering to take medications, remembering to attend physician appointments, and managing blood pressure with a home monitoring unit (patients are encouraged to remember to monitor blood pressure twice in the morning and evening, though they often fail to remember to do so; Niiranen, Johansson, Reunanen, & Jula, 2011; van der Hoeven, van den Born, Cammenga, & van Montfrans, 2009). Three characteristics distinguish prospective memory from the more traditionally studied retrospective memory (e.g., memory for a list of studied words). First, prospective memory is future-focused rather than past-focused. Second, prospective memory retrieval must be self-initiated; individuals must “remember to remember” rather than be prompted to recall information. Third, prospective memory intentions (e.g., remembering to monitor blood pressure) must typically be remembered while one is already engaged in other attention-demanding activities (e.g., getting ready to leave for work). Laboratory tests of prospective memory were designed to reflect these three characteristics (Einstein & McDaniel, 1990): participants are first engaged in an ongoing task (e.g., categorizing words based on their semantic meaning), and then they are instructed to remember to perform a specific action (e.g., press F1) if they ever see a target cue (e.g., the word tortoise) during the ongoing task. Successful prospective remembering depends on detecting that the target cue (tortoise) is related to the prospective memory intention and is not solely a stimulus for ongoing semantic categorization processing.
The multiprocess theory of prospective memory (McDaniel & Einstein, 2000; 2007; 2011) proposes that different cognitive processes can support prospective remembering and that the appearance of these processes depend on the degree of overlap between the prospective memory cue and the ongoing task context. In focal prospective memory tasks, the ongoing task (e.g., semantic categorization of words) directs individuals to process the pertinent features of the prospective memory cue (e.g., a word cue), and consequently, spontaneous retrieval processes can support prospective memory. Spontaneous retrieval is a nonstrategic, relatively automatic process (McDaniel, Guynn, Einstein, & Breneiser, 2004) that allows intentions to be retrieved in the absence of effortful monitoring processes (Einstein & McDaniel, 2010). Furthermore, spontaneous retrieval depends on the integrity of medial temporal lobe structures (Gordon, Shelton, Bugg, McDaniel, & Head, 2011) and is introspectively experienced as a memory “popping” into mind (Einstein & McDaniel, 1990; Meier, von Wartburg, Matter, Reber, & Rothen, 2011).
In nonfocal prospective memory tasks, the processing of the ongoing task and prospective memory cue do not overlap. For example, individual letters would be nonfocal cues during a semantic categorization task, because that ongoing task focuses processing on the semantic meaning of whole words, not on individual letters. Therefore, participants must strategically monitor the environment for the nonfocal cue (Scullin, McDaniel, Shelton, et al., 2010). Strategic monitoring is an effortful, executive control process that neuroimaging studies have consistently linked to anterior prefrontal cortex engagement (e.g., Burgess, Quayle, & Frith, 2001; Reynolds, West, & Braver, 2009; for a review, see Burgess, Gonen-Yaacovi, & Volle, 2011).
Spontaneous retrieval and monitoring processes are typically distinguished by testing whether adding a focal versus nonfocal prospective memory task incurs a response time cost to ongoing task performance on non-target trials (see McDaniel & Einstein, 2007, for a review of focal/nonfocal effects). Because spontaneous retrieval is a relatively automatic retrieval process that does not depend on executive control (Brewer, Knight, Marsh, & Unsworth, 2010; McDaniel et al., 2004), ongoing task performance should not be impaired during focal prospective memory tasks (except if participants are encouraged to monitor; Harrison & Einstein, 2010). In contrast, monitoring is an effortful process that depends on allocating executive control resources toward cue detection and away from performing the ongoing task (Smith, 2003). Therefore, ongoing task performance should suffer during nonfocal prospective memory tasks to the extent that participants sustain monitoring. In a series of experiments, Einstein et al. (2005) observed ongoing task cost during nonfocal, but not during focal, prospective memory tasks (see also, e.g., Marsh, Hicks, Cook, Hansen, & Pallos, 2003). Furthermore, monitoring (ongoing task cost) has been causally linked to support nonfocal, but not focal, prospective memory (Scullin, McDaniel, & Einstein, 2010; see also Meier et al., 2011). Importantly, though focal and nonfocal prospective memory tasks require different degrees of attentional resources (to detect the cue), focal/nonfocal effects do not simply reflect differences in the intrinsic difficulty of the particular cues assigned to focal and nonfocal conditions. For example, Scullin, McDaniel, Shelton, and Lee (2010) identified two cue types (word and initial-letter cues) that were detectable with similar ease in non-prospective memory (but dual-task) contexts (Experiments 1–2). When these same cues were subsequently embedded as prospective memory cues in an ongoing task that promoted focal processing of one cue type but not the other, the typical focal/nonfocal effects obtained (Experiments 3–4). Furthermore, Bugg, McDaniel, Scullin, and Braver (2011, Experiment 2) demonstrated that focal/nonfocal effects obtain when holding constant cue type (word cue) and varying the degree by which the ongoing task (Stroop) promoted processing of the word prospective memory cue (i.e., by varying proportion of word-color congruence to focus processing on word reading or color naming). Thus, the literature suggests that spontaneous retrieval processes can support focal prospective remembering but monitoring is important to successfully perform a nonfocal prospective memory task.
Hypertension and Prospective Memory
The association between hypertension and prospective memory has received little attention (Insel, Einstein, Morrow, & Hepworth, 2012). Investigating hypertension-related changes in prospective memory may have health implications, however, because prospective memory has both conceptually and empirically been linked to health behaviors including remembering medications (Woods et al., 2008) and treatment adherence (Liu & Park, 2004). Accordingly, it is possible that hypertension-related prospective memory impairments could contribute to poor disease self-management (e.g., decreased medication adherence, missed physician appointments, sporadic home blood pressure monitoring, etc.) and continued hypertensive-related health risks (for an analogue in the HIV literature, see Paul, Cohen, & Stern, 2002). To begin to examine the possible association between hypertension and prospective memory, we compared participants with a history of hypertension (hereafter, hypertension-history group) to those without a history of hypertension (hereafter, control group) on focal and nonfocal prospective memory tasks (using cues previously suggested to be similar in detection difficulty; Scullin, McDaniel, Shelton, et al., 2010). The hypertension and cognition literature in concert with the above theoretical analysis of prospective memory suggests four possible outcomes.
Hypertension and Cognition: Predictions for Prospective Memory
First, early research suggested that hypertension produces global cognitive impairments (Boller, Vrtunski, Mack, & Kim, 1977), and according to this view, the hypertension-history group will demonstrate deficits in both focal and nonfocal prospective memory (i.e., both spontaneous retrieval and monitoring processes will be impaired). Researchers have recently argued, however, that the effects of hypertension in (otherwise) healthy older adults who have never had a stroke and show no signs of dementia, might be more pronounced in (or possibly selective to) some cognitive domains. For example, one conceptualization is that hypertension primarily impairs basic memory processes (e.g., Swan, Carmelli, & Larue, 1998). Based on this memory impairment view, a second possible prospective memory outcome is that the hypertension-history group will perform worse than the control group on a focal prospective memory task that relies on spontaneous memory retrieval processes. If so, then the results would be similar to our previous observation that patients in the early stages of dementia (of the Alzheimer’s type) who demonstrated low medial temporal lobe volumes also showed focal, but not nonfocal, prospective memory impairments (Gordon et al., 2011; McDaniel, Shelton, Breneiser, Moynan, & Balota, 2011).
A third conceptualization is that hypertension particularly affects executive control (e.g., Novak & Hajjar, 2010). The executive control view predicts impairments on the nonfocal prospective memory task in the hypertension-history group because nonfocal performance is dependent on the engagement of executive control processes to monitor for the target cue (for theoretical elaboration on monitoring as an executive control process, see Shallice & Burgess, 1996; Smith, 2003). Nonfocal prospective memory impairments would emerge behaviorally as fewer prospective-memory hits or lower ongoing task cost (indicating insufficient monitoring). Finally, it remains possible that hypertension history will have little bearing on prospective memory in general (cf. Farmer et al., 1987).
To complement the behavioral analyses and refine the above predictions, we obtained structural magnetic-resonance imaging (MRI) scans that were available for a subset of the participants to determine whether behavioral effects were mirrored by group differences in regional grey or white matter volumes in the medial temporal lobe (MTL), anterior prefrontal cortex (aPFC), ventral/dorsal-lateral prefrontal cortex (VL/DLPFC), and lateral parietal cortex. Focal prospective memory is associated with the MTL (Gordon et al., 2011), and nonfocal prospective memory is most often connected to aPFC (e.g., Burgess et al., 2001). Nonfocal prospective memory has additionally sometimes been associated with parietal regions (Burgess et al., 2011). Following previous research (Gordon et al.), we selected the VL/DLPFC as a region that has been commonly associated with cognitive control (Corbetta & Shulman, 2002) but rarely associated with prospective memory (Burgess et al., 2011) with the intent of examining the specificity of neural effects. Thus, we expected that if hypertension-history was associated with lower focal or nonfocal prospective memory performance, then such effects would be paralleled by MTL or aPFC (possibly parietal) volume differences, respectively.
Experimental data arising from non-human animal studies (e.g., Moore, Killiany, Rosene, Prusty, Hollander, & Moss, 2002) as well as correlational data emerging from human neuroimaging studies (e.g., Raz et al., 2003) suggest that hypertension might affect the prefrontal cortex earlier or more severely than other regions. Following these studies, we predicted that in our sample of healthy (non-stroke), cognitively normal older adults, that hypertension status would be more likely to be associated with impaired monitoring (nonfocal prospective memory) than impaired spontaneous retrieval (focal prospective memory), and that such effects would be paralleled by aPFC structural differences.
Method
Participants
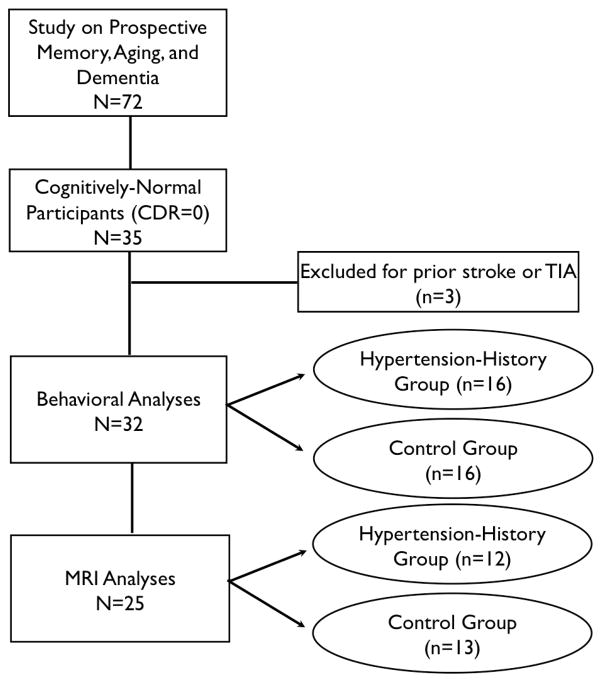
Thirty-five older adults (ages 64–83) were recruited from the Knight Alzheimer’s Disease Research Center (ADRC) at Washington University in St. Louis where these individuals undergo longitudinal assessments. For recruitment flow chart see Figure 1 and for participant demographic information see Table 1. Data were collected as part of a larger ongoing study on prospective memory, aging, and dementia (Shelton, Lee, Scullin, Rose, Rendell, & McDaniel, 2012). Inclusion criteria included normal or corrected vision and hearing and absence of dementia as measured by the rigorous Clinical Dementia Rating scale (i.e., all participants received a CDR score of 0.0; Morris, 1993). Exclusion criteria included reported previous history of stroke or transient ischemic attack because we were interested in the impact of hypertension prior to major cerebrovascular events (Tsivgoulis et al., 2009). These criteria resulted in the elimination of data from three participants, though including these participants does not alter the prospective memory results. In addition, none of the included participants reported recent history of tobacco use, heart attack, Parkinson’s disease, or Huntington’s disease.
Figure 1.
Flow chart of study recruitment and sample size for behavioral and MRI analyses. Abbreviations: CDR=Clinical Dementia Rating; MRI: magnetic resonance imaging; TIA: transient ischemic attack.
Table 1.
Demographic information across hypertension-history and control groups. Several variables are self-report scales. WAIS measures are age-adjusted scale scores. Where applicable, we present p values (t, chi square, or Fisher’s exact tests) and standard deviations are in parentheses.
| Control Group | Hypertension-History Group | p value | |
|---|---|---|---|
|
|
|||
| Age | 70.69 (5.12) | 75.44 (6.12) | .02 |
| Female (%) | 44% | 56% | .72 |
| Caucasian (%) | 87.5% | 68.75% | .39 |
| African American (%) | 6.25% | 31.25% | .17 |
| Native American (%) | 6.25% | 0.0% | ns |
| SES (Hollingshead) | 2.0 (.97) | 3.0 (1.03) | .008 |
| Years of Education | 14.94 (2.54) | 14.38 (2.78) | .56 |
| Clinical Dementia Rating | 0.0 | 0.0 | ns |
| MMSE | 28.88 (1.63) | 29.00 (1.41) | .82 |
| WAIS Information | 14.00 (2.13) | 13.44 (3.14) | .56 |
| WAIS Block Design | 13.81 (2.61) | 12.81 (2.64) | .29 |
| Body Mass Index | 27.34 (6.89) | 28.81 (6.43) | .52 |
| Diabetes (%) | 0.0% | 19% | .23 |
| Alcohol Abuse (%) | 0.0% | 13% | .48 |
| Previous Seizures (%) | 6% | 0.0% | ns |
| Kidney Disease (%) | 6% | 0.0% | ns |
Abbreviations: n.s. = not significant; SES = socioeconomic status; WAIS = Wechsler Adult Intelligence Scale (Wechsler, 1955); MMSE = mini mental state examination (Folstein, Folstein, & McHugh, 1975).
History of hypertension was determined during the annual ADRC assessment via clinical self-report. Given the experimental (factorial design) focus of the present study and the limited available sample size, no effort was made to quantify or evaluate the severity of hypertension (e.g., duration, blood pressure levels, treatment, etc.). Sixteen participants were classified with a history of hypertension (active/recent), whereas sixteen participants had no history of hypertension. Clinical self-report agrees highly with objective measures of hypertension (Alonso, Beunza, Delgado-Rodriguez, & Martinez-Gonzalez, 2005; Giles, Croft, Keenan, Lane, & Wheeler, 1995; Haapanen, Miilunpalo, Pasanen, Oja, & Vuori, 1997; Kehoe, Wu, Leske, & Chylack, 1994; Okura, Urban, Mahoney, Jacobsen, & Rodeheffer, 2004; Vargas, Burt, Gillum, & Pamuk, 1997). For example, in a sample of 942 patients, Kehoe et al. found that self-reported hypertension demonstrated 91% sensitivity and 88% specificity when compared with medical records, and such high levels of agreement have been confirmed in several other large-scale validation studies (Giles et al.; Okura et al.; Vargas et al.). It is noteworthy that self-report is not always a valid indicator of history of a particular disease (Kehoe et al.; Okura et al.); presumably, the validity of hypertension self-report, at least in developed countries, is due to clear diagnostic criteria for physicians (Chobanian et al., 2003) and the ease with which the diagnosis can be communicated to patients (Haapanen et al.). Though some studies have reported similar levels of agreement between self-report and objective measures of hypertension history regardless of age, gender, and years of education (Kehoe et al.; Okura et al.), other validation studies have limited their sample to well-educated adults (e.g., Alonso et al.), have found higher validity in females than males (Vargas et al.; Giles et al.), or observed that agreement between self-report and objective measures is greater with increasing age (Giles et al.; see also Goldman, Lin, Weinstein, & Lin, 2003). Given these possibilities we statistically controlled for age, education, and gender in evaluating differences between the hypertension-history and control groups in the current work.
Design
The experiment was a 2 × 3 mixed factorial design in which group (hypertension-history, control) was a between subjects factor and ongoing task block (control, focal prospective memory, nonfocal prospective memory) was a within subjects factor. The Institutional Review Board at Washington University in St. Louis approved the protocol and all participants provided written consent prior to participating.
Materials
Based on the materials from Einstein et al.’s (2005) study we constructed three semantic categorization lists and counterbalanced the assignment of lists across focal, nonfocal, and control blocks. Nontarget word length (range: 4–11 letters) and frequency (log-transformed Hyperspace–Analogue-to-Language range: 4.42–11.58) were similar across lists (according to the English Lexicon Project database; Balota et al., 2007).
Procedure
Participants performed three experimental semantic categorization blocks, and the order of these blocks was counterbalanced such that half of the participants performed the control block first whereas the other half performed the two prospective memory blocks first. The order of the two prospective memory blocks was further counterbalanced. For clarity purposes, we present the procedure in the context of the focal-nonfocal-control block order counterbalance.
Participants first received the semantic categorization task instructions. They were told that they would see two words on the screen (e.g.: baseball SPORT), and to press the keys labeled Y or N (1 and 2 on the number pad, respectively) depending on whether the lowercase word on the left indicated an exemplar of the uppercase category word on the right. Participants were given a 20-trial practice block that included response time and accuracy feedback. Following the semantic categorization practice, participants were given the prospective memory task instructions. They were instructed that there was a secondary interest in the ability to remember to perform an action in the future and to press the Q key if they ever saw the target item lawyer (or orange, in a counterbalanced condition). To ensure successful encoding, participants verbally explained the instructions to the experimenter before continuing. For some participants, we attempted to boost encoding by having them repeat the instructions three times and mentally imagine performing the prospective memory task. The repeated encoding condition is theorized to boost automatic processes, which would amplify focal, but presumably not nonfocal, prospective memory (Gollwitzer, 1999; McDaniel, Howard, & Butler, 2008). However, this manipulation produced no significant effects, and therefore, all data are presented collapsed across encoding conditions.
Following encoding, participants performed a 200-trial semantic categorization block that included six target trials (trials 30, 60, 90, 120, 150, and 180). We presented category pairs two, four, or six times to control for possible effects related to repeating the prospective memory cues.
Participants were then told that they no longer had to remember the word lawyer (or orange) and were given the nonfocal prospective memory task instructions to press the Q key if they ever saw a word beginning with the letter o (or l, in a counterbalanced condition). To prevent interference, or other unexpected target set effects, the initial-letter of the target items did not overlap between focal and nonfocal blocks (Set-1: lawyer, initial-letter o; Set-2: orange, initial-letter l). During the following 200-trial semantic categorization block, which was identical in structure to the focal block, we repeated three nonfocal target items twice (olive, opera, orange; or, linen, lion, lawyer).
Upon completing the nonfocal prospective memory block, participants were told that they would no longer have to remember to press the Q key. They then completed a 100-trial semantic categorization control block in which no prospective memory target items appeared. After completing the third semantic categorization block, participants were asked to recognize the focal target, nonfocal target, and prospective memory response key amongst lure items. Recognition performance was 98.9% correct and eliminating the individual with an error did not change the behavioral results.
Imaging Protocol
The ADRC database had MRI data available for 25 of this study’s participants (Figure 1). For each participant two T1-weighted sagittal MP-RAGE scans (TR=2400ms, TE=3.08ms, flip angle=8°, TI=1000ms, 1mm × 1mm × 1mm resolution) were acquired using a Siemens 3 Tesla Trio scanner. The multiple scans were aligned using a rigid body transform and averaged for each individual. There were on average 20.48 months (SD=11.73) between scan acquisition and behavioral testing, and there was not a significant difference in this interval between the hypertension-history group and the control group (t(23)=1.59, p = .13).
Image Analysis
Estimates of regional grey and white matter volume were obtained using the Freesurfer image analysis suite. This process utilizes an automated labeling procedure based on probabilistic information from a manually labeled training set (Desikan et al., 2006; Fischl et al., 2004), it corresponds very well with manually-generated label estimates (Fischl et al.), and it has high scan-rescan reliability (Morey et al., 2010). Quality control included reviews of cortical surface reconstructions and volumetric segmentations performed by trained technicians. Standard minimal manual edits were performed to correct failures in boundary detection in 5 of 25 scans. Regions-of-interest (ROIs) were obtained from the Desikan-Killiany atlas (see Desikan et al., 2006, for details on anatomical boundaries) and were selected to approximate brain regions implicated in the prospective memory literature (e.g., Burgess et al., 2011; Gordon et al., 2011; McDaniel & Einstein, 2011; Reynolds et al., 2009). We utilized an ROI approach rather than a whole brain approach to be more conservative analytically, given the limited sample size available. The a priori selected ROIs (Gordon et al., 2011) were aPFC, ventral/dorsal-lateral prefrontal cortex (VL/DLPFC; combined caudal middle frontal gyrus and inferior frontal gyrus), lateral parietal cortex (combined superior and inferior parietal cortex), and MTL (combined parahippocampal gyrus, entorhinal cortex, and hippocampus). Freesurfer does not generate hippocampal white matter estimates; therefore, the MTL white matter composite was generated from the parahippocampal and entorhinal regions.
Volumes were adjusted for estimated total intracranial volume (Buckner et al., 2004) using a covariance approach (Jack, Twomey, Zinsmeister, Sharbrough, Petersen, & Cascino, 1989) and summed across hemispheres as no a priori effects of hemisphere were expected.
Statistical Analyses
The probability of a type I error was set to .05, a level that was maintained throughout the study because prospective memory analyses were based on a priori predictions. We conducted t-tests, analyses of variance (ANOVA), and correlations to determine statistical significance. We estimated effect sizes using Cohen’s d or partial eta squared. Where we identified group differences in demographic variables (e.g., age, socioeconomic status) we conducted a follow-up test that statistically controlled for the variable (analysis of covariance[ANCOVA] and partial correlations).
Results
Prospective Memory Performance
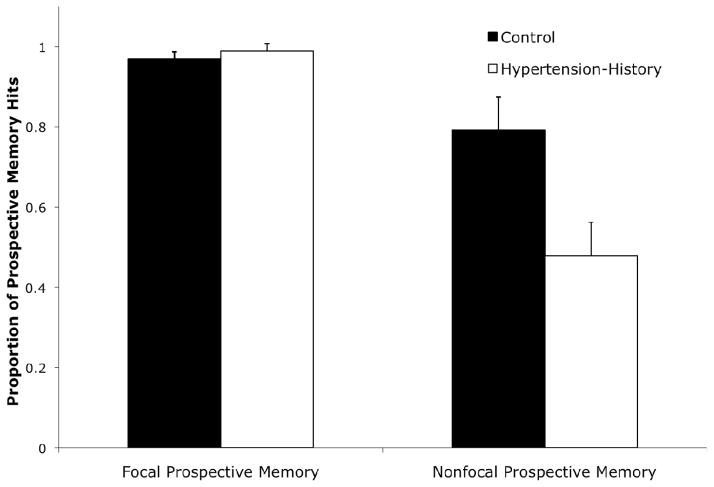
A prospective memory hit occurred when the Q key was pressed on the target trial or the following trial (e.g., Einstein et al., 2005). As illustrated in Figure 2, hypertension-history was associated with a deficit in nonfocal prospective memory, but not in focal prospective memory performance. A 2 × 2 mixed ANOVA that included the within-subjects variable of prospective memory cue (focal, nonfocal) and the between-subjects variable of group (hypertension-history, control) confirmed this observation with a significant cue by group interaction, F(1, 30) = 7.72, MSE = .057, ηp2 = .21. Focal prospective memory was nominally, but not significantly, higher in the hypertension-history group than in the control group (t < 1). By contrast, for the nonfocal prospective memory task, there was significantly worse performance in the hypertension-history group than the control group, t(30) = 2.66, d = .97. The above interaction remained significant after controlling for age, years of education, and gender, F(1, 27) = 6.61, MSE = .062, ηp2 = .20, as well as after excluding the individuals with a history of diabetes or seizures (Table 1), F(1, 26) = 8.40, MSE = .060, ηp2 = .24. Even though socioeconomic status (Hollingshead, 1975) significantly differed between the hypertension-history group and the control group (Table 1), socioeconomic status was not significantly correlated with nonfocal prospective memory performance (r(30) = −.294, p = .102). Moreover, an ANCOVA demonstrated that the prospective memory cue (focal, nonfocal) by group (hypertension-history, control) interaction was still significant after controlling for socioeconomic status and age, F(1, 28) = 4.54, MSE = .060, ηp2 = .14. Therefore, the prospective memory behavioral results cannot be explained by group differences in demographic variables. The omnibus ANOVA also revealed that in general prospective memory performance was better during focal blocks than nonfocal blocks, F(1, 30) = 33.01, MSE = .057, ηp2 = .52 (Einstein et al., 2005; Scullin, McDaniel, Shelton, et al., 2010; the main effect of group was also significant, F(1, 30) = 5.92, MSE = .057, ηp2 = .17).
Figure 2.
Focal and nonfocal prospective memory performance across control (n = 16) and hypertension-history (n = 16) groups. These results replicated when restricted to participants who also have MRI data. Error bars represent standard errors.
Semantic Categorization Ongoing Task Cost
We further investigated whether the above hypertension-history versus control group patterns reflected impaired monitoring during nonfocal blocks but reliance on spontaneous retrieval during focal blocks. Specifically, we examined ongoing task response time cost, which is the most typical measure for distinguishing cognitive processes in prospective memory (Einstein et al., 2005; Smith, 2003). Following Einstein et al. (2005), we examined mean trimmed semantic categorization response times on correct non-target trials that were no greater than two standard deviations above the individual’s mean. Trimming was done separately for each block (focal, nonfocal, control). Mean trimmed response times were contrasted between the control block and each prospective memory block within each group. Consistent with the idea that participants relied on spontaneous retrieval to support focal prospective remembering, there was not significant evidence for cost in the focal prospective memory block (t < 1, p = .67), and this pattern was similar for the hypertension-history group (MFocal = 1631 ms, MControl = 1655 ms; t < 1, d = .13) and the control group (MFocal = 1543 ms, MControl = 1474 ms; t(15) = 1.88, d = .97). As expected, we observed significant (p < .001) ongoing task cost during the nonfocal block in the control group (MNonfocal = 1650 ms), t(15) = 4.30, d = 2.22, indicating that these participants sustained monitoring for nonfocal cues. Interestingly, there was not significant (p = .42) ongoing task cost during the nonfocal block in the hypertension-history group (MNonfocal = 1737 ms; t < 1, d = .42). These results paralleled the nonfocal prospective-memory hits pattern and suggest monitoring deficits in the hypertension-history group.
Grey and White Matter Volume
The volumetric means are presented in Table 2. We first examined correlations between ROIs and relevant demographic variables (Table 1). As expected, there were significant correlations between age and several of the ROIs: DLPFC (white: r(23) = −.478, p = .016; grey: r(23) = −.548, p = .005), MTL (grey: r(23) = −.412, p =. 041), and parietal (white: r(23) = −.421, p =. 036; grey: r(23) = −.463, p = .020). No significant associations were observed with years of education or socioeconomic status (all ps > .05).
Table 2.
Raw volume means (standard deviations in parentheses) for regions of interest across hypertension-history and control groups.
| White aPFC | White MTL | White DLPFC | White Parietal | Grey aPFC | Grey MTL | Grey DLPFC | Grey Parietal | |
|---|---|---|---|---|---|---|---|---|
| Control Group | 642.30 (97.10) | 4822.10 (669.95) | 26806.46 (2949.53) | 45103.38 (4663.84) | 1750.42 (204.87) | 15817.26 (1089.46) | 29236.99 (3258.63) | 49074.32 (3698.04) |
| Hypertension-History Group | 544.73 (54.59) | 4635.47 (720.49) | 25960.40 (3087.29) | 44361.13 (3945.55) | 1549.76 (124.83) | 14720.26 (1180.56) | 27921.78 (2547.33) | 48380.58 (3617.64) |
Abbreviations: aPFC = anterior prefrontal cortex; MTL = medial temporal lobe; DLPFC: ventral/dorsal-lateral prefrontal cortex
With regard to the question of volumetric differences between the hypertension-history and control groups, as illustrated in Table 3, after controlling for age, gender, and years of education, the ROI analyses demonstrated significant group differences in white matter aPFC (see Raz et al., 2003, for similar structural findings). The other ROI analyses did not reach statistical significance (all ps > .05). Moreover, the association between hypertension-history and aPFC white matter was significant after controlling for socioeconomic status and age, rp(21)= −.503, p = .014.
Table 3.
Partial correlations (controlling for age, gender, and years of education) between regions-of-interest, hypertension-history status, and nonfocal prospective memory (PM) performance. Negative correlations with hypertension-history status indicate smaller volumes in the hypertension-history group.
| White aPFC | White MTL | White DLPFC | White Parietal | Grey aPFC | Grey MTL | Grey DLPFC | Grey Parietal | |
|---|---|---|---|---|---|---|---|---|
| Hypertension-history Status | −.49* | .04 | .09 | .29 | −.42† | −.32 | .04 | .28 |
| Nonfocal PM Performance | .44* | .23 | .17 | .29 | .12 | .25 | .05 | .10 |
Abbreviations: aPFC = anterior prefrontal cortex; MTL = medial temporal lobe; DLPFC: ventral/dorsal-lateral prefrontal cortex
indicates p < .05,
indicates p < .10.
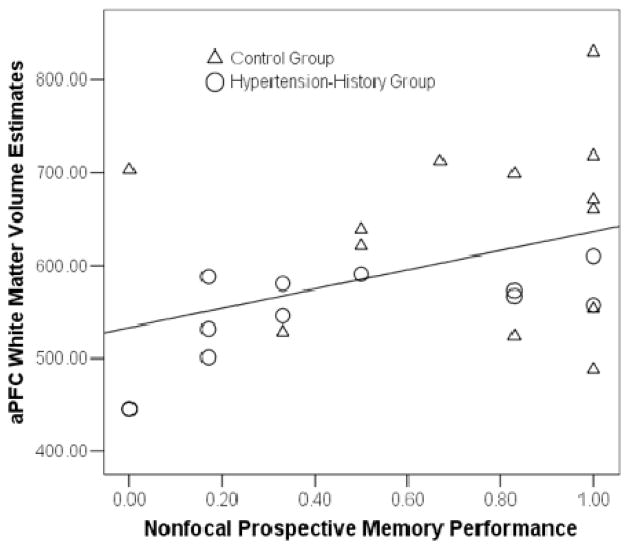
There was insufficient focal prospective memory variability to conduct correlational analyses. To further elucidate the relationship between structure, hypertension status, and nonfocal prospective memory performance, we conducted a series of partial correlations (using the entire sample) that controlled for age, gender, and education (see Table 3). Nonfocal prospective memory performance was significantly correlated only with aPFC white matter volume, and that relationship is illustrated in Figure 3. This association between nonfocal prospective memory and aPFC white matter was still significant when controlling for both age and socioeconomic status, rp(21) = .432, p = .039.
Figure 3.
Scatterplot of the association between white matter anterior prefrontal cortex (aPFC) volume and nonfocal prospective memory performance.
Discussion
The present research investigated whether hypertension-history was associated with prospective memory impairments, and specifically, if monitoring, spontaneous retrieval, or both processes are impaired. In our sample of cognitively normal (i.e., no dementia), non-stroke older adult participants, we observed hypertension-related impairments in proportion of prospective-memory hits and ongoing task cost during a nonfocal, but not during a focal, prospective memory task. These findings may have theoretical and translational implications.
Hypertension and Prospective Memory
First consider the nonfocal prospective memory task. The hypertension-history group (relative to the control group) showed fewer nonfocal prospective-memory hits, thereby suggesting deficits in monitoring. To confirm monitoring impairments, levels of nonfocal prospective-memory hits must be considered along with levels of ongoing task cost (e.g., Scullin, McDaniel, & Einstein, 2010), because cost indicates presence of sustained, effortful monitoring (Einstein et al., 2005; Scullin, McDaniel, Shelton, et al., 2010). Consistent with the conceptualization that hypertension is associated with executive control impairments (e.g., Novak & Hajjar, 2010), ongoing task cost was not observed in the hypertension-history group but was observed in the control group, as expected (McDaniel, Einstein, & Rendell, 2008).
In contrast to the impairments in monitoring, behavior in the focal prospective memory task was suggestive of relatively preserved spontaneous retrieval in the hypertension-history group. First, focal prospective memory performance did not significantly differ between the groups. This result may be contrasted with our previous finding in MTL-deficient patients with very mild dementia who showed pronounced focal prospective memory impairments in the absence of nonfocal prospective memory impairments (McDaniel et al., 2011). Note, however, that the critical pattern for signaling successful reliance on spontaneous retrieval is not simply prospective-memory hits in the focal condition; instead, one must consider whether focal prospective remembering occurred in the absence of ongoing task cost (Einstein et al., 2005; Scullin, McDaniel, & Einstein, 2010). In the hypertension-history group, ongoing task performance was, if anything, in the opposite direction of cost (i.e., response times were nominally faster during the focal block than during the control block). The possible finding of spontaneous retrieval in the hypertension-history group is consistent with the conceptualization that hypertension might be most strongly associated with executive control impairments (Novak & Hajjar, 2010; cf. global cognitive decline and memory impairments conceptualizations; Boller et al., 1977; Swan et al., 1998). However, a limitation to this conclusion was the different levels of focal and nonfocal prospective memory performance, with hit rate in the focal condition at ceiling-level. A reasonable alternative explanation for the present findings is that as cognitive task demands increase hypertension history will be more likely to be associated with cognitive impairments. Thus, it is possible that both focal and nonfocal prospective memory are affected in hypertension conditions and future work should compare hypertension-history and control groups on highly difficult focal prospective memory tasks (Scullin & McDaniel, 2010). Our prediction in that context is the same (i.e., no hypertension-history effect on focal prospective memory). But, we would predict a focal prospective memory deficit in hypertension samples that are associated with MTL deficits (e.g., severe, untreated hypertension; Gentile et al., 2009; patients with combined hypertension and mild cognitive impairment; Wysocki et al., 2012).
The brain—behavior analyses provided complementary evidence for the potential mechanisms underlying behavioral impairments in nonfocal, but not focal, prospective memory performance in the hypertension-history group. The multiprocess theory (McDaniel & Einstein, 2011) predicts that the aPFC supports monitoring (e.g., Burgess et al., 2001; Reynolds et al., 2009), whereas the MTL supports spontaneous retrieval (Gordon et al., 2011). Consistent with this distinction, in the present study we did not observe any group differences in spontaneous retrieval, or significant group differences in MTL volume. Importantly, we found that white matter aPFC volume was significantly smaller in the hypertension-history group. This finding converges with several studies demonstrating associations between hypertension and white matter decrements (e.g., de Leeuw et al., 2002; Raz et al., 2003; but see Jacobs et al., 2011). Because hypertension history was associated with structural differences in the aPFC but not the VL/DLPFC it is possible that hypertension-history-associated cognitive performance decrements may be observed more readily in some executive tests (e.g., nonfocal prospective memory) than in other cognitive control tests (Corbetta & Shulman, 2002).
The purpose of the current work was to investigate the possibility of an association between hypertension-history and worsened prospective memory performance. This study adds to the prospective memory field, which is only beginning to examine prospective memory under conditions of “abnormal” aging (e.g., Duchek, Balota, & Cortese, 2006; Katai, Maruyama, Hashimoto, & Ikeda, 2003; McDaniel et al., 2011). The current work may also add to the hypertension and cognition literature by employing a theoretically-guided, parametrically-manipulated prospective memory test to assess the particular cognitive processes that are impaired in individuals with a history of hypertension. The current work does not inform whether levels of blood pressure on the day of testing relate to prospective memory performance or whether there is a longitudinal benefit of antihypertension medications on prospective remembering. With regard to the medication issue, recent research suggests that angiotensin II receptor antagonists might both lower blood pressure and improve prospective remembering. Mechaeil, Gard, Jackson, and Rusted (2011) reported that an acute 50mg dose of losartan increased nonfocal prospective memory performance, though this effect was observed in normotensive younger adults. However, in a group of hypertensive older adults, Hajjar et al. (2012) found that 12-months of taking 32mg of candesartan controlled blood pressure and improved executive control performance.
Study Limitations
Some limitations of the current study include the size of the sample and a lack of detailed medical history for hypertension classification. Both of these limitations would have, however, worked against the observation of a significant association between prospective memory and hypertension, via insufficient statistical power and failure to identify participants in the control group who might have unrecognized hypertension (Roger et al., 2012). Despite the potential for these to be limiting factors, significant relationships were observed between hypertension-history status, nonfocal prospective memory, and aPFC volume; importantly, these results were consistent with a priori theoretical predictions derived from integrating the prospective memory (e.g., McDaniel & Einstein, 2007) and hypertension (e.g., Novak & Hajjar, 2010) literatures. Furthermore, self-report of hypertension shows high agreement with objective measures (Alonso et al., 2005; Giles et al., 1995; Haapanen et al., 1997; Kehoe et al., 1994; Okura et al., 2004; Vargas et al., 1997). The sample size for the MRI analyses may have led to an underestimation of the influence of hypertension on other brain regions, from the perspective that smaller effect sizes outside of the aPFC may not have been statistically detectable (but see Raz et al., 2003, for comparable findings). As we took a hypothesis-driven approach to the complementary brain analyses, we did not correct for multiple comparisons in these analyses. Given the expected medium-sized effects (e.g., Raz et al., 2003), it would not have been feasible to detect statistically significant effects if we had employed Bonferroni corrections with our available sample size. It is encouraging though that the next largest group difference to aPFC white matter was aPFC grey matter (p < .10). However, it will be important to replicate the current results in larger samples.
We used a cross-sectional, not a longitudinal, approach to associating hypertension history, prospective memory, and aPFC volume. Though there are developing animal and human literatures to suggest that hypertension causes neural and cognitive impairments it is also important to consider the other causal direction for the present results. For example, individuals who have poor prospective memory ability could be at an increased risk for adhering to health-related behaviors (e.g., adhering to healthy diet and exercising). Decreased adherence to health-related behaviors would be expected to lead to increased blood pressure, which would result in a hypertension diagnosis.
Conclusions and Implications
The present study suggested that individuals who have previously been given a hypertension diagnosis show poorer prospective memory functioning. Laboratory measures of prospective memory appear to relate to medication management and performance of daily activities in patients (Pirogovsky, Woods, Filoteo, & Gilbert, 2012; Woods et al., 2008); failing to remember the prospective memory intention of taking antihypertensive medications, attending physician appointments, or monitoring blood pressure at home could potentially perpetuate the effects of hypertension (Gard, 2010; see Paul et al., 2002, for an analogue in the HIV literature). As a step toward improving prospective remembering, during hypertension diagnosis, healthcare providers might educate patients on the importance of encoding the context in which a prospective memory intention needs to be executed, leaving salient reminder cues, and routinizing their prospective memory intention of taking medication (McDaniel & Einstein, 2007; Insel et al., 2012). The idea here, underscored by the present findings, would be to engineer health self-management situations to support prospective remembering with spared spontaneous retrieval processes rather than with hypertension-related impaired monitoring processes. Communicating the neurocognitive outcomes of hypertension along with the cognitive strategies that improve prospective memory functioning, may be particularly worthwhile to attempt in some minority groups (e.g., Kountz, 2004; Perez, 2011) as well as in developing countries (Parker, Nagar, Thomas, Badri, & Ntusi, 2011; Prince et al., 2012), in which poor self-management and minimal awareness of hypertension have been consistently observed. Such efforts might help diminish potentially negatively cascading effects of “the silent killer” on prospective memory functioning.
Acknowledgments
National Institute on Aging Grants P50AG05681 and P01AG03991 helped support this research. Furthermore, M.K.S. and B.A.G. were partially supported by National Institute on Aging Grant T32AG00030 (as well as F32AG041543 and an Emory University Cottrell Fellowship to M.K.S). We thank the Clinical Core of the Knight Alzheimer’s Disease Research Center for participant assessments and the Imaging Core for structural MRI data. We are also highly appreciative of the specific assistance that Dr. John Morris, Betsy Grant, Mary Coates, and Becky Fierberg provided at the Knight Alzheimer’s Disease Research Center. We further thank Kwan Woo Paik for his assistance with analyses.
Footnotes
Portions of this project were presented at the 2012 Cognitive Aging Conference in Atlanta, GA.
References
- Alonso A, Beunza JJ, Delgado-Rodriguez M, Martinez-Gonzalez MA. Validation of self reported diagnosis of hypertension in a cohort of university graduates in Spain. BMC Public Health. 2005;5 doi: 10.1186/1471-2458-5-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balota DA, Yap MJ, Cortese MJ, Hutchison KA, Kessler B, Loftis B, Treiman R. The English Lexicon Project. Behavior Research Methods. 2007;39:445–459. doi: 10.3758/BF03193014. [DOI] [PubMed] [Google Scholar]
- Boller F, Vrtunski B, Mack JL, Kim Y. Neuropsychological correlates of hypertension. Archives of Neurology. 1977;34:701–705. doi: 10.1001/archneur.1977.00500230071012. [DOI] [PubMed] [Google Scholar]
- Brewer GA, Knight JB, Marsh RL, Unsworth N. Individual differences in event-based prospective memory: Evidence for multiple processes supporting cue detection. Memory & Cognition. 2010;38:304–311. doi: 10.3758/MC.38.3.304. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Bugg JM, McDaniel MA, Scullin MK, Braver TS. Revealing list- level control in the stroop task by uncovering its benefits and a cost. Journal of Experimental Psychology: Human Perception and Performance. 2011;37:1595–1606. doi: 10.1037/a0024670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Gonen-Yaacovi G, Volle E. Functional neuroimaging studies of prospective memory: What have we learnt so far? Neuropsychologia. 2011;49:2246–2257. doi: 10.1016/j.neuropsychologia.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Quayle A, Frith CD. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39:545–555. doi: 10.1016/s0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. Journal of the American Medical Association. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Cooper T. Hypertension; The silent killer. The Journal of Practical Nursing. 1973;23:23–25. [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- de Leeuw FE, de Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, Breteler MM. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125:765–772. doi: 10.1093/brain/awf077. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Duchek JM, Balota DA, Cortese M. Prospective memory and apolipoprotein E in healthy aging and early Alzheimer’s disease. Neuropsychology. 2006;20:633–644. doi: 10.1037/0894-4105.20.6.633. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA. Normal aging and prospective memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1990;16:717–726. doi: 10.1037//0278-7393.16.4.717. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Thomas R, Mayfield S, Shank H, Morrisette N, Breneiser J. Multiple processes in prospective memory retrieval: Factors determining monitoring versus spontaneous retrieval. Journal of Experimental Psychology: General. 2005;134:327–342. doi: 10.1037/0096-3445.134.3.327. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA. Prospective memory and what costs do not reveal about retrieval processes: A commentary on Smith, Hunt, McVay, and McConnell (2007) Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36:1082–1088. doi: 10.1037/a0019184. [DOI] [PubMed] [Google Scholar]
- Elias MF, Goodell AL, Dore GA. Hypertension and cognitive functioning A perspective in historical context. Hypertension. 2012;60:260–268. doi: 10.1161/HYPERTENSIONAHA.111.186429. [DOI] [PubMed] [Google Scholar]
- Farmer ME, White LR, Abbott RD, Kittner SJ, Kaplan E, Wolz MM, Wolf PA. Blood pressure and cognitive performance: The Framingham study. American Journal of Epidemiology. 1987;126:1103–1114. doi: 10.1093/oxfordjournals.aje.a114749. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Dale AM. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein S, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatry Reseach. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gard PR. Non-adherence to antihypertensive medication and impaired cognition: Which comes first? International Journal of Pharmacy Practice. 2010;18:252–259. doi: 10.1111/j.2042-7174.2010.00045.x. [DOI] [PubMed] [Google Scholar]
- Gentile MT, Poulet R, Di Pardo A, Cifelli G, Maffei A, Vecchione C, Lembo G. Beta-amyloid deposition in brain is enhanced in mouse models of arterial hypertension. Neurobiology of Aging. 2009;30:222–228. doi: 10.1016/j.neurobiolaging.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Giles WH, Croft JB, Keenan NL, Lane MJ, Wheeler FC. The validity of self-reported hypertension and correlates of hypertension awareness among blacks and whites within the stroke belt. American Journal of Preventative Medicine. 1995;11:163–69. [PubMed] [Google Scholar]
- Goldman N, Lin I-F, Weinstein M, Lin YH. Evaluating the quality of self-reports of hypertension and diabetes. Journal of Clinical Epidemiology. 2003;56:148–154. doi: 10.1016/s0895-4356(02)00580-2. [DOI] [PubMed] [Google Scholar]
- Goldstein IB, Bartzokis G, Hance DB, Shapiro D. Relationship between blood pressure and subcortical lesions in healthy elderly people. Stroke. 1998;29:765–772. doi: 10.1161/01.str.29.4.765. [DOI] [PubMed] [Google Scholar]
- Gollwitzer PM. Implementation intentions: Strong effects of simple plans. American Psychologist. 1999;54:493–503. [Google Scholar]
- Gordon BA, Shelton JT, Bugg JM, McDaniel MA, Head D. Structural correlates of prospective memory. Neuropsychologia. 2011;49:3795–3800. doi: 10.1016/j.neuropsychologia.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapanen N, Miilunpalo S, Pasanen M, Oja P, Vuori I. Agreement between questionnaire data and medical records of chronic diseases in middle-aged and elderly Finnish men and women. American Journal of Epidemiology. 1997;145:762–769. doi: 10.1093/aje/145.8.762. [DOI] [PubMed] [Google Scholar]
- Hajjar I, Hart M, Chen YL, Mack W, Milberg W, Chui H, Lipsitz L. Effect of antihypertensive therapy on cognitive function in early executive cognitive impairment: A double-blind randomized clinical trial. Archives of Internal Medicine. 2012;172:442–444. doi: 10.1001/archinternmed.2011.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison TL, Einstein GO. Prospective memory: Are preparatory attentional processes necessary for a single focal cue? Memory & Cognition. 2010;38:860–867. doi: 10.3758/MC.38.7.860. [DOI] [PubMed] [Google Scholar]
- Hollingshead ADB. Four factor index of social status. Yale University; New Haven, CT: 1975. [Google Scholar]
- Igase M, Kohara K, Miki T. The association between hypertension and dementia in the elderly. International Journal of Hypertension. 2012 doi: 10.1155/2012/320648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel KC, Einstein GO, Morrow DG, Hepworth JT. A multifaceted prospective memory intervention to improve medication adherence: Design of a randomized control trial. Contemporary Clinical Trials. 2012 doi: 10.1016/j.cct.2012.09.005. http://dx.doi.org/10.1016/j.cct.2012.09.005. [DOI] [PMC free article] [PubMed]
- Jack CR, Jr, Twomey CK, Zinsmeister AR, Sharbrough FW, Petersen RC, Cascino GD. Anterior temporal lobes and hippocampal formations: Normative volumetric measurements from MR images in young adults. Radiology. 1989;172:549–554. doi: 10.1148/radiology.172.2.2748838. [DOI] [PubMed] [Google Scholar]
- Jacobs HIL, Leritz EC, Williams VJ, Van Boxtel MPJ, van der Elst W, Jolles J, Salat DH. Association between white matter microstructure, executive functions, and processing speed in older adults: The impact of vascular health. Human Brain Mapping. 2011 doi: 10.1002/hbm.21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katai S, Maruyama T, Hashimoto T, Ikeda S. Event based and time based prospective memory in Parkinson’s disease. Journal of Neurology, Neurosurgery and Psychiatry. 2003;74:704–709. doi: 10.1136/jnnp.74.6.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- Kehoe R, Wu SY, Leske MC, Chylack LT. Comparing self-reported and physician-reported medical history. American Journal of Epidemiology. 1994;139:813–818. doi: 10.1093/oxfordjournals.aje.a117078. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Research. 2009;10:41–56. doi: 10.1016/j.brainres.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kountz DS. Hypertension in ethnic populations: Tailoring treatments. Clinical Cornerstone. 2004;6:39–46. doi: 10.1016/s1098-3597(04)80063-2. [DOI] [PubMed] [Google Scholar]
- Liu LL, Park DC. Aging and medical adherence: the use of automatic processes to achieve effortful things. Psychology and Aging. 2004;19:318–325. doi: 10.1037/0882-7974.19.2.318. [DOI] [PubMed] [Google Scholar]
- Marsh RL, Hicks JL, Cook GI, Hansen JS, Pallos AL. Interference to ongoing activities covaries with the characteristics of an event-based intention. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:861–870. doi: 10.1037/0278-7393.29.5.861. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO. Strategic and automatic processes in prospective memory retrieval: A multiprocess framework. Applied Cognitive Psychology. 2000;14:S127–S144. [Google Scholar]
- McDaniel MA, Einstein GO. Prospective memory: An overview and synthesis of an emerging field. Thousand Oaks, CA: Sage; 2007. [Google Scholar]
- McDaniel MA, Einstein GO. The neuropsychology of prospective memory in normal aging: A componential approach. Neuropsychologia. 2011;49:2147–2155. doi: 10.1016/j.neuropsychologia.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO, Rendell PG. The puzzle of inconsistent age- related declines in prospective memory: A multiprocess explanation. In: Kliegel M, McDaniel MA, Einstein GO, editors. Prospective memory: Cognitive, neuroscience, developmental, and applied perspectives. Mahwah, NJ: Erlbaum; 2008. pp. 141–160. [Google Scholar]
- McDaniel MA, Guynn MJ, Einstein GO, Breneiser J. Cue-focused and reflexive-associative processes in prospective memory retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2004;30:605–614. doi: 10.1037/0278-7393.30.3.605. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Howard DC, Butler KM. Implementation intentions facilitate prospective memory under high attention demands. Memory & Cognition. 2008;36:716–724. doi: 10.3758/mc.36.4.716. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Shelton JT, Breneiser JE, Moynan S, Balota DA. Focal and nonfocal prospective memory performance in very mild dementia: A signature decline. Neuropsychology. 2011;25:387–396. doi: 10.1037/a0021682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechaeil R, Gard P, Jackson A, Rusted J. Cognitive enhancement following acute losartan in normotensive young adults. Psychopharmacology. 2011;217:51–60. doi: 10.1007/s00213-011-2257-9. [DOI] [PubMed] [Google Scholar]
- Meier B, von Wartburg P, Matter S, Reber R, Rothen N. Performance predictions improve prospective memory and influence retrieval experience. Canadian Journal of Experimental Psychology. 2011;65:12–18. doi: 10.1037/a0022784. [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Rosene DL, Prusty S, Hollander W, Moss MB. Impairment of executive function induced by hypertension in the rhesus monkey (Macaca mulatta) Behavioral Neuroscience. 2002;116:387–396. doi: 10.1037//0735-7044.116.3.387. [DOI] [PubMed] [Google Scholar]
- Morey RA, Selgrade ES, Wagner HR, II, Huettel SA, Wang L, McCarthy G. Scan-rescan reliability of subcortical brain volumes derived from automated segmentation. Human Brain Mapping. 2010;31:1751–1762. doi: 10.1002/hbm.20973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The clinical dementia rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Niiranen TJ, Johansson JK, Reunanen A, Jula AM. Optimal schedule for home blood pressure measurement based on prognostic data: The Finn-Home study. Hypertension. 2011;57:1081–1086. doi: 10.1161/HYPERTENSIONAHA.110.162123. [DOI] [PubMed] [Google Scholar]
- Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nature Reviews Cardiology. 2010;7:686–698. doi: 10.1038/nrcardio.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. Journal of Clinical Epidemiology. 2004;57:1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Paul RH, Cohen RA, Stern RA. Neurocognitive manifestations of Human Immunodeficiency Virus. CNS Spectrums. 2002;7:860–866. doi: 10.1017/s1092852900022471. [DOI] [PubMed] [Google Scholar]
- Parker A, Nagar B, Thomas G, Badri M, Ntusi NB. Health practitioners’ state of knowledge and challenges to effective management of hypertension at primary level. Cardiovascular Journal of Africa. 2011;22:186–190. doi: 10.5830/CVJA-2010-066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez A. Self-management of hypertension in Hispanic adults. Clinical Nursing Research. 2012;20:347–365. doi: 10.1177/1054773811411582. [DOI] [PubMed] [Google Scholar]
- Pirogovsky E, Woods SP, Filoteo JV, Gilbert PE. Prospective memory deficits are associated with poorer everyday functioning in Parkinson’s disease. Journal of the International Neuropsychological Society. 2012;18:1–10. doi: 10.1017/S1355617712000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince MJ, Ebrahim S, Acosta D, Ferri CP, Guerra M, Huang Y, Liu Z. Hypertension prevalence, awareness, treatment and control among older people in Latin America, India, and China: A 10/66 cross-sectional population-based survey. Journal of Hypertension. 2012;30:177–187. doi: 10.1097/HJH.0b013e32834d9eda. [DOI] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurology. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodgrigue KM, Acker JD. Hypertension and the brain: Vulnerability of the prefrontal regions and executive functions. Behavioral Neuroscience. 2003;117:1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Reynolds JR, West R, Braver T. Distinct neural circuits support transient and sustained processes in prospective memory and working memory. Cerebral Cortex. 2009;19:1208–1221. doi: 10.1093/cercor/bhn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Turner MB. Executive summary: Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- Scullin MK, McDaniel MA. Remembering to execute a goal sleep on it! Psychological Science. 2010;21:1028–1035. doi: 10.1177/0956797610373373. [DOI] [PubMed] [Google Scholar]
- Scullin MK, McDaniel MA, Einstein GO. Control of cost in prospective memory: Evidence for spontaneous retrieval processes. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36:190–203. doi: 10.1037/a0017732. [DOI] [PubMed] [Google Scholar]
- Scullin MK, McDaniel MA, Shelton JT, Lee JH. Focal/nonfocal cue effects in prospective memory: Monitoring difficulty or different retrieval processes? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36:736–749. doi: 10.1037/a0018971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Burgess P. The domain of supervisory processes and temporal organization of behaviour. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 1996;351:1405–1412. doi: 10.1098/rstb.1996.0124. [DOI] [PubMed] [Google Scholar]
- Shelton JT, Lee JH, Scullin M, Rose N, Rendell P, McDaniel M. Implementation intentions boost prospective memory in very mildly demented older adults. Paper presented at the Cognitive Aging Conference; Atlanta, GA. 2012. Mar, [Google Scholar]
- Smith RE. The cost of remembering to remember in event-based prospective memory: Investigating the capacity demands of delayed intention performance. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:347– 361. doi: 10.1037/0278-7393.29.3.347. [DOI] [PubMed] [Google Scholar]
- Swan GE, Carmelli D, Larue A. Systolic blood pressure tracking over 25 to 30 years and cognitive performance in older adults. Stroke. 1998;29:2334–2340. doi: 10.1161/01.str.29.11.2334. [DOI] [PubMed] [Google Scholar]
- Tsivgoulis G, Alexandrov AV, Wadley VG, Unverzagt FW, Go RCP, Moy CS, Kissela B, Howard G. Association of higher diastolic blood pressure levels with cognitive impairment. Neurology. 2009;73:589–595. doi: 10.1212/WNL.0b013e3181b38969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unverzagt FW, McClure LA, Wadley VG, Jenny NS, Go RC, Cushman M, Howard G. Vascular risk factors and cognitive impairment in a stroke-free cohort. Neurology. 2011;77:1729–1736. doi: 10.1212/WNL.0b013e318236ef23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoeven NV, van den Born BJ, Cammenga M, van Montfrans GA. Poor adherence to home blood pressure measurement schedule. Journal of Hypertension. 2009;27:275–279. doi: 10.1097/hjh.0b013e328319917e. [DOI] [PubMed] [Google Scholar]
- Vargas CM, Burt VL, Gillum RF, Pamuk ER. Validity of self-reported hypertension in the national health and nutrition examination survey III, 1988–1991. Preventative Medicine. 1997;26:678–685. doi: 10.1006/pmed.1997.0190. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual: Wechsler Adult Intelligence Scale. New York: Psychological Corporation; 1955. [Google Scholar]
- Woods SP, Moran LM, Carey CL, Dawson MS, Iudicello JE, Gibson S, Atkinson JH. Prospective memory in HIV infection: Is “remembering to remember” a unique predictor of self-reported medication management? Archives of Clinical Neuropsychology. 2008;23:257–270. doi: 10.1016/j.acn.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki M, Luo X, Schmeidler J, Dahlman K, Lesser GT, Grossman H, Beeri MS. Hypertension is associated with cognitive decline in elderly people at high risk for dementia. American Journal of Geriatric Psychiatry. 2012;20:179–187. doi: 10.1097/JGP.0b013e31820ee833. [DOI] [PMC free article] [PubMed] [Google Scholar]