Abstract
Background
Both emotion regulation and impulsivity are core aspects of borderline personality disorder (BPD) pathology. Although both problems may be combined specifically in BPD, few studies to date have investigated the emotional modulation of impulsivity in BPD.
Methods
Women with BPD and matched healthy controls performed go/no-go tasks after induction of anger, joy or a neutral mood by vocally presented short stories. Dependent variables were the behavioural results and functional magnetic resonance imaging data.
Results
We included 17 women with BPD and 18 controls in our study. No behavioural group differences were found. However, patients with BPD showed stronger activation of the left amygdala and weaker activation of the subgenual anterior cingulate during anger induction than controls. Inhibition in the go/no-go task after anger induction increased activity in the left inferior frontal cortex in controls, but not in women with BPD, who, in turn, showed increased activation in the subthalamic nucleus.
Limitations
Findings cannot be generalized to men, and 4 patients were taking antidepressant medication (selective serotonin reuptake inhibitors). In addition, no patient control group was investigated, thus we do not know whether findings are specific to BPD compared with other disorders.
Conclusion
Our findings are consistent with the view that a disturbed amygdala–prefrontal network in patients with BPD is compensated by a subcortical loop involving the subthalamic nucleus, leading to normal behavioural inhibition in these patients.
Introduction
Impulsivity and emotion regulation problems are core traits of borderline personality disorder (BPD) and are related to other symptoms and problematic behaviours.1 With respect to emotion dysregulation, BPD is characterized by elevated levels of all negative emotions.2 Neuroimaging studies and psychophysiological research into emotional responses have indicated that BPD is characterized by elevated and prolonged levels of negative emotional responses, especially to threatening stimuli associated with abuse or abandonment.3,4 In neuroimaging studies, the experience of strong negative emotions in patients with BPD is reflected by frontolimbic dysfunctions5 (i.e., increased amygdala and hippocampal responses to threatening stimuli,6–9 reduced anterior cingulate [ACC] and prefrontal cortical activations9–11).
Most studies, particularly functional magnetic resonance imaging (fMRI) studies, have not investigated specific emotions; emotional stimuli are usually stressful and negatively valenced pictures from the International Affective Picture System.12 In particular, the emotion of anger has rarely been investigated, which is surprising given that exaggerated anger is a diagnostic criterion of BPD. To our knowledge, no fMRI study investigating anger has been conducted so far in patients with BPD. In healthy participants, the experience of anger is associated with activations in the medial (MPFC), ventromedial (vmPFC) and lateral prefrontal cortex; ACC; posterior cingulate cortex (PCC); and thalamus.13
Impulsivity in BPD is defined by the disinhibition of behaviours that are usually highly restricted, such as uncontrolled eating, excessive spending, sex or aggression. Accordingly, self-reports of impulsivity are generally elevated in patients with BPD compared with both healthy and clinical control groups.14–16 However, well-controlled studies investigating inhibitory control with experimental tasks, such as the Stroop test, stop or go/no-go tasks, generally render mixed results. Some studies have reported performance deficits in go/no-go tasks,17,18 while most others did not show impairments in patients with BPD.16,19,20 Recent behavioural studies suggest that general deficits of BPD regarding impulsive behaviours may occur more on the motivational level in domains such as decision-making and delay of gratification.20–22
Clinically, emotion dysregulation and impulsivity are closely linked in BPD.23 Some experimental studies showed significant interactions between impulse control and negative emotions in patients with BPD. Chapman and colleagues24 found that negative emotional state moderated the reponses of participants with severe BPD features in a passive avoidance learning task. In an fMRI study, Silbersweig and colleagues8 found decreased vmPFC activity (including medial orbitofrontal and subgenual anterior cingulate) in patients with BPD compared with healthy controls under conditions associated with the interaction of behavioural inhibition and BPD-related negative emotion in an emotional go/no-go task. In patients with BPD, decreasing vmPFC and increasing extended amygdalar–ventral striatal activity correlated highly with negative emotion. However, results regarding the interaction between impulsivity and emotion in patients with BPD are mixed, since 2 studies did not find (behavioural) group effects in the performance of a Stroop test using emotional stimulus materials.15,20
Note that all these studies investigated negative emotions in general. To our knowledge, no study has investigated motor inhibition in the context of anger yet, although both problems are prominent in patients with BPD. Furthermore, anger and impulsivity are closely linked in different conditions, such as conduct disorders,25 suicidality26 and bulimia nervosa.27 In the present study, we aimed to investigate how inhibition is modulated by the emotion of anger in women with BPD. An anger induction task was followed by an emotionally neutral letter-based go/no-go task assessing motor inhibition. In accordance with the findings of Silbersweig and colleagues,8 we expected group differences in brain activation in the go/no-go task after anger induction, with reduced prefrontal activation in women with BPD compared with healthy controls. For anger induction itself, we expected an increase in amygdala activity in women with BPD.
Methods
Participants
We recruited female patients with BPD from the inpatient and outpatient services of the Department of Psychiatry and Psychotherapy of the University Hospital Freiburg, Germany; healthy female control participants matched for age and years of education were recruited by means of advertisement in a local paper. Exclusion criteria for the BPD group were a current psychotropic medication besides selective serotonin resuptake inhibitors (SSRIs), a current diagnosis of depression or substance dependence, a lifetime diagnosis of schizophrenia or bipolar disorder, male sex, vision or auditory problems and language problems. A further exclusion criterion for the control group was any current Axis I or II psychiatric diagnosis. Participants signed written informed consent before study participation. The study was approved by the local ethics committee, Ethik Kommission der Albert-Ludwigs-Universitaet.
Current and lifetime psychiatric diagnoses were confirmed with SCID interviews both for Axis I and Axis II disorders.28,29 We assessed mean intelligence using the Mehrfachwahl-Wortschatz-Intelligenztest, a linguistic intelligence test commonly used in the German-speaking region.30
Assessments
The UPPS impulsiveness scale31 comprises 45 items with 4-point answering formats measuring a total of 4 scales. Urgency is characterized by a lack of control over action impulses. It covers the experience of strong impulses, often associated with negative emotions. Lack of premeditation describes a lack of anticipating the consequences of one’s own actions. Participants with high scores on this scale typically act very spontaneously. Lack of perseverance measures difficulties in continuing tasks that are boring or difficult. Sensation seeking comprises a tendency to enjoy and seek exciting activities and an openness to new experiences, even if they are risky. The UPPS shows good psychometric properties.31
We assessed state and trait anxiety and anger using the State-Trait Anxiety Inventory (STAI) and State-Trait Anger Expression Inventory (STAXI).32 Severity of BPD symptoms was assessed using the Borderline Personality Disorder Severity Index (BPDSI).33 This semistructured interview consists of 71 items assessesing frequency and severity of the 9 DSM-IV criteria of BPD and has excellent psychometric properties.33
Experimental procedures
During the scanning sessions, participants listened to 3 different short stories, which robustly induced anger, joy and a neutral mood, respectively, as previously described.34 We chose joy as an additional control condition because we expected no particular interaction between joy and inhibitory control. Each story lasted 4 minutes and was followed by a go/no-go task. The experimental procedure is described in Appendix 1, Figure S1, available at cma.ca/jpn. The order of story presentation was counterbalanced to control for habituation and time effects. Before each story, a relaxation instruction was given.
Emotion induction
All 3 stories covered everyday plots. In the anger condition, the protagonist is treated disrespectfully: a friend borrows the protagonist’s bike, but does not return it in time despite the protagonist needing it urgently. In the joy condition, a friend wins a cruise in a lottery and invites the protagonist to join her. In the neutral condition, the protagonist follows her morning routine, gets the newspaper and prepares breakfast. Participants were instructed to put themselves into the protagonist’s perspective while listening to the story. In prior studies, the stories had been shown to induce anger, joy and a neutral mood, respectively.34 Participants rated the intensity of anger and joy before and after each story and after the go/no-go task on a visual analogue scale via mouse click; the scale was later transformed to values between 0 and 10.
Go/no-go task
In go/no-go tasks, participants are confronted with a rapid, continous series of stimuli inducing a so-called prepotent motor response. Most stimuli require an active reaction (usually a key press), whereas for some stimuli the reaction has to be suppressed. Go/no-go tasks reliably assess inhibitory components of impulse control.35,36 The go/no-go task used in the present study was a modified version of the task used by Garavan and colleagues37 with a mixed blocked/event-related design comparable to that used in the study by Silbersweig and colleagues.8 The task was rather simple to ensure a high rate of correct responses, since the aim of the study was to investigate brain activation during successful motor inhibition. Participants were presented a series of letters, each appearing on the screen for 1.5 seconds. They were instructed to press the button corresponding to the letter shown as fast as possible for all letters except “X”; they were instructed to inhibit this reaction when “X” appeared as the stimulus. Each go/no-go session after an emotion induction consisted of 5 go blocks with 16 go stimuli and 5 no-go blocks with 10 go and 6 no-go stimuli. Between blocks, a fixation cross was presented for 20 seconds. The order of letters was randomized except for no-go stimuli, which were pseudeoranomized. Each no-go block started with a minimum of 2 go stimuli to induce a strong prepotent reaction tendency; for the same reason, a maximum of 2 no-go stimuli was presented in sequence. For the stimulus presentation, we used Presentation software version 11.0 (Neurobehavioural Systems Inc.).
Functional MRI data acquisition
Imaging data were acquired using a 3 T Magnetom TIM Trio scanner (Siemens Medical Systems) at the University Hospital Freiburg. Functional T2*-weighted echo-planar imaging (EPI) scans were acquired under the following parameters: repetition time (TR) 2000 ms, echo time (TE) 30 ms, flip angle 90°, 40 slices (slice thickness 3 mm), field of view (FOV) 192 × 192 mm, isotopic voxel size of 3 × 3 × 3 mm. We applied fully automated prospective acquisition correction for motion38 and distortion correction based on point spread function mapping39 to reduce susceptibility artifacts in mesiotemporal and orbitofrontal regions. We acquired 375 images per session. After the EPI acquisition, a reference T1-weighted anatomic image was assessed with a magnetization prepared rapid acquisition gradient echo sequence (TR 2200 ms, TR 4.11 ms, flip angle 12°, FOV 25 mm, voxel size 1 × 1 × 1 mm).
Image processing and data analysis
Data preprocessing was conducted using Statistical Parametric Mapping software (SPM5, Wellcome Trust Centre for Neuroimaging) and based on MATLAB software version 7 (MathWorks).
The first 5 functional images of each run were discarded to allow for equilibrium effects. First, images were manually reoriented to the T1 template of SPM5. Then, several preprocessing steps were carried out on the remaining functional images for each task separately. To spatially correct for residual interscan movement artifacts, images were realigned to the first image of the first run using a rigid body transformation with 6 degrees of freedom. The realigned functional images were coregistered to T1-weighted anatomic images. Subsequently, both the realigned functional images and the anatomic images were spatially normalized (linear and nonlinear transformations) into the reference system of the Montreal Neurological Institute’s (MNI) reference brain, and normalization parameters were applied to the functional images. Finally, the normalized functional data were smoothed with a 3-dimensional isotropic Gaussian kernel (12 mm full-width at half-maximum) to enhance signal-to-noise ratio and to account for residual differences in functional neuroanatomy among participants that persisted after normalization.
Statistical analysis
We analyzed psychometric and behavioural data using SPSS software version 14. We calculated t tests for questionnaire data to compare the 2 groups, with Bonferroni corrections including all scales. Emotion ratings were analyzed with repeated-measures multivariate analysis of variance (MANOVA) for each emotion with condition (anger, joy, neutral) and time (t1, t2, t3) as within-subjects factors and group (control v. BPD) as a fixed factor, with a significance threshold of 5%. We analyzed performance in the go/no-go tasks using repeated-measures analysis of variance (ANOVA) for both error types (commission v. omission) separately with condition as a within-subject factor, error type as a dependent variable and group as a fixed factor.
Statistical analyses of imaging data were performed with SPM5. We used a 2-stage general linear model to examine the effect sizes of the key group/condition contrasts. First, 2 voxel-wise multiple linear regression models were used at the individual participant level for mood induction and go/no-go parts of the experiment, respectively. These models included the principal regressors of interest, which consisted of the onset times and length (i.e., blocked modelling) of conditions of interest (anger, joy and neutral for the mood induction phase, i.e., 3 conditions; and go and no-go for each mood, i.e., 6 conditions) convolved with a prototypical hemodynamic response function and constant for each run. Data were high-pass filtered with a cut-off period of 128 seconds to remove low-frequency artifacts in the time-series. We analyzed differences in brain activation with appropriate t test contrasts. We used corresponding SPM full factorial models (2-way ANOVA design with group [control v. BPD] as a between-subjects factor and condition as the within-subjects factor) to assess voxel activation changes depending on condition and group in the second-level group analysis. Contrasts of interest were chosen following the method of Silbersweig and colleagues.8 In the first second-level full factorial model, we compared anger induction with neutral mood (or joy) between patients and controls: [patients (anger–neutral) minus controls (anger–neutral)]. In the second second-level full factorial model, no-go alone (inhibition not controlled for motor activity) and no-go minus go (inhibition controlled for motor activity) was compared between patients and controls either across mood inductions [patients (no-go – go) minus controls (no-go – go)] or between moods [patients (no-goanger – no-goneutral) minus controls (no-goanger – no-goneutral) and patients (no-goanger – goanger × no-goneutral – goneutral) minus controls (no-goanger – goanger × no-goneutral – goneutral)].
Based on a priori hypotheses, regions of interest (ROI) were the amygdala, subgenual ACC (sgACC), orbitofrontal cortex, inferior frontal cortex (IFC), dorsolateral prefrontal cortex and nucleus subthalamicus. We defined ROIs on the basis of a previously reported fMRI study concerned with impulse control and affect regulation in patients with BPD.8 We used ROI masks from the study by Tzourio-Mazoyer and colleagues.40 For the nucleus subthalamicus, a sphere-based correction was used based on the method of Aron and Poldrack,36 although we defined it more restrictively at MNI space x, y, z = 0, −15, −5, with a sphere size of 5.5 mm.
Using small volume correction (SVC) for ROIs, we considered results to be significant at p < 0.05, family wise error (FWE)–corrected. In regions outside the small-volume ROIs, FWE whole brain correction was used.
Results
Participants
A total of 24 female patients with BPD and 23 controls were recruited for participation. Of the patients with BPD, 1 was excluded owing to current alcohol dependency, 1 did not finish fMRI scanning owing to claustrophobia, and 5 were excluded owing to head movements during fMRI scanning, leaving 17 women with BPD for our analyses. Of the controls, 1 was excluded owing to severe anorexia nervosa in her youth with possible remaining cerebral changes, and 4 were excluded owing to head movements, leaving 18 controls for our analyses. The mean age of the total group was 28.4 years (BPD 28.9 [standard deviation (SD) 7.7] yr v. control 28.0 [SD 6.9] yr; p = 0.70). The mean number of years of education was 12.1 (SD 1.5) in both groups (p = 0.92). Mean intelligence of the total group, as assessed with the Mehrfachwahl-Wortschatz-Intelligenztest, was 104 with no group differences (p = 0.83). All participants were right-handed, and all but 5 (4 women with BPD, 1 control) were free of psychotropic medication except SSRIs. All participants had sufficient vision, and none had severe medical conditions.
All participants with BPD fulfilled DSM-IV diagnostic criteria. Comorbid current Axis I disorders were alcohol abuse (n = 1), panic disorder (n = 1), obsessive–compulsive disorder (n = 4), generalized anxiety disorder (n = 1), posttraumatic stress disorder (n = 5), bulimia nervosa (n = 1), binge eating disorder (n = 1) and attention-deficit/hyperactivity disorder (n = 4). No patient fulfilled criteria for current depression, since this was an exclusion criterion. However, 9 patients reported a lifetime diagnosis of depression. Comorbid Axis II disorders in the BPD group were avoidant personality disorder (n = 7), dependent personality disorder (n = 5), obsessive–compulsive personality disorder (n = 1) and paranoid personality disorder (n = 1).
Psychometric data
Psychometric data are given in Table 1. The BPD group scored significantly higher on most negative emotion and impulsivity scales.
Table 1.
Psychometric data of 17 patients with borderline personality disorder and 18 healthy controls
| Group, mean (SD) | |||
|---|---|---|---|
|
|
|||
| Variable | BPD | Control | p value |
| Beck Depression Inventory41 | 26.3 (11.0) | 5.6 (5.0) | < 0.001* |
| STAI, STAXI | |||
| State anxiety | 53.2 (8.7) | 38.1 (7.2) | < 0.001* |
| Trait anxiety | 62.6 (6.3) | 41.0 (10.2) | < 0.001* |
| State anger | 15.5 (7.8) | 11.8 (2.4) | 0.07 |
| Trait anger | 26.3 (7.1) | 18.4 (4.7) | 0.001† |
| UPPS impulsivity questionnaire | |||
| Premediation | 39.1 (6.9) | 28.3 (7.7) | < 0.001* |
| Urgency | 49.0 (5.8) | 32.5 (9.8) | < 0.001* |
| Sensation seeking | 39.8 (8.8) | 35.7 (9.7) | 0.20 |
| Lack of perseverance | 30.0 (4.2) | 24.1 (5.1) | 0.001* |
| BPDSI | |||
| Abandonment | 3.33 (1.60) | — | — |
| Interpersonal relationships | 3.42 (1.45) | — | — |
| Identity | 2.30 (1.03) | — | — |
| Impulsivity | 2.09 (1.00) | — | — |
| Parasuicidal behaviour | 1.37 (1.30) | — | — |
| Affective instability | 7.19 (1.94) | — | — |
| Emptiness | 6.04 (3.02) | — | — |
| Outbursts of anger | 3.16 (2.00) | — | — |
| Dissociation and paranoid ideation | 2.30 (2.15) | — | — |
| Total score | 35.36 (6.57) | — | — |
BPD = borderline personality disorder; BPDSI = Borderline Personality Disorder Severity Index;33 SD = standard deviation; STAI = State-Trait Anxiety Inventory;32 STAXI = State-Trait Anger Expression Inventory;32 UPPS = Urgency, Premeditation, Lack of Perseverance, Sensation Seeking.31
5% α error significance after Bonferroni correction for multiple comparisons, in which all tests (scales) were included.
1% α error significance.
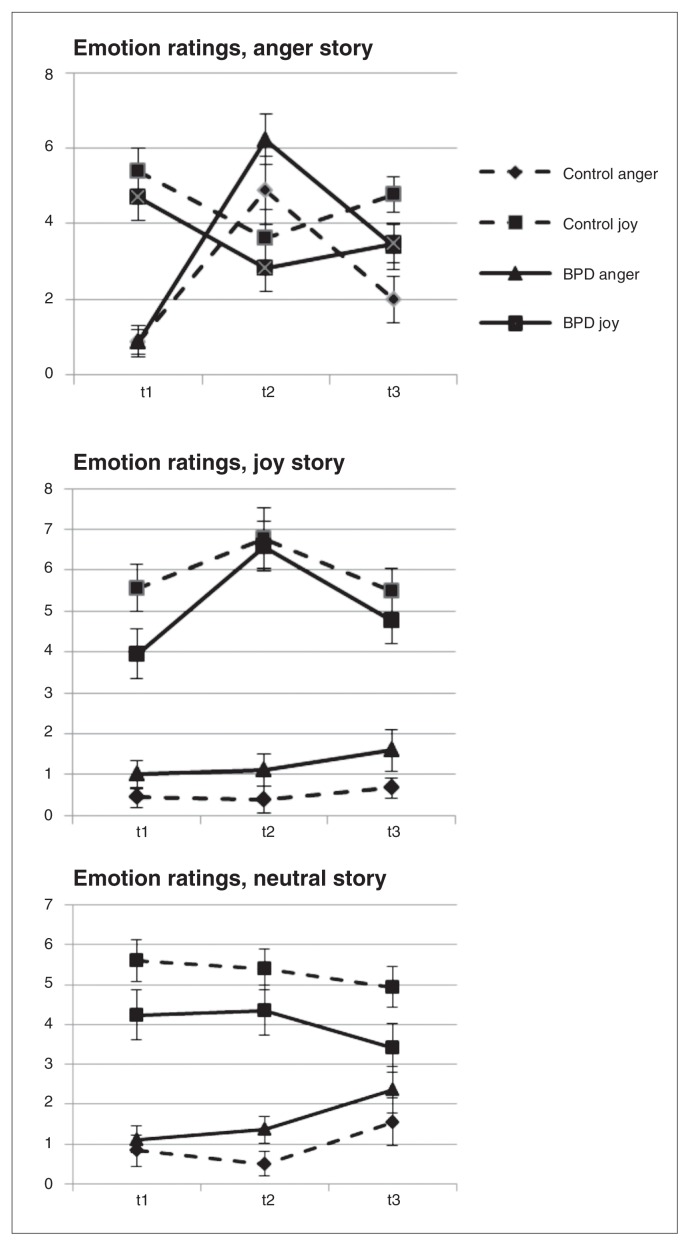
Emotion ratings
Figure 1 depicts the self-ratings of joy and anger on visual analogue scales (range 0–10) in the 2 groups for the 3 different conditions. The MANOVA results were as follows. For anger, multivariate tests showed strong and significant effects for condition (F2,32 = 31.5, ηρ2 = 0.663, p < 0.001), time (F2,32 = 37.8, ηρ2 = 0.702, p < 0.001) and condition × time (F4,30 = 16.4, ηρ2 = 0.686, p < 0.001). For joy, multivariate tests showed significant effects for condition (F2,36 = 21.4, ηρ2 = 0.572, p < 0.001) and condition × time (F4,30 = 7.9, ηρ2 = 0.512, p < 0.001) and a weaker but still significant effect of time (F2,32 = 6.0, ηρ2 = 0.273, p = 0.006).
Fig. 1.
Ratings of joy and anger (mean and standard error of the mean) in 17 patients with borderline personality disorder (BPD) and 18 healthy controls before (t1) and after (t2) different emotional conditions and after the go/no-go task (t3)
No main effect for group, group × time interaction, group × condition interaction or group × time × condition interaction became significant for either emotion. Anger was significantly greater in the anger condition than the control conditions, as reflected in the contrasts for story (neutral story v. anger story: F1,33 = 55.7, ηρ2 = 0.628, p < 0.001; joy story v. anger story: F1,33 = 56.5, ηρ2 = 0.631, p < 0.001).
Behavioural data during the go/no-go task
The number of commission errors (go reaction in a no-go trial) and omission errors (no-go reaction in a go trial) after different conditions in the 2 groups are summarized in Table 2. Higher numbers of commission errors is typical of go/no-go tasks. In the repeated-measures ANOVA, neither condition nor group nor the condition × group interaction were significant.
Table 2.
Omission and commission errors during the go/no-go tasks in 17 patients with borderline personality disorder and 18 healthy controls after induction of joy, anger and a neutral mood
| Group, mean (SD) | ||
|---|---|---|
|
|
||
| Error | BPD | Control |
| Commissions | ||
| Anger | 3.01 (1.85) | 2.2 (2.39) |
| Joy | 3.35 (3.59) | 1.72 (1.99) |
| Neutral mood | 2.94 (2.30) | 2.44 (2.15) |
| Omissions | ||
| Anger | 0.47 (1.01) | 0.61 (1.33) |
| Joy | 0.94 (3.13) | 0.22 (0.94) |
| Neutral mood | 1.35 (3.72) | 0.44 (1.46) |
BPD = borderline personality disorder; SD = standard deviation.
Functional MRI results
Regarding fMRI data, only the results of the anger versus neutral mood comparison are reported. Corresponding results comparing anger versus joy are omitted for the purpose of greater clarity and owing to space limitations. Comparisons of anger versus joy resembled the results reported for anger versus neutral mood (Appendix 1, Fig. S2).
Emotion induction
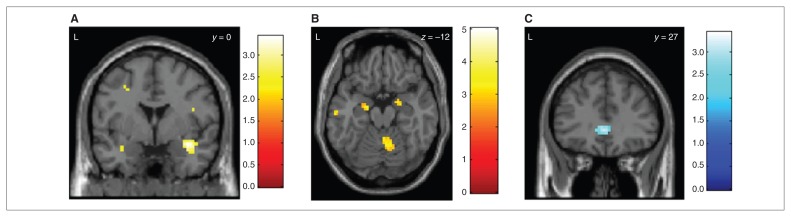
The group comparison showed stronger activation of the right amygdala in women with BPD than controls (right amygdala MNI space x, y, z = 33, 0, −15, SVC pFWE = 0.033; left amygdala MNI space x, y, z = −33, −3, −21, SVC pFWE = 0.08; Fig. 2A). Women with BPD also showed stronger activation of the right nucleus subthalamicus than controls (MNI space x, y, z = 3, −18, −9, SVC pFWE = 0.032). Correspondingly, patients with BPD showed a correlation with symptom severity in the right amygdala (BPDSI, within-group correlation: right amygdala MNI space x, y, z = 21, −3, −18, SVC pFWE = 0.035; left amygdala MNI space x, y, z = −24, −6, −12, SVC pFWE = 0.06; Fig. 2B). The control group, however, showed a stronger activation of the subgenual anterior cingulate (MNI space x, y, z = 0, 27, 0, SVC pFWE = 0.044; Fig. 2C).
Fig. 2.
Group differences during anger induction. (A) Stronger activation of the right amygdala (pFWE = 0.033; the left amygdala showed a trend: SVC pFWE = 0.08) in 17 patients with borderline personality disorder (BPD) compared to 18 healthy controls when participants listened to an anger-inducing story compared to a neutral story. Colour bars indicate t values. (B) Stronger activation of the right amygdala (SVC pFWE = 0.035; the left amygdala showed a trend: Montreal Neurological Institute space x, y, z = −24, −6, −12, SVC pFWE = 0.06) in 17 patients with BPD in correlation to symptom severity (Borderline Personality Disorder Severity Index33) when participants listened to an anger-inducing story compared to a neutral story. (C) Stronger activation of the subgenual anterior cingulate (SVC pFWE = 0.044) in 18 healthy controls compared to 17 patients with BPD when participants listented to an anger-inducing story compared to a neutral story. FWE = family-wise error; SVC = small volume correction.
Go/no-go task
Brain activation during the go/no-go task independent of emotional context (contrast no-go – go over all 3 sessions) was mostly identical with the activations found by Garavan and colleagues,37 for a comparable paradigm. Both groups activated regions of the inferior and middle frontal cortex, the insula and the parietal cortex (Appendix 1, Table S1). No significant group differences were found in ROI or whole brain analyses.
Emotional modulation of the go/no-go task
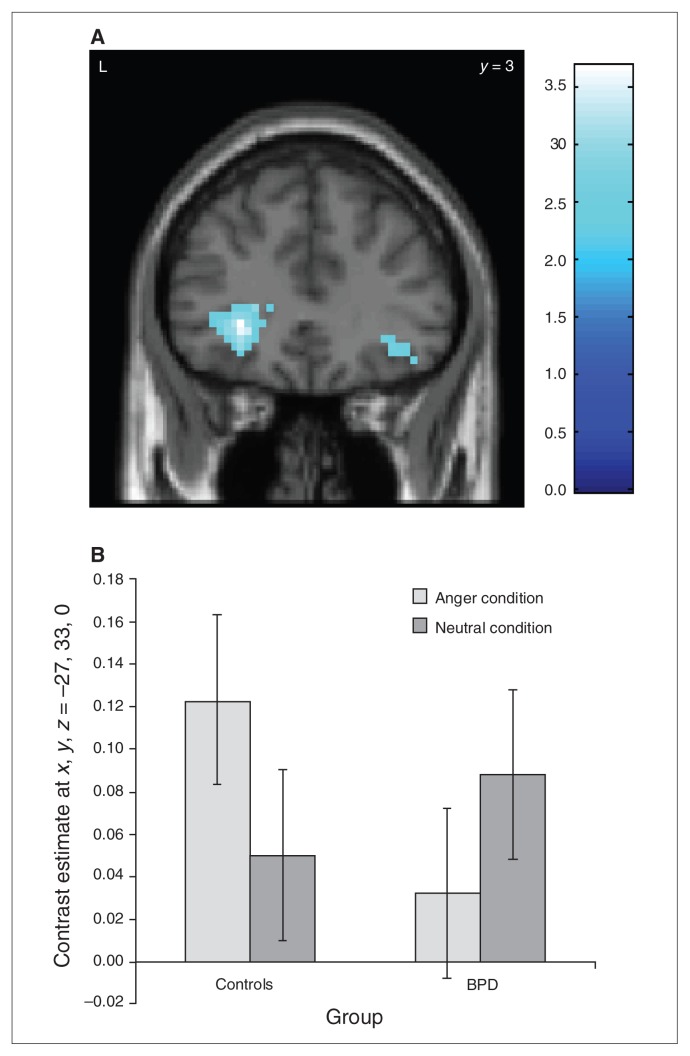
In the contrast no-go after anger induction – no-go after neutral, only controls activated the left inferior frontal cortex more strongly (MNI space x, y, z = −27, 33, −3, SVC pFWE = 0.05). Accordingly, the group comparison shows stronger left inferior frontal cortex activation in controls in no-go after anger (Fig. 3A and B; control – BPD × no-go [anger] – no-go [neutral]: MNI space x, y, z = −27, 33, 0, SVC pFWE = 0.026).
Fig. 3.
Group differences during the go/no-go task after anger induction. (A) Reducted left inferior frontal cortex activation (SVC pFWE = 0.026) in 17 patients with borderline personality disorder (BPD) compared to 18 healthy controls during go/no-go blocks after an anger condition compared to a neutral condition. Colour bar indicates t values. (B) Bar plot (mean and standard error of the mean) of the left inferior frontal cortex activity in the no-go tasks for the anger and neutral conditions in 17 patients with BPD compared to 18 healthy controls. FWE = family-wise error; SVC = small volume correction. The x, y, z coordinates refer to Montreal Neuroligical Institute space.
When impulsivity was further corrected for motor processes by analyzing the interaction contrast (HC – BPD × (no-go [anger] – go [anger]) – no-go [neutral] – go [neutral]), only the patient group showed a substantial modulation in the (right) nucleus subthalamicus (MNI space x, y, z = 3, −18, −3, SVC pFWE = 0.001), which was stronger than the respective reaction in the control group (group comparison: MNI space x, y, z = 0, −18, −6, SVC pFWE = 0.05; Fig. 4). No further significant group differences were found in other ROIs or whole brain analyses.
Fig. 4.
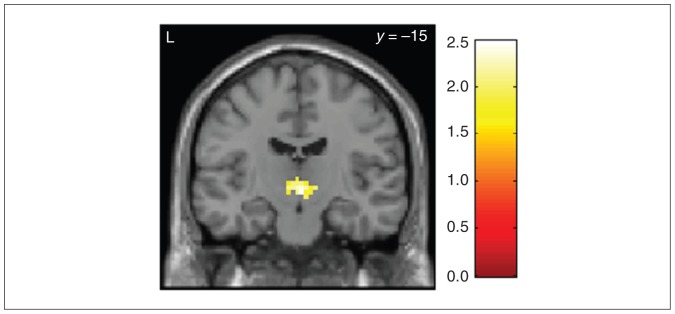
Relative hyperactivation of the nucleus subthalamicus (SVC pFWE = 0.05) in the interaction contrast (no-go – go × anger – neutral) in the go/no-go task after anger induction in 17 patients with borderline personality disorder compared to 18 healthy controls during go/no-go blocks after an anger condition compared to a neutral condition. Colour bar indicates t values. FWE = family-wise error; SVC = small volume correction.
Discussion
To our knowledge, the present study is the first to investigate how brain activation during an impulse control task is modulated by anger. A group of women with BPD and a matched healthy control group performed a go/no-go task after listening to an anger-inducing story or a neutral or joyful control story. Earlier studies investigating the interplay between impulsivity and emotions in patients with BPD usually used emotional stimulus material for the impulsivity task.8,15 The design of the present study might have the advantage of greater ecological validity, since in everyday life, an angry affect may be particularly dysfunctional when it has been stimulated by an earlier event that is unrelated to the present situation. The analysis of emotion ratings showed a successful anger induction, with anger lasting until the end of the impulsivity task.
With regard to behavioural results in the go/no-go tasks, no significant group differences were found. This is is in line with previous studies that showed a tendency toward higher error rates in motor impulsiveness in patients with BPD than controls, although this difference was not significant.19,42 Other studies have found significant group differences in go/no-go tasks between patients with BPD and healthy control participants.17,18 It should be noted that for the present study we chose a relatively simple go/no-go task since brain activation during successful motor inhibition was the aim of our investigation. However, with a relatively simple task one would not expect group differences, especially given the mixed results of previous studies. Inhibition was associated with an increased activation of the IFC and the premotor, parietal and insular cortex in both groups. Earlier studies similarly found activation of the IFC,36,43,44 the premotor and parietal cortex35–37 and the insular cortex.37,44,45 These findings imply that behavioural inhibition is controlled in both groups by a network including the frontal and subthalamic regions.46
While listening to the anger story, both groups showed increased activation of the temporal lobe, medial frontal regions and the PCC. Medial prefrontal activations have been found in other studies investigating brain correlates of anger.13,47 This activation may be due to (automatic) emotion regulation, since Ochsner and colleagues48 found activations in the lateral and mPFC regions when participants reappraised negative emotions. Similarly, PCC activations have been found as correlates of anger processing49 and of processing threat-related words.50
Group comparisons showed stronger activation of the right amygdala and the right nucleus subthalamicus (with similar trends on the left side) in patients with BPD compared with controls, whereas controls showed stronger sgACC activation. Increased amygdalar activity has repeatedly been shown in patients with BPD.6–8 It may be regarded as a correlate of negative emotions in general7 rather than a correlate of anger specifically.51 Prior studies have shown an increase of other negative emotions besides anger after listening to the anger story.34
In line with a study investigating brain activity during anger induction in depressive patients with and without anger attacks that reported a lack of vmPFC activation during anger induction in patients with anger attacks,52 and in line with the finding of relatively decreased vmPFC activity in the context of an inhibition task with emotional stimuli,8 we found decreased sgACC activity in women with BPD. Previous findings of prefrontal dysfunction in patients with BPD8,9,53–55 suggest that missing inhibitory control by prefrontal areas over the amygdala are a possible mechanism of exaggerated and/or prolonged amygdala response to emotional stimuli in patients with BPD.56 Our findings support this assumption.
When the impulsivity task was performed in the anger condition, only control participants showed stronger IFC activation compared with the neutral condition; this is in accordance with our a priori hypothesis. However, the BPD group showed an increase of nucleus subthalamicus activation instead. Increased IFC activation is consistent with an increase of the inhibitory network, as discussed previously. The importance of the IFC for successful inhibition has been demonstrated in previous studies.46,57,58 The modulating effect of anger on this region speaks in favour of dynamic models of emotion regulation and impulse control.59,60
The relatively weaker IFC activation in the BPD group in the no-go/anger condition is in line with previous studies showing generally decreased prefrontal activation in patients with BPD.9,11 The increase in nucleus subthalamicus activity in patients with BPD, in turn, might be interpreted as a compensatory mechanism, since the nucleus subthalamicus plays an important role in the inhibitory network, as described by Aron.46 This may explain why patients with BPD often do not show impairments in inhibition tasks, even when emotional stimulus material is used.15,20 Together with the nucleus subthalamicus over-reactivity during anger induction, this finding might suggest a more general attempt of subcortical compensation for missing prefrontal control.
Limitations
This study had several limitations. Our sample included only women; therefore, results cannot be generalized to men. Four patients took antidepressant medication (SSRIs). Although SSRI treatment may influence the results on several levels (anger induction, go/no-go task, blood oxygen level–dependent activity), it probably would reduce effects (i.e., as shown by Murphy and colleagues61 for amygdala activity immediately after SSRI treatment). However, despite this possible confound, group differences could be found. No additional patient control group was included, although comparisons with patient groups that have other disorders with impulse-control deficits are needed.
Conclusion
Behavioural disinhibition is not impaired in a neutral go/no-go task in women with BPD compared with healthy female controls. While listening to an anger-inducing story, women with BPD had stronger activation of the right amygdala and the right nucleus subthalamicus and less activation of the sgACC than controls. When performing the go/no-go task in the anger condition, only controls showed stronger IFC activation. Patients with BPD, however, showed increased nucleus subthalamicus activation, and this may have compensated for their lack of prefrontal activation.
Acknowledgements
This work was funded by the German Research Foundation (grant JA1785/1-1), a fellowship grant from the Freiburg University Medical School to O. Tüscher, and a grant from the German Federal Ministry of Research and Education (grant 01GV0606) to L. Tebartz van Elst.
Footnotes
Contributors: G.A. Jacob, K. Zvonik, L. Tebartz van Elst, K. Lieb and O. Tüscher designed the study. K. Zvonik, S. Kamphausen, S. Maier, A. Philipsen and O. Tüscher acquired the data. G.A. Jacob, K. Zvonik, A. Sebastian, S. Maier, A. Philipsen, K. Lieb and O. Tüscher analyzed the data. G.A. Jacob and O. Tüscher wrote the article. K. Zvonik, S. Kamphausen, A. Sebastian, S. Maier, A. Philipsen, L. Tebartz van Elst, K. Lieb and O. Tüscher reviewed the article. All authors approved its publication.
Competing interests: G.A. Jacob receives royaltees from Beltz, Elsevier and Junfermann. A. Philipsen sits on the advisory boards of and has received lecture fees from Lilly, Medice and Shire. L. Tebartz van Elst declares having received lecture and travels fees from Elli Lilly, Medtronic, Pfizer and UCB. O. Tüscher received support through his institution from the German Federal Ministry of Research and Education (grant 01GW0730). None declared for K. Zvonik, S. Kamphausen, A. Sebastian, S. Maier and K. Lieb.
References
- 1.Lieb K, Zanarini MC, Schmahl C, et al. Borderline personality disorder. Lancet. 2004;364:453–61. doi: 10.1016/S0140-6736(04)16770-6. [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal MZ, Gratz KL, Kosson DS, et al. Borderline personality disorder and emotional responding: a review of the research literature. Clin Psychol Rev. 2008;28:75–91. doi: 10.1016/j.cpr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Arntz A, Klokman J, Sieswerda S. An experimental test of the schema mode model of borderline personality disorder. J Behav Ther Exp Psychiatry. 2005;36:226–39. doi: 10.1016/j.jbtep.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Lobbestael J, Arntz A. Emotional, cognitive, and physiological correlates of abuse-related stress in borderline and antisocial personality disorders. Behav Res Ther. 2010;48:116–24. doi: 10.1016/j.brat.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Salavert J, Gasol M, Vieta E, et al. Fronto-limbic dysfunction in borderline personality disorder: a 18F-FDG positron emission tomography study. J Affect Disord. 2011;131:260–7. doi: 10.1016/j.jad.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Herpertz SC, Dietrich TM, Wenning B, et al. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biol Psychiatry. 2001;50:292–8. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- 7.Donegan NH, Sanislow CA, Blumberg HP, et al. Amygdala hyper-reactivity in borderline personality disorder: implications for emotional dysregulation. Biol Psychiatry. 2003;54:1284–93. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- 8.Silbersweig D, Clarkin JF, Goldstein M, et al. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. Am J Psychiatry. 2007;164:1832–41. doi: 10.1176/appi.ajp.2007.06010126. [DOI] [PubMed] [Google Scholar]
- 9.Minzenberg MJ, Fan J, New AS, et al. Fronto-limbic dysfunction in response to facial emotion in borderline personality disorder: an event-related fMRI study. Psychiatry Res. 2007;155:231–43. doi: 10.1016/j.pscychresns.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmahl CG, Elzinga BM, Vermetten E, et al. Neural correlates of memories of abandonment in women with and without borderline personality disorder. Biol Psychiatry. 2003;54:142–51. doi: 10.1016/s0006-3223(02)01720-1. [DOI] [PubMed] [Google Scholar]
- 11.Schmahl CG, Vermetten E, Elzinga BM, et al. A PET study of memories of childhood abuse in borderline personality disorder. Biol Psychiatry. 2004;55:759–65. doi: 10.1016/j.biopsych.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Gainesville (FL): University of Florida; 2008. [Google Scholar]
- 13.Phan KL, Wager T, Taylor SF, et al. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 14.Berlin HA, Rolls ET, Iversen SD. Borderline personality disorder, impulsivity, and the orbitofrontal cortex. Am J Psychiatry. 2005;162:2360–73. doi: 10.1176/appi.ajp.162.12.2360. [DOI] [PubMed] [Google Scholar]
- 15.Domes G, Winter B, Schnell K, et al. The influence of emotions on inhibitory functioning in borderline personality disorder. Psychol Med. 2006;36:1163–72. doi: 10.1017/S0033291706007756. [DOI] [PubMed] [Google Scholar]
- 16.Jacob GA, Gutz L, Bader K, et al. Impulsivity in borderline personality disorder: impairment in self-report measures, but not behavioral inhibition. Psychopathology. 2010;43:180–8. doi: 10.1159/000304174. [DOI] [PubMed] [Google Scholar]
- 17.Leyton M, Okazawa H, Diksic M, et al. Brain regional a-[11C] methyl-l-tryptophan trapping in impulsive subjects with borderline personality disorder. Am J Psychiatry. 2001;158:775–82. doi: 10.1176/appi.ajp.158.5.775. [DOI] [PubMed] [Google Scholar]
- 18.Rentrop M, Backenstrass M, Jaentsch B, et al. Response inhibition in borderline personality disorder: performance in a go/no go task. Psychopathology. 2008;41:50–7. doi: 10.1159/000110626. [DOI] [PubMed] [Google Scholar]
- 19.Lampe K, Konrad K, Kroener S, et al. Neuropsychological and behavioural disinhibition in adult ADHD compared to borderline personality disorder. Psychol Med. 2007;37:1717–29. doi: 10.1017/S0033291707000517. [DOI] [PubMed] [Google Scholar]
- 20.Völker KA, Spitzer C, Limberg A, et al. [Executive dysfunctions in female patients with borderline personality disorder with regard to impulsiveness and depression]. Psychother Psychosom Med Psychol. 2009;59:264–72. doi: 10.1055/s-2008-1067437. [Article in German] [DOI] [PubMed] [Google Scholar]
- 21.Lawrence KA, Allen JS, Chanen AM. Impulsivity in borderline personality disorder: reward-based decision-making and its relationship to emotional distress. J Pers Disord. 2010;24:786–99. doi: 10.1521/pedi.2010.24.6.785. [DOI] [PubMed] [Google Scholar]
- 22.Schuermann B, Kathmann N, Stiglmayr C, et al. Impaired decision making and feedback evaluation in borderline personality disorder. Psychol Med. 2011;41:1917–27. doi: 10.1017/S003329171000262X. [DOI] [PubMed] [Google Scholar]
- 23.Clarkin JF, Posner M. Defining the mechanisms of borderline personality disorder. Psychopathology. 2005;38:56–63. doi: 10.1159/000084812. [DOI] [PubMed] [Google Scholar]
- 24.Chapman AL, Leung DW, Lynch TR. Impulsivity and emotion dysregulation in borderline personality disorder. J Pers Disord. 2008;22:148–64. doi: 10.1521/pedi.2008.22.2.148. [DOI] [PubMed] [Google Scholar]
- 25.Colder CR, Stice E. A longitudinal study of the interactive effects of impulsivity and anger on adolescent problem behavior. J Youth Adolesc. 1998;27:255–74. [Google Scholar]
- 26.Giegling I, Olgiati P, Harmann AM, et al. Personality and attempted suicide. Analysis of anger, aggression and impulsivity. J Psychiatr Res. 2009;43:1262–71. doi: 10.1016/j.jpsychires.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Engel SG, Boseck JJ, Crosby RD, et al. The relationship of momentary anger and impulsivity to bulimic behavior. Behav Res Ther. 2007;45:437–47. doi: 10.1016/j.brat.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV), User’s Guide. Washington: American Psychiatric Press, Inc; 1997. [Google Scholar]
- 29.First MB, Spitzer RL, Gibbon M, et al. User’s Guide for the Structured Clinical Interview for DSM-IV Personality Disorders (SCID-II) Washington: American Psychiatric Press; 2006. [Google Scholar]
- 30.Lehrl S. Mehrfachwahl-Wortschatz-Intelligenztest. Balingen (Germany): Spitta; 1999. [Google Scholar]
- 31.Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Pers Individ Dif. 2001;30:669–89. [Google Scholar]
- 32.Spielberger CD, Sydeman SJ, Owen AE, et al. Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI) In: Maruish ME, editor. The use of psychological testing for treatment planning and outcomes assessment. Mahwah (NJ): Lawrence Erlbaum; 1999. pp. 993–1021. [Google Scholar]
- 33.Arntz A, van den Hoorn M, Cornelis J, et al. Reliability and validity of the border-line personality disorder severity index. J Pers Disord. 2003;17:45–59. doi: 10.1521/pedi.17.1.45.24053. [DOI] [PubMed] [Google Scholar]
- 34.Jacob GA, Hellstern K, Ower N, et al. Emotional reactions to standardized stimuli in women with borderline personality disorder: stronger negative affect, but no differences in reactivity. J Nerv Ment Dis. 2009;197:808–15. doi: 10.1097/NMD.0b013e3181bea44d. [DOI] [PubMed] [Google Scholar]
- 35.Hester R, Fassbender C, Garavan H. Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cereb Cortex. 2004;14:986–94. doi: 10.1093/cercor/bhh059. [DOI] [PubMed] [Google Scholar]
- 36.Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–33. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96:8301–6. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thesen S, Heid O, Mueller E, et al. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44:457–65. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 39.Zaitsev M, Hennig J, Speck O. Point spread function mapping with parallel imaging techniques and high acceleration factors: fast, robust, and flexible method for echo-planar imaging distortion correction. Magn Reson Med. 2004;52:1156–66. doi: 10.1002/mrm.20261. [DOI] [PubMed] [Google Scholar]
- 40.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 41.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 42.Ruchsow M, Groen G, Kiefer M, et al. Response inhibition in borderline personality disorder: event-related potentials in a Go/Nogo task. J Neural Transm. 2008;115:127–33. doi: 10.1007/s00702-007-0819-0. [DOI] [PubMed] [Google Scholar]
- 43.Floden D, Stuss DT. Inhibitory control is slowed in patients with right superior medial frontal damage. J Cogn Neurosci. 2006;18:1843–9. doi: 10.1162/jocn.2006.18.11.1843. [DOI] [PubMed] [Google Scholar]
- 44.Swick D, Ashley V, Turken AU. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008;9:102. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garavan H, Ross TJ, Murphy K, et al. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17:1820–9. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- 46.Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–28. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- 47.Denson TF, Pedersen WC, Ronquillo J, et al. The angry brain: neural correlates of anger, angry rumination, and aggressive personality. J Cogn Neurosci. 2009;21:734–44. doi: 10.1162/jocn.2009.21051. [DOI] [PubMed] [Google Scholar]
- 48.Ochsner KN, Bunge SA, Gross JJ, et al. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 49.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–44. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maddock RJ, Buonocore MH, Kile SJ, et al. Brain regions showing increased activation by threat-related words in panic disorder. Neuroreport. 2003;14:325–8. doi: 10.1097/00001756-200303030-00006. [DOI] [PubMed] [Google Scholar]
- 51.Blair RJ, Morris JS, Frith CD, et al. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–93. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- 52.Dougherty DD, Rauch SL, Deckersbach T, et al. Ventromedial prefrontal cortex and amygdala dysfunction during an anger induction positron emission tomography study in patients with major depressive disorder with anger attacks. Arch Gen Psychiatry. 2004;61:795–804. doi: 10.1001/archpsyc.61.8.795. [DOI] [PubMed] [Google Scholar]
- 53.Tebartz van Elst L, Hesslinger B, Thiel T, et al. Frontolimbic brain abnormalities in patients with borderline personality disorder: a volumetric magnetic resonance imaging study. Biol Psychiatry. 2003;54:163–71. doi: 10.1016/s0006-3223(02)01743-2. [DOI] [PubMed] [Google Scholar]
- 54.New AS, Hazlett EA, Buchsbaum MS, et al. Amygdala–prefrontal disconnection in borderline personality disorder. Neuropsychopharmacology. 2007;32:1629–40. doi: 10.1038/sj.npp.1301283. [DOI] [PubMed] [Google Scholar]
- 55.Mauchnik J, Schmahl C. The latest neuroimaging findings in borderline personality disorder. Curr Psychiatry Rep. 2010;12:46–55. doi: 10.1007/s11920-009-0089-7. [DOI] [PubMed] [Google Scholar]
- 56.Berdahl CH. A neural network model of borderline personality disorder. Neural Netw. 2010;23:177–88. doi: 10.1016/j.neunet.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Swick D, Ashley V, Turken AU. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–65. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- 58.Chiu PH, Holmes AJ, Pizzagalli DA. Dissociable recruitment of rostral anterior cingulate and inferior frontal cortex in emotional response inhibition. Neuroimage. 2008;42:988–97. doi: 10.1016/j.neuroimage.2008.04.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lewis MD. Bridging emotion theory and neurobiology through dynamic systems modeling. Behav Brain Sci. 2005;28:169–94. doi: 10.1017/s0140525x0500004x. [DOI] [PubMed] [Google Scholar]
- 60.Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9:148–58. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- 61.Murphy SE, Norbury R, O’Sullivan U, et al. Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry. 2009;194:535–40. doi: 10.1192/bjp.bp.108.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]