INTRODUCTION
Bone marrow derived progenitor cells have been shown to participate in lung injury repair and integrate in the lungs as both epithelial and endothelial cells (1). These bone marrow derived progenitors include hematopoietic, endothelial and mesenchymal progenitors.
The intra-tracheal (2) or intravenous administration of mesenchymal stem cells or MSC conditioned media (3) can improve lung vascular and alveolar growth in a rodent model of bronchopulmonary dysplasia (BPD). Intravenous administration of angiogenic hematopoietic cells also contributes to improved alveolarization in a hyperoxic model of BPD (4). Experimental studies have shown that intravenous infusion of bone marrow derived or peripheral blood derived endothelial progenitor cells (EPC) attenuates endotoxin induced acute lung injury (5,6) and improves survival (6) in animal models.
In human adults, increases in circulating EPC are described with bacterial pneumonia (7), severe sepsis (8), and following acute ischemic stroke (9). Furthermore, increases in circulating EPC in patients with acute lung injury was associated with improved survival outcome (10).
Studies in human cord blood documented that endothelial colony forming cells (ECFC) counts in preterm cord blood are either higher (11) or equivalent in number (12) when compared to term cord blood, but that lower levels of ECFC, a subset of EPC, are present in cord blood from preterm infants who later developed BPD than from those who did not (13). Others have shown that maternal factors such as diabetes and pre-eclampsia are associated with reduced cord blood ECFC (14) and lower cord blood EPC compared to normal pregnancies (15,16). Because many preterm infants are delivered early for maternal indications such as pregnancy induced hypertension with pre-eclampsia, cord blood ECFC may reflect the in-utero environment rather than the infant’s ability to respond to the postnatal environment. Studies that examine postnatal changes in circulating EPC in preterm neonates may help determine whether preterm infants can mobilize EPC in response to clinical stress, and whether there is an association between low EPC and BPD risk.
Animal models of BPD showed that impaired microvascular development leads to failure of alveolar development and strategies that promote vascular development result in improved lung alveolarization (17). In addition, low levels of bone marrow and circulating EPC are observed in the blood of hyperoxia exposed neonatal mice (18) that developed BPD, suggesting that EPC may play a role in neonatal lung development and repair. Erythropoietin treatment during hyperoxia exposure in the neonatal rat model of BPD resulted in increased microvascular density and improved alveolar development compared to placebo (17). Erythropoietin can stimulate EPC mobilization in vivo (19); thus, evidence from animal studies suggests that EPC may be involved in lung repair and vascular development.
We recently demonstrated an association between erythropoietin treatment for anemia of prematurity in the first 4 weeks of life and a reduced incidence of BPD in preterm infants below 1500 grams birth weight, as compared to non-erythropoietin treated infants (20). The possible mechanisms are unknown and may involve anti-apoptotic, anti-inflammatory and/or endothelial cell mobilization.
Various studies have quantified circulating EPC using either colony assays or flow cytometry to identify EPC by cell surface markers. Although the exact cell surface phenotype for circulating EPC remains unsettled (21), some authors have used CD34+VEGFR2+ cells, CD34+CD133+VEGFR2+ cells or CD34+CD45-VEGFR2+ cells. Others have suggested that CD34+CD133+VEGFR2+ cells are not endothelial progenitors (22,23) but are primitive hematopoietic progenitor cells and that ECFC are restricted to the rare CD34+CD45-cell population (23). Recent literature refers to CD34+CD45-VEGFR2+ cell population as circulating EPC in adults (24,25) and children (26). However, there is no data on CD34+CD45-VEGFR2+ cell phenotype in neonatal blood. In this pilot study we examined whether the circulating hematopoietic (CD34+) and endothelial progenitor cell (CD34+CD45-VEGFR2+) frequency on the first day of life may be different between term and preterm infants, and examined the pattern of change in preterm infants during the first 3 weeks of life.
METHODS
Subjects and blood samples acquisition
This study was approved by the Institutional Review Boards at Children’s Hospital Los Angeles and LAC+USC Medical Center and by the Research Oversight Committee at Hollywood Presbyterian Medical Center. Newborn infants admitted to the nursery or NICU were enrolled after obtaining informed consent from each infant’s mother. All infants were eligible if born at 25 weeks gestation or more and less than 36 hours old at the time of enrollment. Preterm infants were below 37 weeks gestation and term infants between 37 and 41 weeks gestation. Infants born at 32 weeks gestation or less were considered at risk for BPD. We excluded infants with congenital anomalies that could impact their respiratory outcome, and patients enrolled in other clinical studies that also involved blood sampling. The first blood sample (1 mL) was collected between 12 and 36 hours of age and subsequent samples were collected at weekly intervals for 3 weeks while the infant was still in the hospital.
Erythropoietin measurement
The plasma was separated from each sample for the measurement of erythropoietin concentration by ELISA assay using the Quantikine IVD kit (R&D Systems, Minneapolis, MN) following the manufacturer’s protocol.
Quantitation of EPC
The cellular fraction of each sample was utilized to quantify CD34+CD45-VEGFR2+ circulating EPC by multicolor flow cytometry on a FACS Aria cytometer (BD Biosciences, San Jose, CA) with Diva v6.1.3 software (BD Biosciences). Aliquots of whole blood leukocytes or mononuclear cells (MNC) obtained by Ficoll-gradient centrifugation were used to set up unstained, isotypic and fluorescence minus one (FMO) controls and test samples. Cells were blocked with IVIG, stained with fluorochrome-conjugated monoclonal antibodies for 30 minutes at 4°C, and washed with PBS. Three color combinations of fluorescent antibodies were used: mouse monoclonal antibodies against human CD34 conjugated to either phycoerythrin (PE) or allophycocyanin (APC) (BD Pharmingen, San Jose, California), human CD45 conjugated to peridinin-chlorophyll-protein (PerCP) (BD Biosciences), and human VEGFR2/KDR conjugated to either APC or PE (R&D Systems). For whole blood samples, the red cells were lysed twice using 1X Pharmlyse (BD Biosciences) for 15 minutes incubation at room temperature in the dark followed by PBS wash. Fresh cord blood samples served as positive controls to evaluate the sensitivity of detection of CD34+CD45-VEGFR2+ cells. Comp Beads (BD Biosciences) or peripheral blood leukocytes were used to set up compensation controls (CD45-PE, CD3-FITC, CD3-APC, and CD45-PerCP). Isotypes (IgG-PE, IgG-PerCP, IgG-FITC, and IgG-APC) (BD Biosciences) were used at the same concentrations as the primary antibodies. FMO controls, CD34-FITC with CD45-PerCP, CD34-FITC with VEFR2-PE, CD34-PE with CD45-PerCP, and CD34-PE with VEGFR2-APC were used to set the gates precisely for different populations. Test samples were analyzed in duplicate when there were sufficient cell numbers. In such cases, the mean of duplicates was reported. A minimum of 1 million events was acquired per test sample.
Collection of clinical data
Clinical data abstracted from the medical charts included birth-weight, gestational age, complications of pregnancy, medical diagnoses, treatment modalities, bronchopulmonary dysplasia (BPD) (27) and survival outcome.
Statistical methods
The outcome measures of interest were summarized as median and interquartile range for preterm and term infants. Comparisons between these two groups on day one were made using Wilcoxon rank sum test. Associations with gestational age were examined with Spearman correlation coefficients. Patterns of change from day 1 through week 3 were examined for preterm infants only using general estimating equation (GEE) methods. All statistical tests were 2-sided at a significance level of 0.05. Data analysis was performed using SAS ® software, version 9.2, copyright 2002-2008 (SAS Institute Inc, Cary, NC, USA).
RESULTS
Forty five patients were enrolled between July 2010 and March 2012. One patient was excluded from analysis due to inadequate sample. The study population included 15 term and 29 preterm infants. Of the 14 premature infants born at 32 weeks gestation or less, 4 had BPD: 3 of them developed moderate/severe BPD (defined as oxygen requirement at 36 weeks postmenstrual age (PMA) and one was on a ventilator for 30 days but weaned off oxygen before 36 weeks PMA (mild BPD). One hundred and three blood samples were collected for determination of CD34+CD45-VEGFR2+ cell frequency by flow cytometry analysis. Among the 15 term infants, 6 subjects had more than one blood sample tested. Of the 29 preterm infants, 6 had a single sample and 23 had 2 or more samples tested. One preterm infant expired from a lethal congenital metabolic disorder before a week of age.
Flow cytometry results
1) Gating strategy
CD34 subsets were quantitated in whole blood and MNC fraction. Of the 103 blood samples, analysis of both whole blood and MNC fraction was carried out in 82 samples, 10 samples were analyzed only in whole blood and 11 analyzed only in MNC fraction. Using the combination of CD34-FITC with CD45-PerCP and VEGFR2-PE, we achieved a better separation of the CD34+CD45-VEGFR2+ cells than the combination CD34-PE with CD45-PerCP and VEGFR2-APC. Therefore, we used this preferred combination for samples 11 to 103. After doublet discrimination, cells were first gated on CD34+, then CD45 negative and then VEGFR2+ signals, as shown in Figure 1.
Figure 1. Gating strategy.
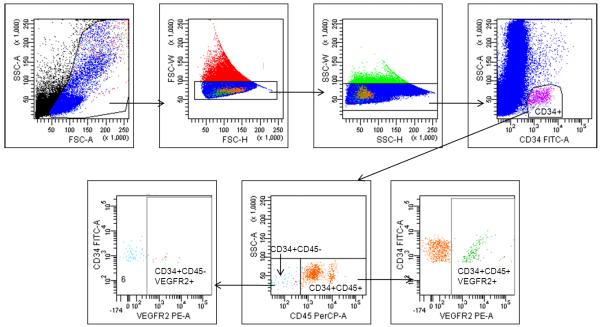
Following doublet discrimination, cells were sequentially gated on CD34, then CD45 and VEGFR2 to identify CD34+CD45-VEGFR2+ population as EPC. The CD34+VEGFR2+ cell number is the sum of CD34+CD45-VEGFR2+ and CD34+CD45+VEGFR2+ cells for each sample.
2) Evaluation of CD34+, CD34+CD45-, CD34+VEGFR2+ and CD34+CD45-VEGFR2+ cell frequency in preterm and term infants on day one
On day 1, the frequencies of CD34+ cells (median 4969 versus 1071 per million, p <0.0001), and CD34+VEGFR2+ cells (median 51 versus 16 per million, p=0.019) in whole blood leukocytes were significantly higher in preterm than in term infants (Table 1). There was a significant inverse correlation, i.e. a decrease in CD34+ cells (r= −0.72, p<0.0001, n=40) and CD34+VEGFR2+ cells (r= −0.32, p=0.045, n=39) in whole blood with advancing gestational age but not with CD34+CD45-cells (r= −0.19, p=0.24) or CD34+CD45-VEGFR2+ cells (r= 0.15, p=0.37).
Table 1.
| WHOLE BLOOD | - |
Preterm (N =29) |
Term (n=15) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Median | Q1 | Q3 | N | Median | Q1 | Q3 | P-value | |
| CD34+ | 25 | 4969.00 | 3004.04 | 7040.49 | 15 | 1071.00 | 500.01 | 1595.00 | <0.0001 |
| CD34+CD45− | 24 | 49.73 | 23.76 | 151.00 | 15 | 27.20 | 7.22 | 66.60 | 0.0939 |
| CD34+VEGFR2+ | 24 | 51.35 | 19.45 | 87.86 | 15 | 16.40 | 0.00 | 46.00 | 0.0194 |
| CD34+CD45-VEGFR2+ | 24 | 0.00 | 0.00 | 3.47 | 15 | 0.90 | 0.00 | 3.60 | 0.3930 |
| MNC | |||||||||
| N | Median | Q1 | Q3 | N | Median | Q1 | Q3 | ||
| CD34+ | 27 | 3642.26 | 1472.12 | 4797.88 | 11 | 1127.76 | 660.02 | 2203.00 | 0.0035 |
| CD34+CD45− | 27 | 130.14 | 74.41 | 225.50 | 11 | 73.53 | 42.30 | 92.60 | 0.0498 |
| CD34+VEGFR2+ | 27 | 76.06 | 50.00 | 130.00 | 11 | 64.43 | 48.60 | 83.50 | 0.8106 |
| CD34+CD45-VEGFR2+ | 27 | 2.22 | 0.20 | 6.50 | 11 | 3.79 | 0.65 | 10.60 | 0.7973 |
The frequencies of CD34+ cells in the MNC fraction (median 3642 versus 1127 per million, p=0.0035) and CD34+CD45-cells (median 130 versus 74 per million, p=0.049) were also higher in preterm than in term infants as shown in Table 1 with observed inverse correlation between CD34+ cells and gestational age (r= −0.40, p=0.01, n=38). The median frequency of CD34+CD45-VEGFR2+ cells was not significantly different between term and preterm infants (2.2 versus 3.8 per million MNC, p=0.79).
3) Effect of postnatal age and clinical stress on CD34+cell subsets
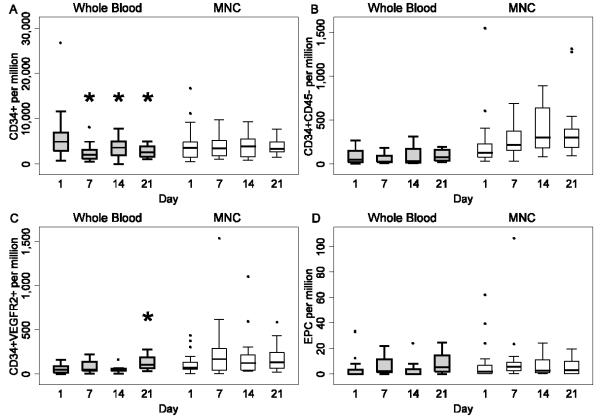
Only 6 term infants had more than one blood sample collected, therefore, we restricted our analysis to preterm infants <37 weeks. The quantitative data for CD34+, CD34+CD45-, CD34+VEGFR2+ and CD34+CD45-VEGFR2+ cell frequency in whole blood leukocytes and MNC fraction of preterm infants at 1, 7, 14 and 21 days are shown in Table 2A, Table 2B and Figure 2. The CD34+ cell frequency in whole blood decreased with postnatal age; values on day 7, 14 and 21 were lower than on day 1 (p=0.0001), panel A. The CD34+VEGFR2+ cell frequency in whole blood was higher on day 21 compared to day 1 (p=0.0002), panel C. There was a trend towards higher CD34+VEGFR2+ cells on weeks 1 and 2 than on day 1 in the MNC fraction (p=0.071).
Table 2 A. CD34+ cell subsets in whole blood of preterm infants for the first 3 weeks of life.
| Whole blood | Day | N | Median | Q1 | Q3 | Min | Max |
|---|---|---|---|---|---|---|---|
| CD34+ | 1 | 25 | 4969.00 | 3004.04 | 7040.49 | 745.00 | 26782.61 |
| 7 | 17 | 2105.00 | 1179.00 | 3160.00 | 549.00 | 8102.00 | |
| 14 | 13 | 3653.00 | 1987.09 | 5019.34 | 59.00 | 7849.00 | |
| 21 | 10 | 2621.50 | 1634.00 | 3991.00 | 1134.14 | 5008.64 | |
| P | 0.0001* | ||||||
| CD34+CD45− | 1 | 24 | 49.78 | 23.76 | 151.00 | 6.12 | 272.00 |
| 7 | 12 | 32.72 | 25.77 | 94.65 | 12.42 | 187.00 | |
| 14 | 11 | 35.50 | 16.60 | 175.00 | 13.20 | 315.00 | |
| 21 | 8 | 78.30 | 38.16 | 163.83 | 23.70 | 200.00 | |
| P | 0.2268 | ||||||
| CD34+VEGFR2+ | 1 | 24 | 51.35 | 19.45 | 87.86 | 0.00 | 165.00 |
| 7 | 12 | 52.74 | 37.87 | 138.34 | 6.31 | 222.26 | |
| 14 | 9 | 45.53 | 36.97 | 61.87 | 8.33 | 161.57 | |
| 21 | 8 | 105.36 | 67.10 | 193.30 | 33.67 | 280.00 | |
| P | 0.0002* | ||||||
| CD34+CD45−VEGFR2+ (EPC) |
1 | 24 | 0.00 | 0.00 | 3.47 | 0.00 | 33.70 |
| 7 | 12 | 2.53 | 1.48 | 11.70 | 0.00 | 22.10 | |
| 14 | 11 | 0.00 | 0.00 | 4.00 | 0.00 | 24.10 | |
| 21 | 8 | 5.52 | 2.15 | 14.75 | 0.00 | 24.70 | |
| P | 0.379 |
Data presented as events per million leukocytes gated.
p<0.05
Table 2B. CD34+ cell subsets in MNC fraction of preterm infants for the first 3 weeks of life.
| MNC | Day | N | Median | Q1 | Q3 | Min | Max |
|---|---|---|---|---|---|---|---|
| CD34+ | 1 | 27 | 3642.26 | 1492.12 | 4797.88 | 428.50 | 16744.01 |
| 7 | 22 | 3516.27 | 1756.70 | 5136.00 | 1068.00 | 9788.02 | |
| 14 | 15 | 4000.00 | 1527.74 | 5546.00 | 842.00 | 9308.00 | |
| 21 | 15 | 3453.69 | 2644.00 | 4818.00 | 1412.00 | 7622.58 | |
| P | 0.7632 | ||||||
| CD34+CD45− | 1 | 27 | 130.14 | 74.41 | 225.50 | 27.36 | 1547.00 |
| 7 | 22 | 219.70 | 155.42 | 371.30 | 32.97 | 691.00 | |
| 14 | 15 | 303.00 | 181.00 | 638.66 | 79.20 | 893.48 | |
| 21 | 14 | 306.29 | 186.00 | 396.00 | 93.00 | 1311.99 | |
| P | 0.0882 | ||||||
| CD34+VEGFR2 | 1 | 27 | 76.06 | 50.00 | 130.00 | 2.70 | 436.00 |
| 7 | 21 | 170.94 | 38.44 | 284.13 | 2.35 | 1536.82 | |
| 14 | 15 | 126.84 | 42.70 | 213.34 | 29.31 | 1102.06 | |
| 21 | 13 | 133.81 | 63.74 | 239.60 | 20.56 | 585.60 | |
| P | 0.0715 | ||||||
| CD34+CD45−VEGFR2+ (EPC) |
1 | 27 | 2.22 | 0.20 | 6.50 | 0.00 | 62.10 |
| 7 | 22 | 5.75 | 2.33 | 9.14 | 0.00 | 106.60 | |
| 14 | 15 | 2.70 | 0.80 | 11.20 | 0.00 | 24.10 | |
| 21 | 14 | 3.25 | 0.00 | 9.78 | 0.00 | 19.60 | |
| P | 0.307 |
Data presented as number of events per million MNC gated
Figure 2. Variations in CD34+ cell subsets with postnatal age for preterm infants.
Box plots represent median, quartiles and range for the different CD34+ cell subsets in whole blood and MNC fraction. Panel A: CD34+ cells, panel B: CD34+CD45neg cells, panel C: CD34+VEGFR2+ cells, panel D: EPC. Whole blood data are shown in grey boxes and MNC data in the white boxes. CD34+ frequency in whole blood was lower at 7, 14 and 21 days compared to day 1 (p= 0.0001), *panel A and CD34+VEGFR2+ cell frequency in whole blood was higher on day 21 compared to day 1 (p= 0.0002), *panel C.
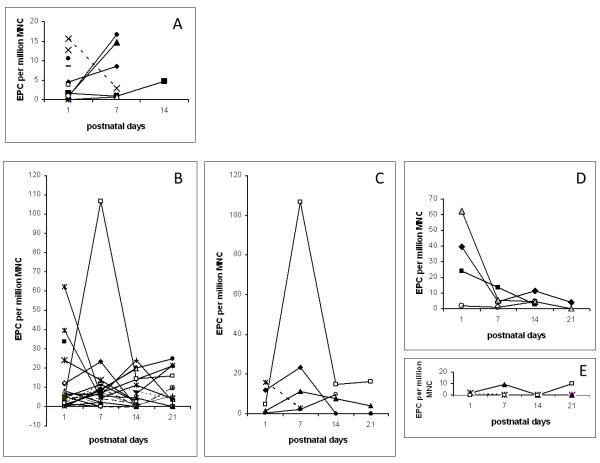
In Figure 3, panel A shows EPC data for all term infants and panel B shows the EPC data over the study period for all preterm infants. There were trends towards higher EPC in infants who developed postnatal infections (panel C) or who were born to mothers with chorioamnionitis (panel D), whereas lower EPC were noted in infants who later developed BPD (panel E). One preterm infant who developed necrotizing enterocolitis (NEC) on day 6 showed a 24-fold increase in EPC frequency on day 7 compared to day 1 (panel C, open square). Modest 2-fold and 6-fold increases in EPC frequency at 1 week were also observed in 3 patients who were treated for sepsis. Three of four patients, with chorioamnionitis or presumed infection due to prolonged rupture of fetal membranes, had initial elevation of EPC with a subsequent decline over the following weeks (panel D).
Figure 3. Pattern of change over time of EPC frequency by clinical subgroups.
Panel A: EPC frequency in 15 term infants. Three infants required mechanical ventilation on day 1 and showed an increase in EPC at 1 and 2 weeks (2 meconium aspiration, one transient tachypnea of the newborn who was treated for omphalitis). One infant with bacteremia at birth showed a decrease in EPC at 7 days compared to day 1 (dotted line). The other two did not show changes in EPC at 1 week. Panel B: data for all preterm infants 25 to 36 weeks GA. Panel C: EPC frequency for 5 patients with infections (one term infant with bacteremia shown in dotted line, and 4 preterm infants: 2 treated for clinical sepsis with CSF pleiocytosis (solid symbols), one developed NEC on day 6 of life and had a 24-fold increase in EPC number on day 7 (open square), and one preterm infant who had late onset yeast urinary infection (open circle)). Panel D: EPC frequency in 4 preterm infants born to mothers with chorioamnionitis and treated for suspected infection. Initial EPC was high in 3 of the 4 infants and decreased over time. Panel E: EPC in 4 preterm infants who developed BPD showing undetectable to low levels over a 3 weeks period.
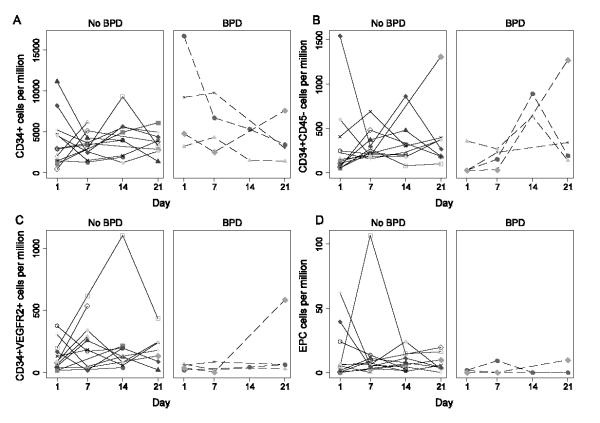
Figure 4 shows the individual data for the different CD34+ cell subsets (CD34+, CD34+CD45-, CD34+VEGFR2+, CD34+CD45-VEGFR2+) in MNC for the 14 preterm infants born at 32 weeks GA or less with and without BPD.
Figure 4. CD34+, CD34+CD45−, CD34+VEGFR2+, and EPC frequencies in MNC for preterm infants ≤32 weeks GA.
Panel A: CD34+ cells, panel B: CD34+CD45neg cells, panel C: CD34+VEGFR2+ cells, panel D: EPC. Patients with BPD are represented in broken lines and no BPD in solid lines. EPC were either undetectable or very low in the patients with BPD.
Plasma erythropoietin concentrations
1) Plasma erythropoietin levels relative to postnatal age
The erythropoietin concentrations were determined by ELISA assay on 71 samples. The assay normal range for erythropoietin in the ELISA kit (R&D Quantikine IVD kit) was 3 to 15 mIU/mL. Most of the blood samples tested within the normal range of the assay with only 3 samples above the normal range. Increased erythropoietin levels were detected in 2 premature infants: one had severe metabolic acidosis, the other was born with presumed infection after prolonged rupture of fetal membranes for 2 weeks and subsequently developed sepsis and NEC. Plasma erythropoietin levels did not increase with postnatal age. Plasma erythropoietin concentrations expressed as median (1st and 3rd quartile; range) were: 5.3 mIU/mL (2.4-9.1; range 0.08-29.8) on day one, 4.3 mIU/mL (2.9-6.8; range 1.6-19) at 1 week, 5.14 mIU/mL (4.7-8.6; range 2.3-13.4) at 2 weeks and 5.8 mIU/mL (3.4-6.7; range 2.2-10.6) at 3 weeks of age.
2) Plasma erythropoietin concentrations relative to CD34+ cell subsets
No correlation was observed between plasma erythropoietin and either CD34+ cell frequency or EPC frequency.
DISCUSSION
This study is the first to quantify circulating EPC defined as CD34+CD45-VEGFR2+ cells (24-26) in newborn infants over the first 3 weeks of life. We provide data on both term and preterm neonates using the combination of cell surface markers currently used to identify endothelial progenitor cells in the adult and pediatric literature (24-26). CD34+CD45-VEGFR2+ cell number was very low in postnatal blood samples with a median of 4 per million MNC and overall represented less than 0.1% of the CD34+ cell population.
Li’s group showed that circulating CD34+ stem cell frequencies drop rapidly within the first 6 hours after birth in term infants (28) but increase in preterm infants in the first 8 hours followed by a gradual decline (29) such that levels at 24 and 48 hours were lower than values at birth. Thus we chose to collect the first blood sample between 12 and 36 hours (day 1) when CD34+ fluctuations would be minimized. We quantitated the CD34+ cells and subsets of CD34+CD45-, CD34+VEGFR2+ and CD34+CD45-VEGFR2+ in neonatal peripheral blood for up to 3 weeks.
Qi’s group reported an association between high levels of CD34+ cells in the first 72 hours and survival outcome with reduced ventilator support days in preterm infants with respiratory distress syndrome (30). Whereas Bizzaro’s study showed no correlation with CD34+ cell count measured either at birth or in the first 4 weeks of life and any measures of pulmonary function in preterm infants of 24 to 32 weeks of gestation (31).
Earlier studies have used less comprehensive surface markers to study EPC in postnatal blood or have reported the phenotype of cultured endothelial cells isolated from endothelial cell colonies derived from peripheral or cord blood. Ingram’s group (32) suggested that cord blood endothelial cell colonies may derive from a different progenitor population than those previously described in adult blood. Hence those data may not accurately reflect the properties of EPC present in the peripheral blood of newborn infants. Our study revealed that the peripheral blood from preterm infants on the first day of life had a significantly higher proportion of circulating CD34+ cells and CD34+VEGFR2+ cells than blood from term infants. The CD34+ and CD34+ VEGFR2+ cell number in whole blood decreased with advancing gestational age. The inverse correlation between CD34+ cell count and gestational age was also described in other studies (29-31) that used postnatal peripheral blood, whereas in cord blood, a higher percentage of CD34+ cells (11) was reported in term compared to preterm samples. Prior studies in cord blood showed no significant difference in CD34+VEGFR2+ cells between term and preterm samples (11) in contrast to our findings in postnatal peripheral blood. The CD34+ and CD34+VEGFR2+ cell frequencies in whole blood in our study are in line with the range previously reported by Paviotti et al (33). The CD34+ VEGFR2+ cell frequency in whole blood in our study was statistically higher in preterm than in term infants (Table 1). In contrast, the frequency of CD34+VEGFR2+ in MNC was not statistically different between the two groups, although there was a trend toward a higher frequency in preterm infants. The discordance observed between the whole blood and MNC fraction could likely be explained by small sample size. In some infants EPC were detected in higher frequency in the whole blood than in MNC fraction of the same sample. In other samples, no EPC were detected in the whole blood tube but were detected in the MNC fraction of the same sample most likely due to concentration of cellular component in the specimen.
Several clinical studies have used CD34+KDR+ (33,34) (also referred to as CD34+VEGFR2+) or CD34+CD133+VEGFR2+ combinations (9, 33) as surface markers for circulating EPC. Approximately 70% of CD34+ cells from peripheral blood co-express CD133, a marker of more immature progenitors (35). A recent study of 36 preterm neonates (33) at birth and a subset of 18 neonates at 36 weeks PMA reported that the levels of CD34+KDR+ cells and CD34+CD133+KDR+ cells did not differ between infants who later developed BPD compared to infants who did not develop BPD. These authors also reported that the frequency of CD34+CD133+KDR+ cells was the same or slightly higher at 36 weeks PMA compared to values at birth. However, it should also be noted that other studies have shown that CD34+CD133+ VEGFR2+ cells are not EPC but are, in fact, primitive hematopoietic progenitor cells (22). Highly purified CD133+ cells are CD45+, and these cells do not give rise to endothelial colonies (23). For this reason, some investigators have excluded the CD45+ (common leukocyte marker) cells from the CD34+ cell fraction and considered CD34+CD45-VEGFR2+ cells as EPC (24, 25).
Borghesi et al (13) reported a lower number of ECFC in the cord blood of 9 preterm infants who later developed BPD compared to 23 preterm infants who did not develop BPD, and flow cytometry analyses on postnatal blood samples from 98 preterm infants of 24 to 32 weeks gestation showed a negative correlation between CD34+CD45-cell frequency and gestational age. The percentage of CD34+CD45-cells was lower at 48 hours and 7 days of life compared to values at birth. Both CD34+CD45+ cells and CD34+CD45-cells were higher at birth in infants who later developed BPD. The CD34+CD133+VEGFR2+ triple positive cells were comparable in infants with and without BPD (13), a finding similar to that of Paviotti’s group (33). The CD34+CD133-VEGFR2+ cell frequency was lower at birth in infants who later developed BPD, but after adjusting for gestational age differences, there was no longer a correlation between any of the CD34+ cell subsets and development of BPD.
The median frequency of CD34+CD45-cell subsets in our present study was 158 per million MNC in preterm infants, or six fold lower than values reported by Borghesi et al (13) (median of 0.10%) but the gestational age of the patients in their study was 24 to 32 weeks, whereas the gestational age of preterm infants in the present study was 25 to 36 weeks.
In our study, we went one step further by quantifying a subset of CD34+VEGFR2+ cells that lacked the expression of CD45. The CD34+CD45-VEGFR2+ cells in our study only accounted for about 2% of the CD34+CD45-cells and about 7% of the CD34+VEGFR2+ cells. The majority of CD34+VEGFR2+ cells expressed the CD45 common leukocyte marker. These CD34+CD45+VEGFR2+ cells may be angiogenic hematopoietic cells as the VEGFR2/KDR is expressed in 0.1 to 0.5% of CD34+ pluripotent hematopoietic stem cells (34).
The pattern of change with postnatal age in our present study revealed that the CD34+ and CD34+CD45-VEGFR2+ cells tended to be higher in infants with infection and decreased over time. In particular, one preterm infant who developed NEC at 6 days of age showed a 24 fold increase in CD34+CD45-VEGFR2+ cell frequency in the blood sample drawn at day 7 compared to day 1 of life. The EPC frequency subsequently decreased at 2 and 3 weeks of age. This suggests that newborn infants can mobilize EPC in response to stimuli such as infection. Three term infants who required mechanical ventilation on day 1 showed an increase in EPC at 1 week of life when they were extubated and breathing room air.
Plasma erythropoietin levels measured in this study were comparable to erythropoietin levels in other studies. Brown et al reported a mean erythropoietin level of 9.7 mU/mL in preterm infants in the first month of life (36). Others reported serum erythropoietin levels of 11.3 ± 6.1 mU/mL at 1 week and 15.9 ± 13.4 mU/mL at 4 weeks of age in preterm infants (37), which are lower than cord blood erythropoietin levels (25 to 72 mU/mL) (38). Studies involving both preterm and term infants show serum erythropoietin levels vary widely in the first week (39). Levels are lowest (<20 mU/mL) between 7 and 50 days of age (39) and increase during the anemic phase (40). There was no correlation between plasma erythropoietin and CD34+ cells or EPC number in the present study. This is not surprising considering that the erythropoietin values were not elevated. A prior randomized study in preterm infants using recombinant erythropoietin at a dose of 300 IU/kg body weight subcutaneously three times a week for 4 weeks or placebo showed no significant change in CD34+ cells at 14 days of treatment compared to pretreatment values or to values in the placebo control group (41). Whether or not erythropoietin can mobilize EPC, as a rare subset of CD34+ progenitor cells in human neonates is unknown. However with ongoing clinical trials of erythropoietin for neuroprotection this question may be addressed by other investigators.
In summary, an inverse correlation was observed in CD34+ cells and in CD34+VEGFR2+ cells between cell number and gestational age on day one. CD34+CD45-VEGFR2+ (EPC) frequency was low, on the order of 4 per million MNC, and was not statistically different between term and preterm infants in this pilot study which may represent a limitation in sample size. A postnatal increase in EPC frequency was observed in neonates with infection and term infants with respiratory distress. We observed an interesting trend with undetectable to very low levels of circulating CD34+CD45-VEGFR2+ cells over a 3 week period in preterm infants who later developed BPD, which is a distinct pattern compared to preterm infants who did not develop BPD. Our analysis is, however, limited due to the small sample size. Larger studies that involve smaller premature infants at highest risk for BPD are needed to confirm our findings. Plasma erythropoietin levels were low, did not increase with postnatal age and did not correlate with EPC frequency. Thus the results of this pilot feasibility study provide reference values for term and preterm infants and a foundation for a larger definitive study.
Acknowledgments
We thank Linda Dukes for technical assistance with the erythropoietin ELISA assay, and Drs. Istvan Seri and Parviz Minoo for their support and critical advice.
Funding source: This work was supported in part by Grant Number 1UL1RR031986, Children’s Hospital Los Angeles Clinical Translational Science Institute, with funds provided by the National Center for Research Resources (NCRR), NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have no conflict of interest to disclose.
References
- 1.Yamada M, Kubo H, Kobayashi S, Ishizawa K, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol. 2004;172:1266–72. doi: 10.4049/jimmunol.172.2.1266. [DOI] [PubMed] [Google Scholar]
- 2.Van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M, Rey-Parra GJ, Galipeau J, Haromy A, Eaton F, Chen M, Hashimoto K, Abley D, Korbutt G, Archer SL, Thebaud B. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009;180(11):1131–42. doi: 10.1164/rccm.200902-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, Kourembanas S. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180(11):1122–30. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balasubramaniam V, Ryan SL, Seedorf GJ, Roth EV, Heumann TR, Yoder MC, Ingram DA, Hogan CJ, Markham NE, Abman SH. Bone marrow derived angiogenic cells restore lung alveolar and vascular structure after neonatal hyperoxia in infant mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L315–23. doi: 10.1152/ajplung.00089.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao JP, He XY, Xu HT, Zou Z, Shi XY. Autologous transplantation of peripheral blood-derived circulating endothelial progenitor cells attenuates endotoxin-induced acute lung injury in rabbits by direct endothelial repair and indirect immunomodulation. Anesthesiology. 2012;116(6):1278–87. doi: 10.1097/ALN.0b013e3182567f84. [DOI] [PubMed] [Google Scholar]
- 6.Mao M, Wang SN, Lv XJ, Wang Y, Xu JC. Intravenous delivery of bone marrow derived endothelial progenitor cells improves survival and attenuates lipopolysaccharide-induced lung injury in rats. Shock. 2010;34(2):196–204. doi: 10.1097/SHK.0b013e3181d49457. [DOI] [PubMed] [Google Scholar]
- 7.Yamada M, Kubo H, Ishizawa K, Kobayashi S, Shinkawa M, Sasaki H. Increased circulating endothelial progenitor cells in patients with bacterial pneumonia: evidence that bone marrow derived cells contribute to lung repair. Thorax. 2005;60(5):410–3. doi: 10.1136/thx.2004.034058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlichting DE, Waxman AB, O’Brien LA, Wang T, Naum CC, Rubeiz GJ, Um SL, Williams M, Yan SC. Circulating endothelial and endothelial progenitor cells in patients with severe sepsis. Microvasc Res. 2011;81(2):216–21. doi: 10.1016/j.mvr.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Yip HK, Tsai TH, Lin HS, Chen SF, Sun CK, Leu S, Yuen CM, Tan TY, Lan MY, Liou CW, Lu CH, Chang WN. Effect of erythropoietin on level of circulating endothelial progenitor cells and outcome in patients after acute ischemic stroke. Crit Care. 2011;15(1):1–11. doi: 10.1186/cc10002. R40. Epub 2011 Jan 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnham E, Taylor W, Quyyumi A, Rojas M, Brigham K, Moss M. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med. 2005;172:854–60. doi: 10.1164/rccm.200410-1325OC. [DOI] [PubMed] [Google Scholar]
- 11.Baker CD, Ryan SL, Ingram DA, Seedorf GJ, Abman SH, Balasubramanian V. Endothelial colony-forming cells from preterm infants are increased and more susceptible to hyperoxia. Am J Respir Crit Care Med. 2009;180(5):454–61. doi: 10.1164/rccm.200901-0115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Javed MJ, Mead LE, Prater D, Bessler WK, Foster D, Case J, Goebel WS, Yoder MC, Haneline LS, Ingram DA. Endothelial colony forming cells and mesenchymal stem cells are enriched at different gestational ages in human umbilical cord blood. Pediatr Res. 2008;64(1):68–73. doi: 10.1203/PDR.0b013e31817445e9. [DOI] [PubMed] [Google Scholar]
- 13.Borghesi A, Massa M, Campanelli R, Bollani L, Tzialla C, Figar T, Ferrari G, Bonetti E, Chiesa G, de Silvestri A, Spinillo A, Rosti V, Stronati M. Circulating endothelial progenitor cells in preterm infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2009;180(6):540–6. doi: 10.1164/rccm.200812-1949OC. [DOI] [PubMed] [Google Scholar]
- 14.Ingram DA, Lien IZ, Mead LE, Estes M, Prater DN, Derr-Yellin E, DiMeglio LA, Haneline LS. In vitro hyperglycemia or a diabetic intrauterine environment reduces neonatal endothelial colony forming cell numbers and function. Diabetes. 2008;57(3):724–31. doi: 10.2337/db07-1507. [DOI] [PubMed] [Google Scholar]
- 15.Kwon JY, Maeng YS, Kwon YG, Kim YH, Kang MH, Park YW. Decreased endothelial progenitor cells in umbilical cord blood in severe preeclampsia. Gynecol Obstet Invest. 2007;64(2):103–8. doi: 10.1159/000100081. [DOI] [PubMed] [Google Scholar]
- 16.Xia L, Zhou XP, Zhu JH, Xie XD, Zhang H, Wang XX, Chen JZ, Jian S. Decrease and dysfunction of endothelial progenitor cells in umbilical cord blood with maternal pre-eclampsia. J Obstet Gynaecol Res. 2007;33(4):465–74. doi: 10.1111/j.1447-0756.2007.00555.x. [DOI] [PubMed] [Google Scholar]
- 17.Ozer EA, Kumral A, Ozer E, Yilmaz O, Duman N, Ozkal S, Koroglu T, Ozkan H. Effects of erythropoietin on hyperoxic lung injury in neonatal rats. Pediatr Res. 2005;58(1):38–41. doi: 10.1203/01.PDR.0000163391.75389.52. [DOI] [PubMed] [Google Scholar]
- 18.Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH. Hyperoxia reduces bone marrow, circulating and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1073–84. doi: 10.1152/ajplung.00347.2006. [DOI] [PubMed] [Google Scholar]
- 19.Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, Mildner-Rihm C, Martin H, Zeiher A, Dimmeler S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–6. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 20.Rayjada N, Barton L, Chan LS, Plasencia S, Biniwale M, Bui KC. Decrease in incidence of bronchopulmonary dysplasia with erythropoietin administration in preterm infants: A retrospective study. Neonatology. 2012;102:287–92. doi: 10.1159/000341615. [DOI] [PubMed] [Google Scholar]
- 21.Richardson MR, Yoder MC. Endothelial progenitor cells: Quo Vadis? J Mol Cell Cardiol. 2011;50(2):266–72. doi: 10.1016/j.yjmcc.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA. Human CD34+AC133+VEGFR2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35(7):1109–18. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Timmermans F, Van Hauwermeiren F, De Smedt M, Raedt R, Plasschaert F, De Buyzere ML, Gillebert TC, Plum J, Vanderkerckhove B. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol. 2007;27:1572–9. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 24.Pelliccia F, Cianfrocca C, Rosano G, Mercuro G, Speciale G, Pasceri V. Role of endothelial progenitor cells in restenosis and progression of coronary intervention: a prospective study. JACC Cardiovasc Interv. 2010;3(1):78–86. doi: 10.1016/j.jcin.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Hristov M, Schmitz S, Schuhmann C, Leyendecker T, von Hundelshausen P, Krotz F, Sohn HY, Nauwelaers FA, Weber C. An optimized flow cytometry protocol for analysis of angiogenic monocytes and endothelial progenitor cells in peripheral blood. Cytometry A. 2009;75(10):848–53. doi: 10.1002/cyto.a.20772. [DOI] [PubMed] [Google Scholar]
- 26.Taylor M, Rossler J, Geoerger B, Laplanche A, Hartmann O, Vassal G, Farace F. High levels of circulating VEGFR2+ bone marrow-derived progenitor cells correlate with metastatic disease in patients with pediatric solid malignancies. Clin Cancer Res. 2009;15(14):4561–71. doi: 10.1158/1078-0432.CCR-08-2363. [DOI] [PubMed] [Google Scholar]
- 27.Jobe A. Bancalari E: Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;173:1723–9. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 28.Li K, Liu J, Fok TF, Yau FW, Wong A, Li CK, Yang M, So KW, Tsang KS, Shing MM, Yuen PM. Human neonatal blood: stem cell content, kinetics of CD34+ cell decline and ex vivo expansion capacity. Br J Haematol. 1999;104(1):178–85. doi: 10.1046/j.1365-2141.1999.01147.x. [DOI] [PubMed] [Google Scholar]
- 29.Li K, Yau FW, Fok TF, So KW, Li CK, Yuen PM. Haematopoietic stem and progenitor cells in human term and preterm neonatal blood. Vox Sang. 2001;80(3):162–9. doi: 10.1046/j.1423-0410.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- 30.Qi Y, Qian L, Sun B, Chen C, Cao Y. Circulating CD34+ cells are elevated in neonates with respiratory distress syndrome. Inflamm Res. 2010;59:889–95. doi: 10.1007/s00011-010-0201-9. [DOI] [PubMed] [Google Scholar]
- 31.Bizzarro MJ, Bhandari V, Krause DS, Smith BR, Gross I. Circulating stem cells in extremely preterm infants. Acta Paediatr. 2007;96(4):521–5. doi: 10.1111/j.1651-2227.2007.00194.x. [DOI] [PubMed] [Google Scholar]
- 32.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 33.Paviotti G, Fadini GP, Boscaro E, Agostini C, Avogaro A, Chiandetti L, Baraldi E, Filippone M. Endothelial progenitor cells, bronchopulmonary dysplasia and other short-term outcomes of extremely preterm birth. Early Hum Dev. 2011;87:461–5. doi: 10.1016/j.earlhumdev.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Ziegler BL, Valtieri M, Porada GA, et al. KDR receptor: a key marker defining hematopoietic stem cells. Science. 1999;285(5433):1553–8. doi: 10.1126/science.285.5433.1553. [DOI] [PubMed] [Google Scholar]
- 35.Zubair AC, Malik S, Paulsen A, Ishikawa M, McCoy C, Adams PX, Amrani D, Costa M. Evaluation of mobilized peripheral blood CD34+ cells from patients with severe coronary artery disease as a source of endothelial progenitor cells. Cytotherapy. 2010;1292:178–89. doi: 10.3109/14653240903493409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown MS, Phibbs RH, Garcia JF, Dallman PR. Postnatal changes in erythropoietin levels in untransfused premature infants. J Pediatr. 1983;103(4):612–7. doi: 10.1016/s0022-3476(83)80600-3. [DOI] [PubMed] [Google Scholar]
- 37.Arif B, Ferhan K. Recombinant human erythropoietin therapy in low birthweight preterm infants; a prospective controlled study. Pediatr Int. 2005;47(1):67–71. doi: 10.1111/j.1442-200x.2005.02007.x. [DOI] [PubMed] [Google Scholar]
- 38.Maier RF, Bohme K, Dudenhausen JW, Obladen M. Cord blood erythropoietin in relation to different markers of fetal hypoxia. Obstet Gynecol. 1993;81(4):575–80. [PubMed] [Google Scholar]
- 39.Yamashita H, Kukita J, Ohga S, Nakayama H, Akazawa K, Ueda K. Serum erythropoietin levels in terms and preterm infants during the first year of life. Am J Pediatr Hematol Oncol. 1994;16(3):213–8. doi: 10.1097/00043426-199408000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Tschirch E, Weber B, Koehne P, Guthmann F, Von Gise A, Wauer RR, Rudiger M. Vascular endothelial growth factor as marker for tissue hypoxia and transfusion need in anemic infants: a prospective clinical study. Pediatrics. 2009;123(3):784–90. doi: 10.1542/peds.2007-2304. [DOI] [PubMed] [Google Scholar]
- 41.Meister B, Maurer H, Simma B, Kern H, Ulmer H, Hittmair A, Fink FM. The effect of recombinant human erythropoietin on circulating hematopoietic progenitor cells in anemic premature infants. Stem Cells. 1997;15(5):359–63. doi: 10.1002/stem.150359. [DOI] [PubMed] [Google Scholar]