Abstract
Macrophages lacking connexin43 (Cx43), a gap junction protein, have been reported to exhibit dramatic deficiencies in phagocytosis. In this study, we revisit these findings using well-characterized macrophage populations. Cx43 knock out (Cx43−/−) mice die soon after birth, making the harvest of macrophages from adult Cx43−/− mice problematic. To overcome this obstacle we employed several strategies: Mice heterozygous for the deletion of Cx43 were crossed to produce Cx43+/+ (WT) and Cx43−/− fetuses. Cells isolated from 12–14 day fetal livers were used to reconstitute irradiated recipient animals. Following reconstitution, thioglycollate-elicited macrophages were collected by peritoneal lavage and bone marrow was harvested. Bone marrow cells and, alternatively, fetal liver cells were cultured in media containing M-CSF for 7–10 days, resulting in populations of cells that were greater than 95% macrophages based on flow cytometry. Phagocytic uptake was detected using flow cytometric and microscopic techniques. Quantification of phagocytic uptake of IgG-opsonized sheep erythrocytes, zymosan particles and Listeria monocytogenes failed to show any significant difference between WT and Cx43−/− macrophages. Further, the use of particles labeled with pH sensitive dyes showed equivalent acidification of phagosomes in both WT and Cx43−/− macrophages. Our findings suggest that modulation of Cx43 levels in cultured macrophages does not have a significant impact on phagocytosis.
Introduction
Connexin43 (Cx43) is a multimeric protein conduit that functions to connect the cytoplasms of two cells. Cx43 is expressed in multiple cells and organs of the immune system and has been suggested to contribute to immune function (1–3). For almost a decade, our laboratory has been engaged in the study of Cx43, specifically its contribution to macrophage function. The most extensive of these studies involve the use of radiation chimeras to produce mice lacking Cx43 in cells of hematopoietic origin (4). Equivalent reconstitution from Cx43+/+, +/− and −/− fetal liver cells is commonly observed in these animals and populations of inflammatory macrophages are proportionately high for cells of donor origin (>98%). There are no obvious immune defects in these animals (4). Furthermore, macrophages derived from Cx43+/+, +/− and −/− fetal liver cells have been analyzed for bacterial killing, revealing no obvious defect in bactericidal activity (unpublished result). Throughout these studies, we have found no evidence that Cx43 is required for phagocytosis, or that macrophages generated from mice lacking Cx43 display any impairment of phagocytic uptake, a prerequisite for bactericidal activity, as compared to macrophages derived from wild type animals.
The lack of a role for Cx43 in macrophage phagocytosis is in sharp contrast with a study by Anand et al. published in 2008 (5). In that study a population of adherent cells was isolated from fetal livers of Cx43+/+, +/− and −/− mice. These cells were assessed for phagocytic capacity and the cells displayed differential phagocytosis, leading the authors to propose a “direct role” for Cx43. However, the cells analyzed were only characterized according to their expression of CD45, not macrophage-specific markers; making the proportion of macrophages in the population difficult to determine.
In the present study, we examined phagocytic uptake of three distinct phagocytic target particles (sheep erythrocytes, zymosan, and Listeria monocytogenes (Listeria)), comparing wild type (WT, Cx43+/+) macrophages and Cx43 deleted (Cx43−/−) macrophages. We present data here showing that a well characterized, macrophage population lacking Cx43 is indeed capable of phagocytosis and that no significant differences between WT and Cx43−/− macrophages were apparent in any phagocytic parameter measured.
Materials and Methods
Animals
Mice heterozygously-deficient in connexin43 (Cx43+/−, B6;129-Gja1tm1Kdr/J) were obtained from The Jackson Laboratory. Cx43+/− mice expressing the CD45.1 isoform were generated by first crossing Cx43+/− animals with BALB/c mice (BALB/cAnTac, Taconic) for 11 generations. The resulting BALB/cCx43 strain was then subsequently crossed with BALB/c mice expressing the CD45.1 isoform of CD45 (CBy.SJL(B6)-Ptprca/J, Jackson). All animals were maintained at the Department of Laboratory Animal Resources at SUNY Upstate Medical University. For each animal, Cx43 status was confirmed by PCR-based genotyping. All experiments and procedures in this study were approved by the Institutional Animal Care and Use Committee at SUNY Upstate.
Cell culture
WT or Cx43−/− fetuses were generated by crossing two Connexin43 heterozygous (Cx43+/−) mice. At 12 to 14 days of gestation, pregnant females were euthanized and the fetuses were explanted. Fetal livers were then isolated from each fetus for derivation to macrophages, while the posterior portion of each fetus was removed for genotyping.
Livers were placed in DMEM (HyClone) supplemented with 25 mM HEPES buffer and dissociated by gentle pipetting. Fetal liver cell suspensions were cultured in complete medium (DMEM supplemented with 10% FBS and 5% penicillin/streptomycin) for 24 h to allow for selective adherence of contaminating cells (such as fibroblasts and fetal liver resident macrophages).
Following genotyping, non-adherent fetal liver cells were collected, pooled according to genotype, and plated at a concentration of 1×106 nucleated cells per plate in non-tissue culture treated 100 mm Petri dishes (BD Falcon). Cells were grown in complete medium supplemented with supernatant from L929 cells, a source of M-CSF, for 7 to 10 days (6). Mature macrophages, which were adherent to the dishes, were harvested after incubation with cold PBS + 2mM EDTA.
Generation of radiation chimeric mice
Radiation chimeric mice were generated by as previously described. (4) Cx43+/− mice on a CD45.1 background were mated to produce CD45.1+ Cx43+/+ and Cx43−/− fetuses from which fetal liver cell suspensions were prepared as described above. 1×106 fetal liver cells were transferred to an irradiated (950 cGy) CD45.2+ BALB/c host by retro-orbital injection. After allowing 10 weeks for reconstitution, peripheral blood was analyzed for the donor marker (CD45.1). Greater than 97% of peripheral blood leukocytes were donor derived (Cx43+/+ 99.1±1.0 vs. Cx43−/− 97.5±.5).
Preparation of phagocytic targets
Sheep erythrocytes (Innovative Labs) were washed in PBS and labeled with CFSE (Cell Trace, Invitrogen) according to manufacturer’s specifications. Immediately before use in a phagocytosis assay, labeled erythrocytes were incubated for 30 min with fractionated rabbit anti-sheep red blood cell antisera (Sigma) diluted 1:10 followed by washing with PBS before addition to macrophages.
Zymosan A from Saccharomyces cerevisiae (Sigma) was fluorescently labeled using the DyLight 649 labeling kit (Thermo Scientific) or using pHrodo red succinimidyl ester kit (Invitrogen) according to manufacturer’s instructions. Phagocytic target concentration and fluorescence was determined using a hemocytometer and fluorescence microscope.
Listeria monocytogenes
Listeria expressing a non-secreted form of GFP and a secreted recombinant protein containing the amino acid sequence SIINFEKL (Lm-PASFLAG) was generated previously (7).
Phagocytosis Assays
Fetal liver-derived and bone marrow-derived macrophages were plated in non-tissue culture treated 24-well plates (CytoOne) at a density of 5×105 cells per well in complete medium. Cells were allowed to adhere for at least 12 h and DMEM was replenished 30 min before assessment of phagocytosis.
Phagocytosis of sheep erythrocytes (sRBCs)
IgG-opsonized sheep erythrocytes (sRBCs) were added to wells at a target to macrophage ratio of 100:1 (to ensure an abundance of available targets) or 10:1. During incubation, cultures were maintained at 37°C and 5% CO2. At 20, 40, and 60 min time points, external sRBCs were lysed by a 1 min incubation with distilled water, cultures were washed with PBS to remove remaining sRBC fragments, and macrophages in PBS + 2mM EDTA were placed on ice for several minutes to allow release from the surface of the dish. Fetal liver-derived macrophage suspensions were filtered using 70 µm nylon mesh, transferred to round-bottomed tubes (BD Falcon), and kept on ice until flow cytometric analysis.. The bone marrow-derived macrophages from radiation chimeric animals were not filtered prior to analysis.
Phagocytosis of sRBCs by fluorescence microscopy
Fetal liver-derived macrophages or thioglycollate-elicited macrophages, from radiation chimeric animals, were allowed to adhere to 18 mm diameter glass cover slips (Fisherbrand) placed in the wells of a 12 well plate at a density of 5×104 cells per well. The following day, IgG-opsonized fluorescently labeled sRBCs were added to each well. After 20 min of incubation, wells were washed three times with PBS and placed on ice.
For macrophages derived from fetal livers, fixation was achieved using 1% paraformaldehyde. Non-specific secondary antibody binding to macrophage Fcγ receptors was blocked by incubation with 2.4G2 hybridoma supernatant (8). Incompletely internalized sRBCs were labeled using Alexa Fluor 594-conjugated goat anti-rabbit IgG (Invitrogen). After several washes, cells were permeabilized using 0.5% Triton X-100 in PBS and mounted to slides using Prolong Gold with DAPI (Invitrogen). Images were taken at 200× magnification using a Nikon Eclipse E800 fluorescence microscope equipped with a Spot RT Slider camera. Images were randomized and analyzed in a blinded fashion for the number of internalized sRBCs per macrophage.
For thioglycollate macrophages, non-internalized sRBCs were lysed using distilled water, followed by several washes in PBS. Cells were fixed in 1% paraformaldehyde and placed in a randomized configuration in the wells of a new 12 well plate. An inverted fluorescence microscope (Olympus IX51) was used to count the number of sRBCs per cell.
To compare the phagocytic capacity of WT and Cx43−/− macrophages, histograms were prepared showing the number of particles per cell on the horizontal axis. To normalize for unequal numbers of cells from each group, a relative cell number is reported on the vertical axis. Relative cell number = (number of macrophages in each bin/total number of cells counted)×100. At least 100 cells were counted for each experiment. For both sets of data, averages were analyzed using Student’s t-test. No significant differences (p<.05) were observed in any parameter.
Phagocytosis of labeled Zymosan
Either DyLight 649 (DL649)-labeled or pHrodo-labeled zymosan particles were added to cultured macrophages at a target to macrophage ratio of 100:1. For each Cx43 genotype studied, two identical 24-well plates were used: one plate that was incubated at 37°C and 5% CO2 to measure phagocytic uptake and a second plate incubated on ice at 4°C for the same period. The 4°C plate was used to establish a baseline measurement of particle adherence to macrophages for comparison to actual phagocytic uptake. After 60 min, the wells of both the 37°C and 4°C plates were washed three times to remove unbound/unengulfed zymosan particles. Macrophages were incubated on ice with PBS + 2mM EDTA, lifted by pipetting, filtered, and kept on ice until flow cytometric analysis.
Phagocytosis of Listeria monocytogenes and antigen presentation assay
Listeria uptake and antigen presentation assays were performed as previously described (9). Briefly, overnight Listeria cultures were used to inoculate BHI broth and grown to log phase. WT or Cx43−/− fetal liver-derived macrophages were suspended at a concentration of 2×106 cells/ml in IMDM supplemented with 10% FBS but without the addition of antibiotic. Macrophages were infected with mid-log phase Listeria at an MOI= 20 for 1 h at 37°C, followed by washing and resuspension in IMDM containing 5 µg/ml gentamicin (Cellgro) to kill any remaining extracellular bacteria. Infected cells were incubated at 37 °C and aliquots were harvested for analysis at 30 min intervals.
To identify Listeria-derived SIINFEKL presented by macrophage MHC class I molecules (surface Kb-SIINFEKL), macrophages were incubated in 2.4G2 supernatant, followed by staining with Alexa Flour 647 (AF647, Invitrogen)-conjugated 25-D1.16 mAb (10). Flow cytometry was used to identify infected cells using GFP fluorescence. Additionally, the mean fluorescence intensity (MFI) of AF647 was used to quantify surface Kb-SIINFEKL expression in GFP positive cells.
Flow cytometry
Flow cytometry was performed using an LSRII or LSRFortessa cytometer (Becton Dickinson). Phenotypic analysis of cultured fetal liver derived macrophages was achieved by pre-incubation with 2.4G2 supernatant followed by staining using anti-CD11b-APC/Cy7 and anti-F4/80-PE antibodies (BioLegend). Analysis of thioglycollate-elicited and bone marrow-derived macrophages was performed using anti-CD45.1-BrilliantViolet605, anti-CD11b-APC/Cy7 and anti-F4/80-BrilliantViolet421 (BioLegend). For macrophage phenotyping and analysis of phagocytic activity at least 1×104 live cell events were recorded. Analysis was performed using FlowJo software (Tree Star). Data was analyzed using Student’s T test and no significant differences (p<.05) were found.
Results
Derivation of Cx43-deficient macrophages from cultured fetal liver and bone marrow cells
Connexin43 deficient (Cx43−/−) mice die soon after birth, making the generation of macrophages from bone marrow impractical (11). Therefore, we employed several approaches to obtain wild type (WT) and Cx43−/− macrophages: 1) derivation from hematopoietic cells harvested from fetal livers; 2) the harvest of thioglycollate-elicited peritoneal macrophages from radiation chimeric mice consisting of irradiated wild type animals reconstituted with WT or Cx43−/− fetal liver cells; and 3) production of bone marrow-derived macrophages from radiation chimeric animals.
After 7–10 days in culture in the presence of M-CSF containing media, fetal liver-derived cells from WT and Cx43−/− fetuses were found to be predominantly macrophages, as demonstrated by their expression of the macrophage markers CD11b and F4/80 (Fig. 1A). Similarly, macrophages prepared in an identical manner from bone marrow cells collected from radiation chimeric mice were overwhelmingly both donor-derived, as identified by the marker CD45.1, and expressed both CD11b+ and F4/80+ (Fig. 1B). Importantly, no discrepancy in the proportion of positively stained cells was observed between WT or Cx43−/− macrophages.
FIGURE 1.
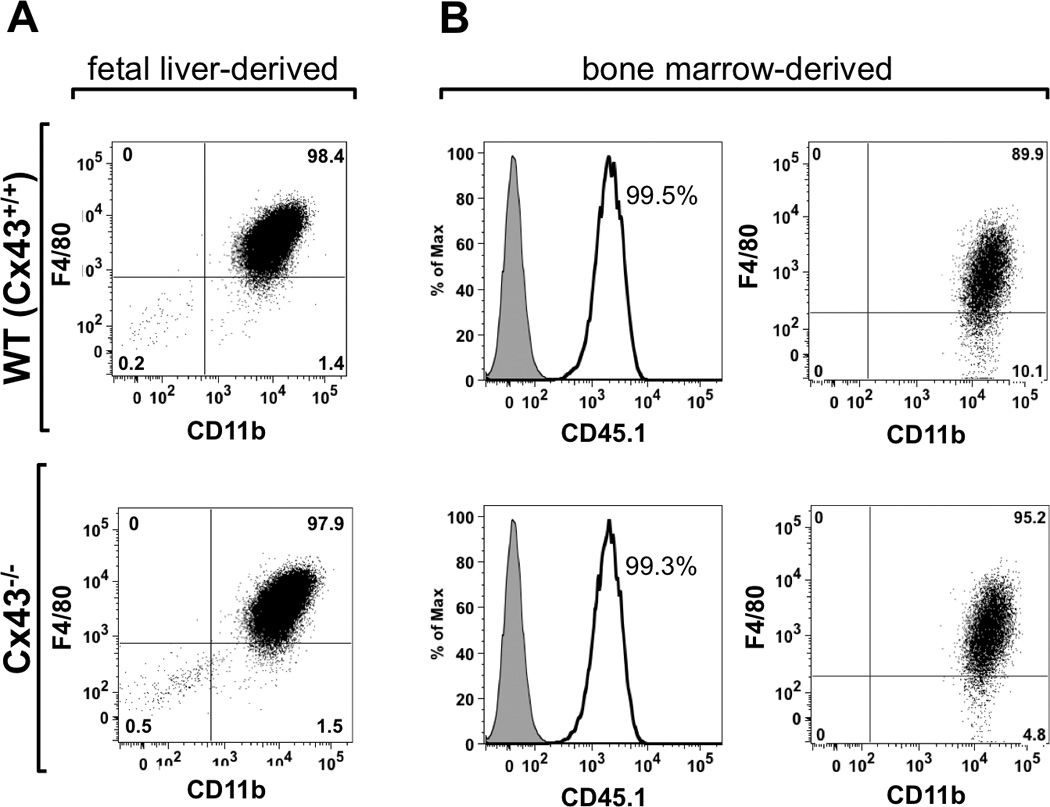
Characterization of the macrophages populations used in this study. (A) Wild type (WT, top panel) and Cx43−/− (bottom panel) macrophages derived from fetal liver cells were stained for the macrophage markers F4/80 and CD11b. In both cases, cultures contained a high proportion of double-positive cells, indicating that these cultures were predominantly comprised of macrophages. (B) WT (top panel) and Cx43−/− (bottom panel) bone marrow-derived macrophages from chimeric mice were stained for the donor marker, CD45.1, as well as CD11b and F4/80. Nearly all of the cultured macrophages were donor derived, and of these a high proportion were positive for macrophage markers, suggesting that WT and Cx43−/− populations were essentially pure and faithful to their respective genotypes.
Cx43 deficient macrophages phagocytose sheep erythrocytes
We next sought to characterize the phagocytic capacity of WT and Cx43−/− macrophages. First, the Fcγ receptor-mediated phagocytic capacity of macrophages was assayed using IgG-opsonized sRBCs as a target. WT and Cx43−/− fetal liver-derived macrophages were equally capable of sRBC uptake (Fig. 2A). Importantly, there was no difference in fluorescence intensity between these two populations (MFI = 54017±2122 WT vs. 54899±2018 Cx43−/−), as is evidenced by the overlapping fluorescence histograms. The vast majority (94.6% WT vs. 95.6% Cx43−/−) of cultured macrophages from both genotypes were positive for uptake of sRBCs after 20 min of incubation (Fig 2B), increasing to nearly 100% of cells after 60 min (data not shown). As evidence that this uptake was Fc receptor-dependent, non-opsonized sRBCs were taken up at a rate approximately 20 fold less (<5% of cells at 20 min) than that of opsonized targets.
FIGURE 2.
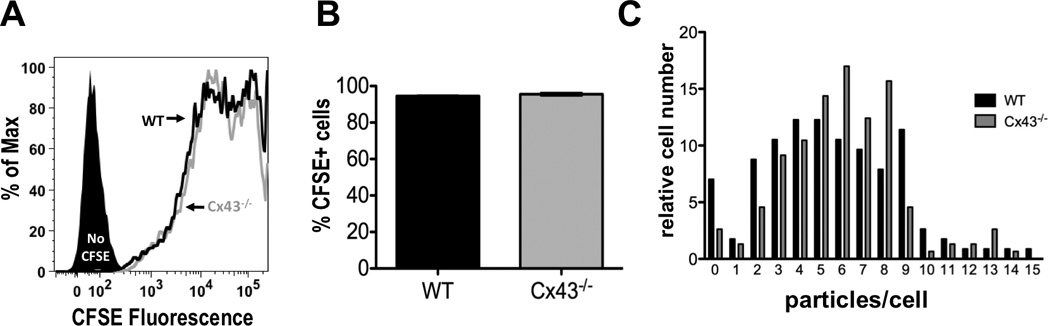
WT and Cx43−/− macrophages are equally capable of phagocytosing opsonized sRBCs. (A) Wild type (black line) and Cx43−/− (gray line) macrophages were incubated for 20 min with IgG-opsonized CFSE-labeled sRBCs and analyzed by flow cytometry (shaded black peak represents macrophages incubated with unlabeled sRBCs). (B) Percentage of cells positive for CFSE fluorescence after 20 minutes of incubation with opsonized sRBCs (average and S.E.M. of 8 experiments). (C) Histogram of phagocytosis by WT (black bars) and Cx43−/− (grey bars) fetal liver-derived macrophages after 20 min of incubation with sRBCs. Histograms were normalized for cell counts by dividing the number of cells in each bin by the total number of cells counted. At least 100 cells were counted for each genotype.
The phagocytic capacity of WT and Cx43−/− fetal liver-derived macrophages after 20 min of incubation with sRBCs was also quantified microscopically. The resulting histograms demonstrate the phagocytic capabilities of WT and Cx43−/− macrophages were comparable (5.5±0.3 particles per cell WT vs. 6.1±0.2 particles per cell Cx43−/−, Fig 2C). A similar assay was also performed on thioglycollolate-elicited macrophages from radiation chimeric animals and again, no major difference in phagocytic ability was observed (4.3±0.4 WT vs. 3.6±0.3 Cx43−/−, on average, Supplemental Fig. 1).
Bone marrow-derived macrophages were produced from radiation chimeric mice reconstituted with WT or Cx43−/− fetal liver cells. These cells were greater than 99% donor derived. Both WT and Cx43−/− macrophages were exposed to with fluorescently labeled opsonized sRBCs and were phagocytic. This phagocytosis was dependent on time and target concentration. Both populations exhibited identical mean fluorescence intensities at 20, 40 and 60 min time points when treated when treated with a specific amount of sRBCs. This identity was observed at a particle to macrophage ratio of 100:1 as well as 10:1 (Fig. 3).
FIGURE 3.
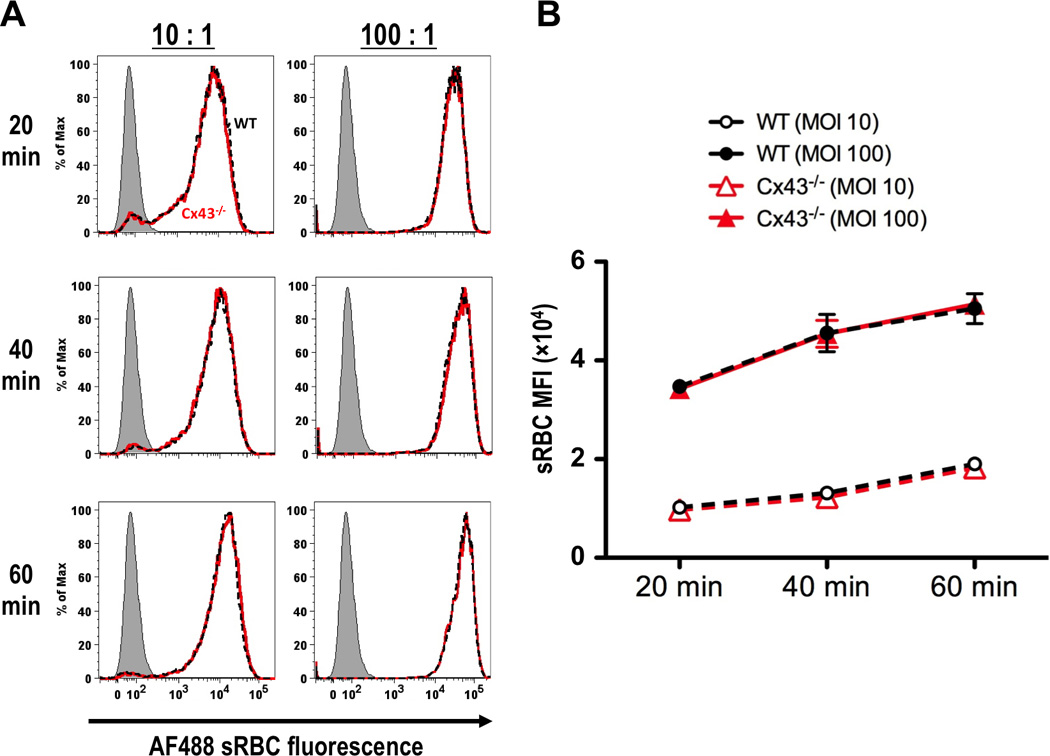
Bone marrow-derived macrophages from radiation chimeric mice reconstituted with fetal liver cells from WT and Cx43−/− mice are equally capable of phagocytosis of sRBCs. (A) Fluorescence histograms comparing sRBC uptake of WT (black dashed lines) and Cx43−/− (red lines) bone marrow-derived macrophages after 20, 40 and 60 min co-incubation. Macrophages were treated with particles at 10:1 (left panels) and 100:1 (right panels) particle-to-macrophage ratios. Grey shaded peaks represent non-fluorescent sRBCs. (B) Kinetic plot of phagocytosis by bone marrow-derived macrophages from radiation chimeric animals reconstituted with WT (black dashed lines) and Cx43−/− (red lines) fetal liver cells. Macrophages were incubated with 100:1 (closed symbols) and 10:1 (open symbols) sRBC to macrophage ratios for 20, 40 and 60 min time points before analysis by flow cytometry (average and S.E.M. for 3 experiments).
Cx43 deficient macrophages can phagocytose zymosan and the resultant phagosomes acidify normally
We next sought to measure the ability of Cx43−/− fetal liver-derived macrophages to phagocytose zymosan, another commonly used target for in vitro phagocytosis. In contrast to targets opsonized with IgG, uptake of zymosan by macrophages is mediated not by Fc receptors, but by several different receptors, including the mannose receptor (12–13). In the present study, zymosan was labeled with either a fluorescent marker DL649 or with the pH sensitive label pHrodo.
We first measured the binding of zymosan to the plasma membrane. WT and Cx43−/− macrophages were incubated with DL649-zymosan for 60 min at 4°C to allow for binding while inhibiting phagocytosis. When assayed by flow cytometry, similar percentages (45.6% of WT vs. 40.9% of Cx43−/−) of macrophages from both genotypes were positive for zymosan (Fig. 4). This indicates that WT and Cx43−/− macrophages bind zymosan at comparable levels. When macrophages were incubated with DL649-zymosan at 37°C for the same time period, 98.1% of WT and 98.4% of Cx43−/− macrophages were positive for zymosan, suggesting that this measured increase represents phagocytosis, not simply the presence of externally bound particles. Furthermore, the histograms of fluorescence intensity overlap, indicating that the populations had similar rates of uptake and similar numbers of particles per cell. Clearly, there is no deficiency in zymosan phagocytosis among Cx43−/− macrophages as compared to their WT counterparts.
FIGURE 4.
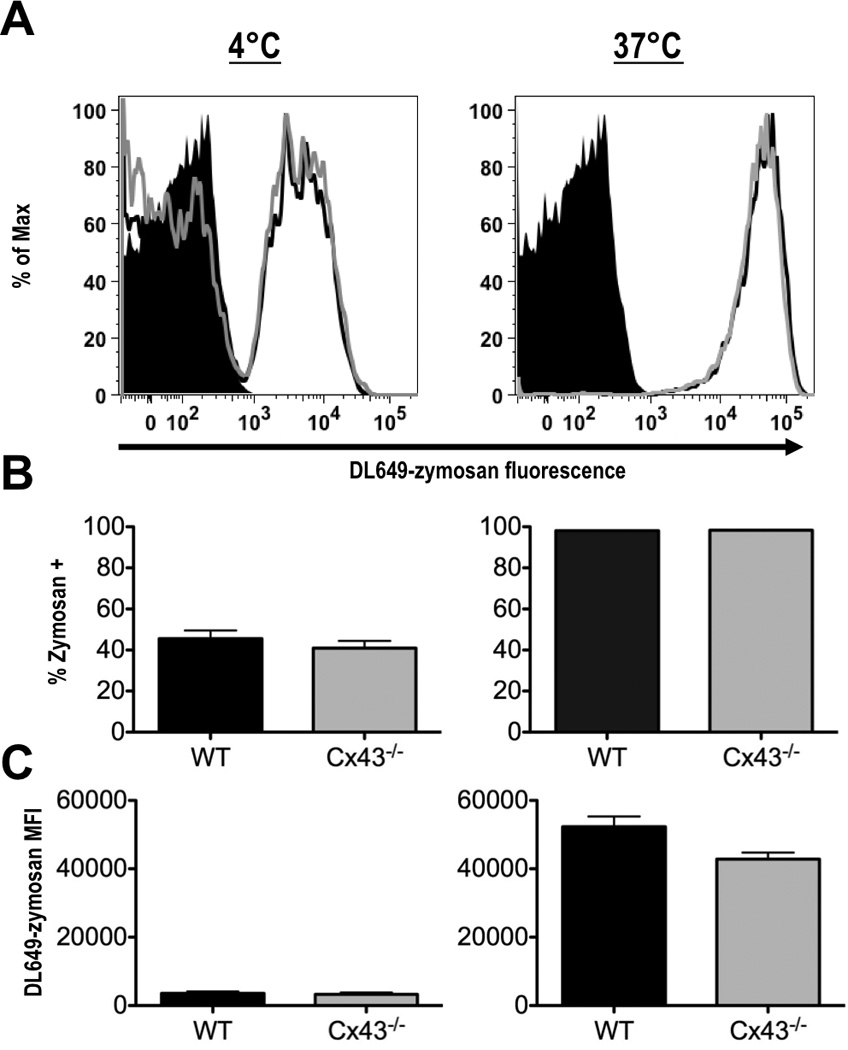
Fetal liver-derived macrophages phagocytose zymosan, independently of Cx43 genotype. (A) Wild type (black line) and Cx43−/− (gray line) macrophages were incubated with DL649-labeled zymosan particles for 60 min at 4°C (left panel), or at 37°C (right panel), (black peak represents macrophages without zymosan). (B) Percentage of cells positive for labeled zymosan after incubation on at 4°C (left panel), or at 37°C (right panel). (C) Mean fluorescence intensity (MFI) of DL649 signal in macrophages incubated at 4°C (left panel), or at 37°C (right panel), (averages from 3 experiments, +/− S.E.M).
To confirm that Cx43 is not required for phagocytosis of zymosan, WT and Cx43−/− macrophages were next treated with zymosan particles labeled with pHrodo. pHrodo is a pH sensitive dye which increases in fluorescence intensity several fold in response to decreasing pH, as occurs during the acidification of a maturing phagosome. Again, the majority of cells were found to be positive for pHrodo-zymosan after 60 min of incubation at 37°C (98.6% of WT vs. 98.9% of Cx43−/−, Fig. 5). Importantly, the dramatic increase (71.1 fold WT vs. 72.2 fold Cx43−/−) in pHrodo fluorescence at 37°C as compared to cells incubated at 4°C indicates that the zymosan particles were successfully incorporated into maturing phagosomes. No significant difference in the percentage of pHrodo-zymosan positive cells or in the fluorescence intensity of live cells was observed between WT and Cx43−/− fetal liver-derived macrophages. Together with the results using DL649-zymosan, this is strong evidence that Cx43 is dispensable for the phagocytosis of zymosan particles.
FIGURE 5.
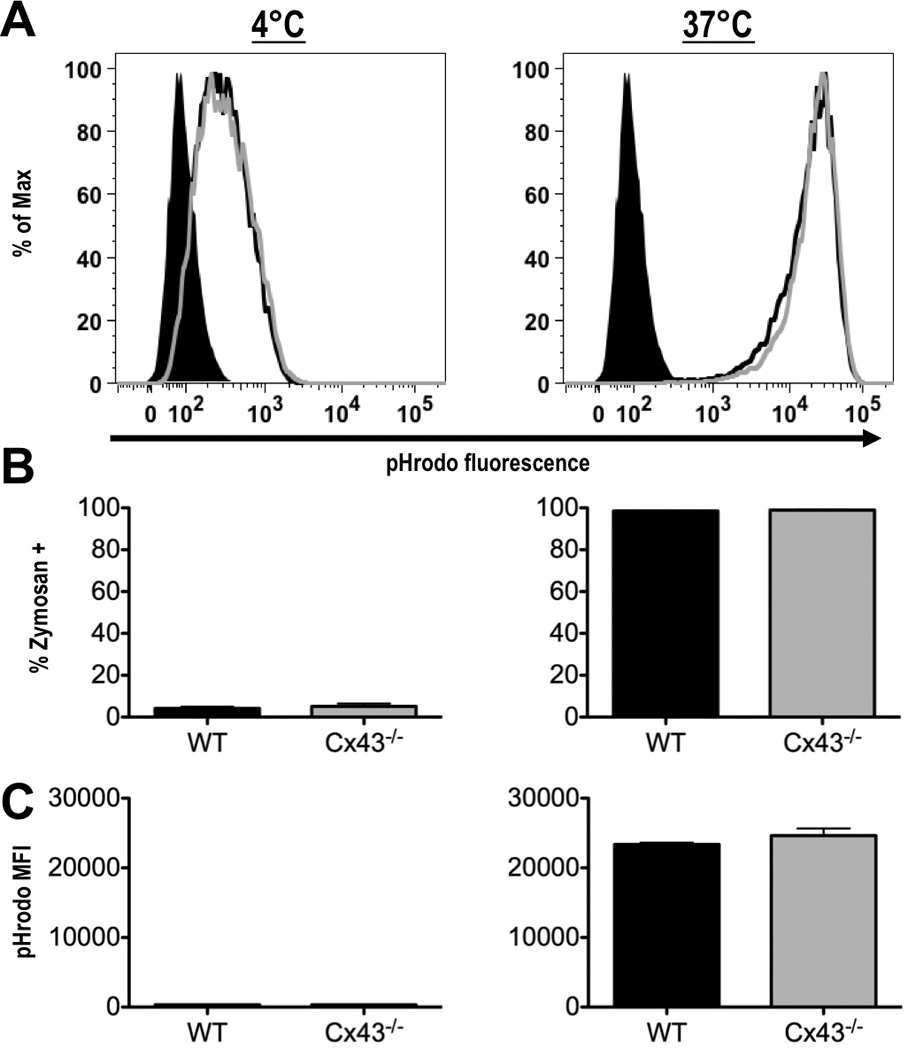
Fetal liver-derived macrophages from wild type and Cx43−/− mice can phagocytose zymosan, and are capable of phagosome acidification. (A) Wild type (black line) and Cx43−/− (gray line) macrophages were incubated with zymosan particles labeled with pHrodo for 60 min at 4°C (left panel), or at 37°C (right panel), (shaded black peak represents macrophages not treated with zymosan). (B) Percentage of cells positive for labeled zymosan after incubation at 4°C (left panel), or at 37°C (right panel). (C) Mean fluorescence intensity (MFI) of pHrodo signal in macrophages incubated at 4°C (left panel), or at 37°C (right panel), (averages from 3 experiments, error bars depict S.E.M.).
Cx43 deficient macrophages are able to phagocytose Listeria and present Listeria-derived antigens on MHC Class I molecules
We next measured the ability of Cx43−/− macrophages to phagocytose a physiologically relevant target: pathogenic bacteria. We chose the gram-positive facultative intracellular bacterium Listeria for these assays. This is an ideal target because immunity to Listeria is dependent on phagocytosis and subsequent killing by activated macrophages (14). For these studies, we utilized a recombinant Listeria strain (Lm-PASFLAG) that expresses a non-secreted form of GFP as well as a secreted protein that includes the model MHC class I binding peptide SIINFEKL. The non-secreted GFP was used to identify macrophages that have internalized Listeria, while the secreted SIINFEKL-containing recombinant protein was used to measure the ability of macrophages to process and present foreign antigens (7). Notably, internalization of Listeria in this model system requires phagocytosis, since mice lack the receptors required for non-phagocytic uptake of these bacteria (15).
WT and Cx43−/− macrophages were infected with Listeria and analyzed by flow cytometry. No differences were observed in either the percentage of cells positive for Listeria (63.1% in WT vs. 63.3 % in Cx43−/−, Fig. 6B), or in the number of Listeria ingested as displayed in the histogram of GFP fluorescence (Figure 6A). There was also no difference in the kinetics of surface expression of Kb-SIINFEKL (Fig 6C) between WT and Cx43−/− cells. This suggests that there are no major differences in the abilities of WT and Cx43−/− macrophages to ingest Listeria and process Listeria-derived proteins for presentation on MHC class I molecules.
FIGURE 6.
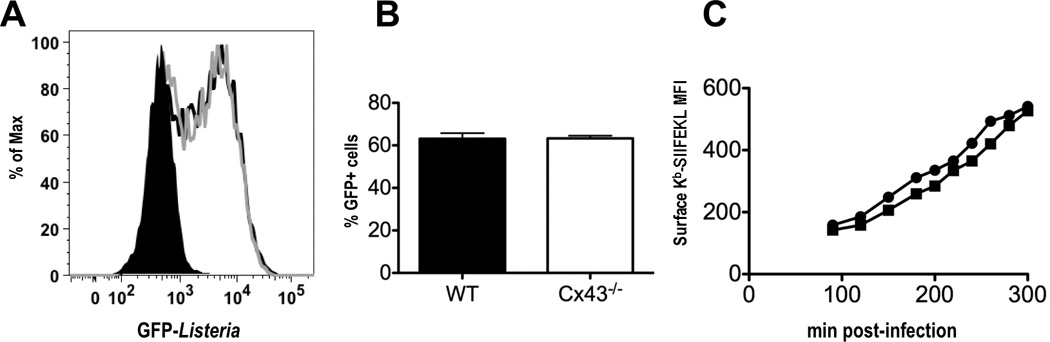
Cx43 deficient macrophages are capable of phagocytosis of Listeria monocytogenes and presentation of a foreign antigen. (A) Flow cytometry histogram of gated live cells demonstrating engulfment of GFP-positive Listeria at 90 min post-infection; black line represents Listeria uptake by WT macrophages, gray line represents uptake by Cx43−/− cells. (B) Percentage of WT and Cx43−/− macrophages positive for GFP fluorescence at 90 min post-infection (graph representative of three independent experiments. (C) Kinetics of surface Kb-SIINFEKL production in WT (circle) and Cx43−/− (square). Analysis of Kb-SIINFEKL was limited to live, GFP-positive cells only. One representative experiment of three is shown.
Discussion
In contrast to a previous report (5), we provide evidence here that modulation of Cx43 levels does not have a major impact on macrophage phagocytosis of opsonized sRBC, zymosan particles, or Listeria. The discrepancies between the results reported by Anand et al. and the present study may lie in the nature of the model systems studied:
As animals unable to express Cx43 die soon after birth, the direct harvest of knock-out macrophages or the culture of bone marrow derived macrophages is essentially impossible (11). To circumvent this issue, Anand et al. harvested mouse fetal livers as a source of macrophages. In their protocol, macrophages were selected by adherence to cover slips, based on the method of Morris et al. (16). These resident fetal cells were identified as mostly (>95%) macrophages based upon expression of CD45. Although it was used as a macrophage marker in the study by Anand et al., it is widely accepted that CD45, also known as leukocyte common antigen, is present on many cell types derived from hematopoietic cells.
That the adherent population of fetal liver cells contains such a high proportion of macrophages is at odds with Morris et al., who found that the resident fetal liver population was only composed of 50% macrophages, based on the more specific marker F4/80. Our own analysis of this adherent, resident cell population from fetal liver found that only a limited number of the cells could be characterized as macrophages, making analysis of phagocytosis by flow cytometry impossible. Therefore, it is possible that the results from Anand et al. can be explained by contamination of cultures by non-macrophage constituents of fetal livers. It is also not clear whether there may be a difference in the maturation of resident macrophages within the fetal liver of a Cx43−/− mouse.
In the model system employed in this study, pluripotent cells derived from fetal livers or bone marrow harvested from radiation chimeric mice were differentiated to the monocyte/macrophage lineage by culture in an M-CSF containing medium. Thus, our system has the advantage that cells from WT and Cx43−/− fetuses were cultured under controlled and identical conditions. This resulted in a population of cultured cells from wild type and Cx43−/− fetuses that were >95% macrophages, as assessed by the markers CD11b and F4/80 (Supplementary Fig. 1). Although CD11b is found on a range of myeloid cell types, F4/80 is specific for murine macrophages (17). The technique used here for deriving macrophages in this manner is based on an established protocol for the production of macrophages from bone marrow suspensions that has been adapted to the use of fetal livers for studying Cx43 deletion due to perinatal lethality (18–19). Under these conditions, cells displayed typical phenotypic macrophage properties with no defect in phagocytosis.
Phagocytosis is essential for the survival of metazoan species. In this study, mice were generated with immune cells lacking Cx43 through the use of radiation chimeric animals (4). Recipients of Cx43−/− fetal liver cells appeared healthy overall. Peritoneal macrophages elicited by injection of thioglycollate broth into animals reconstituted by WT and Cx43−/− donors were >98% donor derived and had similar proportions of CD11b positive cells. Furthermore, peritoneal macrophages from WT and Cx43−/− donors were equally capable of phagocytosis (Supplemental Fig. 1). In previous experiments with Cx43−/− chimeric mice, animals have survived for prolonged periods, in some cases greater than 6 months, without morbidity or mortality; a feat that would not be expected of animals that have been immunocompromised by reconstitution with poorly phagocytic macrophages. That result and the data presented here, clearly demonstrate that there is no primary role for Cx43 in macrophage phagocytosis and phagosome maturation.
Supplementary Material
Acknowledgements
We would like to thank Wanda Coombs for her critical reading of this manuscript.
Footnotes
This study was funded by the following: American Heart Association Grant 5340008 (S.M.T.), American Heart Association Scientist Development Grant AHA0830010N (M.F.P) and National Institutes of Health Grant 1R21AI090516 (M.F.P.).
References
- 1.Mendoza-Naranjo A, Bouma G, Pereda C, Ramirez M, Webb KF, Tittarelli A, Lopez MN, Kalergis AM, Thrasher AJ, Becker DL, Salazar-Onfray F. Functional gap junctions accumulate at the immunological synapse and contribute to T cell activation. J Immunol. 2011;187:3121–3132. doi: 10.4049/jimmunol.1100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pang B, Neijssen J, Qiao X, Janssen L, Janssen H, Lippuner C, Neefjes J. Direct antigen presentation and gap junction mediated cross-presentation during apoptosis. J Immunol. 2009;183:1083–1090. doi: 10.4049/jimmunol.0900861. [DOI] [PubMed] [Google Scholar]
- 3.Neijssen J, Pang B, Neefjes J. Gap junction-mediated intercellular communication in the immune system. Prog Biophys Mol Biol. 2007;94:207–218. doi: 10.1016/j.pbiomolbio.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen TD, Taffet SM. A model system to study Connexin 43 in the immune system. Mol Immunol. 2009;46:2938–2946. doi: 10.1016/j.molimm.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 5.Anand RJ, Dai S, Gribar SC, Richardson W, Kohler JW, Hoffman RA, Branca MF, Li J, Shi XH, Sodhi CP, Hackam DJ. A role for connexin43 in macrophage phagocytosis and host survival after bacterial peritoneal infection. J Immunol. 2008;181:8534–8543. doi: 10.4049/jimmunol.181.12.8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanley ER, Cifone M, Heard PM, Defendi V. Factors regulating macrophage production and growth: identity of colony-stimulating factor and macrophage growth factor. J Exp Med. 1976;143:631–647. doi: 10.1084/jem.143.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf BJ, Princiotta MF. Processing of Recombinant Listeria monocytogenes Proteins for MHC Class I Presentation Follows a Dedicated, High-Efficiency Pathway. J Immunol. 2013 doi: 10.4049/jimmunol.1201660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unkeless JC. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf BJ, Princiotta MF. Viral and bacterial minigene products are presented by MHC class I molecules with similar efficiencies. Mol Immunol. 2011;48:463–471. doi: 10.1016/j.molimm.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 11.Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 12.Speert DP, Silverstein SC. Phagocytosis of unopsonized zymosan by human monocyte-derived macrophages: maturation and inhibition by mannan. J Leukoc Biol. 1985;38:655–658. doi: 10.1002/jlb.38.5.655. [DOI] [PubMed] [Google Scholar]
- 13.Underhill DM. Macrophage recognition of zymosan particles. J Endotoxin Res. 2003;9:176–180. doi: 10.1179/096805103125001586. [DOI] [PubMed] [Google Scholar]
- 14.Shaughnessy LM, Swanson JA. The role of the activated macrophage in clearing Listeria monocytogenes infection. Front Biosci. 2007;12:2683–2692. doi: 10.2741/2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lecuit M, Dramsi S, Gottardi C, Fedor-Chaiken M, Gumbiner B, Cossart P. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 1999;18:3956–3963. doi: 10.1093/emboj/18.14.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris L, Crocker PR, Gordon S. Murine fetal liver macrophages bind developing erythroblasts by a divalent cation-dependent hemagglutinin. J Cell Biol. 1988;106:649–656. doi: 10.1083/jcb.106.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 18.Crowley MT, Costello PS, Fitzer-Attas CJ, Turner M, Meng F, Lowell C, Tybulewicz VL, DeFranco AL. A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. J Exp Med. 1997;186:1027–1039. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Underhill DM, Chen J, Allen LA, Aderem A. MacMARCKS is not essential for phagocytosis in macrophages. J Biol Chem. 1998;273:33619–33623. doi: 10.1074/jbc.273.50.33619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


