Abstract
Manganese (Mn) is an essential trace element that is required for maintaining proper function and regulation of numerous biochemical and cellular reactions. Despite its essentiality, at excessive levels Mn is toxic to the CNS. Increased accumulation of Mn in specific brain regions, such as the substantia nigra, globus pallidus and striatum, triggers neurotoxicity resulting in a neurological brain disorder, termed manganism. Mn has been also implicated in the pathophysiology of several other neurodegenerative diseases. Its toxicity is associated with disruption of the glutamine (Gln)/glutamate (Glu)-γ-aminobutyric acid (GABA) cycle (GGC) between astrocytes and neurons, thus leading to changes in Glu-ergic and/or GABAergic transmission and Gln metabolism. Here we discuss the common mechanisms underlying Mn-induced neurotoxicity and their relationship to CNS pathology and GGC impairment.
Keywords: γ-aminobutyric acid (GABA), astrocytes, glutamate (Glu), glutamine (Gln), manganese, neurodegeneration, neurotransmission
Manganese
Essentiality, transporting characteristics and toxicity of manganese
Manganese (Mn) is a trace metal commonly found in the environment. It is essential for maintaining the proper function and regulation of many biochemical and cellular reactions [1]. It functions as a cofactor for multiple enzymes, including among others glutamine synthetase (GS), arginase, pyruvate decarboxylase and mitochondrial superoxide dismutase. Despite its essentiality, at excessive levels Mn is toxic to the CNS. In the general population, exposure to Mn occurs largely from consumption of well water containing high levels of the metal [2], from soy-based infant formulas [3] and from Mn released into the atmosphere as a result of combustion of methylcyclopentadienyl manganese tricarbonyl (MMT), a petrol antiknock additive [4]. Occupational exposure to Mn has been identified as a health hazard for miners, welders, ferroalloy workers, battery manufacturers and car mechanics [5–9] (Table 1). Excessive accumulation of Mn in the CNS triggers neurotoxicity resulting in a neurological brain disorder, referred to as manganism. It is characterized by early psychotic symptoms and is frequently followed by chronic symptoms analogous to those of idiopathic Parkinson’s disease (PD) [10]. Additionally, elevated levels of manganese in serum and/or cerebrospinal fluid (CSF) have been found in different neurodegenerative diseases, including PD [11–13] (Table 1).
Table 1.
Concentrations of Mn in blood, serum and CSF
| Study subjects | Mn concentration (μg/L) | References |
|---|---|---|
| Control subjects (n=33) | 3.04±1.40a | Wang et al. 2008 [7] |
| Welders (n=49) | 4.45±2.17a | |
| Control subjects (n=36) | 6.78 4.8–10.9 |
Lucchini et al. 1997 [8] |
| Ferroalloy workers (n=33) | 9.8 4.6–23.4 |
|
| Control subjects (n=90) | 8.7 4.1–19.0 |
Lander et al. 1999 [9] |
| Foundry workers (n=13) | 13.1 8.1–20.4 |
|
| Control subjects (n=37) | 1.22±0.59a 0.88±0.8b |
Jimenez-Jimenez et al. 1998 [11] |
| PD (n=37) | 0.93±0.9a 1.2±0.98b |
|
| Control subjects (n=15) | 1.9±1.1b | Hozumi et al. 2011 [13] |
| ALS (n=52) | 2.2±1.5b | |
| Control subjects (n=46) | 13.75±5.5 | Takagi et al. 2002 [12] |
| Adults receiving HPN(n=12) | 41.25±22 |
Data are mean ± SD or mean and range concentrations of Mn in whole blood, except where indicated:
in serum;
in CSF. HPN, home parenteral nutrition.
In the early stages of the disease, patients with manganism display psychotic symptoms, which progress to chronic disturbances in extrapyramidal circuits, leading to postural instability, dystonia and bradyskinesia, micrographia, mask-like facial expression, and speech disturbance [14–19] (Table 2). Morphologically, Mn causes neuronal loss and gliosis in the globus pallidus, the substantia nigra pars reticulata and the striatum [14].
Table 2.
The clinical effects of manganism
| Symptoms of increased Mn levels | References |
|---|---|
| The psychological symptoms of manganism: hallucinations, psychoses and a myriad of behavioural disturbances | Olanow 2004 [14] |
| Dystonia | Olanow et al. 1996 [16] |
| Bradyskinesia | Olanow et al. 1996 [16] |
| Motor deficits | Mergler and Baldwin 1997 [17] |
| Postural instability | Kim et al. 2011 [18] |
| Micrographia | Olanow 2004 [14] |
| Mask-like facial expression | Olanow 2004 [14] |
| Speech disturbances | Olanow 2004 [14] |
| Rydigity | Olanow et al. 1996 [16] |
| Memory and intellectual deficits | Bowler et al. 2006 [19] |
| Mood changes | Mergler and Baldwin 1997 [17] |
| Symptoms of toxicity mimicking PD: tremors, stiff muscles | Lucchini et al. 2009 [10] |
Mn is transported to the CNS either as a free ion or as a non-specific protein-bound species [20]. Transport of Mn in the 2+ oxidation state is mediated, at least in part, by the family of natural resistance-associated macrophage proteins (Nramps), the divalent metal transporter-1 (DMT-1) [21, 22]. In the 3+ oxidation state Mn complexes with transferrin (Tf) and, in a similar manner to iron (Fe), is transported by a Tf receptor-mediated mechanism [23]. Additionally, the divalent metal/bicarbonate ion symporters ZIP8 and ZIP14, various calcium channels, the solute carrier-39 (SLC39) family of zinc transporters, park9/ATP13A2, the magnesium (Mg) transporter hip14 and the transient receptor potential melastatin 7 (TRPM7) channels/transporters have been identified as Mn-transporting carriers [24, 25]. Optimal tissue levels of Mn are maintained through the involvement of all of the above carriers; however, the role(s) assumed by these transporters may differ depending on conditions associated with Mn deficiency or overdose.
Mitochondrial dysfunction commonly occurs in response to elevated Mn tissue burden [5]. In vitro, astrocytic cultures are highly sensitive to Mn and undergo apoptotic cell death secondary to mitochondrial dysfunction [26]. Mn preferentially and rapidly enters the mitochondrial matrix via the calcium (Ca) uniporter. Clearance of Mn from mitochondria is slow, leading to the accumulation of intramitochondrial Ca2+ [27], formation of reactive oxygen species (ROS) by the electron transport chain and inhibition of aerobic respiration [28]. Mn also promotes mitochondrial respiratory dysfunction, inducing ROS and inhibiting the antioxidant system by depleting glutathione and glutathione peroxidase [29–32]. Additionally, it has been demonstrated that oxidative stress-sensitive kinases and transcription factors, including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), are activated in cells exposed to Mn [26, 33].
Mn and neurodegenerative diseases
Manganism shares multiple characteristics with PD (Olanow 2004). In studies in non-human primates, decreased levels of dopamine were observed upon exposure to Mn in the caudate [34], striatum and midbrain [35] and the globus pallidus [36],. Mn can directly increase fibril formation by α-synuclein, an intra-cytoplasmic marker of disorders classified as synucleinopathies, including idiopathic PD, dementia with Lewy bodies and multiple system atrophy. Mn has also been shown to increase the in vitro expression of α-synuclein [37] and to cause α-synuclein aggregation in vivo in neurons and glial cells in non-human primates [38]. PD and Mn-induced neurotoxicity also have numerous common mechanistic features including mitochondrial impairment and oxidative stress. However, manganism is morphologically distinct from PD [38] and is characterized by neuronal loss and reactive gliosis in the globus pallidus and substantia nigra pars reticulata in the absence of Lewy bodies, the intraneuronal protein aggregates that characterize PD [14]. Furthermore, unlike manganism, idiopathic PD is associated with neuronal loss in the substantia nigra pars compacta [15].
A link between Mn overload and amyotrophic lateral sclerosis (ALS) has also been reported. For example, Mn smelters and miners have a propensity to develop both occupational manganism and ALS [39]. In addition, it has been shown that ALS is common in patients with liver cirrhosis, a condition known to sensitize to Mn overload due to impaired biliary excretion of Mn [40]. Chronic Mn treatment in macaques has been shown to upregulate amyloid-like protein 1, a marker of Alzheimer’s disease, with diffuse amyloid-beta plaques in the frontal cortex [41] Together, these observations suggest a potential link between advanced-stage manganism and several forms of neurodegenerative disorders and dementia [42].
Chronic exposure to Mn in animal models leads to significant accumulation in the striatum, an area that is affected in Huntington’s disease (HD). Several Mn-dependent enzymes, including arginase, GS, pyruvate decarboxylase and Mn superoxide dismutase, are altered in post-mortem brains of HD patients and pharmacological models of the disease [43–45]. Intracellular Mn levels are significantly elevated in a striatal cell line expressing mutant HTT [46], and it has recently been demonstrated that changes in the striatal proteome of HD mice exhibit gene environment interactions between mutant HTT and Mn. Identification of altered proteins revealed novel markers of Mn toxicity, including proteins involved in excitotoxicity, glycolysis and cytoskeletal dynamics [47]. These findings suggest that the interaction between Mn and mutant HTT may suppress proteomic phenotypes of HD mice, which could provide potential targets for HD treatment strategies.
Glutamine
Role in CNS function and transporting systems for glutamine
Glutamine (Gln) constitutes one-fifth of all amino acids present in plasma (at 600–800 μmol/L) and two-thirds in brain and the CSF [48–50]. Gln has a role in general metabolism by supporting tissue homeostasis as an intercellular substrate cycler. In the brain, Gln is important as a precursor of several neurotransmitter amino acids, including the excitatory glutamate (Glu) and aspartate (Asp) and the inhibitory γ-aminobutyric acid (GABA) [50]. Gln exhibits extremely rapid cellular turnover rates and shares multiple transport pathways with many other neutral amino acids both in neurons and astrocytes [51]. Neutral amino acid transport systems in the CNS are characterized by their overlapping substrate specificities, substrate affinities and cellular distribution, and demonstrate varied affinities for Gln [52]. Both in astrocytes and neurons, the so-called Systems N, ASC, L and A are responsible for most of the Gln transport [53]. System N is an Na+-dependent transporter with a narrow substrate specificity that is limited to amino acids containing side-chain nitrogen, such as asparagine, Gln and histidine, and it exhibits the highest specificity for Gln [54]. System ASC mediates wide-spectrum Na+-dependent transport of neutral amino acids with preference for shorter-chain substrates, such as alanine, serine, cysteine and threonine. System L mediates the Na+-independent transport of branched-chain and bulky neutral amino acids, and has a low affinity and high capacity for Gln transport both in astrocytes and neurons [55]. System A preferentially recognizes short-chain neutral amino acids [56].
Gln/Glu-GABA cycle
Intercellular compartmentation of Gln and Glu, the so-called Gln/Glu-GABA cycle (GGC), is critical for optimal CNS function.13C NMR studies have demonstrated that the ratio of Gln/Glu is extremely high and increases with brain activity [57]. Thus the GGC gives rise to the amino acid neurotransmitters Glu and GABA via dynamic astrocyte neuron interactions.
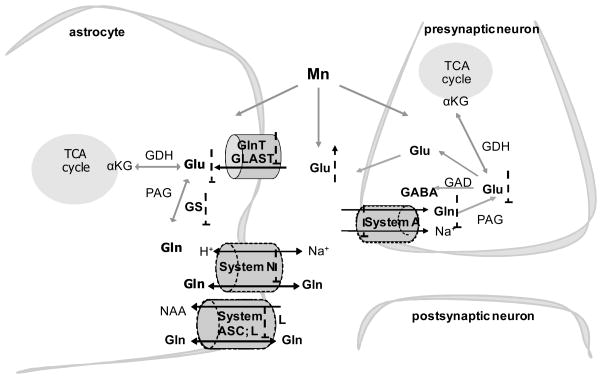
Glu released at synaptic terminals is taken up by surrounding astrocytes via the glutamate transporter 1 (GLT-1) and glutamate aspartate transporter (GLAST) [58, 59]. A small proportion of the astrocytic formed Gln via a reaction mediated by GS is transported into the extracellular space by Gln carriers, with a predominant role for System N/A transporter (SNAT)3, which belongs to the bidirectional transporter System N [60]. In addition to System N, release of Gln from astrocytes is mediated by other transport systems, including Systems L (LAT2) and ASC (ASCT2). Extracellular Gln is taken up into GABAergic and Glu-ergic neurons by the unidirectional System A transporters SNAT1 [61] and SNAT2 [62]. Once in neurons, Gln is converted to Glu by the mitochondrial enzyme phosphate-activated glutaminase [63]. Additionally, Glu is packaged into synaptic vesicles by the vesicular VGLUT transporter [64], released into the extracellular space and taken up by astrocytes where it is converted back to Gln by GS, thus completing the GGC (Fig. 1).
Fig 1.
Schematic representation of Glu and Gln transport systems related to the GGC and potential sites for interference by Mn. For details see text. αKG, α-ketoglutaric acid; GAD, glutamic acid decarboxylase; GDH, glutamate dehydrogenase; NAA, neutral amino acids; PAG, phosphate-activated glutaminase; TCA, tricarboxylic acid;
 , stimulation;
, stimulation;
 , inhibition.
, inhibition.
Mn and the GGC
Given the inability of neurons to perform de novo synthesis of Glu and GABA from glucose, they are metabolically dependent upon astrocytes [50]. The importance of the GGC is dramatically illustrated by the deregulation of the astrocytic Glu and Gln carriers in various neuropathological conditions [65]. Impairment of the GGC was previously described in response to Mn exposure [66, 67]. Glial cells possess a high capacity for transporting Glu from the synaptic cleft, supporting juxtaposed neurons by maintaining low extracellular concentrations of this amino acid [58]. Within the context of a supportive role of neurons, astrocytes maintain optimal Glu-ergic neurotransmission, synaptic Glu homeostasis, synapse formation and plasticity [68]. However, compared with neurons, astrocytes have a greater tendency to accumulate Mn [69]. Thus, Mn neurotoxicity is associated with altered glial function and secondary impairment of astrocyte-dependent neuronal functions (Fig. 1).
Mn and Glu transport
In the mammalian CNS, Glu is the main transmitter for most excitatory neurons [50]. Given this role, highly efficient and specific transport system must operate to optimally control its extracellular concentration, thus reducing excitotoxic injury. Five high-affinity Glu transporters have been identified and cloned, and termed GLAST, GLT-1, EAAC1, EAAT4 and EAAT5 [70–72]. EAAC1 and EAAT4 are restricted to neurons, while EAAT5 is found only in the retina [65]. Glu uptake in the CNS is mediated primarily (80% of synaptic Glu) by astrocytes via the high-affinity transporters GLAST and GLT-1 [50]. The inward transport of one Glu molecule is coupled to co-transport of one H+ and three Na+ ions, with one K+ ion being counter-transported [73]. Rapid removal of Glu from the extracellular space is required for survival and proper neuronal function.
Several studies have established the propensity of Mn to disrupt Glu transporting systems, thus impairing components of the GGC and leading to both a reduction in Glu uptake and elevation in extracellular Glu level [67] (Table 3). Chinese hamster ovary cells transfected with GLAST or GLT-1 show attenuated Glu transport after Mn exposure [74]. In non-human primate brain, long-term airborne Mn exposure leads to downregulation of GLAST and GLT-1, both at the mRNA and protein levels [75] (Table 3). Activation of the lysosomal, rather than the proteasomal pathway is probably responsible for downregulation of Glu transporter activity after Mn exposure [76]. Similarly, studies by Susarla and Robinson [77] revealed that lysosomal proteolysis is responsible for GLT-1 degradation.
Table 3.
Gln and Glu transporters of the GGC are affected by Mn
| Principal cellular localization | Targets of Mn action | |
|---|---|---|
| Gln transporter (System) | ||
| SNAT3 (System N) | Astrocytes [81] | mRNA and protein expression; function (uptake and efflux) [81] |
| SNAT2 (System A) | Astrocytes/neurons [81] | mRNA and protein expression [81] |
| LAT2 (System L) | Astrocytes/endothelial cells [81, 82] | mRNA and protein expression; function (uptake and efflux) [81] |
| ASCT2 (System ASC) | Astrocytes [81] | protein expression; function (uptake and efflux) [81] |
| Glu transporter | ||
| GLAST | Astrocytes [67, 76] | protein expression; function (uptake) [76] |
| GLT-1 | Astrocytes [76] | mRNA and protein expression; function (uptake) [67,76] |
Recent studies have identified a role of protein kinase C (PKC) signalling in Mn-induced downregulation of Glu turnover [76]. PKC stimulation by α-phorbol 12-myristate (PMA) significantly decreased astrocytic Glu uptake, whereas treatment with the general PKC inhibitor bisindolylmaleimide II (BIS II) protected astrocytes from the Mn-induced downregulation of Glu transport. Mn-dependent downregulation of astrocytic Glu uptake was reversed by rottlerin (ROT) and Go6976, specific inhibitors of the PKCδ and PKCα isozymes, respectively. In addition, inhibition of caspase-3 by Z-VAD-FMK blocked the Mn-induced disruption of Glu transport.
In an analogous fashion to the uptake study [76], Mn-induced downregulation of the level of GLT-1 protein was reversed by BIS II, ROT, Gö6976 and Z-VAD-FMK. Of note, co-immunoprecipitation studies have demonstrated associations between GLT-1 and the PKCδ and PKCα isoforms, and a Mn-induced specific increase in the PKCδ GLT-1 interaction. By contrast, the Glu transporter GLAST is regulated by Mn in a different manner. Reversion of Mn-induced downregulation of GLAST protein expression was observed only in the presence of PKCα and casapase-3 inhibitors. Finally, no interaction between GLAST and the PKC isoforms was observed in control conditions or upon Mn exposure in primary cultures of astrocytes [76]. Several groups have studied the effects of PKCs on GLAST-mediated transport with contradictory results, showing that activation of PKC increases, decreases or has no effect on GLAST expression and activity [78]. Other studies have established that activation of PKC rapidly decreases GLT-1 cell surface expression in C6 glioma cells stably transfected with GLT-1 and in primary cultures that endogenously express GLT-1 [79, 80]. It appears that both GLAST and GLT-1 are regulated via the PKC-dependent pathway; however, the contribution of the α and δ PKC isoforms is distinct for each of these transporters (Fig. 2).
Fig 2.
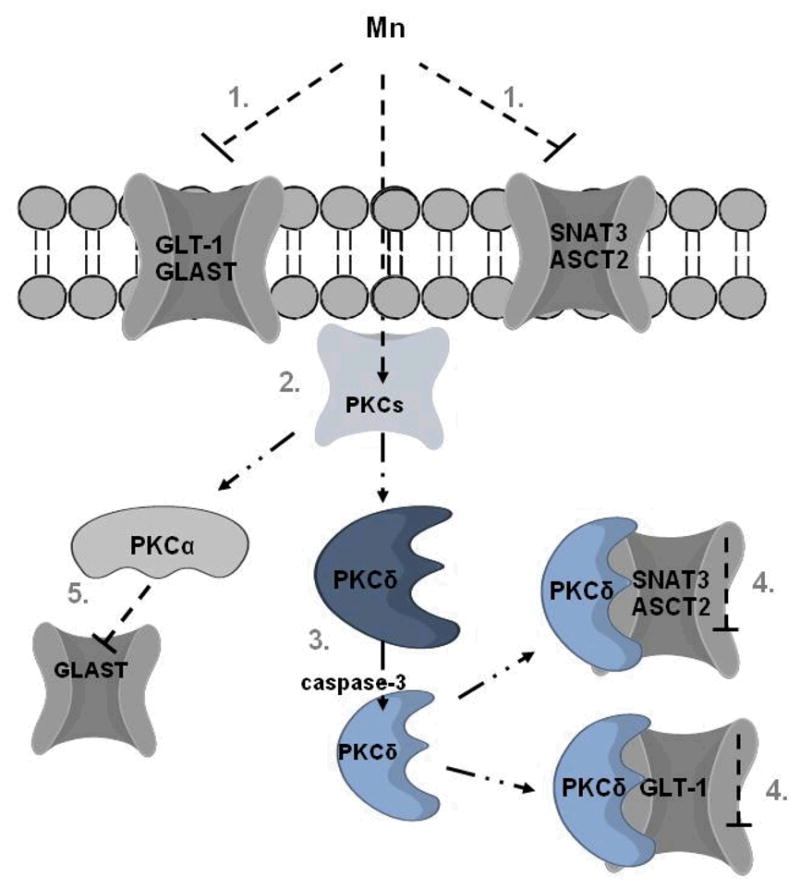
Proposed model of the role of PKC signaling in Mn-induced disruption of the GGC. 1. Mn-induced impairment of expression and function of Glu and Gln transporters; 2. activation of PKCs, including PKCδ and PKCα isoforms; 3. Mn mediates activation of PKCδ by caspase-3-dependent proteolytic cleavage; 4. Mn increases PKCδ binding to ASCT2, SNAT3 and GLT-1 transporters, leading to their inactivation; 5. Mn-dependent activation of PKCα triggers downregulation of GLAST transporter without direct interaction.
 , stimulation;
, stimulation;
 , inhibition.
, inhibition.
Mn and Gln turnover
Compared to Glu, the turnover of Gln in the GGC is more complex, because transport-mediated efflux from astrocytes must be met by transport-mediated influx in neurons. Mn toxicity is associated with the dysfunction of both of these GGC-associated processes. It was shown that pretreatment of neonatal rat cortical primary astrocytes with Mn inhibits the initial net uptake of Gln in a concentration-dependent manner [66]. Mn treatment also significantly reduced the expression of astrocytic mRNA for SNAT3, SNAT2 and the heterodimeric large amino acid transporter2 (LAT2). The decrease in the mRNA of these transporters was also accompanied by a decrease in protein levels. Additionally, astrocytes treated with Mn displayed a significant decrease in Gln uptake by the principal Gln-transporting Systems N and ASC. Moreover, Gln export via Systems N, ASC and L were attenuated in response to Mn exposure [81] (Table 3). SNAT3, which is characterized by the highest specificity for Gln of all the transporters [82], was the most sensitive to Mn exposure. Moreover, SNAT3 protein was completely degraded in response to a relatively short exposure to Mn [81]. It has also been reported that the ubiquitin-mediated proteolytic system may be involved in the Mn-mediated downregulation of SNAT3 [83]. Mn exposure induced ubiquitin B mRNA overexpression, increased free ubiquitin levels and triggered general protein hyperubiquitination. Similarly, gene expression profiling experiments in non-human primates have shown that Mn exposure increases the expression of genes associated with protein folding and turnover via the ubiquitin/proteasome system [84]. A selective interaction between SNAT3 and the ubiquitin ligase neuronal precursor cell expressed, developmentally downregulated 4-2 (Nedd4-2), as well as an increase in Nedd4-2 expression after Mn exposure, was observed in astrocytes [83].
Nedd4-2 is involved in the internalization and degradation of many plasma membrane channels and transporters by promoting their ubiquitination and proteasomal-dependent degradation [85, 86]. In addition, Nedd4-2 is negatively controlled by serum glucocorticoid-inducible kinase (SGK1) [87, 88]. Nedd4-2/SGK1 signalling regulates the expression and function of many membrane channels and transporters [89]. It is noteworthy that Mn decreases SGK1 expression and phosphorylation, suggesting a possible mechanism for Nedd4-2-induced SNAT3 downregulation [85]. Furthermore, Mn increases caspase-like activity of the proteasome; inhibitors of the proteasome and lysosme prevent the Mn-induced loss of SNAT3. Accordingly, Mn may mediate SNAT3 disruption via the ubiquitin-mediated proteolytic system, with a possible additional involvement of the proteasomal and/or lysosomal pathways [83].
Recent findings have shown that Mn-dependent disruption of Glu turnover involves PKC signalling [119]. It was demonstrated that BIS II reverses the Mn-dependent decrease in astrocytic Gln uptake, while PKC stimulation deregulates Systems ASC- and N-mediated specific Gln uptake and decreases the levels of ASCT2 and SNAT3 protein in cell lysates and in plasma membranes [119]. It is interesting that both SNAT3 and ASCT2 were found to contain putative PKC phosphorylation sites, which are conserved in humans, rats and mice [90, 91]. SNAT3 is downregulated by PKC, not by the direct phosphorylation of the transporter, but in a caveolin-dependent manner [90]. On the other hand, it was shown in a recent in situ study that PKC activation induces phosphorylation and internalization of SNAT3 [92]. Exposure to Mn increases the binding of PKCδ to ASCT2 and SNAT3, suggesting a prominent role for PKCδ in Mn-mediated disruption of Gln turnover (Fig. 2).
GABA and Mn
The most abundant inhibitory neurotransmitter in the adult brain, GABA [93, 94], was originally identified in medium spiny neurons of the striatum, where it mediates dopaminergic activity [95]. Altered GABAergic function is associated with manganism pathology. Manganism is associated with neurodegenerative changes in the globus pallidus, a region that is rich in GABA projections and preferentially accumulates Mn [22]. In vitro and in vivo studies have identified an association between Mn accumulation and alterations in GABA concentration and metabolism [96]; however, studies on the effects of Mn on GABA levels have been inconsistent. In non-human primates, chronic Mn exposure failed to alter total GABA levels [84, 97]. By contrast, striatal GABA levels were reduced in Mn-exposed rodents [98, 99], resulting in motor deficits [100]. In a more recent study, increased GABA levels were demonstrated in the brain of Chinese smelters exposed to airborne Mn [101]. Other studies have shown decreased [102–104] or no discernable effects of Mn exposure on GABA levels [105]. Anderson et al. observed a significant concentration-dependent decrease in GABA uptake in rat striatal synaptosomes [106], and these authors have also shown an increase in extracellular GABA concentrations in the striatum of Mn-exposed rats. Additionally, altered expression of GABA transporter 1 (GAT1), GABAA and GABAB proteins have been observed, suggesting that Mn may mediate changes in GABA concentrations via altered expression of transport and receptor proteins [107].
GS and Mn
The Mn-dependent enzyme GS catalyses the formation of Gln from Glu in a process considered to be the primary Glu-recycling pathway in the brain [108]. GS is exclusively localized in astrocytes [109]. GS protein levels were altered in the brain of monkeys exposed to airborne Mn [31], especially in the cerebellum and the globus pallidus [30]. Decreased GS activity and expression was also observed in Mn-treated cultured astrocytes [110]. The Mn-dependent downregulation of GS expression and activity may be due at least in part to the sensitivity of the enzyme to oxidative stress, a known outcome of Mn exposure [30]. Of note, GS is a sensitive marker of ROS as it is highly susceptible to oxidation and subsequent rapid degradation [111]. Inhibition of GS activity can have serious consequences for neuronal functioning through an impaired GGC and thus disrupted synaptic homeostasis and Glu-ergic transmission.
Mn and PKC signalling
Ubiquitination of various transport proteins that lead to their endocytosis and lysosomal targeting requires PKC activation. This mechanism was previously shown to be involved in the degradation of the dopamine transporter (DAT) [112, 113] as well as GLT-1 [80]. Additionally, ubiquitination of transmembrane proteins was implicated in the regulation of endocytosis, post-endocytic trafficking and turnover of various transporters [114]. For example, activation of PKC was found to affect GAT1-mediated transport. In a Xenopus laevis oocyte expression system, activation of PKC caused a rapid redistribution of the GAT1 transporter from the intracellular compartment to the cell surface with a concomitant increase in its transport activity [115, 116]. PKC-dependent ubiquitination is mediated either by direct or indirect interaction of the target protein with E3 ubiquitin ligase(s) [117], and knockdown of the ubiquitin ligase Nedd4-2 results in pronounced reduction in the PKC-dependent ubiquitination of DAT [118]. As discussed above, Mn exposure increases the expression of Nedd4-2 as well as the global polyubiquitination of proteins; both events precede the activation of PKC signalling [119]. Of interest, Mn has been shown to promote the specific phosphorylation of PKCα and PKCδ isozymes, thus increasing PKC activity. Mn also promotes the nuclear translocation of PKCδ, suggesting Mn-dependent specific regulation of PKC pathways and in particular PKCδ [76]. Knockdown of the PKCδ isoform has recently established the involvement of the PKCδ pathway in Mn-induced deregulation of Glu turnover. Astrocytes transfected with shRNA to silence PKCδ expression were less sensitive to Mn compared to those transfected with control shRNA. Furthermore, as mentioned above, Mn treatment has been shown to promote PKCδ and GLT-1co-expression, indicating PKCδ involvement in the Mn-dependent downregulation of Glu turnover [76]. Together, these findings along with the observed increase in PKCδ and its interaction with ASCT2 suggest that the latter may play a prominent role in modulating Mn-induced GGC dyshomeostasis.
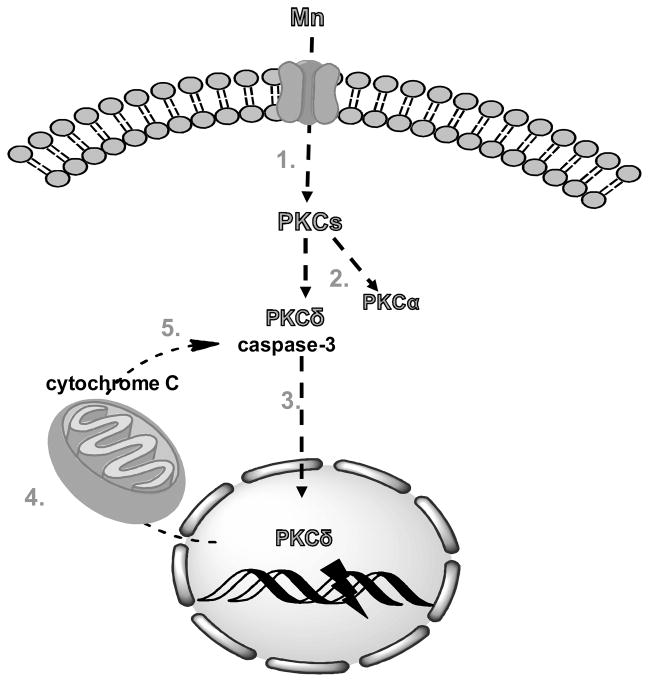
In contrast to other PKC isozymes, which are considered prosurvival mediators, PKCδ has been described as a proapoptotic isoform, as it is activated by a variety of apoptotic stimuli [120]. It was proposed that proteolytic cleavage, mediated by caspase-3, could be a mechanism of PKCδ activation [121]. Proteolytic activation of PKCδ was shown to promote its nuclear translocation as well as PKCδ-dependent mediation of DNA fragmentation. Furthermore, nuclear translocation of PKCδ was found to be dependent on its caspase-3-mediated cleavage [120]. It is noteworthy that, Mn toxicity is associated with caspase-3 activation in several in vitro and in vivo models [121–124] and with increased nuclear translocation of PKCδ [76]. In addition, it has been proposed that the Mn-mediated disruption of Glu uptake may involve caspase-3 and PKCδ inhibition [76]. Combined, these findings suggest that caspase-3-dependent PKC activation precedes PKCδ/Mn-induced deregulation of glutamate turnover.
Previous studies have indicated that caspase-3-dependent PKCδ activation not only contributes to neuronal apoptosis, but also has a regulatory role in amplifying the apoptotic cascade during Mn-dependent neurotoxic stress via a positive feedback loop between PKCδ and caspase-3 activation [121, 125]. Sun et al. demonstrated that mitochondrial translocation/accumulation of the proteolytically cleaved PKCδ triggers the mitochondria-mediated apoptotic cascade during proteasome dysfunction in dopaminergic neuronal cells [126]. Together, these findings suggest that Mn can regulate the GGC and other cellular processes in a PKCδ-/caspase-3-dependent manner (Fig. 3).
Fig 3.
Proposed model of the sequence of PKC signalling in Mn-induced apoptosis and mitochondrial dysfunction. 1. Mn treatment activates signalling of PKCs, including PKCδ and PKCα isoforms; 2. activation of PKCδ by Mn requires its proteolytic cleavage which is mediated by active caspase-3; 3. proteolytically activated PKCδ translocates to the nucleus and mediates DNA fragmentation; 4. nuclear PKCδ regulates key apoptotic events, particularly in mitochondria, resulting in cytochrome c release into the cytosol; 5. cytosolic cytochrome c activates caspase-3 cascade via a positive feedback loop, which leads to amplification of apoptotic signals.
 , stimulation;
, stimulation;
 , inhibition.
, inhibition.
Concluding remarks
Mn has been implicated in the impairment of the GGC at two key regulatory steps, the turnover of both Glu and Gln. Several studies have identified Mn-dependent disruption of Gln uptake, release and metabolism within astrocytes. It has been shown that Mn exposure causes aberrant Glu replenishment/transport in astrocytes, leading to elevated extracellular Glu concentrations. Additionally, Mn causes downregulation of GS expression and activity. These changes may trigger depletion of Gln synthesis/metabolism in glia and consequently diminish astrocyte-derived Gln and/or mediate dyshomeostasis in neurotransmission of juxtaposed neurons (Figs 1, 2). Future studies should focus on development of novel treatment strategies to reverse these effects and restore an optimal GGC in the astrocyte neuron unit. As discussed, manganism and other neurological conditions show distinct neurological symptoms at the clinical, physiological, cellular and molecular levels, suggesting the potential involvement of Mn in the pathophysiology of these disorders. Elucidating the relationships between Mn and GGC function and neurodegeneration may offer better understanding of specific targets that mediate Mn-induced brain pathology.
Acknowledgments
The authors wish to acknowledge partial support from the National Institute of Environmental Health Sciences (NIEHS) (grant numbers ES R01 10563 and ES P30 000267).
Footnotes
Conflict of interest statement
No conflict of interest was declared.
References
- 1.Erikson KM, Syversen T, Aschner JL, Aschner M. Interactions between excessive manganese exposures and dietary iron-deficiency in neurodegeneration. Environ Toxicol Pharmacol. 2005;19:415–21. doi: 10.1016/j.etap.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 2.Wasserman GA, Liu X, Parvez F, et al. Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2006;114:124–9. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krachler M, Rossipal E. Concentrations of trace elements in extensively hydrolysed infant formulae and their estimated daily intakes. Ann Nutr Metab. 2000;44:68–74. doi: 10.1159/000012823. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein MM, Jerrett M. A study of the relationships between Parkinson’s disease and markers of traffic-derived and environmental manganese air pollution in two Canadian cities. Environ Res. 2007;104:420–32. doi: 10.1016/j.envres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Cotzias GC, Greenough JJ. The high specificity of the manganese pathway through the body. J Clin Invest. 1958;37:1298–305. doi: 10.1172/JCI103718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth JA, Garrick MD. Iron interactions and other biological reactions mediating the physiological and toxic actions of manganese. Biochem Pharmacol. 2003;66:1–13. doi: 10.1016/s0006-2952(03)00145-x. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, Du X, Zheng W. Alteration of saliva and serum concentrations of manganese, copper, zinc, cadmium and lead among career welders. Toxicol Lett. 2008;176:40–7. doi: 10.1016/j.toxlet.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucchini R, Bergamaschi E, Smargiassi A, Festa D, Apostoli P. Motor function, olfactory threshold, and hematological indices in manganese-exposed ferroalloy workers. Environ Res. 1997;73:175–80. doi: 10.1006/enrs.1997.3702. [DOI] [PubMed] [Google Scholar]
- 9.Lander F, Kristiansen J, Lauritsen JM. Manganese exposure in foundry furnacemen and scrap recycling workers. Int Arch Occup Environ Health. 1999;72:546–50. doi: 10.1007/s004200050414. [DOI] [PubMed] [Google Scholar]
- 10.Lucchini RG, Martin CJ, Doney BC. From manganism to manganese-induced parkinsonism: a conceptual model based on the evolution of exposure. Neuromolecular Med. 2009;11:311–21. doi: 10.1007/s12017-009-8108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jimenez-Jimenez FJ, Molina JA, Aguilar MV, et al. Cerebrospinal fluid levels of transition metals in patients with Parkinson’s disease. J Neural Transm. 1998;105:497–505. doi: 10.1007/s007020050073. [DOI] [PubMed] [Google Scholar]
- 12.Takagi Y, Okada A, Sando K, Wasa M, Yoshida H, Hirabuki N. Evaluation of indexes of in vivo manganese status and the optimal intravenous dose for adult patients undergoing home parenteral nutrition. Am J Clin Nutr. 2002;75:112–8. doi: 10.1093/ajcn/75.1.112. [DOI] [PubMed] [Google Scholar]
- 13.Hozumi I, Hasegawa T, Honda A, et al. Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J Neurol Sci. 2011;303:95–9. doi: 10.1016/j.jns.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Olanow CW. Manganese-induced parkinsonism and Parkinson’s disease. Ann N Y Acad Sci. 2004;1012:209–23. doi: 10.1196/annals.1306.018. [DOI] [PubMed] [Google Scholar]
- 15.Pal PK, Samii A, Calne DB. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology. 1999;20:227–38. [PubMed] [Google Scholar]
- 16.Olanow CW, Good PF, Shinotoh H, et al. Manganese intoxication in the rhesus monkey: a clinical, imaging, pathologic, and biochemical study. Neurology. 1996;46:492–8. doi: 10.1212/wnl.46.2.492. [DOI] [PubMed] [Google Scholar]
- 17.Mergler D, Baldwin M. Early manifestations of manganese neurotoxicity in humans: an update. Environ Res. 1997;73:92–100. doi: 10.1006/enrs.1997.3710. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y, Bowler RM, Abdelouahab N, Harris M, Gocheva V, Roels HA. Motor function in adults of an Ohio community with environmental manganese exposure. Neurotoxicology. 2011;32:606–14. doi: 10.1016/j.neuro.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, Roels HA. Manganese exposure: neuropsychological and neurological symptoms and effects in welders. Neurotoxicology. 2006;27:315–26. doi: 10.1016/j.neuro.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Aschner M, Gannon M. Manganese (Mn) transport across the rat blood-brain barrier: saturable and transferrin-dependent transport mechanisms. Brain Res Bull. 1994;33:345–9. doi: 10.1016/0361-9230(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 21.Aschner M, Vrana KE, Zheng W. Manganese uptake and distribution in the central nervous system (CNS) Neurotoxicology. 1999;20:173–80. [PubMed] [Google Scholar]
- 22.Fitsanakis VA, Au C, Erikson KM, Aschner M. The effects of manganese on glutamate, dopamine and gamma-aminobutyric acid regulation. Neurochem Int. 2006;48:426–33. doi: 10.1016/j.neuint.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Aschner M, Aschner JL. Manganese neurotoxicity: cellular effects and blood-brain barrier transport. Neurosci Biobehav Rev. 1991;15:333–40. doi: 10.1016/s0149-7634(05)80026-0. [DOI] [PubMed] [Google Scholar]
- 24.He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, Nebert DW. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol Pharmacol. 2006;70:171–80. doi: 10.1124/mol.106.024521. [DOI] [PubMed] [Google Scholar]
- 25.Crossgrove JS, Allen DD, Bukaveckas BL, Rhineheimer SS, Yokel RA. Manganese distribution across the blood-brain barrier. I. Evidence for carrier-mediated influx of managanese citrate as well as manganese and manganese transferrin. Neurotoxicology. 2003;24:3–13. doi: 10.1016/s0161-813x(02)00089-x. [DOI] [PubMed] [Google Scholar]
- 26.Yin Z, Aschner JL, dos Santos AP, Aschner M. Mitochondrial-dependent manganese neurotoxicity in rat primary astrocyte cultures. Brain Res. 2008;1203:1–11. doi: 10.1016/j.brainres.2008.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavin CE, Gunter KK, Gunter TE. Manganese and calcium efflux kinetics in brain mitochondria. Relevance to manganese toxicity. Biochem J. 1990;266:329–34. doi: 10.1042/bj2660329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowaltowski AJ, Castilho RF, Vercesi AE. Ca(2+)-induced mitochondrial membrane permeabilization: role of coenzyme Q redox state. Am J Physiol. 1995;269:C141–7. doi: 10.1152/ajpcell.1995.269.1.C141. [DOI] [PubMed] [Google Scholar]
- 29.Liccione JJ, Maines MD. Selective vulnerability of glutathione metabolism and cellular defense mechanisms in rat striatum to manganese. J Pharmacol Exp Ther. 1988;247:156–61. [PubMed] [Google Scholar]
- 30.Erikson KM, Dorman DC, Fitsanakis V, Lash LH, Aschner M. Alterations of oxidative stress biomarkers due to in utero and neonatal exposures of airborne manganese. Biol Trace Elem Res. 2006;111:199–215. doi: 10.1385/BTER:111:1:199. [DOI] [PubMed] [Google Scholar]
- 31.Erikson KM, Dorman DC, Lash LH, Aschner M. Manganese inhalation by rhesus monkeys is associated with brain regional changes in biomarkers of neurotoxicity. Toxicol Sci. 2007;97:459–66. doi: 10.1093/toxsci/kfm044. [DOI] [PubMed] [Google Scholar]
- 32.Zhang P, Hatter A, Liu B. Manganese chloride stimulates rat microglia to release hydrogen peroxide. Toxicol Lett. 2007;173:88–100. doi: 10.1016/j.toxlet.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prabhakaran K, Ghosh D, Chapman GD, Gunasekar PG. Molecular mechanism of manganese exposure-induced dopaminergic toxicity. Brain Res Bull. 2008;76:361–7. doi: 10.1016/j.brainresbull.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Neff NH, Barrett RE, Costa E. Selective depletion of caudate nucleus dopamine and serotonin during chronic manganese dioxide administration to squirrel monkeys. Experientia. 1969;25:1140–1. doi: 10.1007/BF01900234. [DOI] [PubMed] [Google Scholar]
- 35.Chandra S, Srivastava R, Shukla G. Regional distribution of metals and biogenic amines in the brain of monkeys exposed to manganese. Toxicol Lett. 1979;4:189–192. [Google Scholar]
- 36.Bird ED, Anton AH, Bullock B. The effect of manganese inhalation on basal ganglia dopamine concentrations in rhesus monkey. Neurotoxicology. 1984;5:59–65. [PubMed] [Google Scholar]
- 37.Cai T, Yao T, Zheng G, et al. Manganese induces the overexpression of alpha-synuclein in PC12 cells via ERK activation. Brain Res. 2010;1359:201–7. doi: 10.1016/j.brainres.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 38.Guilarte TR. APLP1, Alzheimer’s-like pathology and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. Neurotoxicology. 2010;31:572–4. doi: 10.1016/j.neuro.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Penalver R. Diagnosis and treatment of manganese intoxication; report of a case. AMA Arch Ind Health. 1957;16:64–6. [PubMed] [Google Scholar]
- 40.Hauser RA, Zesiewicz TA, Rosemurgy AS, Martinez C, Olanow CW. Manganese intoxication and chronic liver failure. Ann Neurol. 1994;36:871–5. doi: 10.1002/ana.410360611. [DOI] [PubMed] [Google Scholar]
- 41.Guilarte TR, Burton NC, Verina T, Prabhu VV, Becker KG, Syversen T, Schneider JS. Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. J Neurochem. 2008;105:1948–59. doi: 10.1111/j.1471-4159.2008.05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banta RG, Markesbery WR. Elevated manganese levels associated with dementia and extrapyramidal signs. Neurology. 1977;27:213–6. doi: 10.1212/wnl.27.3.213. [DOI] [PubMed] [Google Scholar]
- 43.Hurlbert MS, Zhou W, Wasmeier C, Kaddis FG, Hutton JC, Freed CR. Mice transgenic for an expanded CAG repeat in the Huntington’s disease gene develop diabetes. Diabetes. 1999;48:649–51. doi: 10.2337/diabetes.48.3.649. [DOI] [PubMed] [Google Scholar]
- 44.Butterworth J. Changes in nine enzyme markers for neurons, glia, and endothelial cells in agonal state and Huntington’s disease caudate nucleus. J Neurochem. 1986;47:583–7. doi: 10.1111/j.1471-4159.1986.tb04539.x. [DOI] [PubMed] [Google Scholar]
- 45.Andreassen OA, Dedeoglu A, Friedlich A, Ferrante KL, Hughes D, Szabo C, Beal MF. Effects of an inhibitor of poly(ADP-ribose) polymerase, desmethylselegiline, trientine, and lipoic acid in transgenic ALS mice. Exp Neurol. 2001;168:419–24. doi: 10.1006/exnr.2001.7633. [DOI] [PubMed] [Google Scholar]
- 46.Williams BB, Li D, Wegrzynowicz M, et al. Disease-toxicant screen reveals a neuroprotective interaction between Huntington’s disease and manganese exposure. J Neurochem. 2010;112:227–37. doi: 10.1111/j.1471-4159.2009.06445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wegrzynowicz M, Holt HK, Friedman DB, Bowman AB. Changes in the striatal proteome of YAC128Q mice exhibit gene-environment interactions between mutant huntingtin and manganese. J Proteome Res. 2012;11:1118–32. doi: 10.1021/pr200839d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGale EH, Pye IF, Stonier C, Hutchinson EC, Aber GM. Studies of the interrelationship between cerebrospinal fluid and plasma amino acid concentrations in normal individuals. J Neurochem. 1977;29:291–7. doi: 10.1111/j.1471-4159.1977.tb09621.x. [DOI] [PubMed] [Google Scholar]
- 49.Hamberger A, Nystrom B. Extra- and intracellular amino acids in the hippocampus during development of hepatic encephalopathy. Neurochem Res. 1984;9:1181–92. doi: 10.1007/BF00973033. [DOI] [PubMed] [Google Scholar]
- 50.Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98:641–53. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- 51.Darmaun D, Matthews DE, Bier DM. Glutamine and glutamate kinetics in humans. Am J Physiol. 1986;251:E117–26. doi: 10.1152/ajpendo.1986.251.1.E117. [DOI] [PubMed] [Google Scholar]
- 52.Collarini EJ, Oxender DL. Mechanisms of transport of amino acids across membranes. Annu Rev Nutr. 1987;7:75–90. doi: 10.1146/annurev.nu.07.070187.000451. [DOI] [PubMed] [Google Scholar]
- 53.Tamarappoo BK, Raizada MK, Kilberg MS. Identification of a system N-like Na(+)-dependent glutamine transport activity in rat brain neurons. J Neurochem. 1997;68:954–60. doi: 10.1046/j.1471-4159.1997.68030954.x. [DOI] [PubMed] [Google Scholar]
- 54.Kilberg MS, Handlogten ME, Christensen HN. Characteristics of an amino acid transport system in rat liver for glutamine, asparagine, histidine, and closely related analogs. J Biol Chem. 1980;255:4011–9. [PubMed] [Google Scholar]
- 55.Nagaraja TN, Brookes N. Glutamine transport in mouse cerebral astrocytes. J Neurochem. 1996;66:1665–74. doi: 10.1046/j.1471-4159.1996.66041665.x. [DOI] [PubMed] [Google Scholar]
- 56.Oxender DL, Christensen HN. Evidence for two types of mediation of neutral and amino-acid transport in Ehrlich cells. Nature. 1963;197:765–7. doi: 10.1038/197765a0. [DOI] [PubMed] [Google Scholar]
- 57.Shen J, Petersen KF, Behar KL, et al. Determination of the rate of the glutamate/glutamine cycle in the human brain by in vivo 13C NMR. Proc Natl Acad Sci U S A. 1999;96:8235–40. doi: 10.1073/pnas.96.14.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rothstein JD, Martin L, Levey AI, et al. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–25. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 59.Rothstein JD, Dykes-Hoberg M, Pardo CA, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–86. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 60.Chaudhry FA, Reimer RJ, Edwards RH. The glutamine commute: take the N line and transfer to the A. J Cell Biol. 2002;157:349–55. doi: 10.1083/jcb.200201070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Melone M, Quagliano F, Barbaresi P, Varoqui H, Erickson JD, Conti F. Localization of the glutamine transporter SNAT1 in rat cerebral cortex and neighboring structures, with a note on its localization in human cortex. Cereb Cortex. 2004;14:562–74. doi: 10.1093/cercor/bhh018. [DOI] [PubMed] [Google Scholar]
- 62.Grewal S, Defamie N, Zhang X, et al. SNAT2 amino acid transporter is regulated by amino acids of the SLC6 gamma-aminobutyric acid transporter subfamily in neocortical neurons and may play no role in delivering glutamine for glutamatergic transmission. J Biol Chem. 2009;284:11224–36. doi: 10.1074/jbc.M806470200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kvamme E, Torgner IA, Roberg B. Kinetics and localization of brain phosphate activated glutaminase. J Neurosci Res. 2001;66:951–8. doi: 10.1002/jnr.10041. [DOI] [PubMed] [Google Scholar]
- 64.Bellocchio EE, Hu H, Pohorille A, Chan J, Pickel VM, Edwards RH. The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic transmission. J Neurosci. 1998;18:8648–59. doi: 10.1523/JNEUROSCI.18-21-08648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 66.Milatovic D, Yin Z, Gupta RC, Sidoryk M, Albrecht J, Aschner JL, Aschner M. Manganese induces oxidative impairment in cultured rat astrocytes. Toxicol Sci. 2007;98:198–205. doi: 10.1093/toxsci/kfm095. [DOI] [PubMed] [Google Scholar]
- 67.Lee ES, Sidoryk M, Jiang H, Yin Z, Aschner M. Estrogen and tamoxifen reverse manganese-induced glutamate transporter impairment in astrocytes. J Neurochem. 2009;110:530–44. doi: 10.1111/j.1471-4159.2009.06105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304–11. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- 69.Aschner M, Gannon M, Kimelberg HK. Manganese uptake and efflux in cultured rat astrocytes. J Neurochem. 1992;58:730–5. doi: 10.1111/j.1471-4159.1992.tb09778.x. [DOI] [PubMed] [Google Scholar]
- 70.Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360:467–71. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- 71.Pines G, Danbolt NC, Bjoras M, et al. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360:464–7. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- 72.Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci U S A. 1997;94:4155–60. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levy LM, Warr O, Attwell D. Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a Chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. J Neurosci. 1998;18:9620–8. doi: 10.1523/JNEUROSCI.18-23-09620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mutkus L, Aschner JL, Fitsanakis V, Aschner M. The in vitro uptake of glutamate in GLAST and GLT-1 transfected mutant CHO-K1 cells is inhibited by manganese. Biol Trace Elem Res. 2005;107:221–30. doi: 10.1385/BTER:107:3:221. [DOI] [PubMed] [Google Scholar]
- 75.Erikson KM, Dorman DC, Lash LH, Aschner M. Duration of airborne-manganese exposure in rhesus monkeys is associated with brain regional changes in biomarkers of neurotoxicity. Neurotoxicology. 2008;29:377–85. doi: 10.1016/j.neuro.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 76.Sidoryk-Wegrzynowicz M, Lee E, Aschner M. Mechanism of Mn(II)-mediated dysregulation of glutamine-glutamate cycle: focus on glutamate turnover. J Neurochem. 2012 doi: 10.1111/j.1471-4159.2012.07835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Susarla BT, Robinson MB. Internalization and degradation of the glutamate transporter GLT-1 in response to phorbol ester. Neurochem Int. 2008;52:709–22. doi: 10.1016/j.neuint.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Conradt M, Stoffel W. Inhibition of the high-affinity brain glutamate transporter GLAST-1 via direct phosphorylation. J Neurochem. 1997;68:1244–51. doi: 10.1046/j.1471-4159.1997.68031244.x. [DOI] [PubMed] [Google Scholar]
- 79.Gonzalez MI, Susarla BT, Robinson MB. Evidence that protein kinase Calpha interacts with and regulates the glial glutamate transporter GLT-1. J Neurochem. 2005;94:1180–8. doi: 10.1111/j.1471-4159.2005.03330.x. [DOI] [PubMed] [Google Scholar]
- 80.Gonzalez-Gonzalez IM, Garcia-Tardon N, Gimenez C, Zafra F. PKC-dependent endocytosis of the GLT1 glutamate transporter depends on ubiquitylation of lysines located in a C-terminal cluster. Glia. 2008;56:963–74. doi: 10.1002/glia.20670. [DOI] [PubMed] [Google Scholar]
- 81.Sidoryk-Wegrzynowicz M, Lee E, Albrecht J, Aschner M. Manganese disrupts astrocyte glutamine transporter expression and function. J Neurochem. 2009;110:822–30. doi: 10.1111/j.1471-4159.2009.06172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bode BP. Recent molecular advances in mammalian glutamine transport. J Nutr. 2001;131:2475S–85S. doi: 10.1093/jn/131.9.2475S. discussion 2486S–7S. [DOI] [PubMed] [Google Scholar]
- 83.Sidoryk-Wegrzynowicz M, Lee ES, Ni M, Aschner M. Manganese-induced downregulation of astroglial glutamine transporter SNAT3 involves ubiquitin-mediated proteolytic system. Glia. 2010;58:1905–12. doi: 10.1002/glia.21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Burton NC, Schneider JS, Syversen T, Guilarte TR. Effects of chronic manganese exposure on glutamatergic and GABAergic neurotransmitter markers in the nonhuman primate brain. Toxicol Sci. 2009;111:131–9. doi: 10.1093/toxsci/kfp124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boehmer C, Okur F, Setiawan I, Broer S, Lang F. Properties and regulation of glutamine transporter SN1 by protein kinases SGK and PKB. Biochem Biophys Res Commun. 2003;306:156–62. doi: 10.1016/s0006-291x(03)00921-5. [DOI] [PubMed] [Google Scholar]
- 86.Jentsch S, Schlenker S. Selective protein degradation: a journey’s end within the proteasome. Cell. 1995;82:881–4. doi: 10.1016/0092-8674(95)90021-7. [DOI] [PubMed] [Google Scholar]
- 87.Bhalla V, Daidie D, Li H, et al. Serum- and glucocorticoid-regulated kinase 1 regulates ubiquitin ligase neural precursor cell-expressed, developmentally down-regulated protein 4-2 by inducing interaction with 14-3-3. Mol Endocrinol. 2005;19:3073–84. doi: 10.1210/me.2005-0193. [DOI] [PubMed] [Google Scholar]
- 88.Zhou R, Snyder PM. Nedd4-2 phosphorylation induces serum and glucocorticoid-regulated kinase (SGK) ubiquitination and degradation. J Biol Chem. 2005;280:4518–23. doi: 10.1074/jbc.M411053200. [DOI] [PubMed] [Google Scholar]
- 89.Debonneville C, Flores SY, Kamynina E, et al. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na(+) channel cell surface expression. EMBO J. 2001;20:7052–9. doi: 10.1093/emboj/20.24.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Balkrishna S, Broer A, Kingsland A, Broer S. Rapid downregulation of the rat glutamine transporter SNAT3 by a caveolin-dependent trafficking mechanism in Xenopus laevis oocytes. Am J Physiol Cell Physiol. 2010;299:C1047–57. doi: 10.1152/ajpcell.00209.2010. [DOI] [PubMed] [Google Scholar]
- 91.Pawlik TM, Souba WW, Sweeney TJ, Bode BP. Phorbol esters rapidly attenuate glutamine uptake and growth in human colon carcinoma cells. J Surg Res. 2000;90:149–55. doi: 10.1006/jsre.2000.5872. [DOI] [PubMed] [Google Scholar]
- 92.Nissen-Meyer LS, Popescu MC, Hamdani el H, Chaudhry FA. Protein kinase C-mediated phosphorylation of a single serine residue on the rat glial glutamine transporter SN1 governs its membrane trafficking. J Neurosci. 2011;31:6565–75. doi: 10.1523/JNEUROSCI.3694-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beleboni RO, Carolino RO, Pizzo AB, Castellan-Baldan L, Coutinho-Netto J, dos Santos WF, Coimbra NC. Pharmacological and biochemical aspects of GABAergic neurotransmission: pathological and neuropsychobiological relationships. Cell Mol Neurobiol. 2004;24:707–28. doi: 10.1007/s10571-004-6913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Olsen RW, DeLore TM. GABA and Glycine. In: Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, editors. Basic Neurochemistry. Lipincott Williams and Wilkins; Philadelphia: 1999. pp. 335–346. [Google Scholar]
- 95.Ade KK, Janssen MJ, Ortinski PI, Vicini S. Differential tonic GABA conductances in striatal medium spiny neurons. J Neurosci. 2008;28:1185–97. doi: 10.1523/JNEUROSCI.3908-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Burton NC, Guilarte TR. Manganese neurotoxicity: lessons learned from longitudinal studies in nonhuman primates. Environ Health Perspect. 2009;117:325–32. doi: 10.1289/ehp.0800035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Struve MF, McManus BE, Wong BA, Dorman DC. Basal ganglia neurotransmitter concentrations in rhesus monkeys following subchronic manganese sulfate inhalation. Am J Ind Med. 2007;50:772–8. doi: 10.1002/ajim.20489. [DOI] [PubMed] [Google Scholar]
- 98.Bonilla E. Increased GABA content in caudate nucleus of rats after chronic manganese chloride administration. J Neurochem. 1978;31:551–2. doi: 10.1111/j.1471-4159.1978.tb02672.x. [DOI] [PubMed] [Google Scholar]
- 99.Gianutsos G, Murray MT. Alterations in brain dopamine and GABA following inorganic or organic manganese administration. Neurotoxicology. 1982;3:75–81. [PubMed] [Google Scholar]
- 100.Gwiazda RH, Lee D, Sheridan J, Smith DR. Low cumulative manganese exposure affects striatal GABA but not dopamine. Neurotoxicology. 2002;23:69–76. doi: 10.1016/s0161-813x(02)00002-5. [DOI] [PubMed] [Google Scholar]
- 101.Dydak U, Jiang YM, Long LL, et al. In vivo measurement of brain GABA concentrations by magnetic resonance spectroscopy in smelters occupationally exposed to manganese. Environ Health Perspect. 2011;119:219–24. doi: 10.1289/ehp.1002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brouillet EP, Shinobu L, McGarvey U, Hochberg F, Beal MF. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Exp Neurol. 1993;120:89–94. doi: 10.1006/exnr.1993.1042. [DOI] [PubMed] [Google Scholar]
- 103.Chandra SV, Malhotra KM, Shukla GS. GABAergic neurochemistry in manganese exposed rats. Acta Pharmacol Toxicol (Copenh) 1982;51:456–8. doi: 10.1111/j.1600-0773.1982.tb01053.x. [DOI] [PubMed] [Google Scholar]
- 104.Erikson KM, Shihabi ZK, Aschner JL, Aschner M. Manganese accumulates in iron-deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biol Trace Elem Res. 2002;87:143–56. doi: 10.1385/BTER:87:1-3:143. [DOI] [PubMed] [Google Scholar]
- 105.Bonilla E, Arrieta A, Castro F, Davila JO, Quiroz I. Manganese toxicity: free amino acids in the striatum and olfactory bulb of the mouse. Invest Clin. 1994;35:175–81. [PubMed] [Google Scholar]
- 106.Anderson JG, Cooney PT, Erikson KM. Brain manganese accumulation is inversely related to gamma-amino butyric acid uptake in male and female rats. Toxicol Sci. 2007;95:188–95. doi: 10.1093/toxsci/kfl130. [DOI] [PubMed] [Google Scholar]
- 107.Anderson JG, Fordahl SC, Cooney PT, Weaver TL, Colyer CL, Erikson KM. Manganese exposure alters extracellular GABA, GABA receptor and transporter protein and mRNA levels in the developing rat brain. Neurotoxicology. 2008;29:1044–53. doi: 10.1016/j.neuro.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ottersen OP, Zhang N, Walberg F. Metabolic compartmentation of glutamate and glutamine: morphological evidence obtained by quantitative immunocytochemistry in rat cerebellum. Neuroscience. 1992;46:519–34. doi: 10.1016/0306-4522(92)90141-n. [DOI] [PubMed] [Google Scholar]
- 109.Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–8. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- 110.Deng Y, Xu Z, Xu B, Xu D, Tian Y, Feng W. The Protective Effects of Riluzole on Manganese-Induced Disruption of Glutamate Transporters and Glutamine Synthetase in the Cultured Astrocytes. Biol Trace Elem Res. 2012 doi: 10.1007/s12011-012-9365-1. [DOI] [PubMed] [Google Scholar]
- 111.Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–4. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 112.Miranda M, Dionne KR, Sorkina T, Sorkin A. Three ubiquitin conjugation sites in the amino terminus of the dopamine transporter mediate protein kinase C-dependent endocytosis of the transporter. Mol Biol Cell. 2007;18:313–23. doi: 10.1091/mbc.E06-08-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Foster JD, Adkins SD, Lever JR, Vaughan RA. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem. 2008;105:1683–99. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hicke L. Gettin’ down with ubiquitin: turning off cell-surface receptors, transporters and channels. Trends Cell Biol. 1999;9:107–12. doi: 10.1016/s0962-8924(98)01491-3. [DOI] [PubMed] [Google Scholar]
- 115.Corey JL, Davidson N, Lester HA, Brecha N, Quick MW. Protein kinase C modulates the activity of a cloned gamma-aminobutyric acid transporter expressed in Xenopus oocytes via regulated subcellular redistribution of the transporter. J Biol Chem. 1994;269:14759–67. [PubMed] [Google Scholar]
- 116.Quick MW, Corey JL, Davidson N, Lester HA. Second messengers, trafficking-related proteins, and amino acid residues that contribute to the functional regulation of the rat brain GABA transporter GAT1. J Neurosci. 1997;17:2967–79. doi: 10.1523/JNEUROSCI.17-09-02967.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vina-Vilaseca A, Bender-Sigel J, Sorkina T, Closs EI, Sorkin A. Protein kinase C-dependent ubiquitination and clathrin-mediated endocytosis of the cationic amino acid transporter CAT-1. J Biol Chem. 2011;286:8697–706. doi: 10.1074/jbc.M110.186858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sorkina T, Miranda M, Dionne KR, Hoover BR, Zahniser NR, Sorkin A. RNA interference screen reveals an essential role of Nedd4-2 in dopamine transporter ubiquitination and endocytosis. J Neurosci. 2006;26:8195–205. doi: 10.1523/JNEUROSCI.1301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sidoryk-Wegrzynowicz M, Lee E, Mingwei N, Aschner M. Disruption of astrocytic glutamine turnover by manganese is mediated by the protein kinase C pathway. Glia. 2011;59:1732–43. doi: 10.1002/glia.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reyland ME. Protein kinase C isoforms: Multi-functional regulators of cell life and death. Front Biosci. 2009;14:2386–99. doi: 10.2741/3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Latchoumycandane C, Anantharam V, Kitazawa M, Yang Y, Kanthasamy A, Kanthasamy AG. Protein kinase Cdelta is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. J Pharmacol Exp Ther. 2005;313:46–55. doi: 10.1124/jpet.104.078469. [DOI] [PubMed] [Google Scholar]
- 122.Oubrahim H, Chock PB, Stadtman ER. Manganese(II) induces apoptotic cell death in NIH3T3 cells via a caspase-12-dependent pathway. J Biol Chem. 2002;277:20135–8. doi: 10.1074/jbc.C200226200. [DOI] [PubMed] [Google Scholar]
- 123.Chun HS, Lee H, Son JH. Manganese induces endoplasmic reticulum (ER) stress and activates multiple caspases in nigral dopaminergic neuronal cells, SN4741. Neurosci Lett. 2001;316:5–8. doi: 10.1016/s0304-3940(01)02341-2. [DOI] [PubMed] [Google Scholar]
- 124.Kitazawa M, Anantharam V, Yang Y, Hirata Y, Kanthasamy A, Kanthasamy AG. Activation of protein kinase C delta by proteolytic cleavage contributes to manganese-induced apoptosis in dopaminergic cells: protective role of Bcl-2. Biochem Pharmacol. 2005;69:133–46. doi: 10.1016/j.bcp.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 125.Anantharam V, Kitazawa M, Wagner J, Kaul S, Kanthasamy AG. Caspase-3-dependent proteolytic cleavage of protein kinase Cdelta is essential for oxidative stress-mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. J Neurosci. 2002;22:1738–51. doi: 10.1523/JNEUROSCI.22-05-01738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sun F, Kanthasamy A, Song C, Yang Y, Anantharam V, Kanthasamy AG. Proteasome inhibitor-induced apoptosis is mediated by positive feedback amplification of PKCdelta proteolytic activation and mitochondrial translocation. J Cell Mol Med. 2008;12:2467–81. doi: 10.1111/j.1582-4934.2008.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]