Abstract
Objectives
To describe and compare incidence of late-onset sepsis (LOS) and demographic and clinical characteristics associated with LOS in very low birth weight (VLBW) infants from singleton and multiple births and to examine the heritability in susceptibility to LOS among VLBW twins by comparing same-sex with unlike-sex twin pairs.
Study design
We studied infants with birth weight 401–1500 grams cared for at clinical centers of the NICHD Neonatal Research Network 2002–2008. Only the first episode of LOS was examined. Stepwise logistic regression models were fitted separately for singleton and multiple pregnancies to examine the maternal and neonatal factors associated with LOS. LOS due to only gram-negative bacteria among singleton and multiple pregnancies was also examined in separate models. The heritability of LOS was estimated by examining concordance of LOS between twins from same-sex and unlike-sex pairs.
Results
LOS occurred in 25.0% (3797/15,178) of singleton and 22.6% (1196/5294) of multiple VLBW infants. Coagulase-negative staphylococci were the most common infecting organisms, accounting for 53.2% of all LOS episodes in singletons and 49.2% in multiples. E. coli and Klebsiella species were the most commonly isolated gram-negative organisms, and Candida albicans was the most commonly isolated fungus. Concordance of LOS was not significantly different between same-sex and unlike-sex twin pairs.
Conclusions
LOS remains a common problem in VLBW infants. The incidence of LOS is similar for singleton and multiple infants. Similar concordance of LOS in same-sex and unlike-sex twin pairs provided no evidence that susceptibility to LOS among VLBW infants is genetically determined.
Keywords: Heredity, preterm infants, twins
Late-onset sepsis (LOS) remains a major cause of mortality and morbidity among VLBW newborns.1 Scientists and clinicians share interest in understanding the factors that influence the risk of LOS and in developing strategies to prevent infection.
We used data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (NRN) to identify demographic and clinical characteristics associated with LOS in singletons and in multiples and to examine the heritability in susceptibility to LOS by comparing VLBW same-sex and unlike-sex twin pairs. Concordance of infecting organisms among twin pairs with LOS was also examined. We hypothesized that same-sex twin pairs would have greater concordance than unlike-sex twins with respect to LOS.
Methods
Infants with birth weight 401–1500 grams born between January 1, 2002 and December 31, 2008 and cared for at NRN clinical centers were studied. Eligibility criteria changed in January 2008 to inborn infants with birth weight 401–1000 grams or gestational age 22–28 6/7 weeks. Trained research nurses entered maternal demographic, pregnancy and delivery information and infant data collected from birth to hospital discharge, death, or 120 days into a registry of VLBW infants maintained by the NRN.2 The institutional review board of each center approved their participation in the registry.
Late-onset sepsis was defined as a positive blood culture obtained after 72 hours of life and intent to treat with antibiotics for 5 days or more. An episode of sepsis was defined if the infant had a positive blood culture due to an identified bacterial (including coagulase-negative staphylococcus) or fungal organism, treated with antibiotics for 5 days or more or treated for a shorter duration if the infant died during treatment. An additional episode of sepsis was identified if a new organism was recognized at any time or if the same organism was cultured after 10 days or more of appropriate antibiotic therapy. For infants with multiple episodes of LOS, only the first episode was considered. Positive blood cultures due to organisms considered to be contaminants, including Corynebacterium, Propionibacterium, and Penicillium species and diphtheroids were excluded. Infants whose cultures grew unclassified bacteria or who had no recorded organisms as well as infants with blood cultures that grew multiple organisms in the first LOS event also were excluded.
Data Analyses
We examined predictors of LOS including center, maternal variables, and neonatal variables, among singletons and multiples (twins or higher-order multiples). LOS was treated as a binary variable. Maternal variables examined included race, duration of rupture of membranes, mode of delivery, and prenatal antibiotics or steroids. The neonatal predictors examined included intrauterine infection, sex, gestational age, small for gestational age (less than the tenth percentile3), rectal or axillary temperature at birth above 38°C, total duration of assisted ventilation, duration of conventional ventilation, duration of high-frequency ventilation, duration of supplemental oxygen, parenteral nutritional support, major surgery, length of hospital stay, age when birth weight was regained, age at first parenteral feeding, age when full feedings were achieved, and postnatal corticosteroid use. Information on all of these variables was collected for the entire study period except for intrauterine infection, which was available only from 2002 through 2005.
Statistical significance for each predictor was individually tested for association with LOS using logistic regression with center included in all models. Significant predictors (p ≤0.05) were then examined in a stepwise logistic regression to predict LOS. Separate models were fitted for singleton and multiple pregnancies. We also examined predictors of LOS due to only gram-negative bacteria among singleton and multiple pregnancies in separate models. Adjusted odds ratios and 95% confidence intervals from these models were calculated. No adjustment was made for multiple testing. All analyses were conducted using SAS 9.2 software (SAS Institute, Inc., Cary, NC).
Concordance rates of LOS among same-sex (M/M or F/F) and unlike-sex (M/F) twin pairs also were examined. Examining same-sex and unlike-sex twin pairs allowed us to identify a group containing a mixture of monozygous and dizygous pairs and another group containing only dizygous pairs. Because some of the same-sex pairs are monozygous, higher concordance of LOS among same-sex twin pair mates compared with unlike-sex twins may indicate an element of heritability. The absence of demonstrable concordance, however, does not preclude a role of genetics in determining infection risk. All higher order multiples were excluded from the heritability analysis. For a twin pair to be considered in the rate calculation, both twins had to survive to 46 days of age, or both twins had to have the event occur before the 46th day of life if one of the twin pairs died. Day 46 of life was chosen after examining the distribution of LOS incidence in our cohort, as 90% of the cases of LOS developed by day 46. Heritability to LOS was then estimated using h2 = 2(CMZ - CDZ) where CMZ is the concordance rate for monozygotic twins (estimated via proxy same-sex twins) and CDZ is the concordance rate for dizygotic twins (estimated via proxy unlike-sex twins).4,5
Organisms associated with the first episode of infection among twin pairs were examined. Expected counts of types of infectious organisms among twin pairs were calculated based on marginal frequencies. The observed and expected numbers of types of pathogens were then compared by Fisher exact test for homogeneity with the p-values reported accordingly.
Results
Incidence of Infection
A total of 16,713 VLBW singleton infants and 5860 VLBW twins or higher-order multiples were admitted to NRN centers between 2002 and 2008. Data on LOS status were available for 15,178 singletons and 5294 multiples meeting the eligibility criteria. The overall rate of LOS was 25.0% (males 27.3%, females 22.8%) among singletons and 22.6% (males 25.2%, females 20.0%) among multiples. Rates of LOS were similar between singletons and multiples in all gestational age and birth weight categories; the highest rates of LOS occurred among the smallest, most immature infants (Table I).
Table I.
Rates of late-onset sepsis by birth weight and gestational age among singletons and multiples in the NICHD Neonatal Research Network, 2002–2008
| Singletons | Multiples | |||
|---|---|---|---|---|
|
| ||||
| Total N (%) |
Late-onset sepsis N (% of cases) |
Total N (%) |
Late-onset sepsis N (% of cases) |
|
| Birth weight (grams) | ||||
| <400 | 6 (< 0.1) | 3 (50.0) | 0 (--) | 0 (--) |
| 400–500 | 223 (1.5) | 146 (65.5) | 55 (1.0) | 36 (65.4) |
| 501–750 | 2680 (17.7) | 1372 (51.2) | 754 (14.2) | 407 (54.0) |
| 751–1000 | 4030 (26.6) | 1309 (32.5) | 1228 (23.2) | 384 (31.3) |
| 1001–1250 | 3983 (26.2) | 648 (16.3) | 1480 (28.0) | 226 (15.3) |
| 1251–1500 | 4256 (28.0) | 319 (7.5) | 1777 (33.6) | 143 (8.0) |
| Total | 15178 | 3797 (25.0) | 5294 | 1196 (22.6) |
|
| ||||
| Gestational age (weeks) | ||||
| <25 | 1570 (10.3) | 971 (61.8) | 438 (8.3) | 284 (64.8) |
| 25–28 | 7239 (47.7) | 2227 (30.8) | 2250 (42.5) | 680 (30.2) |
| 29–32 | 5537 (36.5) | 569 (10.3) | 2259 (42.7) | 214 (9.5) |
| >32 | 830 (5.5) | 30 (3.6) | 345 (6.5) | 18 (5.2) |
| Total | 15176* | 3797 (25.0) | 5292* | 1196 (22.6) |
Gestational age information was missing for 2 singleton infants and 1 set of twins, none
of whom had LOS.
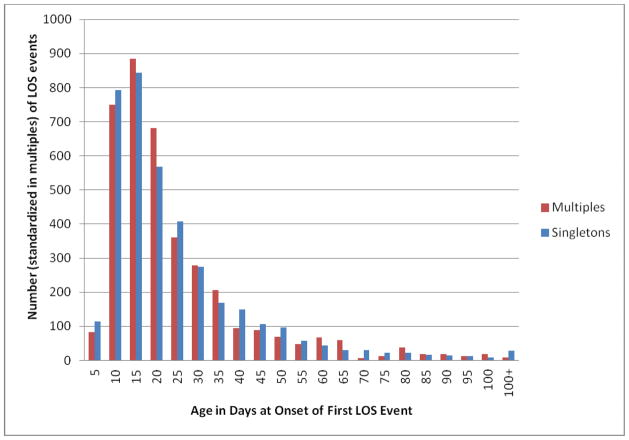
Rates of LOS varied considerably among centers, ranging from 14.0% to 36.4% (data not shown). Half of the multiples who developed LOS during their hospital stay had their first episode by day of life 16, and 90% had their first episode by day 46. Singletons had a similar distribution (Figure).
Figure.
Distribution of age (days) of the first episode of late-onset sepsis among very low birth weight singletons and multiples (including twins) in the NICHD Neonatal Research Network, 2002–2008.
Pathogen Distribution
Gram-positive bacteria were the most common category of infecting organisms. Although the distribution of the organisms was similar among singletons and multiples, small (1–4%) but statistically significant differences between singletons and multiples were noted in the rates of gram-positive, gram-negative, and fungal organisms (Table II). Coagulase-negative staphylococci accounted for the majority of gram positive-organisms, and E. coli and Klebsiella species were the most common gram-negative bacteria among both singletons and multiples.. Candida albicans was the most common fungus isolated with a higher percentage of infections (p < 0.016) among singletons than among multiples. Overall the organisms that affected the infants in a twin pair were not independent. A total of 78 twin pairs were concordant for the same organism versus 46 pairs expected based on the marginal rates of infection (p <0.0003). Individual bacteria with significant same-pair concordance included E. coli (5 pairs concordant vs less than 1 expected, p <0.05) and coagulase-negative staphylococcus (56 observed vs 43 expected, p <0.05) (data not shown).
Table II.
Distribution of pathogens associated with the first episode of late-onset sepsis among singletons and multiples in the NICHD Neonatal Research Network, 2002–2008
| Organism | Singletons N (%) |
Multiples N (%) |
|---|---|---|
| Gram-positive bacteria* | 2916 (76.8) | 905 (75.7) |
| Staphylococcus, coagulase negative* | 2020 (53.2) | 588 (49.2) |
| Staphylococcus aureus | 408 (10.7) | 137 (11.5) |
| Group B streptococcus | 69 (1.8) | 25 (2.1) |
| Other streptococci* | 138 (3.6) | 68 (5.7) |
| Other gram-positive bacteria | 281 (7.4) | 87 (7.3) |
| Gram-negative bacteria* | 597 (15.7) | 222 (18.6) |
| E. coli | 171 (4.5) | 61 (5.1) |
| Klebsiella species | 151 (4.0) | 63 (5.3) |
| Enterobacter species | 102 (2.7) | 42 (3.5) |
| Pseudomonas species | 85 (2.2) | 27 (2.3) |
| Serratia species | 44 (1.2) | 13 (1.1) |
| Other gram-negative bacteria | 44 (1.2) | 16 (1.3) |
| Fungi* | 284 (7.5) | 69 (5.8) |
| Candida albicans* | 172 (4.5) | 35 (2.9) |
| Candida parapsilosis | 73 (1.9) | 23 (1.9) |
| Other fungi | 39 (1.0) | 11 (0.9) |
|
| ||
| Total | 3797 | 1196 |
Indicates a significant difference in rate between singletons and multiples (p <0.05)
Predictors of Late-Onset Sepsis
Significant predictors of LOS among singletons and multiples are shown in Table III. Fewer predictors were found to be significant among multiples, presumably because of the smaller number of infants. When we examined LOS restricted to sepsis caused by gram-negative organisms only, days of ventilation, age when full feedings were achieved, and gestational age were found to be significant and were retained in the multiples model; additional variables retained in the model for singletons included mode of delivery, any surgery, and size for gestational age (data not shown). The effect estimates of the predictors of LOS due to gram-negative organisms were similar to the estimates of LOS due to any organism except for the estimates of surgery and size for gestational age (data not shown). Singletons with surgeries were at higher odds of developing LOS due to gram-negative organisms than singletons with surgeries infected by any type of organism (OR 2.289, 95% CI 1.778–2.948 vs OR 1.425, 95% CI 1.263–1.608). Similarly among small for gestational age (SGA) singletons, the odds of developing LOS due to gram-negative organisms were higher than the odds of developing LOS due to any type of organism (OR 1.523, 95% CI 1.089–2.130 vs OR 1.219, 95% CI 1.058–1.428). Medical center was a significant predictor in all the models we examined.
Table III.
Predictors of late-onset sepsis among infants from singleton and multiple births in the NICHD Neonatal Research Network, 2002–2008
| Variable | Adjusted OR (95% CI) | p-value |
|---|---|---|
|
Singletons
|
||
| Gestational age (weeks) | 0.808 (0.786–0.831) | <0.0001 |
| Age when full feeds were achieved (days) | 1.041 (1.037–1.045) | <0.0001 |
| Length of hospital stay (days) | 1.003 (1.002–1.004) | <0.0001 |
| Any surgery | 1.425 (1.263–1.608) | <0.0001 |
| Small for gestational age | 1.219 (1.058–1.428) | 0.0070 |
| Gender (female/male) | 0.892 (0.812–0.979) | 0.0157 |
| Duration of assisted ventilation (days) | 1.006 (1.003–1.009) | <0.0001 |
| Parenteral Alimentation | 7.663 (3.083–19.046) | < 0.0001 |
|
| ||
|
Multiples
|
||
| Gestational age (weeks) | 0.827 (0.789–0.867) | <0.0001 |
| Duration of assisted ventilation (days) | 1.010 (1.003–1.016) | 0.0006 |
| Length of hospital stay (days) | 1.005 (1.002–1.009) | 0.0023 |
| Age when full feeds were achieved (days) | 1.038 (1.031–1.044) | <0.0001 |
Heritability Estimate
For calculating the concordance/discordance rates for LOS and estimating heritability, only twin pairs with both twins surviving were considered. This excluded higher-order multiples, twins with only one survivor at day of life 46, and twin pairs both of whom died before day 46 without developing LOS. As mentioned previously, this cut-off point was chosen after determining that 90% of LOS occurred by day of life 46. This resulted in a total of 1563 pairs considered for the estimation of heritability. Of these, 33.8% (529/1563) were unlike-sex pairs and 66.2% (1034/1563) were same-sex pairs. Among same-sex twin pairs, 658 pairs (63.6%) were unaffected, 103 pairs (10.0%) were affected, and 273 pairs (26.4%) were discordant. The rate of LOS among same-sex twins was 23.2%. Among the unlike-sex twin pairs, 343 twin pairs (64.8%) were unaffected, 55 pairs (10.4%) were affected, and 131 pairs (24.8%) were discordant for LOS. The rate of LOS among unlike-sex twins was 22.8%. Among twin pairs that had at least one affected infant, 27.4% of same-sex and 29.6% of unlike-sex twin pairs had both infants affected. The concordance among same-sex twin pairs was marginally lower than among unlike-sex twin pairs, yielding a heritability estimate of LOS of 0%. As the unadjusted heritability estimate was not significantly different between same sex and unlike-sex twin pairs, no further adjustment for significant covariates was conducted to reexamine the heritability estimate.
Discussion
Late-onset sepsis continues to be a major cause of mortality and morbidity among VLBW infants. In the current study, 24.4% of VLBW infants in our cohort born between 2002 and 2008 developed at least one episode of sepsis beyond day 3 of life. This rate is higher than reported previously. Using the NICHD NRN database, Stoll et al reported an incidence rate of 21.1% during the period 1998–2000.6 Most of this increase occurred in the group of infants with birth weight 400 to 750 g, for whom LOS increased from 42.8%6 to 52.8%. The change in the clinical centers participating in the NRN between the two periods might explain this increase, as substantial differences in the incidence of LOS were noted among the centers in the current study, ranging from 14.0% to 36.4%. Additionally, during the last year of the study period, the upper birth weight limit for registry qualification decreased from 1500 grams to 1000 grams, which presumably also contributed to the higher rate of LOS in the current cohort. Pathogen distribution did not differ between singletons and twins. However, twin pairs were more likely to be infected by the same organism than expected at random (78 twins concordant vs 46 expected at random p < 0.0003). This observation extended to specific organisms, if one twin had E. coli sepsis, its co-twin was at increased risk of also having E. coli sepsis, as similarly reported by Pai et al.7 This increase in risk for the co-twin was also observed for coagulase-negative staphylococcus. Our cohort however, did not demonstrate the increased risk of group B streptococcus co-sepsis in twins as described by others.8–11
Risk factors for LOS among singletons and multiples were similar. Additional factors were significant in the singletons model, presumably an effect of the large sample size of the cohort in comparison to the multiples subset. Nutrition history and duration of ventilation support were both associated with infection, as found in an earlier NRN study.6 We did not have data on duration of central venous catheters to examine this factor as a predictor of LOS in our models.
When LOS due to only gram-negative organisms was examined, predictors were also similar to the model incorporating all the organisms. The finding that SGA infants were at higher odds of LOS resulting from gram-negative organisms is interesting but hard to explain. Previous studies also have found increased risk of sepsis among SGA infants compared with AGA counterparts.12,13 Thymic atrophy, decreased numbers of T-lymphocyte cells, and impaired levels of immunoglobulins have been reported in SGA infants compared with AGA newborns.14–16 In the present study, coagulase-negative staphylococci constituted the majority of our LOS infections. Infants infected with coagulase-negative staphylococci have been previously found not to be at a higher risk of death in comparison to uninfected infants, suggesting that this organism may be less virulent.6 As a consequence, combining the coagulase-negative staphylococcal infections with the more virulent organisms might have attenuated the impact of being SGA on LOS risk. Although this association might be spurious, further research examining the immunological status and infection risk of SGA infants is warranted.
Our high rates of nosocomial infection with coagulase-negative staphylococci (>49%) could probably be reduced by introducing standardized central catheter care protocols in all participating centers. Several studies have demonstrated the impact of a checklist for line insertion and line maintenance in reducing infection rates.17–20 Studies conducted in NICUs, have proven successful in demonstrating a significant improvement in the rates of central line associated blood stream infection rates.18–20
The significant mortality and morbidity associated with sepsis makes it a good target in examining the role of genetic variants in modifying individual risk. Genetic factors are believed to have a significant contribution to sepsis given the presumed interindividual variation in the risk of developing sepsis and in the response to therapy.21 Polymorphisms involved in cytokines and cytokine receptors, endothelial factors, the recognition of bacterial components, coagulation factors, and the fibrinolysis system have all been investigated as potential candidate genes involved in sepsis in VLBW infants.22 Results across studies have been inconsistent, mainly due to methodological limitations in study design and genotyping methods.21–24 The classical methodology of using twin studies in exploring genetic susceptibility has been used successfully in several major neonatal morbidities including patent ductus arteriosus, retinopathy of prematurity, bronchopulmonary dysplasia, necrotizing enterocolitis, intraventricular hemorrhage, and late-onset sepsis.25–28
In our study, the concordance for LOS between same-sex twin pairs and unlike-sex twin pairs was not significantly different. In a population of newborn twins with known zygosity, Bizzarro et al also found no significant difference in concordance for LOS between monozygous and dizygous twin pairs.28 We did not have specific genotyping information or chorionicity data to classify same-sex twins as monozygotic or dizygotic. Use of same and unlike sex in lieu of zygosity underestimates heritability, and our failure to demonstrate heritability does not preclude a significant influence of genetics on sepsis risk.
Acknowledgments
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
Supported by grants from the National Institutes of Health (NIH) and the Eunice Kennedy Shriver National Institute of Child and Human Development (NICHD) for the Neonatal Research Network’s Generic Database and Follow-up Studies. Data collected at participating sites of the NICHD Neonatal Research Network were transmitted to RTI International, the data coordinating center for the network, which stored, managed, and analyzed the data for this study. R.H. is an employee of NIH, and assisted with the study design, analysis, interpretation of data, writing of the report, and the decision to submit it for publication.
Abbreviations
- E. coli
Escherichia coli
- LOS
late-onset sepsis
- NRN
NICHD Neonatal Research Network
- SGA
small for gestational age
- VLBW
very low birth weight
Footnotes
The authors declare no conflicts of interest.
Reprint Requests: Dr. Bell
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Semin Perinatol. 2003;27:293–301. doi: 10.1016/s0146-0005(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 4.Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral genetics. 4. New York, NY: Worth Publishers; 2001. [Google Scholar]
- 5.Smith C. Concordance in twins: methods and interpretation. Am J Hum Genet. 1974;26:454–66. [PMC free article] [PubMed] [Google Scholar]
- 6.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–91. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 7.Pai JR, Tremlett CH, Clarke P. Late-onset sepsis in a preterm twin may harbinger life-threatening sepsis for the asymptomatic co-twin. Pediatr Infect Dis J. 2010;29:381–2. doi: 10.1097/INF.0b013e3181c42545. [DOI] [PubMed] [Google Scholar]
- 8.Edwards MS, Jackson CV, Baker CJ. Increased risk of group B streptococcal disease in twins. JAMA. 1981;245:2044–6. [PubMed] [Google Scholar]
- 9.Rubin EE, McDonald JC. Group B streptococcal disease in twins: failure of empiric therapy to prevent late onset disease in the second twin. Pediatr Infect Dis J. 1991;10:621–3. [PubMed] [Google Scholar]
- 10.Benitz WE, Gould JB, Druzin ML. Risk factors for early-onset group B streptococcal sepsis: estimation of odds ratios by critical literature review. Pediatrics. 1999;103:e77. doi: 10.1542/peds.103.6.e77. [DOI] [PubMed] [Google Scholar]
- 11.Doran KS, Benoit VM, Gertz RE, Beall B, Nizet V. Late-onset group B streptococcal infection in identical twins: insight to disease pathogenesis. J Perinatol. 2002;22:326–30. doi: 10.1038/sj.jp.7210675. [DOI] [PubMed] [Google Scholar]
- 12.Simchen MJ, Beiner ME, Strauss-Liviathan N, Dulitzky M, Kuint J, Mashiach S, et al. Neonatal outcome in growth-restricted versus appropriately grown preterm infants. Am J Perinatol. 2000;17:187–92. doi: 10.1055/s-2000-9423. [DOI] [PubMed] [Google Scholar]
- 13.Bartels DB, Schwab F, Geffers C, Poets CF, Gastmeier P. Nosocomial infection in small for gestational age newborns with birth weight <1500 g: a multicentre analysis. Arch Dis Child Fetal Neonatal Ed. 2007;92:F449–53. doi: 10.1136/adc.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson AC. Prolonged impairment of cellular immunity in children with intrauterine growth retardation. J Pediatr. 1978;93:52–6. doi: 10.1016/s0022-3476(78)80599-x. [DOI] [PubMed] [Google Scholar]
- 15.Xanthou M. Immunologic deficiencies in small-for-dates neonates. Acta Paediatr Scand Suppl. 1985;319:143–9. doi: 10.1111/j.1651-2227.1985.tb10124.x. [DOI] [PubMed] [Google Scholar]
- 16.Chatrath R, Saili A, Jain M, Dutta AK. Immune status of full-term small-for-gestational age neonates in India. J Trop Pediatr. 1997;43:345–8. doi: 10.1093/tropej/43.6.345. [DOI] [PubMed] [Google Scholar]
- 17.Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–32. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan HC, Lannon C, Walsh MC, Donovan EF Ohio Perinatal Quality Collaborative. Ohio statewide quality-improvement collaborative to reduce late-onset sepsis in preterm infants. Pediatrics. 2011;127:427–35. doi: 10.1542/peds.2010-2141. [DOI] [PubMed] [Google Scholar]
- 19.Schulman J, Stricof R, Stevens TP, Horgan M, Gase K, Holzman IR, et al. Statewide NICU central-line-associated bloodstream infection rates decline after bundles and checklists. Pediatrics. 2011;127:436–44. doi: 10.1542/peds.2010-2873. [DOI] [PubMed] [Google Scholar]
- 20.Wirtschafter DD, Powers RJ, Pettit JS, Lee HC, Boscardin WJ, Ahmad Subeh M, et al. Nosocomial infection reduction in VLBW infants with a statewide quality-improvement model. Pediatrics. 2011;127:419–26. doi: 10.1542/peds.2010-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin MT, Albertso TE. Genomic polymorphisms in sepsis. Crit Care Med. 2004;32:569–79. doi: 10.1097/01.CCM.0000110878.49476.42. [DOI] [PubMed] [Google Scholar]
- 22.Hartel C, Schultz C, Herting E, Gopel W. Genetic association studies in VLBW infants exemplifying susceptibility to sepsis -- recent findings and implications for future research. Acta Paediatr. 2007;96:158–65. doi: 10.1111/j.1651-2227.2007.00128.x. [DOI] [PubMed] [Google Scholar]
- 23.Clark MF, Baudouin SV. A systematic review of the quality of genetic association studies in human sepsis. Intensive Care Med. 2006;32:1706–12. doi: 10.1007/s00134-006-0327-y. [DOI] [PubMed] [Google Scholar]
- 24.Abu-Maziad A, Schaa K, Bell EF, Dagle JM, Cooper M, Marazita ML, et al. Role of polymorphic variants as genetic modulators of infection in neonatal sepsis. Pediatr Res. 2010;68:323–9. doi: 10.1203/PDR.0b013e3181e6a068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhandari V, Bizzarro MJ, Shetty A, Zhong X, Page GP, Zhang H, et al. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. 2006;117:1901–6. doi: 10.1542/peds.2005-1414. [DOI] [PubMed] [Google Scholar]
- 26.Bizzarro MJ, Hussain N, Jonsson B, Feng R, Ment LR, Gruen JR, et al. Genetic susceptibility to retinopathy of prematurity. Pediatrics. 2006;118:1858–63. doi: 10.1542/peds.2006-1088. [DOI] [PubMed] [Google Scholar]
- 27.Bhandari V, Zhou G, Bizzarro MJ, Buhimschi C, Hussain N, Gruen JR, et al. Genetic contribution to patent ductus arteriosus in the premature newborn. Pediatrics. 2009;123:669–73. doi: 10.1542/peds.2008-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bizzarro MJ, Jiang Y, Hussain N, Gruen JR, Bhandari V, Zhang H. The impact of environmental and genetic factors on neonatal late-onset sepsis. J Pediatr. 2011;158:234–8.e1. doi: 10.1016/j.jpeds.2010.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]