Abstract
Background
Increased circulating glucocorticoids are features of both aging and Alzheimer’s disease (AD), and increased glucocorticoids accelerate the accumulation of AD pathologies. Here we analyzed the effects of the glucocorticoid receptor antagonist mifepristone (RU-486) in the 3xTg-AD mouse model at an age where hippocampal damage leads to high circulating corticosterone levels.
Methods
The effects of mifepristone were investigated in 3xTg-AD mice using a combination of biochemical, histological and behaviour analyses.
Results
Mifepristone treatment rescues the pathologically-induced cognitive impairments and markedly reduces Aβ-load and levels, as well as tau pathologies. Analysis of APP processing revealed concomitant decreases in both APP C-terminal fragments C99 and C83 but the appearance of a larger 17-kDa C-terminal fragment. Hence, mifepristone induces a novel C-terminal cleavage of APP that prevents it being cleaved by α- or β- secretase, thereby precluding Aβ generation in the CNS; this cleavage and the production of the 17-kDa APP fragment was generated by a calcium-dependent cysteine protease. In addition, mifepristone treatment also reduced the phosphorylation and accumulation of tau, concomitant with reductions in p25. Notably, deficits in CREB signaling were restored with the treatment.
Conclusions
These preclinical results point to a potential therapeutic role for mifepristone as an effective treatment for AD and further highlight the impact the glucocorticoid system has as a regulator of Aβ generation.
Keywords: Alzheimer disease, mifepristone, glucocorticoids, Aβ, tau, 3xTg-AD
INTRODUCTION
Alzheimer’s disease (AD) is the most prevalent cause of dementia in the elderly, with only palliative treatments available at this time. It has been shown that AD patients display significant elevated levels of the glucocorticoid hormone cortisol in plasma and cerebrospinal fluid (CSF) as well as hypothalamic-pituitary-adrenal axis dysfunction (1-3).
We previously showed that glucocorticoids (GC’s; cortisol in humans and corticosterone in rodents) could drive the formation of AD hallmark pathologies – through increased production of the Aβ peptide, and accumulation of somatodendritic tau (4). In addition, both stress and increased GC exposure have been shown to induce cognitive impairments, trigger APP misprocessing, reduce Aβ clearance by decreasing activity of insulin degrading enzyme and stimulating tau hyperphosphorylation (5-9), together demonstrating a key role for GC’s in the progression of AD pathology and cognitive decline. GC’s are key stress hormones secreted by the adrenal gland and controlled by the hypothalamic-pituitary-adrenal axis, which mediate their effects in the different brain areas through two types of receptors: mineralocorticoid type I (MR) and glucocorticoid type II receptors (GR) (10). Recently the GR has been implicated in epigenetic mechanisms of neurodegeneration, via HDAC2 (11).
This current study investigates the therapeutic potential of the glucocorticoid receptor antagonist mifepristone (RU 3486) on both cognitive and pathological outcomes in the 3xTg-AD mouse model of AD. 3xTg-AD mice show increased circulating GC’s levels from 9 months of age, when hippocampal pathology is advanced (4). Hence, we sought to evaluate if blocking the effects of GC’s could help reduce pathology and cognitive decline in these aged animals. Here, we report that the GR blocker, mifepristone, has unparalleled effects on both outcomes, leading to robust reductions in Aβ levels and plaques, through the induction of a 17-kDa cleavage of APP, precluding Aβ generation. In addition, it restores CREB signaling and reduces tau hyperphosphorylation via reductions in p25 levels. Hence, our results show that compounds targeting the glucocorticoid system could be useful for the treatment of AD, in part through novel disease modifying effects on Aβ generation.
MATERIAL AND METHODS
Transgenic mice and treatment
All animal procedures were performed in accordance with National Institutes of Health and University of California guidelines and Use Committee at the University of California, Irvine. The characterization of 3xTg-AD mice been described previously (12). In this study, 12 month-old homozygous Ntg and 3xTg-AD mice, 8-10 per group (males) were anesthetized with isofluorane and drug pellets containing the glucocorticoid receptor antagonist RU486 (mifepristone;17b-hydroxy-11a-(4-dimethylaminophenyl)-17a-(1-propynyl)-estra-4,9-dien-3-one; Innovative Research of America, Sarasota, FL)) or vehicle pellets were implanted sub-cutaneous according to the manufacturer’s instructions. In addition, 10 month-old homozygous 3xTg-AD mice, 10 per group (male) were anesthetized with isofluorane and drug pellets containing the selective glucocorticoid antagonist Cort-108297 ((R)-4a-Ethoxymethyl-1-(4-fluorophenyl)-6-(4-trifluoromethyl-benzenesulfonyl0-4,4a,5,6,7,8-hexahydro-1H-1,2,6-triaza-cyclopenta[b]naphthalene; Corcept Therapeutics, CA, USA) or vehicle pellets were implanted sub-cutaneously. The animals were treated during 21-days with 25.2mg pellets release a continuous flow of the drug at 1.2mg per day (13-15).
Behavior testing
Novel context, place and object were conducted as described previously (16). Open field was performed as previously described (16). Hidden Morris water maze test were conducted as described previously (17, 18). The passive inhibitory avoidance task was performed as described previously (19, 20).
Tissue preparation
Tissue preparation was performed as previously described (17, 18). For protease analyses, whole brains were homogenized with/without complete protease inhibitor cocktail tablets (Roche) or specific families proteases inhibitor (Leupeptin; 10mg/ml; E64: 10μM; Aprotinin: 10mg/ml; Pepstatin: 1μM; phosphoramidon: 10μM (Sigma)), in the presence or absence of calcium in Assay Buffer (Assay Buffer: 135mM NaCl, 5mM KCl, 1.2mM MgSO4, 5mM Hepes, 10 mM Glucose and 2.5mM CaCl2). The samples were incubated 20 min at 37C with the differences protease inhibitors, and then immunoblotted for APP fragments using C-terminal antibody CT20 (Calbiochem).
Immunohistochemistry
Immunohistochemistry for light microscopy was performed as we described previously (21). The following antibodies were used: anti-6E10 (1:1000, Signet) and PHF1 (1:1000; Pierce Biotechnology), The specificity of the immune reactions was controlled by omitting the primary antibody.
Total Aβ loading
Quantification of total Aβ content was performed as previously (21).
Immunoblotting
Immunoblots analyses were performed as described (4). The following primary antibodies were used: anti-CREB (1:1000; Cell Signaling), anti-p-CREB (ser133) (1:1000; Cell Signaling), anti-CTF20 (1:5000; Calbiochem, San Diego, CA) for C99 and C83, anti-HT7 (1:5000; Pierce Biotechnology), anti-AT8 (1:1000; Pierce Biotechnology), anti-AT180 (1:1000; Pierce Biotechnology), anti-AT270 (1:1000; Pierce Biotechnology), anti-PHF1 (Dr. Peter Davies, Albert Einstein College of Medicine, Manhasset, NY, USA), anti-ADAM10 (1:1000; Calbiochem), anti-BACE (1:1000; Calbiochem), anti-IDE (1:1000; Chemicon), anti-totalGSK3α and β (1:5000; Calbiochem), anti-pGSK3β(ser9) (1:3000; Cell Signaling), anti-Cdk5 (1:1000; Calbiochem), anti-C’-term p35 (1:200; Santa Cruz Biotechnology) for p25 and p35, anti-PP2A (1:1000, Santa Cruz Biotechnology), anti-GAPDH (1:5000, Santa Cruz Biotechnology).
Enzyme-linked immunosorbent assay for Aβ40 and Aβ42
Aß1-40 and Aß1-42 were measured using a sensitive sandwich ELISA system as previously described (4).
Mass Spectrometry of Mifepristone
30 ul of plasma was vortexed with 200 ul of dichloromethane, and the dichloromethane fraction taken and air-dried. The precipitate was dissolved in 50 ul of 50% acetonitrile and then separated on an Acquity UltraPerformance LC and then analysed on a Quatro Premier XE. Mifepristone standards showed fragments of mw 134 and 370, which were positively identified in the plasma samples.
Quantitative and statistical analyses
All immunoblot data were quantitatively analyzed using Image J 1.4 software. Statistical evaluation of the results was performed using Student’s t-test comparison, to compare 2 groups, or Two-way analysis of variance (ANOVA). After significant analyses of variance, multiple post hoc comparisons were performed using Bonferroni’s test. Analysis of MWM acquisition was evaluated via repeated-measure of variance. The significance was set at 95% of confidence. All values are presented as mean ± SEM. All tests were performed using Graphpad Prism software (Graphpad Prism Inc., San Diego, CA, USA).
RESULTS
Mifepristone (RU-486) rescues cognitive deficits in aged 3xTg-AD
12-month old male 3xTg-AD mice were implanted with sub-cutaneous pellets containing 15 mg mifepristone or vehicle pellets designed to release a continuous flow of 10 μg/h for 60 days. This concentration of mifepristone has proven effective to inhibit GR activity in mice (22, 23). To determine whether mifepristone treatment could rescue cognitive deficits, 3xTg-AD mice were tested on a battery of behavioral tasks. Testing occurred during the last 2 weeks of treatment, and probed cortical, hippocampal and amygdala-dependent memory tasks, brain areas that are most severely affected by AD-like pathology in the 3xTg-AD (12, 24).
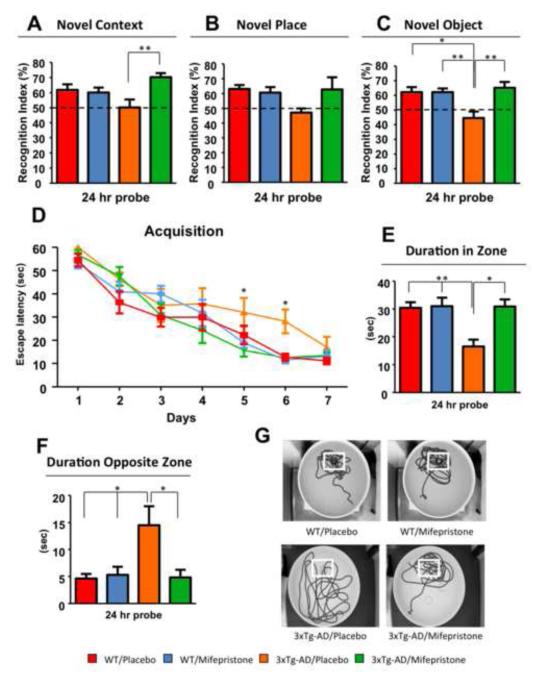
Treated and vehicle-treated 3xTg-AD and Ntg (Non transgenic) mice were first tested on novel context/place/object tasks. These tasks are mainly hippocampal and cortical dependent, respectively. Vehicle-treated 3xTg-AD mice were significantly impaired compared to Ntg mice only on the novel object task, spending 50.17±12.99% of their time exploring the out-of-context object, 47.09±7.56% exploring the out of place object and 44.53±9.87% exploring the new object (Fig. 1A-C), compared to Ntg mice. In contrast, mifepristone-treated 3xTg-AD mice show a significant improvement in performance, spending 71.08±11.30% exploring the new object and 70.92±7.70% of their time exploring the out-of-context object (Fig. 1A and C). No differences were observed between Ntg mifepristone and vehicle-treated mice (Fig. 1A-C).
Figure 1. Mifepristone rescues cognitive impairments in 3xTg-AD mice.
A-C) Significant improvements in memory by novel context (A) and object (C) recognition were observed between mifepristone and vehicle treated 3xTg-AD mice. B) No significant differences were detected in novel place. D) Mice were trained on the spatial reference version of the Morris water maze (MWM; N=8-10 per group). Acquisition curves shown for the 7 days of training on the MWM. Mifepristone treatment reduces spatial memory deficits during training. E-F) Mifepristone-treated mice shown significant improvements in duration spent in the target zone (E) and opposite zone (F). G) Representative path tracings of the probe test session (white box represent localization of platform target). Ntg-vehicle (red); Ntg-mifepristone (blue); 3xTg-AD-vehicle (orange); 3xTg-AD-mifepristone (green). The values represent the mean ± S.E.M. (N = 8-10). *p < 0.05 and **p < 0.01.
3xTg-AD mice were tested on another hippocampal-dependent behavior task, the Morris water maze (MWM). Vehicle-treated 3xTg-AD mice reach criterion after 7 days (escape latency <25 seconds), whereas mifepristone-treated 3xTg-AD and Ntg vehicle and mifepristone-treated mice require only 5 days (Fig. 1D). Furthermore, mifepristone treatment improves long-term memory (24 hours probe) in the 3xTg-AD mice compared to vehicle-treated mice, as determined by the significant increase in the duration of time in the platform quadrant and reduced time in the opposite quadrant (Fig. 1E and F). These data were confirmed by the analysis of the swim patterns in the test session (Fig.1G). Notably, the effects of mifepristone on water maze performance are not directly related to motor alterations, since no significant variations of the swimming speed or total distance traveled in the water maze were observed in mifepristone compared with vehicle-treated mice (Figure S1A-B in Supplement 1).
In addition, no differences between vehicle- and mifepristone-treated mice were seen on the open field test (Figure S1C in Supplement 1). Finally, 3xTg-AD mice were trained in a contextual learning and memory task (passive inhibitory avoidance) primarily dependent on the amygdala (25). A 24-hour memory test revealed no differences between vehicle and mifepristone treated mice (Figure S1D in Supplement 1) pointing to a lack of improvement with mifepristone treatment. We confirmed the presence of mifepristone in the plasma at the end of the experiment via Mass Spectrometry.
Mifepristone regulates CREB signaling in 3xTg-AD, but not wild type, mice
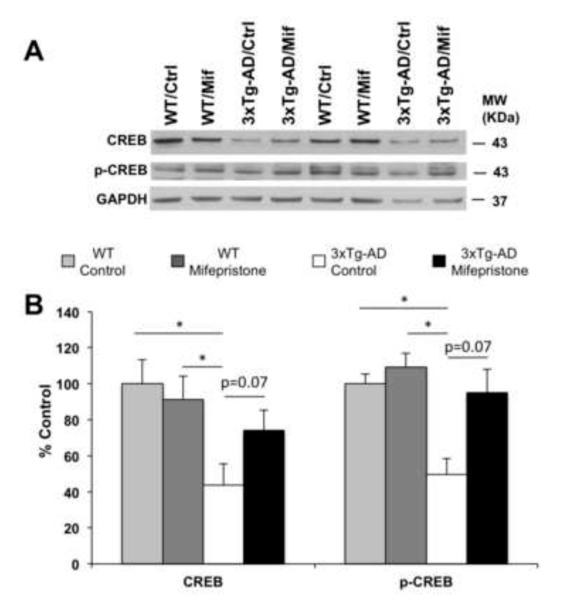
Deficits in CREB signaling have been associated with impaired learning in 3xTg-AD mice (26). Western blot analyses of brain homogenates showed up-regulation in steady-state levels of both CREB (41.17±16.15%, Two-way Anova, Bonferroni post-hoc p=0.07) and phospho-CREB (47.65±9.20%, Two-way Anova, Bonferroni post-hoc p=0.07) with mifepristone treatment compared to vehicle. In addition, no differences were observed in Ntg mice in CREB or phospho-CREB with either vehicle or mifepristone. Notably, significant decreases (for CREB 56.40±11.97%, Two-way Anova, Bonferroni post-hoc, *p<0.05 and for p-CREB 50.28±8.74%, Two-way Anova, Bonferroni post-hoc, *p<0.05) in steady-state level of CREB and p-CREB were observed between Ntg and 3xTg-AD mice (Fig. 2A-B).
Figure 2. Mifepristone regulates CREB signaling in 3xTg-AD.
A) Immunoblot analysis of CREB and p-CREB from whole-brain homogenates of Ntg and 3xTg-AD mice treated for 2 months with either mifepristone (Mif; n=8) or vehicle (Ctrl; n=8) shown as alternating lanes. B) Quantification of A normalized to GAPDH and expressed as a % of control shows significant increases in the steady-state level of CREB (41.17±16.15%, Two-way Anova, Bonferroni post-hoc p=0.07) and p-CREB (47.65±9.20%, Two-way Anova, Bonferroni post-hoc p=0.07) in 3xTg-AD mice treated with mifepristone compare to vehicle. Notably, the quantifications show significant increases in steady-state level of CREB (56.40±11.97%, Two-way Anova, Bonferroni post-hoc, *p<0.05) and p-CREB (50.28±8.74%, Two-way Anova, Bonferroni post-hoc, *p<0.05) in Ntg compared to 3xTg-AD mice. Additionally, no differences in the steady-state level CREB and p-CREB in mifepristone-Ntg treated mice compare to vehicle. The values represent the mean ± S.E.M. *p < 0.05.
Lower Aβ levels and plaque load in mifepristone-treated 3xTg-AD
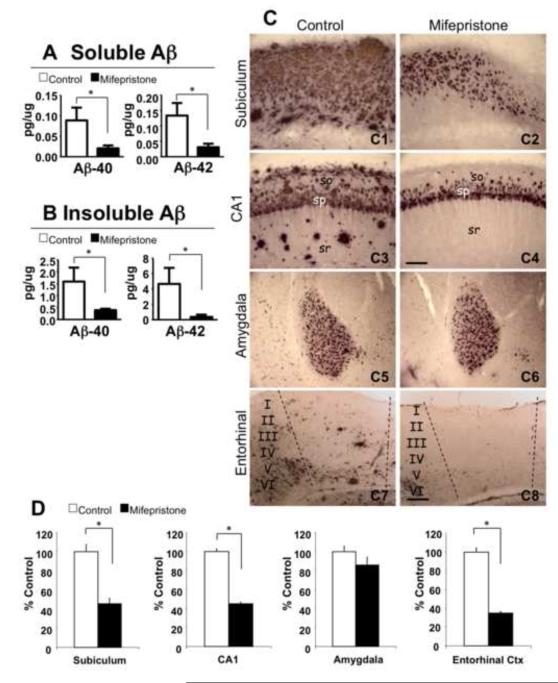
Dramatic reductions in both soluble Aβ40 (73.61±10.56%, *p<0.05, t-test) and Aβ42 (74.06±13.32%, *p<0.05, t-test) are induced by mifepristone- versus vehicle-treatment (Fig. 3A). In addition, insoluble Aβ40 (72.12±2.08%, *p<0.05, t-test) and Aβ42 (89.38±7.92%, *p<0.05, t-test) levels are robustly reduced in mifepristone treatment compared to vehicle mice (Fig. 3B). In agreement with the ELISA data, immunostaining analysis with antibody 6E10 shows intense reduction of extracellular deposits in mifepristone-treated compared to vehicle (Fig. 3C1-8); it should be noted that intracellular staining is likely due to a combination of Aβ as well as intracellular APP. Thus, the results showed a significant decrease in the subiculum (45.18±10.16%, *p<0.05, t-test) and CA1 hippocampal subfields (44.91±7.70%, *p<0.05, t-test), and the entorhinal cortex (64.91±4.01%, *p<0.05, t-test). Of significance to the cognitive data, we did not observe any significant changes in plaque load in the amygdala (Fig. 3D). In addition, intracellular immunolabeling expression patterns were similar in mifepristone and vehicle treated mice in all analyzed brain areas (Fig. 3C).
Figure 3. Mifepristone significantly lowers A levels and plaque load.
A-B) Brain Aβ measurements by sandwich ELISA of both the soluble (Aβ40: 73.61±10.56%, *p<0.05, t-test; Aβ42: 74.06±13.32%, *p<0.05, t-test) and insoluble (Aβ40: 72.12±2.08%, *p<0.05, t-test; Aβ42: 89.38±7.92%, *p<0.05, t-test) fractions of 3xTg-AD mice treated with mifepristone revealed dramatic reductions in Aβ levels. C) Light microscopic images immunostained with anti-A antibody (6E10) in the subiculum (C1 and C2), CA1 (C3 and C4), amygdala (C5 and C6) and entorhinal cortex (C7 and C8) of 3xTg-AD in vehicle (C1, 3, 5 and 7) and mifepristone (C2, 4, 6 and 8) treated mice. D) Quantitative plaque load analysis shows a significant decrease in hippocampal areas: subiculum (45.18 ± 10.16%, *p<0.05, t-test) and CA1 (44.91 ± 7.70%, *p<0.05, t-test); and entorhinal cortex (64.91 ± 4.01%, *p<0.05, t-test). No significant differences were detected in the amygdala. so: stratum oriens; sp: stratum pyramidale; sr: stratum radiatum. Scale bars: 200 μm (C1-C4) 100 μm (C5-C8). The values represent the mean ± S.E.M. *p < 0.05.
Mifepristone induces a novel 17-kDa APP fragment that precludes Aβ generation
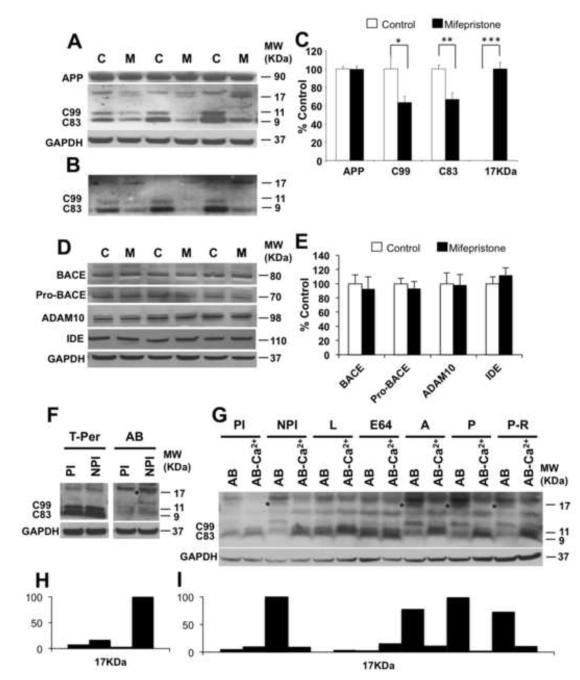
Steady-state levels of full-length APP holoprotein are unaffected by mifepristone treatment, as expected as APP (and tau) are under the control of the thy1.2 transgene promoter in the 3xTg-AD mice. Using a C-terminal APP antibody (CT20; (27)) significant reductions in steady-state levels of both C83 (33.45±7.49%, **p<0.01, t-test) and C99 (36.88±6.86%, *p<0.05, t-test) were observed with mifepristone treatment (Fig. 4A, B and C). Both CTF’s were reduced by the same degree, which is unusual as these fragments are usually mutually exclusive and also given no reductions in the parent holoprotein APP. Instead, we observed the appearance of a 17-kDa APP fragment only in mifepristone-treated animals; the 17-kDa fragment represents the last C’ 17-kDa of APP, which contains both the C99 and C83 sequence. As such, we deduce that this novel fragment is produced with mifepristone-treatment and as such neither C99 nor C83 are produced, thus bypassing Aβ generation and explaining the concomitant reductions in both C99 and C83. We repeated this experiment using a different C-terminal antibody lot (Fig. 4B), as a non-specific band at 18-kDa was observed with the initial lot (Fig. 4A). In confirmation of this pathway in the 3xTg-AD mice, we analyzed steady state levels of endogenous APP and APP C-terminal fragments in NTg mice treated with mifepristone. We found no changes in full length APP with mifepristone treatment, but trended to reductions in the APP CTF’s (27.32±12.07%, p=0.08, t-test) compared to vehicle Ntg mice (Figure S2A-B in Supplement 1).
Figure 4. Mifepristone induces a novel 17-kDa APP fragment and reduces both C99 and C83.
A-B) Immunoblot analysis of APP holoprotein, C99, C83, and a novel 17-kDa C-terminal APP fragment from whole-brain homogenates of 3xTg-AD mice treated for 2 months with either mifepristone (M; n=8) or vehicle (C; n=8) shown as alternating lanes. C) Quantification of A and B normalized to GAPDH and expressed as a % of control shows a significant reduction of C99 (36.88 ± 6.86%, *p<0.05, t-test) and C83 (33.45 ± 7.49%, **p<0.01, t-test) in mifepristone-treated mice compare to vehicle. The appearance of the novel 17-kDa APP fragment was only seen with treatment (***p<0.001, t-test). D) Immunoblot analysis of BACE1, pro-BACE1, ADAM10 and insulin-degrading enzyme (IDE) from whole-brain homogenates of 3xTg-AD mice treated for 2 months with either mifepristone (M; n=8) or vehicle (C; n=8) shown as alternating lanes. E) Quantification of D normalized to GAPDH and expressed as a % of control showed no significant differences in mifepristone-treated mice compare to vehicle. F and H) Whole-brain homogenate were incubated using T-per buffer and Assay Buffer (AB) with and without protease cocktail inhibitor, and immnoblotted with a C-terminal APP antibody. * denotes presence of 17-kDa APP fragment. G and I) Whole-brain homogenate were incubated during 20 min at 37‘C using Assay Buffer with/without proteases inhibitors and specific proteases inhibitor families and immnoblotted with a C-terminal APP antibody. * denotes presence of 17-kDa APP fragment. AB: Assay Buffer; PI: Protease Inhibitors; NPI: Non-Protease Inhibitors; L: Leupeptin; E64: trans-epoxysuccinyl-L-leucylamido-(4-guanidino)-butane; A: Aprotinin; P: Pepstatin; P-R; phospho-ramidon. The values represent the mean ± S.E.M. (N = 8). *p < 0.05, **p < 0.01 and ***p<0.001.
We analyzed steady-state levels of the constitutive protease α- (ADAM10), β–secretase (BACE1) (28-31) and insulin-degrading enzyme IDE (32), and found no differences with treatment (Fig. 4D and E).
We reasoned that the protease responsible for the generation of the 17-kDa APP fragment was probably present in the brain, but unable to interact with APP under normal conditions. To test this we took fresh brains from 3xTg-AD mice, and homogenized them with our usual T-per detergent buffer or a mild Assay Buffer (AB) with (PI) and without (NPI) proteinase inhibitors. We found that brains homogenized in assay buffer in the absence of protease inhibitors generated the 17-kDa APP fragment (Fig. 4F and H), but its production was blocked by the presence of protease inhibitors, or by T-per buffer. We then set to characterize the responsible APP protease further by incubating 3xTg-AD whole-brain homogenates in assay buffer for 20 min with specific proteases inhibitors. Brains incubated with leupeptin (serine and cysteine protease inhibitor) and E64 (cysteine protease inhibitor) blocked the formation of the 17-kDa C-terminal fragment. However, 3xTg-AD brain incubated with Aprotinin (serine protease inhibitor), Pepstatin (aspartyl protease inhibitor) and phosphoramidon (metalloendopeptidase inhibitor) was not able to block the formation of 17-kDa C-terminal fragment (asterix) (Fig. 4G and I). This data indicate that a cysteine protease is involved in the formation of the 17-kDa C-terminal fragment in the 3xTg-AD mice. In addition, when brain samples were incubated in assay buffer without calcium and with EGTA, the generation of the 17-kDa C-terminal fragment was blocked (Fig. 4G and I). These data indicate that a calcium dependent cysteine protease generate the 17-kDa C-terminal fragment in 3xTg-AD mice.
Mifepristone reduces accumulation of tau phosphorylated at epitopes Thr181 and Ser 396/404
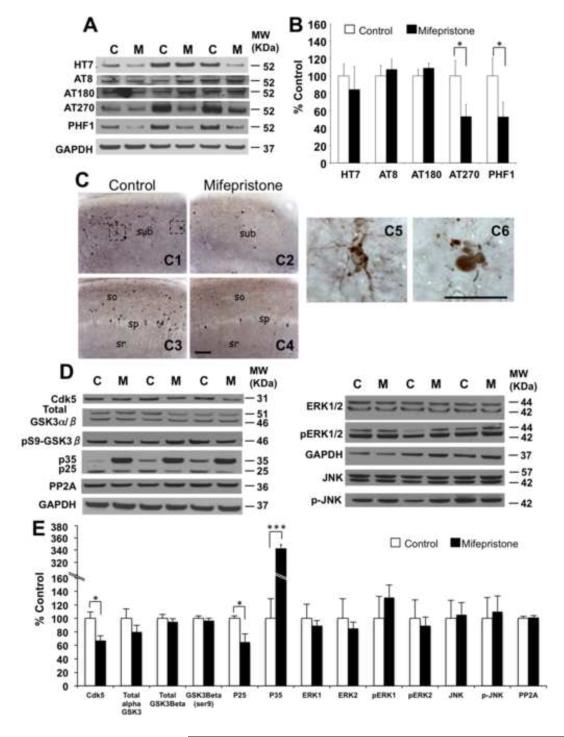
Previous studies showed that glucocorticoids stimulate tau hyperphosphorylation and alter tau trafficking and stability (9, 33). Subsequently, we examined the effects of mifepristone on tau phosphorylation. Western-blot analyses of soluble brain homogenates revealed reductions in tau levels phosphorylated at residues Thr181 (46.89 ± 13.91%, *p<0.05, t-test and recognized by the AT270 antibody) and Ser396/404 (35.32 ± 13.69%, *p<0.05, t-test and recognized by the PHF-1 antibody). Levels of phospho-tau species recognized by antibodies AT8 (Ser199/202) and AT180 (Thr231) were unaffected by mifepristone-treatment versus vehicle-treatment (Fig. 5A and B). In correlation with biochemical analysis, immunohistochemistry of hippocampal sections (CA1 and Subiculum) showed marked reductions in somatodendritic phospho-tau accumulation and aggregation in the pyramidal cell layer in the 3xTg-AD-mifepristone treated mice compared to vehicle (Fig. 5C1-C4 and C5). In addition, reductions in the number of dystrophic neurites positive for PHF-1 were observed in the subiculum area of animals treated with mifepristone versus vehicle (Fig. 5C6).
Figure 5. Mifepristone reduces accumulation of p-tau epitopes Thr181 and Ser396/404.
A) Immunoblot analysis of total tau (HT7) and phospho-tau epitopes including pSer199/202 tau (AT8), pThr231 (AT180), pThr181 (AT270) and pSer396/404 (PHF-1) of protein extracts from whole-brain homogenates of 3xTg-AD mice treated for 2 months with either mifepristone (M; n=8) or vehicle (C; n=8) shown as alternating lanes. B) Quantification of A normalized to GAPDH and expressed as a % of control shows significant reductions in p-tau epitopes Thr181 (46.89 ± 13.91%, *p<0.05, t-test) and Ser 396/404 (47.20 ± 16.88%, *p<0.05, t-test). C) Light microscopic images immunostained with anti-pSer396/404 antibody (PHF-1 antibody) in the hippocampus (subiculum: C1 and C2; CA1: C3 and C4) of 3xTg-AD in control (C1 and C3) and mifepristone (C2 and C4) treated mice at 14 months of age. Intracellular p-tau (pSer396/404) accumulation and aggregation (C1), and a dystrophy neurite positive for pSer396/404 are shown in enlarged images. D) Immunoblot analysis of cdk5, GSK3α/β, inactive GSK3β (phosphorylated at ser9), p25/p35 and PP2A of protein extracts from whole-brain homogenates of 3xTg-AD mice treated for 2 months with either mifepristone (M; n=8) or vehicle (C; n=8) shown as alternating lanes. E)Q uantification of D normalized to GAPDH and expressed as a % of control shows significant reductions in the expression of cdk5 (32.66 ± 6.67%, *p<0.05, t-test) and p25 (35.32 ± 13.69%, *p<0.05, t-test). Furthermore, accumulation of p35 (250.78 ± 8.34%, ***p<0.001, t-test) was observed in 3xTg-AD mifepristone treated mice. sub: subiculum; so: stratum oriens; sp: stratum pyramidale; sr: stratum radiatum. Scale bars: 200μm (C1-C4), 50μm (C5 and C6). GAPDH levels were used as control for protein loading. The values represent the mean ± S.E.M. *p < 0.05 and ***p<0.001.
Finally, to elucidate the cellular mechanisms through which mifepristone reduces tau phosphorylation in the 3xTg-AD, we examined total and activated forms of cyclin-dependent kinase 5 (cdk5) and GSK3-β, two major kinases associated with abnormal tau phosphorylation in the brain (34-37). The results showed significant reductions in the steady-state levels of cdk5 (32.66 ± 6.67%, *p<0.05, t-test) and the cytosolic activators of cdk5, p25 (35.32 ± 13.69%, *p<0.05, t-test) in the mifepristone-treated mice (Fig. 6D and E). Notably, we observed significant increases in steady-state levels of p35 (250.78 ± 8.34%, ***p<0.001, t-test) (Fig. 5D and E). Similar results were observed in the steady-state levels of p25 in Ntg mice treated with mifepristone (46,54 ± 2.82%, **p<0.01, t-test) (Figure S2C-D in Supplement 1) ; the cystein protease calpain mediates the proteolytic cleavage of p35 to p25 (38), suggesting that mifepristone could inhibit calpain activity. Steady-state levels of GSK3-β (glycogen synthase kinase-3), pS9-GSK3-β (inactive GSK3 phosphorylated at ser9), ERK (extracellular signal-regulated kinase), p-ERK, JNK (c-jun amino-terminal kinase), p-JNK and PP2A (tau phosphatase), were unaffected by mifepristone treatment (Fig. 5D and E). In correlation with the cognitive data, immunostaining analysis of the amygdala shows no differences in the expression of total human tau, while similar low numbers of PHF-1 positive cells were observed in both 3xTg-AD vehicle and mifepristone treated mice (Figure S3A-B in Supplement 1). Our results indicate that mifepristone mediates reduction of phospho-tau levels on residues Thr181 and Ser396/404 correlating with inactivation of cdk5/p25 in the 3xTg-AD.
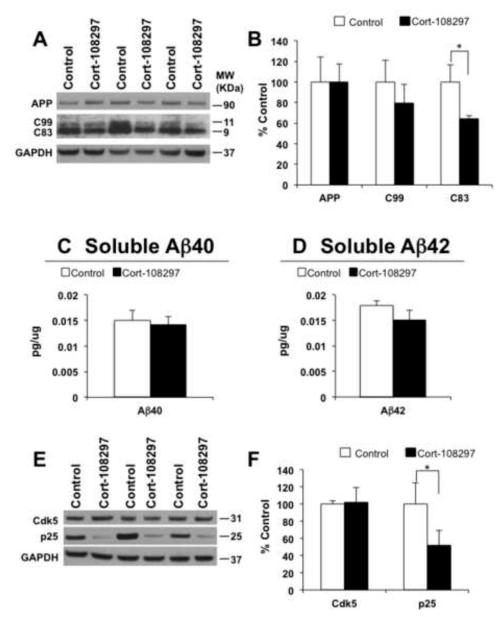
Figure 6. The selective Cort-108297 glucocorticoid antagonist reduces C-terminal APP fragment and p25 expression.
A) Immunoblot analysis of APP holoprotein, C99 and C83 C-terminal APP fragment from whole-brain homogenates of 3xTg-AD mice treated for 21 days with either Cort-108297 (Cort-108297; n=8) or vehicle (Control; n=8) shown as alternating lanes. C) Quantification of A normalized to GAPDH and expressed as a % of control shows a significant reduction of C83 (35.32 ± 8.02%, *p<0.05, t-test) in Cort-108297-treated mice compared to vehicle. In addition, a decrease tendency in steady-state levels was observed with C99. C and D) Brain Aβ measurements by sandwich ELISA of both soluble Aβ40 and Aβ42 of 3xTg-AD mice treated with Cort-108297. E) Immunoblot analysis of cdk5 and p25 of protein extracts from whole-brain homogenates of 3xTg-AD mice treated for 21 days with Cort-108297 (Cort-108297; n=8) or vehicle (C; n=8) shown as alternating lanes. F) Quantification of E normalized to GAPDH and expressed as a % of control shows significant reductions in the expression of p25 (48.02 ± 34.19%, *p<0.05, t-test). GAPDH levels were used as control for protein loading. The values represent the mean ± S.E.M. *p < 0.05.
A specific GR antagonist reduces APP CTF’s and p25
Mifepristone is an antagonist at both the GR and PR. To explore which of these targets were mediating the effects seen in the 3xTg-AD mice, we obtained the specific GR antagonist Cort-108297 (13-15); a generous gift from Corcept Therapeutics). We manufactured slow release pellets and implanted them, or placebo pellets, subcutaneously for 21 days. At the end of this period we analyzed brain homogenates for APP fragments, Aβ and p25 levels (Fig. 6A-F). Consistent with mifepristone treatment, Cort-108297 significantly reduced levels of C83 (35.32 ± 8.02%, *p<0.05, t-test), and trended towards a decrease in C99. Likewise, levels of p25 (48.02 ± 34.19%, *p<0.05, t-test) were significantly reduced with treatment. However, levels of Aβ were not significantly reduced, but this could be related to the shorter treatment duration than the experiments performed with mifepristone.
DISCUSSION
There is ample evidence implicating HPA-axis dysfunction in AD, reflected by markedly elevated basal levels of circulating cortisol, and a failure to show cortisol suppression following a dexamethasone challenge (1-3). We previously demonstrated that elevated circulating glucocorticoids could rapidly increase levels of both Aβ and tau protein in the brains of transgenic mice (4), whereas others showed similar results through changes in Aβ degradation and increases in tau phosphorylation, suggesting that these elevated cortisol levels in AD could drive some of the pathological progression (5-9). Recently, the CRF receptor has also been implicated in the progression of tau pathology, highlighting a link between stress and AD (39). In addition, it is known that prolonged exposure and high levels of GC’s is associated with neuronal damage and cognitive decline in the CNS (40-42). Thus, we investigated if anti-glucocorticoid strategies could offer a viable therapeutic approach.
This study shows clear cognitive improvements in a number of domains with treatment of aged 3xTg-AD mice with the glucocorticoid and progesterone receptor blocker mifepristone. Although these improvements are likely due to the marked reductions in both Aβ and tau pathologies in mifepristone-treated mice, anti-glucocorticoid drugs, through 11- hydroxy steroid dehydrogenase inhibitors, have recently been shown to improve cognition in aged mice of their own (43, 44). Of note, a small clinical trial in AD patients treated with mifepristone for just 6 weeks reported an increase in the ADAS-Cog (45), although this short timeframe is unlikely to have reversed pathology in humans.
Although glucocorticoid-reducing compounds may exhibit cognitive enhancing capabilities on their own, treatment with mifepristone led to profound reductions in both soluble and insoluble Aβ levels in the 3xTg-AD mice. These reductions in Aβ appear to be due to the induction of a novel APP processing pathway in which APP is cleaved 17-kDa from the C-terminal, by an unidentified protease, rather than by either - or - secretase. As such C99 (and C83) formation is prevented, and by extension, so is Aβ formation. We recently described this novel APP processing pathway in the CNS of AD transgenic mice and Cynomolgus primates using a small molecular compound known as ST101, with no known molecular targets or mechanisms of action (46). Here, we show that mifepristone stimulates the same novel APP processing pathway and leads to larger reductions in AD-related pathology as well as robust improvements in cognition. We hypothesize that mifepristone is either modulating the activity of this unknown APP protease, or that it is altering the sub cellular location of either APP or the protease such that they can interact in the presence of mifepristone. To this end we show that the untreated brain has the ability to for this 17-kDa APP fragment in the absence of protease inhibitors, showing that the protease is always present but does not normally cleave APP. Furthermore, we find that only inhibitors of cysteine proteases can block the formation of this APP fragment, and that calcium is necessary. Thus, an APP calcium dependent cysteine protease exists but its identity is unknown. Prominent calcium dependent cysteine proteases include the calpains – here we find that mifepristone down regulates p25 levels, which themselves are a product of calpain activity. Thus it is unlikely that calpains are the unidentified APP protease, and further experiments will be required to fully identify it. Mifepristone also attenuated tau pathology in the 3xTg-AD mice, through reduction of steady-state levels of phospho-tau on residues Thr181 and Ser396/404, concomitant with reductions in p25 in both wild-type and 3xTg-AD mice. We also observed significant reductions in the number of cell and dystrophic neurites positive for PHF1 in 3xTg-AD treated with mifepristone versus vehicle. In agreement with this, previous studies have shown that elevated GC’s can increase tau phosphorylation (9, 33).
In addition, the PR has been implicated in both cognitive improvements (47, 48) and reductions in pathology (49, 50) in mouse models of AD. For example, the progesterone metabolite allopregnanolone improves cognition in the 3xTg-AD mice via increases in neurogenesis (51, 52) and reduces Aβ accumulation (53), and progesterone itself improves cognition in APP mice (49) and also reduces tau hyperphosphorylation in 3xTg-AD mice (50). Notably, mifepristone can be metabolized in both the periphery and CNS (54), and these newly formed metabolites can bind to both the GR and PR (55). Furthermore, treatment with a specific GR antagonist, Cort-108297, was able to reduce APP CTF’s and p25, thus mimicking the some of the effects of mifepristone. These results suggest that the effect observed by mifepristone is related by modulation of GC instead to PR receptor. However, we cannot discard that PR or other steroid receptor could be modulated by mifepristone. Future research using knockout mice for GR, PR or other steroid hormone will be necessary to elucidate the effect of mifepristone to these receptors.
CREB signaling is impaired in 3xTg-AD mice, and restoration of CREB signaling restores cognitive function (26). Hence, we next looked at levels of CREB and phospho-CREB, and found both to be significantly increased in mifepristone treated mice versus vehicle. Notably, no effects were seen in wild type mice. These results suggest that 1) the effects on CREB signaling are mediated by the reductions in pathology rather than a direct effect of mifepristone, as the wild type mice were not affected, and 2) that increases in CREB signaling could be mediating the improved cognition in the 3xTg-AD mice.
Hence, mifepristone, and other GR targeting strategies, may offer potential benefit for the treatment of AD.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by grants from the National Institute of Health AG-021982 to FML. Aβ peptides and anti-Aβ antibodies were provided by the University of California Alzheimer’s Disease Research Center (UCI-ADRC) NIH/NIA Grant P50 AG16573. We thank Corcept Therapeutics for the generous gift of Cort-108297.
Footnotes
FINANTIALS DISCLOSURES All authors declare no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, et al. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. The American journal of psychiatry. 2006;163(12):2164–2169. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoogendijk WJ, Meynen G, Endert E, Hofman MA, Swaab DF. Increased cerebrospinal fluid cortisol level in Alzheimer’s disease is not related to depression. Neurobiol Aging. 2006;275:780 e781–780 e782. doi: 10.1016/j.neurobiolaging.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Umegaki H, Ikari H, Nakahata H, Endo H, Suzuki Y, Ogawa O, et al. Plasma cortisol levels in elderly female subjects with Alzheimer’s disease: a cross-sectional and longitudinal study. Brain Res. 2000;8812:241–243. doi: 10.1016/s0006-8993(00)02847-x. [DOI] [PubMed] [Google Scholar]
- 4.Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;2635:9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catania C, Sotiropoulos I, Silva R, Onofri C, Breen KC, Sousa N, et al. The amyloidogenic potential and behavioral correlates of stress. Mol Psychiatry. 2009;141:95–105. doi: 10.1038/sj.mp.4002101. [DOI] [PubMed] [Google Scholar]
- 6.Jeong YH, Park CH, Yoo J, Shin KY, Ahn SM, Kim HS, et al. Chronic stress accelerates learning and memory impairments and increases amyloid deposition in APPV717I-CT100 transgenic mice, an Alzheimer’s disease model. Faseb J. 2006;206:729–731. doi: 10.1096/fj.05-4265fje. [DOI] [PubMed] [Google Scholar]
- 7.Kulstad JJ, McMillan PJ, Leverenz JB, Cook DG, Green PS, Peskind ER, et al. Effects of chronic glucocorticoid administration on insulin-degrading enzyme and amyloid-beta peptide in the aged macaque. J Neuropathol Exp Neurol. 2005;642:139–146. doi: 10.1093/jnen/64.2.139. [DOI] [PubMed] [Google Scholar]
- 8.Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, et al. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J Neurosci. 2000;20(15):5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sotiropoulos I, Catania C, Riedemann T, Fry JP, Breen KC, Michaelidis TM, et al. Glucocorticoids trigger Alzheimer disease-like pathobiochemistry in rat neuronal cells expressing human tau. J Neurochem. 2008;107(2):385–397. doi: 10.1111/j.1471-4159.2008.05613.x. [DOI] [PubMed] [Google Scholar]
- 10.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117(6):2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 11.Graff J, Rei D, Guan JS, Wang WY, Seo J, Hennig KM, et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483(7388):222–226. doi: 10.1038/nature10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39(3):409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 13.Andrade C, Shaikh SA, Narayan L, Blasey C, Belanoff J. Administration of a selective glucocorticoid antagonist attenuates electroconvulsive shock-induced retrograde amnesia. J Neural Transm. 2011;119(3):337–344. doi: 10.1007/s00702-011-0712-8. [DOI] [PubMed] [Google Scholar]
- 14.Asagami T, Belanoff JK, Azuma J, Blasey CM, Clark RD, Tsao PS. Selective Glucocorticoid Receptor (GR-II) Antagonist Reduces Body Weight Gain in Mice. Journal of nutrition and metabolism. 2011;2011:235389. doi: 10.1155/2011/235389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belanoff JK, Blasey CM, Clark RD, Roe RL. Selective glucocorticoid receptor (type II) antagonist prevents and reverses olanzapine-induced weight gain. Diabetes, obesity & metabolism. 2010;12(6):545–547. doi: 10.1111/j.1463-1326.2009.01185.x. [DOI] [PubMed] [Google Scholar]
- 16.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31(1):47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 17.Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45(5):675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 18.Medeiros R, Kitazawa M, Caccamo A, Baglietto-Vargas D, Estrada-Hernandez T, Cribbs DH, et al. Loss of muscarinic m(1) receptor exacerbates Alzheimer’s disease-like pathology and cognitive decline. Am J Pathol. 2011;179(2):980–991. doi: 10.1016/j.ajpath.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinton LK, Billings LM, Green KN, Caccamo A, Ngo J, Oddo S, et al. Age-dependent sexual dimorphism in cognition and stress response in the 3xTg-AD mice. Neurobiol Dis. 2007;28(1):76–82. doi: 10.1016/j.nbd.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Coria H, Green KN, Billings LM, Kitazawa M, Albrecht M, Rammes G, et al. Memantine improves cognition and reduces Alzheimer’s-like neuropathology in transgenic mice. Am J Pathol. 2010;176(2):870–880. doi: 10.2353/ajpath.2010.090452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baglietto-Vargas D, Moreno-Gonzalez I, Sanchez-Varo R, Jimenez S, Trujillo-Estrada L, Sanchez-Mejias E, et al. Calretinin interneurons are early targets of extracellular amyloid-beta pathology in PS1/AbetaPP Alzheimer mice hippocampus. Journal of Alzheimer’s disease : JAD. 2010;21:119–132. doi: 10.3233/JAD-2010-100066. [DOI] [PubMed] [Google Scholar]
- 22.Kruszewska B, Felten DL, Stevens SY, Moynihan JA. Sympathectomy-induced immune changes are not abrogated by the glucocorticoid receptor blocker RU-486. Brain Behav Immun. 1998;12(3):181–200. doi: 10.1006/brbi.1998.0527. [DOI] [PubMed] [Google Scholar]
- 23.Pastva A, Estell K, Schoeb TR, Schwiebert LM. RU486 blocks the anti-inflammatory effects of exercise in a murine model of allergen-induced pulmonary inflammation. Brain Behav Immun. 2005;19(5):413–422. doi: 10.1016/j.bbi.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiology of aging. 2003;24(8):1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 25.McGaugh JL, McIntyre CK, Power AE. Amygdala modulation of memory consolidation: interaction with other brain systems. Neurobiol Learn Mem. 2002;78(3):539–552. doi: 10.1006/nlme.2002.4082. [DOI] [PubMed] [Google Scholar]
- 26.Caccamo A, Maldonado MA, Bokov AF, Majumder S, Oddo S. CBP gene transfer increases BDNF levels and ameliorates learning and memory deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2010;107:22687–22692. doi: 10.1073/pnas.1012851108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinnix I, Musunuru U, Tun H, Sridharan A, Golde T, Eckman C, et al. Anovel gamma -secretase assay based on detection of the putative C-terminal fragment-gamma of amyloid beta protein precursor. J Biol Chem. 2001;276:481–487. doi: 10.1074/jbc.M005968200. [DOI] [PubMed] [Google Scholar]
- 28.Allinson TM, Parkin ET, Turner AJ, Hooper NM. ADAMs family members as amyloid precursor protein alpha-secretases. J Neurosci Res. 2003;74(3):342–352. doi: 10.1002/jnr.10737. [DOI] [PubMed] [Google Scholar]
- 29.Lammich S, Kojro E, Postina R, Gilbert S, Pfeiffer R, Jasionowski M, et al. Constitutive and regulated alpha-secretase cleavage of Alzheimer’s amyloidprecursor protein by a disintegrin metalloprotease. Proc Natl Acad Sci U S A. 1999;96(7):3922–3927. doi: 10.1073/pnas.96.7.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Postina R, Schroeder A, Dewachter I, Bohl J, Schmitt U, Kojro E, et al. A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J Clin Invest. 2004;113(10):1456–1464. doi: 10.1172/JCI20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 32.Selkoe DJ. Clearing the brain’s amyloid cobwebs. Neuron. 2001;32(2):177–180. doi: 10.1016/s0896-6273(01)00475-5. [DOI] [PubMed] [Google Scholar]
- 33.Rissman RA, Lee KF, Vale W, Sawchenko PE. Corticotropin-releasingfactor receptors differentially regulate stress-induced tau phosphorylation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(24):6552–6562. doi: 10.1523/JNEUROSCI.5173-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. Post-translational modifications of tau protein in Alzheimer’s disease. J Neural Transm. 2005;112(6):813–838. doi: 10.1007/s00702-004-0221-0. [DOI] [PubMed] [Google Scholar]
- 35.Hanger DP, Hughes K, Woodgett JR, Brion JP, Anderton BH. Glycogen synthase kinase-3 induces Alzheimer’s disease-like phosphorylation of tau: generation of paired helical filament epitopes and neuronal localisation of the kinase. Neurosci Lett. 1992;147(1):58–62. doi: 10.1016/0304-3940(92)90774-2. [DOI] [PubMed] [Google Scholar]
- 36.Medeiros R, Baglietto-Vargas D, Laferla FM. The Role of Tau in Alzheimer’s Disease and Related Disorders. CNS Neurosci Ther. 2011;17(5):514–524. doi: 10.1111/j.1755-5949.2010.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noble W, Olm V, Takata K, Casey E, Mary O, Meyerson J, et al. Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron. 2003;38(4):555–565. doi: 10.1016/s0896-6273(03)00259-9. [DOI] [PubMed] [Google Scholar]
- 38.Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405(6784):360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- 39.Carroll JC, Iba M, Bangasser DA, Valentino RJ, James MJ, Brunden KR, et al. Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in 20a transgenic mouse model of tauopathy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:14436–14449. doi: 10.1523/JNEUROSCI.3836-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elgh E, Lindqvist Astot A, Fagerlund M, Eriksson S, Olsson T, Nasman B. Cognitive dysfunction, hippocampal atrophy and glucocorticoid feedback in Alzheimer’s disease. Biological psychiatry. 2006;59(2):155–161. doi: 10.1016/j.biopsych.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 41.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 42.Nichols NR, Zieba M, Bye N. Do glucocorticoids contribute to brain aging? Brain Res Brain Res Rev. 2001;37(1-3):273–286. doi: 10.1016/s0165-0173(01)00131-x. [DOI] [PubMed] [Google Scholar]
- 43.Sooy K, Webster SP, Noble J, Binnie M, Walker BR, Seckl JR, et al. Partial deficiency or short-term inhibition of 11beta-hydroxysteroid dehydrogenase type 1 improves cognitive function in aging mice. J Neurosci. 2010;30(41):13867–13872. doi: 10.1523/JNEUROSCI.2783-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohler EG, Browman KE, Roderwald VA, Cronin EA, Markosyan S, ScottBitner R, et al. Acute inhibition of 11beta-hydroxysteroid dehydrogenase type-1improves memory in rodent models of cognition. The Journal of neuroscience : theofficial journal of the Society for Neuroscience. 2011;31(14):5406–5413. doi: 10.1523/JNEUROSCI.4046-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pomara N, Doraiswamy PM, Tun H, Ferris S. Mifepristone (RU 486) for Alzheimer’s disease. Neurology. 2002;58(9):1436. doi: 10.1212/wnl.58.9.1436. [DOI] [PubMed] [Google Scholar]
- 46.Green KN, Khashwji H, Estrada T, Laferla FM. ST101 induces a nove l17 kDa APP cleavage that precludes Abeta generation in vivo. Annals of neurology. 2011;69:831–844. doi: 10.1002/ana.22325. [DOI] [PubMed] [Google Scholar]
- 47.Gibson CL, Murphy SP. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2004;24(7):805–813. doi: 10.1097/01.WCB.0000125365.83980.00. [DOI] [PubMed] [Google Scholar]
- 48.He J, Hoffman SW, Stein DG. Allopregnanolone, a progesteronemetabolite, enhances behavioral recovery and decreases neuronal loss after traumaticbrain injury. Restorative neurology and neuroscience. 2004;22(1):19–31. [PubMed] [Google Scholar]
- 49.Frye CA, Walf AA. Effects of progesterone administration and APPswe+PSEN1Deltae9 mutation for cognitive performance of mid-aged mice. Neurobiology of learning and memory. 2008;89(1):17–26. doi: 10.1016/j.nlm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, et al. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(48):13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh C, Liu L, Wang JM, Irwin RW, Yao J, Chen S, et al. Allopregnanolone restores hippocampal-dependent learning and memory and neural progenitor survival in aging 3xTgAD and nonTg mice. Neurobiology of aging. 2012;33(8):1493–1506. doi: 10.1016/j.neurobiolaging.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang JM, Singh C, Liu L, Irwin RW, Chen S, Chung EJ, et al. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(14):6498–6503. doi: 10.1073/pnas.1001422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen S, Wang JM, Irwin RW, Yao J, Liu L, Brinton RD. Allopregnanolone promotes regeneration and reduces beta-amyloid burden in a preclinical model of Alzheimer’s disease. PloS one. 2011;6:e24293. doi: 10.1371/journal.pone.0024293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin HL, Zhang H, Hollenberg PF. Metabolic activation of mifepristone[RU486; 17beta-hydroxy-11beta-(4-dimethylaminophenyl)-17alpha-(1-propynyl)-estra-4,9-dien -3-one] by mammalian cytochromes P450 and the mechanism-based inactivation of human CYP2B6. The Journal of pharmacology and experimental therapeutics. 2009;329:26–37. doi: 10.1124/jpet.108.148536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heikinheimo O, Kontula K, Croxatto H, Spitz I, Luukkainen T, Lahteenmaki P. Plasma concentrations and receptor binding of RU 486 and its metabolites in humans. Journal of steroid biochemistry. 1987;26(2):279–284. doi: 10.1016/0022-4731(87)90083-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.