Abstract
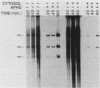
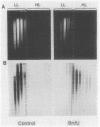
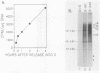
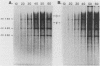
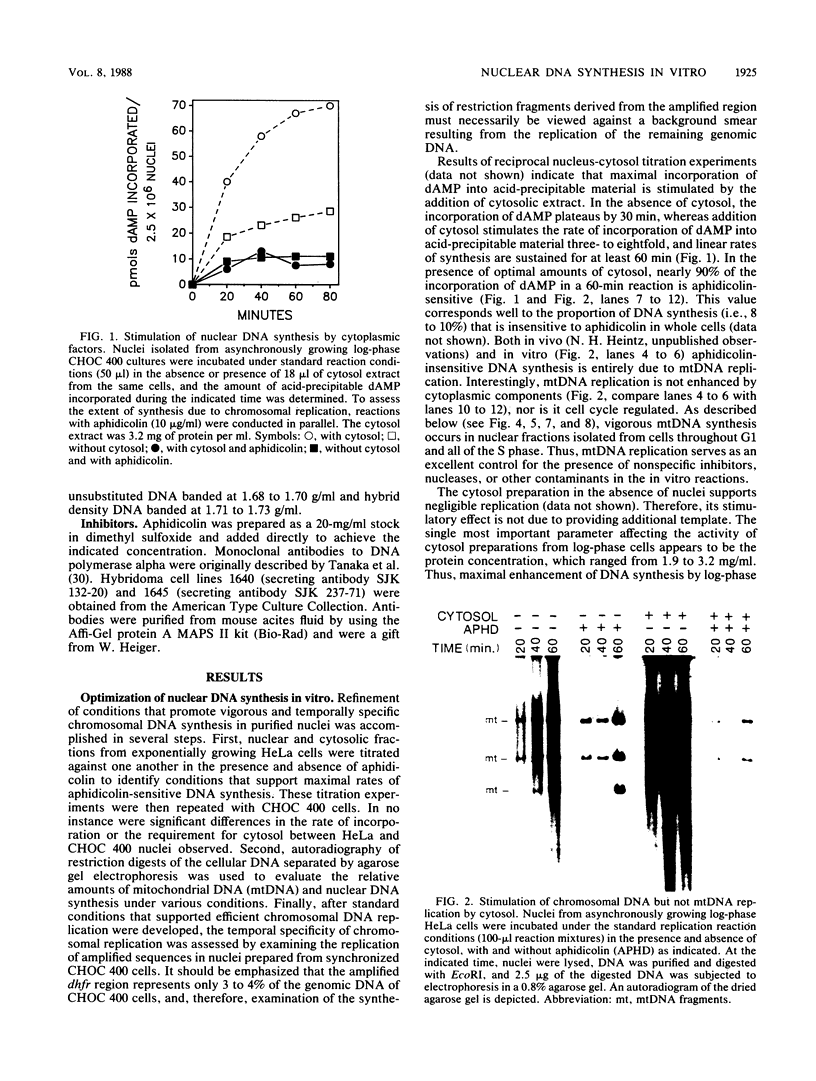
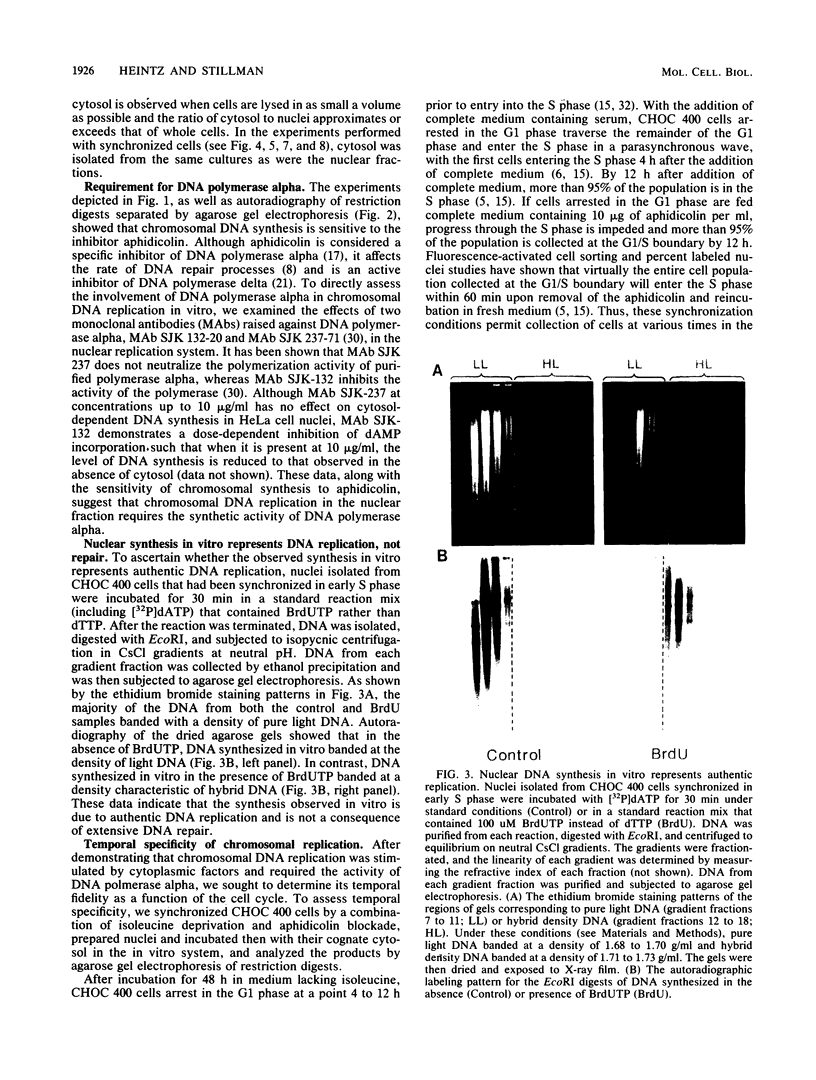
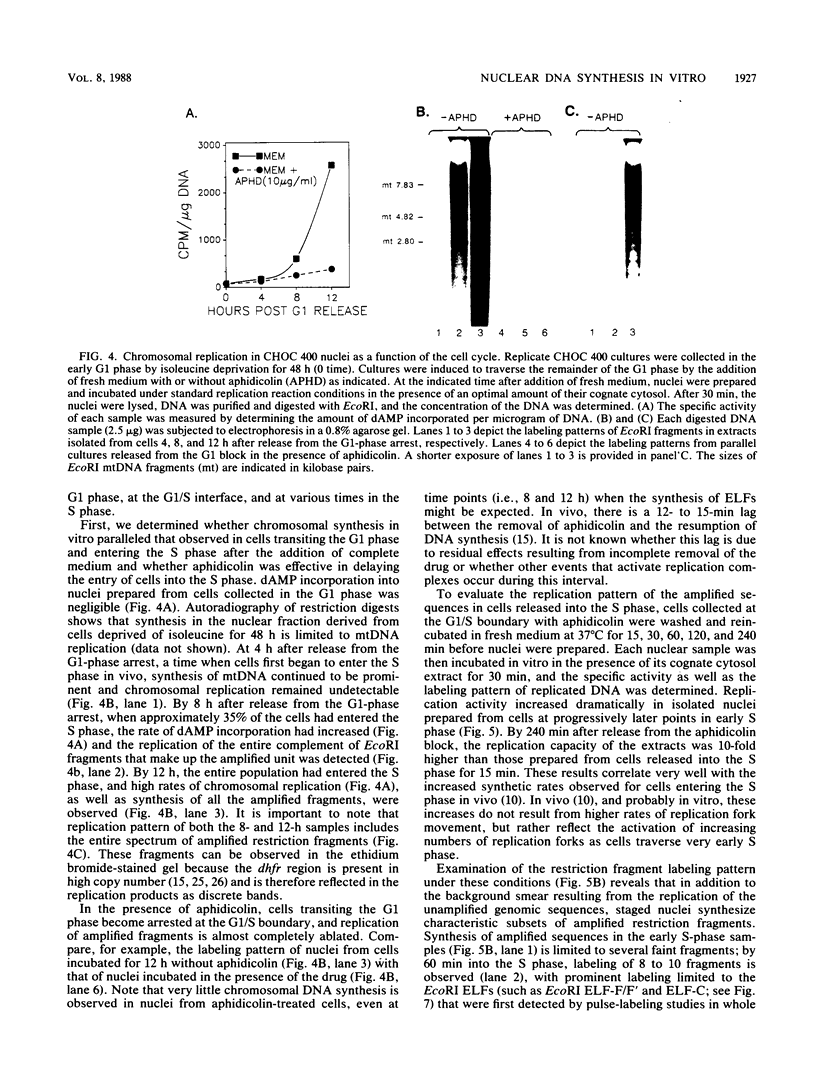
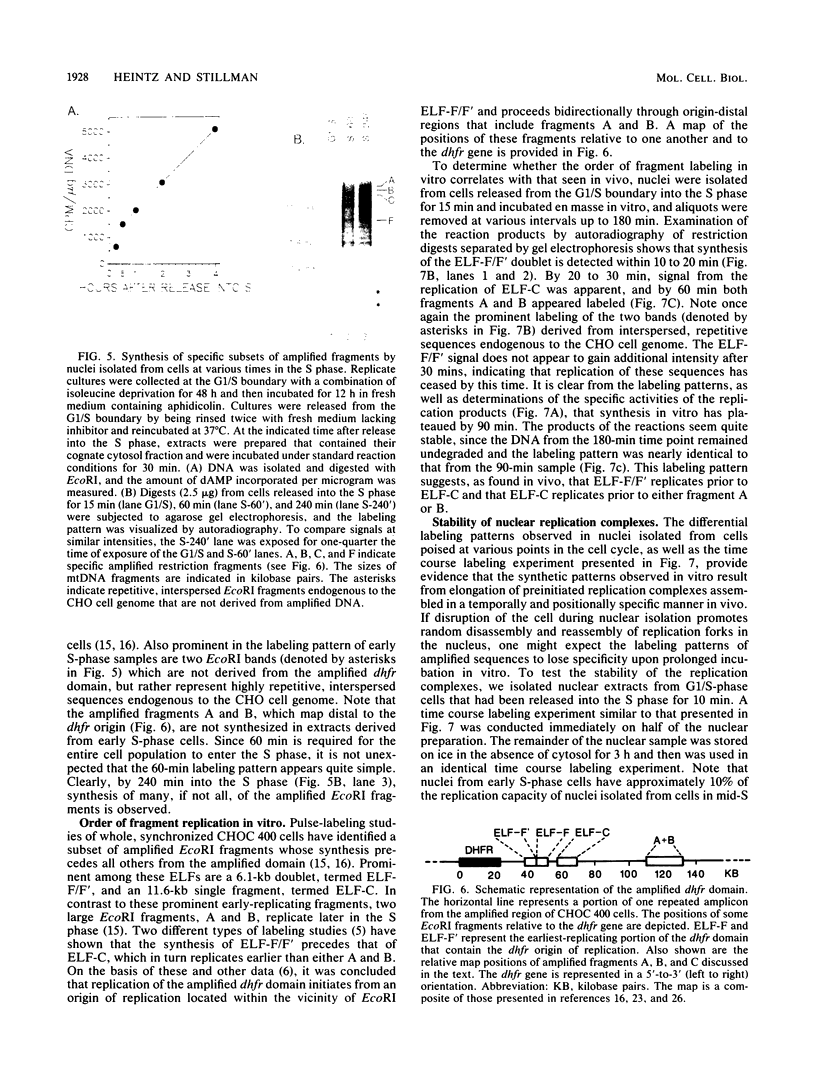
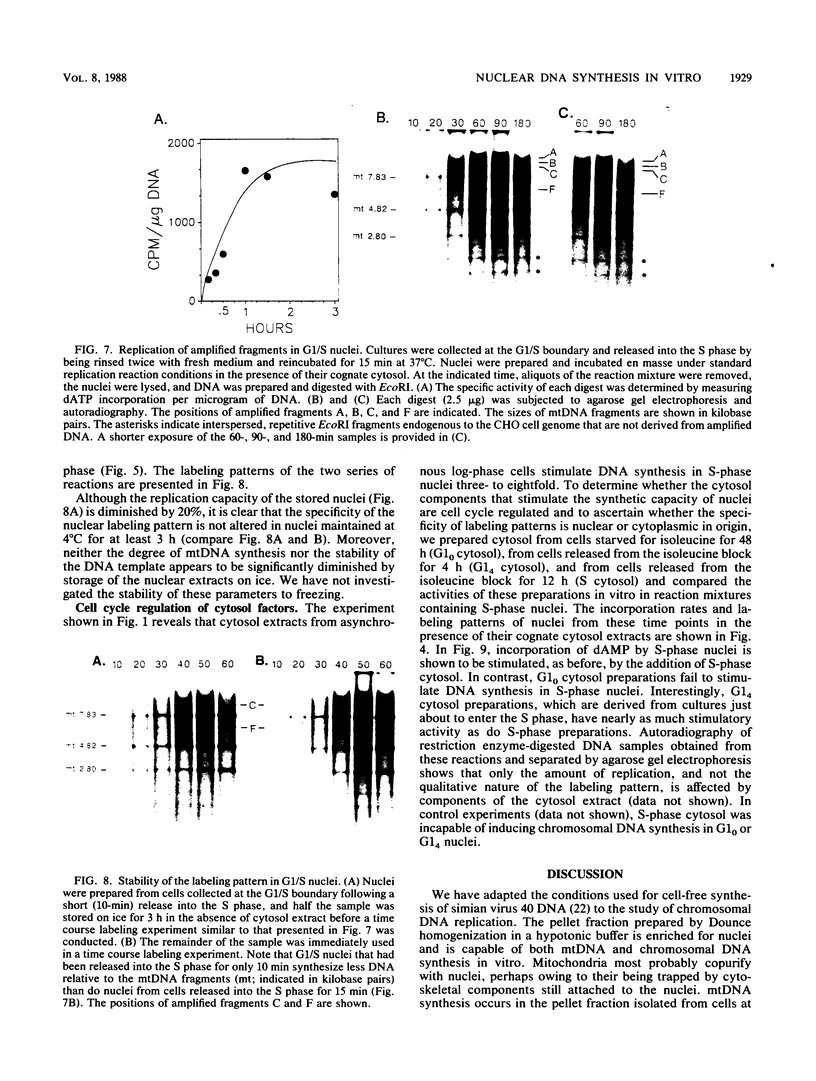
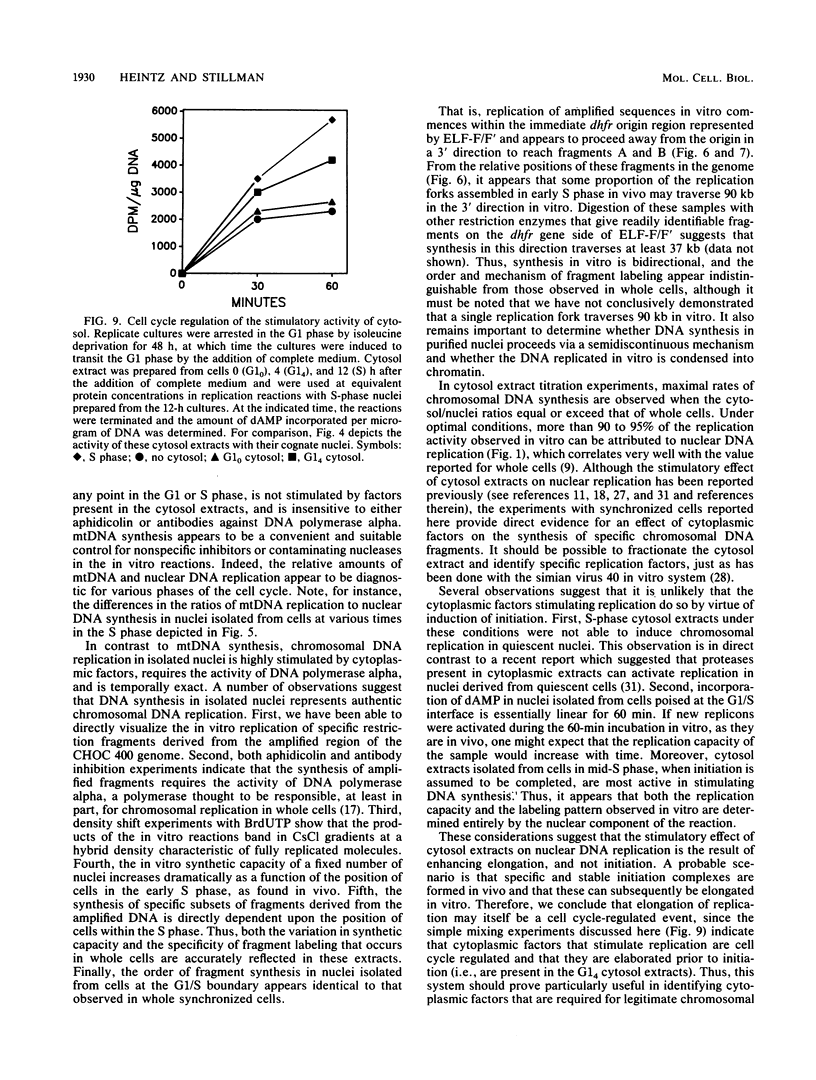
A cell-free nuclear replication system that is S-phase specific, that requires the activity of DNA polymerase alpha, and that is stimulated three- to eightfold by cytoplasmic factors from S-phase cells was used to examine the temporal specificity of chromosomal DNA synthesis in vitro. Temporal specificity of DNA synthesis in isolated nuclei was assessed directly by examining the replication of restriction fragments derived from the amplified 200-kilobase dihydrofolate reductase domain of methotrexate-resistant CHOC 400 cells as a function of the cell cycle. In nuclei prepared from cells collected at the G1/S boundary of the cell cycle, synthesis of amplified sequences commenced within the immediate dihydrofolate reductase origin region and elongation continued for 60 to 80 min. The order of synthesis of amplified restriction fragments in nuclei from early S-phase cells in vitro appeared to be indistinguishable from that in vivo. Nuclei prepared from CHOC 400 cells poised at later times in the S phase synthesized characteristic subsets of other amplified fragments. The specificity of fragment labeling patterns was stable to short-term storage at 4 degrees C. The occurrence of stimulatory factors in cytosol extracts was cell cycle dependent in that minimal stimulation was observed with early G1-phase extracts, whereas maximal stimulation was observed with cytosol extracts from S-phase cells. Chromosomal synthesis was not observed in nuclei from G1 cells, nor did cytosol extracts from S-phase cells induce chromosomal replication in G1 nuclei. In contrast to chromosomal DNA synthesis, mitochondrial DNA replication in vitro was not stimulated by cytoplasmic factors and occurred at equivalent rates throughout the G1 and S phases. These studies show that chromosomal DNA replication in isolated nuclei is mediated by stable replication forks that are assembled in a temporally specific fashion in vivo and indicate that the synthetic mechanisms observed in vitro accurately reflect those operative in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blow J. J., Laskey R. A. Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell. 1986 Nov 21;47(4):577–587. doi: 10.1016/0092-8674(86)90622-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braunstein J. D., Schulze D., DelGiudice T., Furst A., Schildkraut C. L. The temporal order of replication of murine immunoglobulin heavy chain constant region sequences corresponds to their linear order in the genome. Nucleic Acids Res. 1982 Nov 11;10(21):6887–6902. doi: 10.1093/nar/10.21.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. H., Iqbal M. A., Stuart S., Hatton K. S., Valinsky J., Schildkraut C. L. Rate of replication of the murine immunoglobulin heavy-chain locus: evidence that the region is part of a single replicon. Mol Cell Biol. 1987 Jan;7(1):450–457. doi: 10.1128/mcb.7.1.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burhans W. C., Selegue J. E., Heintz N. H. Isolation of the origin of replication associated with the amplified Chinese hamster dihydrofolate reductase domain. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7790–7794. doi: 10.1073/pnas.83.20.7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burhans W. C., Selegue J. E., Heintz N. H. Replication intermediates formed during initiation of DNA synthesis in methotrexate-resistant CHOC 400 cells are enriched for sequences derived from a specific, amplified restriction fragment. Biochemistry. 1986 Jan 28;25(2):441–449. doi: 10.1021/bi00350a025. [DOI] [PubMed] [Google Scholar]
- Campbell J. L. Eukaryotic DNA replication. Annu Rev Biochem. 1986;55:733–771. doi: 10.1146/annurev.bi.55.070186.003505. [DOI] [PubMed] [Google Scholar]
- Ciarrocchi G., Jose J. G., Linn S. Further characterization of a cell-free system for measuring replicative and repair DNA synthesis with cultured human fibroblasts and evidence for the involvement of DNA polymerase alpha in DNA repair. Nucleic Acids Res. 1979 Nov 10;7(5):1205–1219. doi: 10.1093/nar/7.5.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro-Stone M., Kaufman D. G. Kinetics of DNA replication in C3H 10T1/2 cells synchronized by aphidicolin. Biochemistry. 1985 Aug 27;24(18):4815–4822. doi: 10.1021/bi00339a015. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Huberman J. A. Eukaryotic chromosome replication. Annu Rev Genet. 1975;9:245–284. doi: 10.1146/annurev.ge.09.120175.001333. [DOI] [PubMed] [Google Scholar]
- Floros J., Chang H., Baserga R. Stimulated DNA synthesis in frog nuclei by cytoplasmic extracts of temperature-sensitive mammalian cells. Science. 1978 Aug 18;201(4356):651–652. doi: 10.1126/science.675253. [DOI] [PubMed] [Google Scholar]
- Hamlin J. L., Biedler J. L. Replication pattern of a large homogenously staining chromosome region in antifolate-resistant Chinese hamster cell lines. J Cell Physiol. 1981 Apr;107(1):101–114. doi: 10.1002/jcp.1041070112. [DOI] [PubMed] [Google Scholar]
- Hand R. Eucaryotic DNA: organization of the genome for replication. Cell. 1978 Oct;15(2):317–325. doi: 10.1016/0092-8674(78)90001-6. [DOI] [PubMed] [Google Scholar]
- Heintz N. H., Hamlin J. L. An amplified chromosomal sequence that includes the gene for dihydrofolate reductase initiates replication within specific restriction fragments. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4083–4087. doi: 10.1073/pnas.79.13.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N. H., Milbrandt J. D., Greisen K. S., Hamlin J. L. Cloning of the initiation region of a mammalian chromosomal replicon. 1983 Mar 31-Apr 6Nature. 302(5907):439–441. doi: 10.1038/302439a0. [DOI] [PubMed] [Google Scholar]
- Huberman J. A. New views of the biochemistry of eucaryotic DNA replication revealed by aphidicolin, an unusual inhibitor of DNA polymerase alpha. Cell. 1981 Mar;23(3):647–648. doi: 10.1016/0092-8674(81)90426-8. [DOI] [PubMed] [Google Scholar]
- James C. D., Leffak M. Polarity of DNA replication through the avian alpha-globin locus. Mol Cell Biol. 1986 Apr;6(4):976–984. doi: 10.1128/mcb.6.4.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitani T., Yoda K., Okazaki T. Discontinuous DNA replication of Drosophila melanogaster is primed by octaribonucleotide primer. Mol Cell Biol. 1984 Aug;4(8):1591–1596. doi: 10.1128/mcb.4.8.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lee M. Y., Tan C. K., Downey K. M., So A. G. Further studies on calf thymus DNA polymerase delta purified to homogeneity by a new procedure. Biochemistry. 1984 Apr 24;23(9):1906–1913. doi: 10.1021/bi00304a003. [DOI] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney J. E., Hamlin J. L. Isolation of the amplified dihydrofolate reductase domain from methotrexate-resistant Chinese hamster ovary cells. Mol Cell Biol. 1987 Feb;7(2):569–577. doi: 10.1128/mcb.7.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt J. D., Heintz N. H., White W. C., Rothman S. M., Hamlin J. L. Methotrexate-resistant Chinese hamster ovary cells have amplified a 135-kilobase-pair region that includes the dihydrofolate reductase gene. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6043–6047. doi: 10.1073/pnas.78.10.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya-Zavala M., Hamlin J. L. Similar 150-kilobase DNA sequences are amplified in independently derived methotrexate-resistant Chinese hamster cells. Mol Cell Biol. 1985 Apr;5(4):619–627. doi: 10.1128/mcb.5.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. T., Kajiwara K., Mueller G. C. Role of cytosol proteins in DNA chain growth and chromatin replication in Friend erythroleukemia cell nuclei. Biochim Biophys Acta. 1981 May 29;653(3):391–407. doi: 10.1016/0005-2787(81)90195-7. [DOI] [PubMed] [Google Scholar]
- Prelich G., Kostura M., Marshak D. R., Mathews M. B., Stillman B. The cell-cycle regulated proliferating cell nuclear antigen is required for SV40 DNA replication in vitro. Nature. 1987 Apr 2;326(6112):471–475. doi: 10.1038/326471a0. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Hu S. Z., Wang T. S., Korn D. Preparation and preliminary characterization of monoclonal antibodies against human DNA polymerase alpha. J Biol Chem. 1982 Jul 25;257(14):8386–8390. [PubMed] [Google Scholar]
- Wong R. L., Gutowski J. K., Katz M., Goldfarb R. H., Cohen S. Induction of DNA synthesis in isolated nuclei by cytoplasmic factors: inhibition by protease inhibitors. Proc Natl Acad Sci U S A. 1987 Jan;84(1):241–245. doi: 10.1073/pnas.84.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynford-Thomas D., LaMontagne A., Marin G., Prescott D. M. Location of the isoleucine arrest point in CHO and 3T3 cells. Exp Cell Res. 1985 Jun;158(2):525–532. doi: 10.1016/0014-4827(85)90476-8. [DOI] [PubMed] [Google Scholar]