Abstract
Background
The brain levels of glutamate (Glu) and glutamine (Gln) are partially regulated through the Glu–Gln cycle. Astrocytes play a role in regulating the Glu–Gln cycle, and loss of astrocytes has been associated with depressive disorders. We hypothesized that levels of Glu and Gln would be affected by astrocyte loss and dysregulation of the Glu–Gln cycle and that depressive-like behaviours would be closely related to the level of changes in Glu and Gln.
Methods
We used liquid chromatography to measure Glu and Gln concentrations in the prefrontal cortex of male mice infused with L-α aminoadipic acid (L-AAA), a specific astrocyte toxin, in the prelimbic cortex. Methionine sulfoximine, a Gln synthetase inhibitor, and α-methyl-amino-isobutyric acid, a blocker of neuronal Gln transporters, were used to disturb the Glu–Gln cycle. We assessed the behavioural change by drug infusion using the forced swim test (FST) and sucrose preference test.
Results
The Glu and Gln levels were decreased on the fifth day after L-AAA infusion, and the infused mice showed longer durations of immobility in the FST and lower sucrose preference, indicative of depressive-like behaviour. Mice in which Gln synthetase or Gln transport were inhibited also exhibited increased immobility in the FST. Direct infusion of L-Gln reversed the increased immobility induced by astrocyte ablation and Glu–Gln cycle impairments.
Limitations
Genetically modified animal models and diverse behavioural assessments would have been helpful to solidify our conclusions.
Conclusion
Neuronal Gln deficiency in the pre-frontal cortex may cause depressive behaviours.
Introduction
Depression is the most common psychiatric illness, with about 121 million people affected worldwide. Of people who experience a depressive episode, 15% commit suicide.1,2 Although many studies have investigated the pathophysiologic mechanisms of major depressive disorder (MDD) using live brain imaging and postmortem studies, its etiology remains unclear; however, recent progress based on those studies has gradually revealed common features of MDD. Among these features, volume reduction of selective brain regions in patients with MDD is the most remarkable.3–5 This is mainly due to a lower number of glial cells and neuronal atrophy in those regions.6,7 Specifically, the reduction of astrocytes among all glial cells was frequently found in postmortem studies.3,8–10 Another study reported lower levels of glutamine synthetase (GS), one of the astrocyte-specific enzymes involved in the glutamate–glutamine (Glu–Gln) cycle, and its activity levels were decreased in some clinical studies of MDD.11
A variety of preclinical studies have reported findings to support the idea of astrocyte loss and Glu–Gln disruption. Consistent with those studies, several papers have reported decreased gliogenesis and number of astrocytes in the medial prefrontal cortex (mPFC) in animal models of chronic stress-induced depression.7,12–14 These results strongly suggest that a relationship exists between the functions of astrocytes and the behavioural aspects of MDD. To test this issue, a recent study ablated astrocytes in the prelimbic cortex (PLC) using a specific toxin and revealed that depressive-like behaviours could be evoked using only this treatment.14 It remains to be determined how astrocyte loss results in depressive behaviours.
To address this question, we focused on the role of astrocytes in the maintenance of the Glu–Gln cycle,15 because several lines of evidence have indicated a deficit of these amino acids in the brains of patients with MDD and in animal models of depression.12,16,17 We hypothesized that selective astrocyte loss would impair this cycle and change the levels of Glu and Gln. To test this hypothesis, we ablated astrocytes in the mouse PFC and then monitored Glu and Gln levels and depressive-like behaviours.
Methods
Animals
Male, 9-week-old C57BL/6 mice (SPF grade, Hana, Co. Ltd.) were housed in a temperature-controlled (22°C) environment under a 12-hour light/dark cycle (lights on at 6 am), with free access to laboratory chow and water. The animals were habituated for 1 week before the experiments. For the immunohistochemical analysis, mice were transcardially perfused with 4% neutralized paraformaldehyde. For the amino acid analysis, mice were decapitated under CO2 anesthesia. Mice were treated in accordance with the standard Gyeongsang National University Institution Animal Care and Use Committee guidelines for laboratory animal care (GNU IACUC, GLA-100917-M0093).
Guide cannula implantation and L-α aminoadipic acid infusion
We adapted the L-α aminoadipic acid (L-AAA) infusion depression model from a recent report.14 Adult mice were anesthetized using xylazin/tiletamine + zolazepam (0.5/1 μl/g, intraperitoneal), and guide cannulae (PlasticsOne) were bilaterally implanted into the PLC using a stereotaxic frame (Stoelting Inc.) at the coordinates 1.7 mm anteroposterior, −0.25 mm dorsolateral, and depth −2.5 mm from the bregma.18 After 1 week of recovery, we infused L-AAA (100 μg/μl; Sigma) bilaterally using injection cannulae and a microdrive pump. We administered this infusion once daily for 2 days at a rate of 0.1 μl/min for 6 minutes. The same volume of phosphate-buffered saline (PBS) was infused into the PLC of the sham controls.
Time course and experimental design
Experiment A (Appendix 1, Fig. S1, available at cma.ca/jpn) was designed to induce astrocyte ablation using L-AAA infusion and to test depressive behaviours using the open field test (OFT), sucrose preference test (SPT) and forced swim test (FST). In addition, we investigated the effect of astrocyte loss on Glu and Gln levels in the PFC. For this purpose, we implanted guide cannulae 7 days before L-AAA infusion, and then L-AAA was infused for 2 consecutive days. Thereafter, we analyzed Glu and Gln levels in the PFC using liquid chromatography on days 3, 5, 7 and 10. The OFT and the SPT occurred on day 5, and the FST occurred on days 5 and 10. All mice were prepared for each aspect of the experiment, but separate groups of mice were used on different investigation days.
Experiment B (Appendix 1, Fig. S1) aimed to test whether a disturbance of the Glu–Gln cycle affected depressive behaviour. For this purpose, we infused mice with methionine sulfoximine (MSO) or α-methyl-amino-isobutyric acid (MeAIB) on the seventh day after cannulation. The mice were then subjected to the FST. One day after the FST, we analyzed Glu and Gln levels using liquid chromatography.
Experiment C (Appendix 1, Fig. S1) tested whether exogenous Gln could reverse the depressive behaviours caused by L-AAA infusion and by Glu–Gln cycle disturbance. Cannulation and toxin infusion were performed as in experiments A and B. The depressive behaviours were confirmed on the fifth day after L-AAA infusion using the FST. To test the effects of Glu and Gln on L-AAA infusion, Glu and Gln were infused the day after the FST, and the mice repeated the FST so we could re-evaluate depressive behaviours. To evaluate the effect of Gln on Glu–Gln cycle disturbance, Gln was cotreated with MSO or MeAIB, and then the mice were subjected to the FST. We examined the effect of Glu and Gln infusion on the normal mouse behaviour using the the OFT and FST.
Behavioural assessments
We adapted the FST method from Porsolt and colleagues,19 using modifications that have been described elsewhere.20 Briefly, each mouse was placed in a Plexiglas cylinder (height 25 cm, diameter 12 cm) containing water at a temperature of 25°C and a depth of 17 cm so that the mouse could neither escape nor touch the bottom. Mice were subjected to 5 minutes of preswimming the day before the experiment. On the day of the experiment, mice were forced to swim for 6 minutes. The animals were habituated for the first minute, and their behaviour was noted over the next 5 minutes. Mobility was defined as a change of 7% of the recorded pixels (EthoVision; Noldus Information Technology). The water in the chamber was changed between mice. The OFT was carried out as previously described.20 The SPT was performed as previously described,21 with some modifications, to determine symptoms of anhedonia. Briefly, animals were habituated for 48 hours to 0.1M sucrose solution followed by a 12-hour deprivation period. We then determined preference for sucrose solution or water (identical bottles) for 6 hours.
Immunohistochemistry
Immunohistochemistry was performed as previously described,22 with several modifications. Briefly, fixed brains were sectioned (30 μm sections, coordinates 4.7–1.7 mm from the bregma) and incubated with an anti–glial fibrillary acidic protein (GFAP) antibody (1:500; Dako) at 4°C overnight. The positive signals were developed using an ABC kit (VECTA-STAIN Elite) and DAB as a substrate for the peroxidase labelling. The sections were mounted on gelatin-coated slides, dried, dehydrated through a series of graded alcohols, cleared in xylene and then cover-slipped using Permount (Sigma). We obtained and documented digital images (Olympus). We used 9 sections from each animal to count GFAP-positive cells in the PLC (NIH ImageJ).
Analysis of Glu and Gln levels
Mice were anesthetized with CO2 and decapitated. The PFC (including the PLC) was dissected on ice and rapidly frozen using liquid nitrogen. The samples were weighed and then mixed with 700 μl of sodium citrate loading buffer to analyze Glu levels or with a lithium citrate loading buffer to analyze Gln levels. The tissue was homogenized and centrifuged at 13 000g for 60 minutes, and we used the resulting supernatant for analysis. The supernatant was filtered with a 5 μm pore filter before loading (Millex Syringe filter; Millipore). We visualized Glu and Gln using a Biochrom 20 Plus amino acid analyzer (Biochrom Ltd.) with a mobile phase (flow rate 25.0 mL/h). We identified the amino acid peaks in the samples by their retention times compared with external standards. Amino acid concentrations were quantified according to the relative peak height measured and were expressed in micrograms per milligram.
Infusion of L-Gln, L-Glu, MSO and MeAIB
We purchased L-Glu, L-Gln, MSO and MeAIB from Sigma and dissolved them in PBS. The concentrations of Glu used in this study were 0.1 and 1 M; Gln concentrations were 0.1, 1 and 2 M. The MSO solutions for infusion were adjusted to 5 and 10 mM. Experimental concentrations of MeAIB were 5 and 7 mM. We infused all chemical solutions bilaterally into the PLC through guide cannulae. The infused volume for each chemical was 0.2 μl at a rate of 0.1 μl per minute. Mice performed the FST 3 hours after Gln and MSO infusion and 6 hours after Glu and MeAIB infusion. To confirm retention and to prevent an adverse tide of the infused solution, we kept the injection cannulae in place for 5 minutes after completion of the infusion before retracting them. For the sham control procedures, we infused the same volume of PBS.
Statistical analysis
We performed 1-way analysis of variance (ANOVA) and Dunnett post hoc tests for multiple-group comparisons. For between-group comparisons, we used t tests. Statistical analyses were conducted using SigmaStat software version 3.5 (Sigma). We considered results to be significant at p < 0.05.
Results
Mice infused with L-AAA showed significantly greater immobility and lower Glu and Gln levels
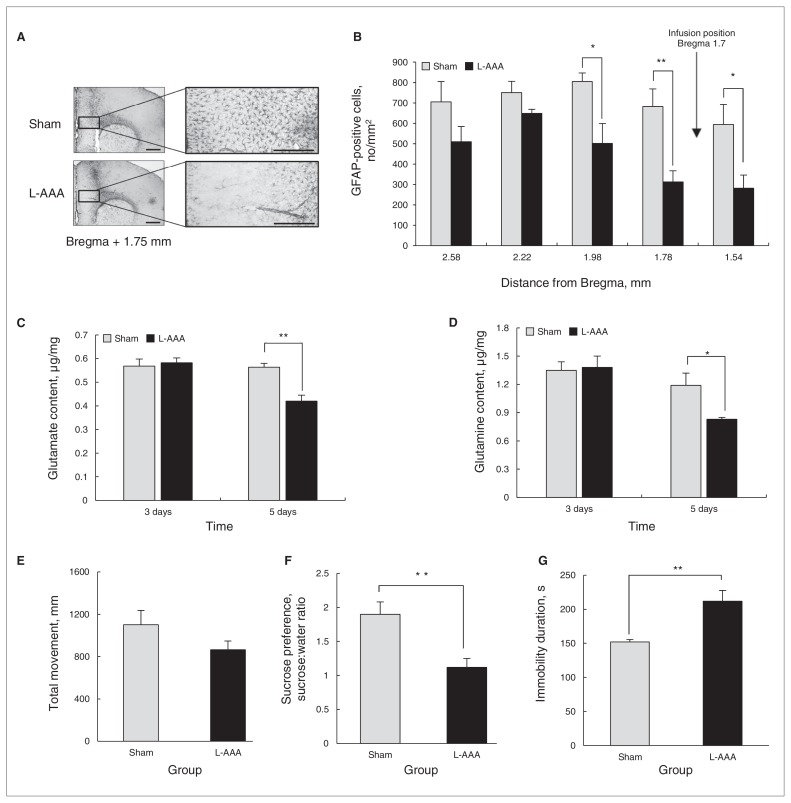
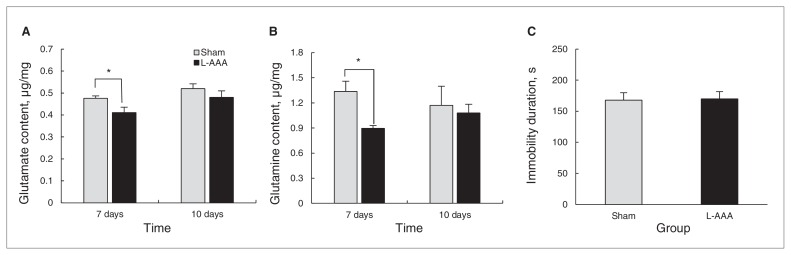
To investigate the role of astrocytes in depressive behaviours, we infused L-AAA into the PLC of mice, and astrocyte ablation was confirmed by immunohistochemistry on the sixth day after L-AAA infusion and following SPT, OFT and FST (Fig. 1A and B). To investigate the changes in Glu and Gln levels, we assayed Glu and Gln concentrations on the third and fifth days after L-AAA infusion (Appendix 1, Fig. S1). The concentrations of Glu and Gln significantly decreased on the fifth day after L-AAA infusion (Fig. 1C and D). L-α aminoadipic acid did not influence the locomotor activity of infused mice (Fig. 1E). Interestingly, mice infused with L-AAA showed lower sucrose preference and longer durations of immobility on the fifth day after infusion, indicating a more depressive-like behaviour (Fig. 1F and G). On the seventh day, the levels of Glu and Gln remained significantly lower in mice infused with L-AAA than in controls (Fig. 2A and B) but returned to sham levels on day 10 after infusion (Fig. 2A and B). At this time point, we found no difference in the duration of immobility (Fig. 2C). From these results, we conclude that astrocyte depletion in the PLC results in lower levels of Glu and Gln, which closely relate to depressive behaviours.
Fig. 1.
L-α aminoadipic acid (L-AAA) infusion into the mouse prelimbic cortex (PLC) led to a reduction of astrocytes compared with the vehicle injection group (sham; A and B, bregma 1.98, t8 = 2.86, p = 0.021; bregma 1.78; t = 3.63, p = 0.007; bregma 1.54, t8 = 2.68, p = 0.028). We performed the immunohistochemical analysis on the sixth day after the first L-AAA infusion. Glutamate (Glu) and glutamine (Gln) levels in the prefrontal cortex (PFC) were measured after L-AAA infusion. There was no change in Glu or Gln levels in the PFC on the third day after the infusion (Glu level of day 3, t8 = −0.840, p = 0.42; Gln level of day 3, t8 = −0.174, p = 0.87); however, Glu and Gln levels in mice infused with L-AAA decreased significantly compared with those of control mice on the fifth day after L-AAA infusion (C and D, Glu level day 5, t8 = 3.488, p = 0.008; Gln level day 5, t8 = 2.596, p = 0.032). L-α aminoadipic acid infusion did not affect the locomotor activity in the open field test (OFT; E, t8 = 1.505, p = 0.17). Sucrose preference from the sucrose preference test (SPT) was significantly decreased (F, t8 = 3.585, p = 0.007) and the duration of immobility from the forced swim test (FST) increased (G, t8 = −3.791, p = 0.005) in mice infused with L-AAA on the fifth day after infusion, indicating a greater degree of depressive behaviour. The OFT and FST were sequentially performed using the same animals each day. Data are presented as means and standard errors of the mean. We used the t test to evaluate the mean difference between the control mice and those infused with L-AAA. *p < 0.05, **p < 0.01, n = 5 per group. Scale bars in A are 500 μm. GFAP = glial fibrillary acidic protein.
Fig. 2.
On the seventh day after L-α aminoadipic acid (L-AAA) infusion, glutamate (Glu) and glutamine (Gln) levels remained significantly lower than those of control mice, but on the tenth day, no significant differences were observed (A and B, Glu level day 7, t8 = 2.460, p = 0.039; Gln level day 7, t8 = 2.634, p = 0.030; Glu level day 10, t8 = 0.951, p = 0.37; Gln level day 10, t8 = 0.215, p = 0.84). Duration of immobility was similar between mice infused with L-AAA and control mice on the tenth day (C). Data are presented as means and standard errors of the mean. *p < 0.05, n = 5 per group. Different mice groups were used for each day of testing. We conducted a t test to evaluate the mean difference between the control and L-AAA groups.
Blockade of Gln synthesis and transport increased the duration of immobility
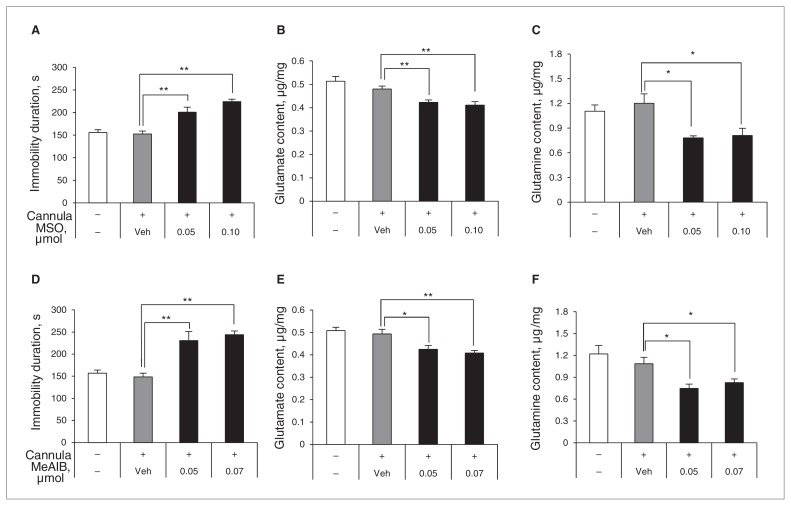
Glutamine is synthesized by GS in astrocytes and is transported into neurons through the system A transporter–2 (SAT2).23 Glutamine synthesis can be inhibited by MSO,24 and Gln transport can be blocked with MeAIB.25 If the depressive behaviours caused by astrocyte ablation are due to lack of Gln supply from astroglia, inhibition of Gln synthesis in the PLC would be sufficient to evoke depressive behaviours. To address this issue, we infused MSO directly into the PLC, and then conducted the FST to evaluate the level of depressive behaviours 3 hours after infusion. Interestingly, mice infused with MSO displayed significantly more depressive behaviours than control mice (Fig. 3A). Athough a sufficient supply of Gln is available from astrocytes, the concentrations of Glu and Gln in neurons decrease when abnormal transport of Gln occurs through SAT2.23 Therefore, we also examined the effect of MeAIB, a competitive blocker of SAT2, on immobility duration using a similar experimental paradigm to that used for MSO (Fig. S1). As expected, mice given MeAIB showed significantly greater immobility than controls (Fig. 3D). The levels of Glu and Gln were also significantly lower in MSO- and MeAIB-treated mice than in control mice (Fig. 3B, C, E and F). These results suggest that dysfunction of the Glu–Gln cycle can trigger depressive behaviours even with sufficient numbers of astrocytes present.
Fig. 3.
Effects of methionine sulfoximine (MSO), an inhibitor of glutamine synthetase, and α-methyl-amino-isobutyric acid (MeAIB), a competitive blocker of the glutamine (Gln) transporter on the neuronal membrane, on the duration of immobility during the forced swim test. The infusion of MSO (A, F3,16 = 22.611, p < 0.001) or MeAIB (D, F3,16 = 15.963, p < 0.001) into the prelimbic cortex increased the duration of immobility, indicating that infused mice exhibit more depressive-like behaviour. Levels of glutamate and Gln decreased significantly after administration of these 2 compounds (B, F3,16 = 5.970, p = 0.006; C, F3,16 = 6.609, p = 0.004; E, F3,16 = 5.916, p = 0.002; F, F3,16 = 8.774, p = 0.001). Data are presented as means and standard errors of the mean. *p < 0.05, **p < 0.01, n = 5 per group. Analyses of variance and Dunnett post hoc tests were used for multiple comparisons between the control and other groups.
Glutamine treatment reversed immobility induced by astrocyte ablation and Glu–Gln cycle impairments
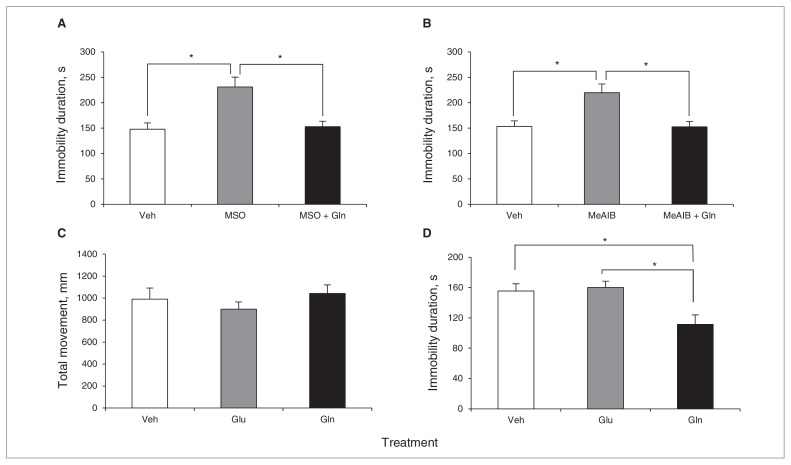
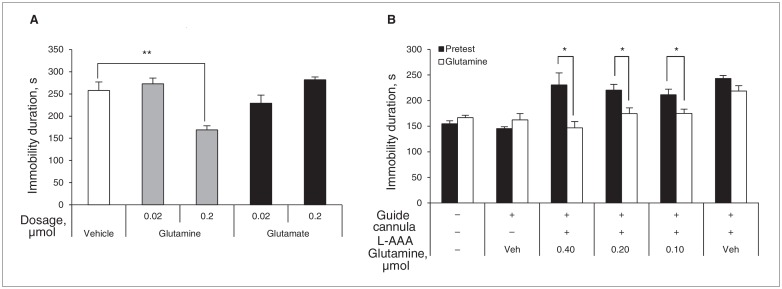
Based on our previous results (Figs. 1–3), we postulated that the reduction of Glu and Gln levels in the PFC might evoke depressive behaviours, and that these amino acids could be the target of new antidepressant development. Therefore, we directly tested 2 substances, L-Glu and L-Gln, as therapeutic agents in our animal models. Astrocyte-ablated mice were directly infused with L-Glu, L-Gln or vehicle (saline), and they were subjected to the FST after this treatment. As shown in Figure 5A, the higher dose of L-Gln (0.2 μmol) reversed the depressive behaviours of mice infused with L-AAA, but neither the doses of L-Glu or the lower dose of L-Gln (0.02 μmol) altered the duration of immobility (Fig. 4A). This result was further verified using different doses of L-Gln in another experiment (Fig. 4B). For this experiment, mice infused with L-AAA were pretested for immobility in the FST and then divided into 4 groups, 3 receiving a different dose of L-Gln and 1 receiving a sham dose. Figure 4B shows the antidepressant effect of the 3 doses of L-Gln tested. Among them, the highest dose (0.4 μmol) of Gln lowered the immobility of mice infused with L-AAA to that of mice that were not infused with L-AAA. As discussed previously (Fig. 3), treatment with MSO or MeAIB to disrupt the Glu–Gln cycle also induced low levels of Glu and Gln in the PFC. Therefore, we also infused Gln with each compound. Interestingly, the depressive behaviours induced by MSO and MeAIB disappeared with Gln treatment (Fig. 5A and B), implying that Gln could also be a potent antidepressant in these models. Furthermore, we investigated the single effect of Glu or Gln on locomotor activity and immobility duration using control mice that were not treated with L-AAA (Fig. 5C and D). As a result, Glu and Gln did not interfere with locomotor activity, and only Gln treatment reduced the duration of immobility, suggesting the potency of Gln as an antidepressant (Fig. 5C and D).
Fig. 5.
Effects of glutamine (Gln) administration on immobility of mice infused with methionine sulfoximine (MSO) or α-methyl-amino-isobutyric acid (MeAIB) and control mice. Glutamine (0.2 μmol) reversed the duration of immobility induced by MSO (0.1 μmol, A, F2,12 = 8.527, p = 0.005; Dunnett post hoc tests were performed to compare MSO and the other 2 groups) and MeAIB (0.07 μmol, B, F2,12 = 8.294, p < 0.005; Dunnett post hoc tests were performed to compare MeAIB and the other 2 groups). Glutamate (Glu; 0.2 μmol) and Gln (0.2 μmol) had no influence on the locomotor activity of control mice (C, F2,12 = 0.758, p = 0.49). Glutamine reduced the duration of immobility of control mice, but Glu did not (D, F2,12 = 6.670, p = 0.011; Dunnett post hoc tests were performed to compare Gln and the other 2 groups). The open field test and forced swim test were performed using the same animals in 1 day. Data are presented as means and standard errors of the mean. *p < 0.05, n = 5 per group.
Fig. 4.
Effects of glutamate (Glu) and glutamine (Gln) administration on immobility induced by L-α aminoadipic acid (L-AAA) infusion. The duration of immobility was significantly decreased by Gln (0.2 μmol), but not by Glu or by a low dose of Gln (A, F4,15 = 11.726, p < 0.001). Analyses of variance and Dunnett post hoc tests were used for multiple comparisons between control and other groups. This result was further confirmed using different doses of Gln, from sham to 0.4 μmol, using 4 groups of mice infused with L-AAA. The antidepressant effect of Gln was verified by the same test after Gln infusion (B, Gln 0.40, t6 = 2.740, p = 0.034; Gln 0.20, t6 = 2.551, p = 0.043; Gln 0.10, t6 = 2.538, p = 0.044). One group of mice was tested using the forced swim test twice, before and after Gln treatment. We conducted a t test between, before and after Gln treatment. Data are presented as means and standard errors of the mean. *p < 0.05, **p < 0.01, n = 4 per group.
Discussion
In this study, we used 3 different mouse models of helplessness, a representative depressive phenotype, to investigate how astrocyte ablation in the PFC evokes depressive behaviours. In one model, we infused the astrocyte-specific toxin, L-AAA, into the PLC of adult mice. In the second model, we infused MSO, an inhibitor of GS, into the PLC. In the third model, we infused MeAIB, a blocker of SAT2, into the PLC. Although we used 3 different chemicals, the phenotype displayed by all 3 animal models was very similar. The typical phenotype displayed in all of these models included longer duration of immobility and lower concentrations of Glu and Gln in the PFC than in control mice. Moreover, intracranial Gln infusion reversed the increased duration of immobility in all 3 models to control levels and also decreased the duration of immobility in control mice that were not treated with L-AAA. These results provide useful clues to understand and overcome MDD.
It has been reported that the effect of L-AAA on astrocyte ablation was most apparent by 48 hours and lasted 7 days following a single injection with robust gliosis.26 In the present study, we performed twice as many infusions as in a previous study14 and expected a longer duration of the effects. In the present study, astrocyte ablation was evident on the sixth day after L-AAA infusion (Fig. 1A), and the effect of astrocyte ablation on the levels of Glu and Gln began to appear on the fifth day and lasted until the seventh day. On the tenth day, significant differences in Glu and Gln levels and duration of immobility between mice infused with L-AAA and control mice were no longer found, implying that the number of astrocytes and their functions may be recovered via gliosis. Further investigation is needed. Our results suggest that the levels of Glu and Gln transiently decreased in the PFC after infusion of L-AAA, and depressive-like phenotypes were found during the time when these 2 amino acid levels dropped. Therefore, the shortage of Glu and Gln in the PFC may be the reason that depressive behaviours occur, because this shortage can alter neuronal activity.16 Moreover, a variety of clinical studies involving patients with depression have indicated that the concentrations of these 2 amino acids fluctuate in the brain and in plasma.27–32 The next step is to consider why this phenomenon occurs when fewer astrocytes are present.
Several lines of reasoning exist to explain why Glu and Gln levels were downregulated by astrocyte ablation. First, we should consider the inter-relationship between neurons and astrocytes from the viewpoint of Glu, a basic amino acid neurotransmitter in the brain. Glutamatergic neurons obtain Glu by 2 routes: de novo synthesis from glucose by the tricarboxylic acid cycle and conversion from Gln by glutaminase.33 Astrocytes protect neurons from Glu excitotoxicity after glutamatergic neurotransmission by clearing excess Glu from the synaptic cleft using the excitatory amino acid transporter (EAAT) 1–2. In astrocytes, Glu is used by GS for Gln synthesis and then transported back to neurons. Neurons make Glu from Gln and pack it into synaptic vesicles for use in their next round of signalling.34 When we infused L-AAA into the PLC, this process, known as the Glu–Gln cycle, was impaired. If fewer astrocytes were present around the cleft, the Glu concentration in the extracellular space would increase temporally, and neighbouring neurons could be damaged by high levels of Glu. It has recently been reported that a high level of extracellular Glu could be a cause of depressive-like behaviours in a genetic rat model of depression.35 However, in the present study, the total Glu level, as well as Gln, decreased after astrocyte ablation, which could be explained by the reduction of materials for Glu synthesis in neurons. This was also supported by the result of the neuronal Gln transport inhibition experiment in this study (Fig. 4). As a result, we hypothesized that increased duration of immobility during the FST and decreased sucrose preference during the SPT (Fig. 1), behaviours typically interpreted as depressive-like in rodents,19,21 are caused by the decreased Glu and Gln levels in the PFC owing to astrocyte ablation. This is supported by other clinical studies that reported decreased Glu, Gln and γ-aminobutyric acid levels in the anterior cingulate cortex and the PFC of depressed patients.28,29,36,37
In addition to the similar phenotype displayed in our 3 animal models, they share another common feature in that all models displayed impairment in the Glu–Gln cycle. Thus, this suggests that any disturbances in the cycle would lead to a nonhomeostatic state for Glu and Gln levels and that depressive behaviours would result from such circumstances. A variety of studies have reported this possibility. It is easier to understand if we consider 6 steps of the Glu–Gln cycle: (1) the release of Glu from presynaptic glutamatergic neurons, (2) the reuptake of Glu by astrocytes via EAAT1–2, (3) the GS-mediated conversion of Glu to Gln, (4) the Gln release from astrocytes into the extracellular space, (5) the Gln transport from the extracellular space into the neuron and (6) reconversion of Gln to Glu.11,38,39 In other words, if one of these steps is blocked or uncontrolled, the levels of Glu and Gln in the brain will be abnormal, which can result in depressive behaviours.
Both preclinical and clinical studies have shown that glutamatergic drugs, such as N-methyl-d-aspartate antagonists40–42 and 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propanoic acid potentiators,43 as well as mGlu2/3 antagonists,44 have antidepressant properties. These reports have suggested that a change in presynaptic Glu release, step 1 of the Glu–Gln cycle, could cause depressive behaviours. Step 2 of the cycle has also been considered as an inducer of depression. The action of EAAT1–2 has at least 2 functions, including clearance of EAA from the synaptic cleft and the provision of Glu to GS for Gln synthesis. In other words, excitotoxicity in the extra-cellular space and decreased synthesis of Gln would occur if there were a problem in the EAAT1–2 system. In accordance with this idea, lower rates of EAAT1–2 activity have been suggested as a possible cause of depressive disorders.33,45 Recently, it has been reported that GS expression was reduced in patients with MDD,11 an effect that may be due to changes in the number or activity of astrocytes. Thus, it has been suggested that Gln synthesis mediated by GS, step 3 of the Glu–Gln cycle, might also be involved in disease onset. This hypothesis is reinforced in our study, where MSO, a specific inhibitor of GS, evoked depressive behaviours (Fig. 3A). Inhibition of step 5 in the Glu–Gln cycle could also cause depressive behaviours, as we demonstrated in the present study using MeAIB (Fig. 3D). This result also suggests that abnormal amino acid transport through the neuronal membrane might lead to depressive behaviours, even when normal astrocyte functions occur. Therefore, step 4 and step 6 of the cycle are worthy of further study, as dysfunction of either of these steps may also evoke depressive behaviours. Collectively, our results suggest that regulation of the Glu–Gln cycle between neurons and astrocytes, especially in the PFC, plays an important role in mood regulation and should be further investigated for the prevention and treatment of depressive disorders.
In the present study, we tested the possibility that Gln could be used as an antidepressant because Gln levels were lower after astrocyte ablation (Fig. 1) and MSO or MeAIB infusion (Fig. 3). The application of an exogenous Gln supplement reduced the duration of immobility in all 3 mouse models (Fig. 4, 5A and B), even in control mice that were not infused with L-AAA (Fig. 5D). The successful use of Gln as a neuropsychologic or nutritional supplement has been in practice since the 1950s. At that time, the benefits of Gln administration were first observed when scientists found that it improved intellectual ability and reduced alcohol consumption and harmfulness.46–48 In the 1970s, the antidepressive property of Gln was suggested,49 and Gln was included in a list of drugs with potential effectiveness in treating psychological dependency.50 Numerous studies have also reported that the use of Gln as a nutrient has advantages, including clinical safety in both healthy individuals and patients, and improvements in nitrogen balance and immune function after surgery, extensive radiation and chemotherapy.51–54 All of these studies indicated that using Gln for human disease treatment exhibited many merits without causing harmful side effects. In our study, Gln was infused directly into the PLC of depressed mice because these mice exhibited low Gln levels. To our knowledge, this is the first report detailing the use of Gln as an antidepressant candidate based on our findings of a low level of Gln in mouse models of depression. Consequently, our results suggest a possibility that Gln may be an effective antidepressant candidate with fewer side effects than traditionally prescribed antidepressants.
Limitations
The present study has some limitations. First, we did not provide evidence that L-AAA infusion in the PFC did not affect neuronal survival; however, previous studies14,26 have confirmed that L-AAA did not affect neuronal survival and that neuronal cell death did not affect depressive-like behaviours. This is the reason that we did not investigate the neuron population. Second, we did not use genetically engineered animal models to test our hypothesis. For example, we conclude that neuronal Gln deficiency would induce depressive behaviour using only chemical inhibitors. However, if we had used genetically modified animals, including the neuronal-specific Gln transporter knockout or astrocyte-specific GS knockout mice, our conclusion may have been more concrete. In addition, the interpretation of our results might have been more clear if we had used other well-defined depression models, such as the chronic stress depression model and diverse behavioural assessments including SPT, tail suspension test or novelty suppression test.
Conclusion
Three points regarding depressive behaviours became evident from the present study. First, a reduced number of astrocytes results in lower levels of Glu and Gln and increased depressive behaviours. Second, a deficient supply of Gln to neurons might cause depressive behaviours, even when normal numbers of astrocytes are present. Third, depressive behaviours caused by lower levels of Glu and Gln can be reversed by the addition of exogenous Gln.
Acknowledgement
This research was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2011-0006200).
Footnotes
Contributors: S.S. Kang, G.J. Cho, W.S. Choi and H.J. Kim designed the study. Y. Lee acquired and analyzed the data. H. Son, G. Kim and S.J. Kim also acquired the data. D.H. Lee, G.S. Roh and H.J. Kim also analyzed the data. Y. Lee, S.S. Kang and H.J. Kim wrote the article, which all other authors reviewed. All authors approved the final version for publication.
Competing interests: H.J. Kim declares having received grant funding for this work as above. None declared for the other authors.
References
- 1.Nemeroff CB. The neurobiology of depression. Sci Am. 1998;278:42–9. doi: 10.1038/scientificamerican0698-42. [DOI] [PubMed] [Google Scholar]
- 2.Hercher C, Turecki G, Mechawar N. Through the looking glass: examining neuroanatomical evidence for cellular alterations in major depression. J Psychiatr Res. 2009;43:947–61. doi: 10.1016/j.jpsychires.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Bremner JD, Vythilingam M, Vermetten E, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–9. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 4.Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–31. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- 5.Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–52. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- 6.Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007;6:219–33. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banasr M, Duman RS. Regulation of neurogenesis and gliogenesis by stress and antidepressant treatment. CNS Neurol Disord Drug Targets. 2007;6:311–20. doi: 10.2174/187152707783220929. [DOI] [PubMed] [Google Scholar]
- 8.Miguel-Hidalgo JJ, Baucom C, Dilley G, et al. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiatry. 2000;48:861–73. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs E, Flugge G. Stress, glucocorticoids and structural plasticity of the hippocampus. Neurosci Biobehav Rev. 1998;23:295–300. doi: 10.1016/s0149-7634(98)00031-1. [DOI] [PubMed] [Google Scholar]
- 10.Cotter D, Mackay D, Chana G, et al. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–94. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- 11.Choudary PV, Molnar M, Evans SJ, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A. 2005;102:15653–8. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banasr M, Valentine GW, Li XY, et al. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry. 2007;62:496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Czéh B, Muller-Keuker JI, Rygula R, et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- 14.Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008;64:863–70. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee Y, Gaskins D, Anand A, et al. Glia mechanisms in mood regulation: a novel model of mood disorders. Psychopharmacology (Berl) 2007;191:55–65. doi: 10.1007/s00213-006-0652-4. [DOI] [PubMed] [Google Scholar]
- 16.Sanacora G, Rothman DL, Mason G, et al. Clinical studies implementing glutamate neurotransmission in mood disorders. Ann N Y Acad Sci. 2003;1003:292–308. doi: 10.1196/annals.1300.018. [DOI] [PubMed] [Google Scholar]
- 17.Walter M, Henning A, Grimm S, et al. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry. 2009;66:478–86. doi: 10.1001/archgenpsychiatry.2009.39. [DOI] [PubMed] [Google Scholar]
- 18.Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- 19.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 20.Joo Y, Choi KM, Lee YH, et al. Chronic immobilization stress induces anxiety- and depression-like behaviors and decreases transthyretin in the mouse cortex. Neurosci Lett. 2009;461:121–5. doi: 10.1016/j.neulet.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 21.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 22.Jeon BT, Shin HJ, Kim JB, et al. Adiponectin protects hippocampal neurons against kainic acid-induced excitotoxicity. Brain Res Rev. 2009;61:81–8. doi: 10.1016/j.brainresrev.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Jenstad M, Quazi AZ, Zilberter M, et al. System A transporter SAT2 mediates replenishment of dendritic glutamate pools controlling retrograde signaling by glutamate. Cereb Cortex. 2009;19:1092–106. doi: 10.1093/cercor/bhn151. [DOI] [PubMed] [Google Scholar]
- 24.Dadsetan S, Bak LK, Sorensen M, et al. Inhibition of glutamine synthesis induces glutamate dehydrogenase-dependent ammonia fixation into alanine in co-cultures of astrocytes and neurons. Neurochem Int. 2011;59:482–8. doi: 10.1016/j.neuint.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Kanamori K, Ross BD. Kinetics of glial glutamine efflux and the mechanism of neuronal uptake studied in vivo in mildly hyperammonemic rat brain. J Neurochem. 2006;99:1103–13. doi: 10.1111/j.1471-4159.2006.04152.x. [DOI] [PubMed] [Google Scholar]
- 26.Khurgel M, Koo AC, Ivy GO. Selective ablation of astrocytes by intracerebral injections of alpha-aminoadipate. Glia. 1996;16:351–8. doi: 10.1002/(SICI)1098-1136(199604)16:4<351::AID-GLIA7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Merkl A, Schubert F, Quante A, et al. Abnormal cingulate and pre-frontal cortical neurochemistry in major depression after electro-convulsive therapy. Biol Psychiatry. 2011;69:772–9. doi: 10.1016/j.biopsych.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Pfleiderer B, Michael N, Erfurth A, et al. Effective electroconvulsive therapy reverses glutamate/glutamine deficit in the left anterior cingulum of unipolar depressed patients. Psychiatry Res. 2003;122:185–92. doi: 10.1016/s0925-4927(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 29.Hasler G, van der Veen JW, Tumonis T, et al. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 30.Ajilore O, Haroon E, Kumaran S, et al. Measurement of brain metabolites in patients with type 2 diabetes and major depression using proton magnetic resonance spectroscopy. Neuropsychopharmacology. 2007;32:1224–31. doi: 10.1038/sj.npp.1301248. [DOI] [PubMed] [Google Scholar]
- 31.Yildiz-Yesiloglu A, Ankerst DP. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: a meta-analysis. Psychiatry Res. 2006;147:1–25. doi: 10.1016/j.pscychresns.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Mitani H, Shirayama Y, Yamada T, et al. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1155–8. doi: 10.1016/j.pnpbp.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 33.Valentine GW, Sanacora G. Targeting glial physiology and glutamate cycling in the treatment of depression. Biochem Pharmacol. 2009;78:431–9. doi: 10.1016/j.bcp.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Márquez J, Tosina M, de la Rosa V, et al. New insights into brain glutaminases: beyond their role on glutamatergic transmission. Neurochem Int. 2009;55:64–70. doi: 10.1016/j.neuint.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 35.Hascup KN, Hascup ER, Stephens ML, et al. Resting glutamate levels and rapid glutamate transients in the prefrontal cortex of the Flinders Sensitive Line rat: a genetic rodent model of depression. Neuropsychopharmacology. 2011;36:1769–77. doi: 10.1038/npp.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Auer DP, Putz B, Kraft E, et al. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305–13. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- 37.Michael N, Erfurth A, Ohrmann P, et al. Metabolic changes within the left dorsolateral prefrontal cortex occurring with electroconvulsive therapy in patients with treatment resistant unipolar depression. Psychol Med. 2003;33:1277–84. doi: 10.1017/s0033291703007931. [DOI] [PubMed] [Google Scholar]
- 38.Zou J, Wang YX, Dou FF, et al. Glutamine synthetase down-regulation reduces astrocyte protection against glutamate excitotoxicity to neurons. Neurochem Int. 2010;56:577–84. doi: 10.1016/j.neuint.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zink M, Vollmayr B, Gebicke-Haerter PJ, et al. Reduced expression of glutamate transporters vGluT1, EAAT2 and EAAT4 in learned helpless rats, an animal model of depression. Neuropharmacology. 2010;58:465–73. doi: 10.1016/j.neuropharm.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Kugaya A, Sanacora G. Beyond monoamines: glutamatergic function in mood disorders. CNS Spectr. 2005;10:808–19. doi: 10.1017/s1092852900010403. [DOI] [PubMed] [Google Scholar]
- 41.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 42.Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 43.Alt A, Nisenbaum ES, Bleakman D, et al. A role for AMPA receptors in mood disorders. Biochem Pharmacol. 2006;71:1273–88. doi: 10.1016/j.bcp.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 44.Pilc A, Chaki S, Nowak G, et al. Mood disorders: regulation by metabotropic glutamate receptors. Biochem Pharmacol. 2008;75:997–1006. doi: 10.1016/j.bcp.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 45.Moghaddam B, Adams B, Verma A, et al. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–7. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers LL, Pelton RB. Effect of glutamine in IQ scores of mentally deficient children. Tex Rep Biol Med. 1957;15:84–90. [PubMed] [Google Scholar]
- 47.Rogers LL, Pelton RB. Glutamine in the treatment of alcoholism; a preliminary report. Q J Stud Alcohol. 1957;18:581–7. [PubMed] [Google Scholar]
- 48.Ravel JM, Felsing B, Lansford EM, Jr, et al. Reversal of alcohol toxicity by glutamine. J Biol Chem. 1955;214:497–501. [PubMed] [Google Scholar]
- 49.Cocchi R. Antidepressive properties of I-glutamine. Preliminary report. Acta Psychiatr Belg. 1976;76:658–66. [PubMed] [Google Scholar]
- 50.Cocchi R, Tornati A. Psychic dependence? A different formulation of the problem with a view to the reorientation of therapy for chronic drug addiction. Acta Psychiatr Scand. 1977;56:337–46. doi: 10.1111/j.1600-0447.1977.tb06675.x. [DOI] [PubMed] [Google Scholar]
- 51.Lowe DK, Benfell K, Smith RJ, et al. Safety of glutamine-enriched parenteral nutrient solutions in humans. Am J Clin Nutr. 1990;52:1101–6. doi: 10.1093/ajcn/52.6.1101. [DOI] [PubMed] [Google Scholar]
- 52.Stehle P, Zander J, Mertes N, et al. Effect of parenteral glutamine peptide supplements on muscle glutamine loss and nitrogen balance after major surgery. Lancet. 1989;1:231–3. doi: 10.1016/s0140-6736(89)91254-3. [DOI] [PubMed] [Google Scholar]
- 53.Ziegler TR, Young LS, Benfell K, et al. Clinical and metabolic efficacy of glutamine-supplemented parenteral nutrition after bone marrow transplantation. A randomized, double-blind, controlled study. Ann Intern Med. 1992;116:821–8. doi: 10.7326/0003-4819-116-10-821. [DOI] [PubMed] [Google Scholar]
- 54.Young LS, Bye R, Scheltinga M, et al. Patients receiving glutamine-supplemented intravenous feedings report an improvement in mood. JPEN J Parenter Enteral Nutr. 1993;17:422–7. doi: 10.1177/0148607193017005422. [DOI] [PubMed] [Google Scholar]