Abstract
Copolymer (Cop)-1, also known as glatiramer acetate, is an active compound of Copaxone, a drug widely used by patients with multiple sclerosis (MS). Copaxone functions in MS through two mechanisms of action, namely immunomodulation and neuroprotection. Because the immune system is suppressed or altered in depressed individuals, and since depression is often associated with neurological conditions, we were interested in examining whether the neuroprotective effect of Copaxone persists under conditions of stress-induced depressive behavior. We exposed mice to unpredictable chronic mild stress for 4 weeks and then treated them with three doses of Copaxone at 3-day intervals, with the last dose given immediately before the mice underwent a crush injury to the optic nerve. Whereas nonstressed mice exhibited a strong neuroprotective response after Copaxone treatment, this effect was completely absent in mice that underwent chronic mild stress. Interestingly, when Copaxone was combined with Prozac, the neuroprotective effect of Copaxone was regained, suggesting that chronic mild stress interferes with the neuroprotective effect of Copaxone. These results may shed a light on mechanism of action of Copaxone and lead to new combined therapies for neurodegenerative and neuroinflammatory disorders.
Introduction
Injury to the central nervous system (CNS) results in a continuous spread of neuronal degeneration beyond the initial damage, often culminating in a much larger final infarct zone than could have been predicted from the initial injury (Faulkner et al., 2004; Popovich et al., 1994; Schwartz and Yoles, 1999; Schwartz et al., 1999; Sofroniew, 2000). Among the numerous and complex molecular pathways that underlie this degeneration are the release of toxic compounds from dying cells, impairment of blood supply to the site of injury, gliosis around the injury site, and an inflammatory response (Gillessen et al., 2002; Jones et al., 2004; Lipton, 1993; Popovich et al., 1999; Schwartz et al., 2003). Most of the current therapeutic interventions are aimed at manipulating the functions of certain molecules. However, given the complex nature of the neurodegenerative process, a more global approach may be required in order to achieve substantial neuronal survival.
A little over a decade ago, T cells were found to be capable of inducing neuronal survival after injury (Hauben et al., 2000a; Hauben et al., 2000b; Kipnis et al., 2002; Kipnis et al., 2001; Moalem et al., 1999; Schwartz and Kipnis, 2001; Schwartz et al., 2000). At about the same time it was also proposed that macrophages, if appropriately activated, were beneficial for neuronal survival (Lazarov-Spiegler et al., 1998; Rapalino et al., 1998). More recently, several groups have demonstrated that whether the immune response (both adaptive and innate) to a CNS injury is protective or destructive will determine the injury outcome in terms of neuronal survival (Corona et al., 2010; Donnelly et al., 2011; Shechter et al., 2009). After injury to the CNS, neuronal survival in immune-deficient mice is lower than in mice with a normal immune system, and this impaired survival can be largely restored to wild-type levels by passive transfer of T cells from wild-type donors into the immune-deficient recipients (Kipnis et al., 2002; Kipnis et al., 2001; Yoles et al., 2001).
Copaxone, an FDA-approved drug used by patients with multiple sclerosis (MS), was designed to compete with pathological peptides and displace them from the MHC II groove, thus attenuating their pathological effect (Aharoni et al., 1997; Arnon et al., 1989; Teitelbaum et al., 1997a; Teitelbaum et al., 1996; Teitelbaum et al., 1997b). While this drug is effective in MS and in experimental autoimmune encephalomyelitis (EAE) (an animal model of neuroinflammation), its mechanism of action is not yet completely understood and appears to be substantially more complex than was initially anticipated. While Copaxone exerts an immune-modulatory effect it is also strongly neuroprotective, as shown in numerous animal models of neurological diseases such as CNS trauma, glaucoma, Alzheimer's disease, Parkinson's disease, and others (Angelov et al., 2003; Gorantla et al., 2007; Gorantla et al., 2006; Kipnis and Schwartz, 2002; Kipnis et al., 2000; Liu et al., 2007; Mosley et al., 2007; Schori et al., 2001).
Major depression is associated with immune malfunction (Choudhry et al., 2007; Frank et al., 2002; Leonard, 2000; Miller, 2010; Miller et al., 2009; Miller et al., 1999; Schuld et al., 2003; Soygur et al., 2007). The outcome of CNS injury in patients with major depression has not been extensively studied, and it is not yet known whether the immune malfunction associated with major depression results in exaggerated neuronal loss after CNS injury or under conditions of disorders such as MS. Moreover, to the best of our knowledge hardly any studies have addressed the question of whether immune-modulatory drugs whose action is neuroprotective, such as Copaxone, maintain their neuroprotective activities under conditions of chronic stress (animal depression model).
Here we examined the effect of unpredicted chronic mild stress on the survival of neurons after CNS injury and on the neuroprotective effects of Copaxone in such mice. We found that neither acute nor chronic stress had any effect on neuronal survival after optic nerve crush injury. Interestingly, however, whereas Copaxone was significantly effective in promoting neuronal survival after optic nerve injury in normal control mice, in depressed mice this neuroprotective effect was absent. Neuroprotective effect of Copaxone was regained when mice were co-treated with Copaxone and the anti-depressant, Fluoxetine (Prozac).
Results
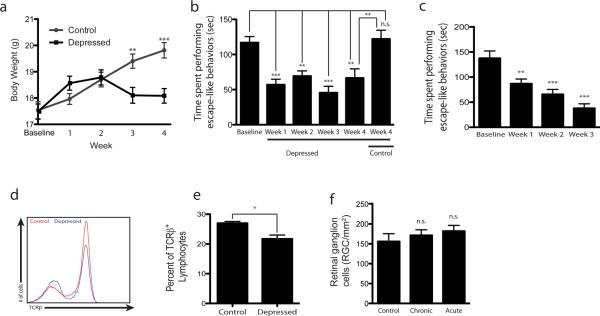
To determine whether neuronal survival after CNS injury is altered by stress, we induced depressive behavior in C57Bl6 mice by exposing them to chronic unpredicted mild stress (Feng et al., 2012; Isingrini et al., 2010) for 4 weeks (Table 1). Both stressed mice and nonstressed controls were weighed weekly, revealing a characteristic progressive loss of growth in the stressed mice (Fig. 1a, F(1,51) = 6.80), p=0.001). In addition, weekly examination for escape-like behaviors by means of the tail suspension test showed a substantial reduction in the escape behaviors of the stressed mice over time (F(5,68) = 11.59, p<0.001, Fig. 1b). Similar results were obtained with forced swim test (F(3,24) = 15.07, p<0.001, Fig. 1c), further demonstrating the depressive phenotype of these mice. We also tested the peripheral immune system in these mice in both the draining (deep cervical) and the nondraining (auxiliary) lymph nodes. As expected, the numbers of TCRβ+ cells and percentage of CD4+ T cells in all tested lymph nodes of the stressed mice were slightly yet significantly reduced (Fig. 1d, e).
Table.
| Frequency | Day0 | Day1 | Day2 | Day3 | Day4 | Day5 | Day6 | Day7 | Day8 | Day9 | Day10 | Day11 | Day12 | Day13 | Day14 | Day15 | Day16 | Day17 | Day18 | Day19 | Day20 | Day21 | Day22 | Day23 | Day24 | Day25 | Day26 | Day27 | Day28 | Day29 | Day30 | Day31 | Day32 | Day33 | Day34 | Day35 | Day36 | Day37 | Day38 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1hr of restraining in a 50mL tube and 24hr of light (no dark period | every 3 days | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||||||||||||||
| 1hr of tail suspension and 12hr of tilting with little bedding; | every 3 days | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||||||||||||||
| 0.5hr of forced swimming | every 3 days | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||||||||||||||
| Social Change | every 3 days | X | X | X | X | X | X | X | X | X | ||||||||||||||||||||||||||||||
| Weight | weekly | X | X | X | X | X | X | |||||||||||||||||||||||||||||||||
| Tail Suspension Test (6 minutes) | weekly | X | X | X | X | X | ||||||||||||||||||||||||||||||||||
| Forced Swim Test (6 minutes) | weekly | X | X | X | X | X | ||||||||||||||||||||||||||||||||||
| Sucrose preference Test | end of 4 week | X | X | X | ||||||||||||||||||||||||||||||||||||
| Fluorogold injection | on day 28 | X | ||||||||||||||||||||||||||||||||||||||
| Optic nerve crush | on day 31 | X | ||||||||||||||||||||||||||||||||||||||
| Retinat's detachment | on day 38 | X |
Figure 1. Depressive behavior does not affect neuronal survival after CNS injury.
C57Bl/6J mice underwent unpredicted chronic mild stress to induce depressive behavior. (a) Weights were recorded weekly and a characteristic drop in weight is seen in a stressed group (tweek3=3.38, tweek4=4.50). (b) Bar graphs represent times the mice spent in escape-like behaviors during the last 4 minutes of 6 minute-long tail suspension (Tweek1=5.09, tweek2=3.97, tweek3=5.83, tweek4=3.55, tcontrol-baseline=0.37). (c) Bar graphs represent times the mice spent in escape-like behaviors during the last 4 minutes of 6 minute-long forced swim test (tweek1=3.31, tweek2=4.69, tweek3=6.50). (d, e) Cervical lymph nodes were examined for T cell levels in control and depressed mice (after 4 weeks of chronic stress). (d) Representative histogram of TCRβ+ cells is shown. (e) Bar graph represents percent of TCRb lymphocytes in the dCLN that are CD4+ by flow cytometry. (t(8) = 3.1, p=0.015), (f) Graphs represent retinal ganglion cell (RGC) survival after optic nerve crush injury in non-stressed, acutely stressed and chronically stressed (depressed) mice (tchronic=0.6, tacute=1.1). For statistical analysis one-way ANOVA with Bonferroni's post-test was used (**p<0.01, ***p<0.001).
After these mice had been exposed to chronic unpredicted mild stress for 4 weeks, we examined their responses to injury. Our hypothesis was that the depressed mice would show signs of exacerbated neuronal degeneration. For this experiment we used two control groups, one consisting of naïve mice (not exposed to stress) and the other comprising acutely stressed mice (exposed to a single stress). The mice in all three groups were subjected to optic nerve crush injury and their neuronal survival was assessed 1 week later. No differences were observed between the groups (F(2,18) = 0.658, p=0.530, Fig. 1f), suggesting that the immune suppression detected earlier in the lymph nodes of the chronically stressed mice was not sufficient to impair neuronal survival in this model.
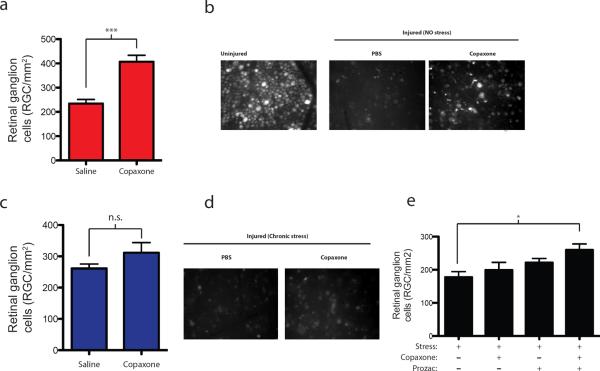
Next, we examined whether therapeutic vaccination with Copaxone (shown in this and other models of neurodegeneration to be neuroprotective) retains its therapeutic effect in the stressed mouse model. To this end, we subjected another group of mice to the 4-week paradigm of chronic unpredicted mild stress, and then injected each mouse with three doses of Copaxone. The first dose was given 6 days before the injury, the next dose 3 days before the injury, and the last on the day of injury. A control group of naïve (nonstressed) mice received similar injections. As expected, Copaxone treatment was highly neuroprotective after optic nerve crush injury in the naïve mice (Fig. 2a, b). In the stressed mice, however, treatment with Copaxone after optic crush injury had no effect on their neuronal survival (Fig. 2c, d), suggesting that the effect of stress on the immune system had rendered it unresponsive to this therapy. Interestingly, however, lymph nodes of the Copaxone-treated stressed mice were found to contain more CD4+ T cells than the lymph nodes of their nonstressed counterparts (data not shown).
Figure 2. Neuroprotective effect of Copaxone lost in depressed mice and is regained with anti-depressant therapy.
(a) Graphs represent retinal ganglion cell (RGC) survival after optic nerve crush injury in control non-stressed mice treated with either PBS or three doses of Copaxone. A significant increase in neuronal survival is evident with Copaxone treatment (t(1)=5.2, p < 0.001; Student's t-test). (b) Representative retinas from uninjured control, injured PBS treated and injured after Copaxone treated mice are shown. (c) Graphs represent RGC survival after optic nerve crush injury in chronically-stressed mice (exhibiting depressive behaviors) treated with either PBS or three doses of Copaxone. No change in neuronal survival is achieved with Copaxone treatment (t(1)=1.4, p > 0.05; Student's t-test). (d) Representative retinas from injured mice after chronic stress (exhibiting depressive behavior), treated with either PBS or Copaxone, are shown. (e) Graphs represent RGC survival after optic nerve crush injury in chronically-stressed mice treated with PBS, Copaxone, Prozac, or Prozac plus Copaxone. One-way ANOVA with Bonferroni's post-test was used for statistical analysis (tcop+proz=3.022, p<0.05). shown. (c) Graphs represent RGC survival after optic nerve crush injury in chronically-stressed mice (exhibiting depressive behaviors) treated with either PBS or three doses of Copaxone. No change in neuronal survival is achieved with Copaxone treatment (t(1)=1.4, p > 0.05; Student's t-test). (d) Representative retinas from injured mice after chronic stress (exhibiting depressive behavior), treated with either PBS or Copaxone, are shown. (e) Graphs represent RGC survival after optic nerve crush injury in chronically-stressed mice treated with PBS, Copaxone, Prozac, or Prozac plus Copaxone. One-way ANOVA with Bonferroni's post-test was used for statistical analysis (tcop+proz=3.022, p<0.05).
To further address the possible interference of chronic mild stress with the neuroprotective function of Copaxone, mice were stressed and either treated with Copaxone only or along with antidepressant, Fluoxetine (Prozac). While Copaxone alone (as well as Prozac alone) did not have any beneficial effect on neuronal survival, a combination of two drugs resulted in a significantly higher neuronal survival (Fig. 2e; one-way ANOVA with Bonferroni's post-test was used for statistical analysis; tcop+proz=3.022, p<0.05).
Discussion
The results of this study showed that although the chronic mild stress in mice led to a reduction in the numbers of T cells, it did not exacerbate the outcome of CNS injury. The basal neuroprotection after CNS injury in stressed mice, however, was not amenable to a therapeutic boost with Copaxone unlike in control non-stressed mice. Interestingly, when Copaxone was combined with antidepressant, Prozac, the neuroprotective effect of Copaxone was regained, suggesting that chronic mild stress interferes with the neuroprotective mechanism of Copaxone.
Compared to normal mice, immune-deficient mice exhibit a lower degree of neuroprotection after CNS injury. This phenomenon has been widely described by numerous groups in several animal models of acute CNS injury or chronic neurodegenerative conditions (Angelov et al., 2003; Gorantla et al., 2007; Gorantla et al., 2006; Kipnis and Schwartz, 2002; Kipnis et al., 2000; Liu et al., 2007; Mosley et al., 2007; Schori et al., 2001). Restoration of the T-cell pool in T cell-compromised animals restores the baseline neuronal survival. Moreover, the protective T-cell response to a CNS injury in immune-competent mice can be boosted by passive transfer of autoimmune T cells or by active vaccination with CNS-specific antigens (Avidan et al., 2004; Hauben et al., 2000a; Kipnis et al., 2004; Schori et al., 2001). Although such boosting of this autoimmune T cell response increases neuronal survival after CNS injury, it has substantial side effects, as exemplified by induction of the neuroinflammatory autoimmune condition EAE, an animal model for MS (Axtell et al., 2010; Ho et al., 2010; Itoh et al., 2010; Man et al., 2007; Moll et al., 2009; Ransohoff, 2005, 2006; Rudick et al., 2009).
In studies seeking a safe way to boost protective immune response after CNS injury, vaccination with Copaxone was assessed and was indeed found to be neuroprotective (Kipnis et al., 2000). The initial studies suggested that the observed beneficial effect of Copaxone in CNS injury was mediated by glatiramer acetate-specific T cells (Kipnis et al., 2000). More recent research suggests, however, that the beneficial Copaxone effect may be exerted either indirectly, via regulation of a protective phenotype of myeloid cells, or directly via upregulation of neurotrophic factors in neurons by a mechanism as yet unknown (Liu et al., 2007). This implies that Copaxone possesses both immune-modulatory and neuroprotective properties, through both of which it confers a high degree of benefit in MS patients who are responsive to it.
The reason why Copaxone evidently loses its neuroprotective activity under conditions of chronic mild stress is not understood. Although the T-cell numbers were found to be slightly reduced in depressed mice, this was not accompanied by a reduction in endogenous neuronal survival to the level seen earlier in CNS-injured T cell-deficient mice. It would seem however, that these changes in T-cell number and possibly also in their function were sufficient to render these depressed mice impervious to Copaxone treatment. It is also possible that the functionality of myeloid cells impaired, at least to some extent, under conditions of chronic mild stress, and hence that Copaxone is also wholly or partially ineffective in inducing neuroprotective monocytes. Interestingly, however, what when Copaxone was combined with anti-depressant drug, fluoxetine (Prozac), its neuroprotective effect was restored. Thus, while we do not yet understand the precise mechanism underlying loss of the Copaxone neuroprotective effect after CNS injury in depressed mice, our studies suggest that its combination with anti-depressants may be a more efficient neuroprotective therapy.
Although MS has long been recognized as an autoimmune disease, its neurodegenerative component was characterized only later (Trapp et al., 1999a; Trapp et al., 1998; Trapp et al., 1999b; Wujek et al., 2002). It has also long been acknowledged that the percentage of MS patients with symptoms of major depression is significantly higher than in the general population (Heesen et al., 2010; Schulz et al., 2006), and our results suggest that the neuroprotective efficacy of Copaxone in these patients cannot be fully expressed. Future studies might throw light on this issue by comparing the impact of Copaxone on MS progression in depressed and in nondepressed MS patients or in combination with anti-depressant drugs. We would expect to find that such studies confirm our contention that while Copaxone retains its immune-modulatory functions in both subpopulations of patients, in patients with depression its neuroprotective aspect is lost.
The effect of Copaxone in models of ALS in both mice and humans is still a matter of debate (Angelov et al., 2003; Gordon et al., 2006; Mosley et al., 2007). The mental state of ALS subjects (or mice) has never been defined, either experimentally in mice or clinically, as a factor in exclusion/inclusion criteria for Copaxone treatment. Our data indicate that Copaxone loses its neuroprotective properties under conditions of chronic mild stress and, thus, possibly in patients with major depression. Future studies should attempt combination therapy of Copaxone with Prozac in neurodegenerative conditions, such as ALs and others. Our findings may have significant implications for the inclusion of Copaxone in combined experimental therapies for different neurological diseases.
Methods
Stereotaxic surgery
Mice are anesthetized with Isoflurane (5% for induction and 1.5% for maintenance) in oxygen from a precision vaporizer (EZ AF9000 Auto Flow System). The head is shaved and scrubbed and washed 3X with an antiseptic solution of iodine followed by 70% ethanol. The mouse is placed in a stereotaxic frame. The scalp is opened with a scalpel, and the skull exposed. Two 1.2-mm-diameter holes are drilled in the skull bilaterally −2.9mm behind and 0.5mm lateral to bregma. 1ul of 4% FluoroGold was be applied (5uL Hamilton syringe with 32G needle) at a rate of 0.5 uL/min in each hemisphere with 1 minute diffusing time before the needle was removed. The scalp is then sutured shut and the mouse allowed to recover on a heating pad until fully awake.
Optic nerve injury
Three days after stereotaxic surgery, mice are anesthetized with a mixture of 1 ml of Ketamine HCL (100mg/ml) with 1 ml of Xylazine 2% and 8ml of Saline. When they are unresponsive to paw pinch, the soft tissue around the eye is dissected to expose the optic nerve. The optic nerve was crushed with an Dumont N5 self-closing forceps (FST; cat# 501203). The mice are allowed to recover on a heating pad until they were fully recovered.
Chronic Mild Stress
The mice were given one stress per day. Stress fell into two categories: physical stress and emotional stress. The stress was alternated between physical and emotional so that mice have 48 hours to recover between physical stressors. a. Physical Stress was one of: 1. Tail suspension – taped to a board suspended off the ground for half an hour. Physical restraint – placed into a 50 mL conical tube with the end cut off and ventilation holes poked for half an hour. 24-hour water deprivation – water bottles are removed from the cages for 24 hours. b. Emotional Stress was one of: Lights on during the dark cycle – cages were placed into the behavior room at 7 pm and the lights remain on until 7 am the next morning, when the cages are returned to the vivarium. Cage tilting – clean cages had all but two handfuls of bedding removed. Mice were placed in the cages and the cages were propped up on Styrofoam blocks and tilted to a 45-degree angle for 12 hours. Social changes – the mice were randomized to new clean cages within an experimental group so they have new cage mates. This chronic mild stress is continued for up to 4 weeks (see table 1 for further detail).
Acute Stress
For acute stress group of mice will undergo only one stress condition (tail suspension for 0.5 hr) day before injection of 4% FluoroGold.
Behavior Readouts
Forced Swim – mice were placed into large clear glass 4000 mL beakers with 2500 mL of room temperature water for 6 minutes while being video taped. Water was changed in between mice. Escape-like behaviors vs. complete immobility were assessed by a blind investigator from the obtained movies. Tail suspension – mice were taped to a board suspended off the ground for 6 minutes while being video taped. Escape-like behaviors vs. complete immobility were assessed by a blind investigator from the obtained movies.
Copaxone and Prozac treatments
A purchased Copaxone (Teva, Israel) formulation that is used for MS patients was used in this study. Mice received usually three injections (unless differently indicated) six and three days before the injury and on the day of injury. Each injection of 150 mg in 150 ml is introduced sub-cutaneously in flanks. Control mice are injected with similar regimen and volume of PBS.
When Copaxone was combined with Prozac, mice were stressed for 4 weeks and then while pre-treated with Copaxone as described above, were also treated daily with oral gavage of 10mg/kg Fluoxetine.
Statistical analysis
For comparisons involving two groups, Student's t-test was used. For comparisons involving more than two groups, One-way ANOVA with Bonferroni's post-test was used. For comparing weight of mice undergoing chronic mild stress over time, two-way ANOVA with Bonferroni's post-test was used. All statistical analyses were preformed using the GraphPad Prism software
Highlights.
Neuroprotective activity of Copaxone is lost under chronic mild stress condition and is regained when co-administered with Prozac.
Acknowledgments
This work was primarily supported by a grant from the National Institute on Aging, NIH (award NS061973 to JK) and in part by a grant from the National Institute of Mental health, NIH (MH096484 to JK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aharoni R, Teitelbaum D, Sela M, Arnon R. Copolymer 1 induces T cells of the T helper type 2 that crossreact with myelin basic protein and suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 1997;94:10821–10826. doi: 10.1073/pnas.94.20.10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelov DN, Waibel S, Guntinas-Lichius O, Lenzen M, Neiss WF, Tomov TL, Yoles E, Kipnis J, Schori H, Reuter A, Ludolph A, Schwartz M. Therapeutic vaccine for acute and chronic motor neuron diseases: implications for amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2003;100:4790–4795. doi: 10.1073/pnas.0530191100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon R, Teitelbaum D, Sela M. Suppression of experimental allergic encephalomyelitis by COP1--relevance to multiple sclerosis. Isr J Med Sci. 1989;25:686–689. [PubMed] [Google Scholar]
- Avidan H, Kipnis J, Butovsky O, Caspi RR, Schwartz M. Vaccination with autoantigen protects against aggregated beta-amyloid and glutamate toxicity by controlling microglia: effect of CD4+CD25+ T cells. Eur J Immunol. 2004;34:3434–3445. doi: 10.1002/eji.200424883. [DOI] [PubMed] [Google Scholar]
- Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P, Naves R, Han M, Zhong F, Castellanos JG, Mair R, Christakos A, Kolkowitz I, Katz L, Killestein J, Polman CH, de Waal Malefyt R, Steinman L, Raman C. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry MA, Bland KI, Chaudry IH. Trauma and immune response--effect of gender differences. Injury. 2007;38:1382–1391. doi: 10.1016/j.injury.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona AW, Huang Y, O'Connor JC, Dantzer R, Kelley KW, Popovich PG, Godbout JP. Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. Journal of neuroinflammation. 2010;7:93. doi: 10.1186/1742-2094-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DJ, Longbrake EE, Shawler TM, Kigerl KA, Lai W, Tovar CA, Ransohoff RM, Popovich PG. Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+ macrophages. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:9910–9922. doi: 10.1523/JNEUROSCI.2114-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng SF, Shi TY, Fan Y, Wang WN, Chen YC, Tan QR. Long-lasting effects of chronic rTMS to treat chronic rodent model of depression. Behavioural brain research. 2012;232:245–251. doi: 10.1016/j.bbr.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Frank MG, Wieseler Frank JL, Hendricks SE, Burke WJ, Johnson DR. Age at onset of major depressive disorder predicts reductions in NK cell number and activity. Journal of affective disorders. 2002;71:159–167. doi: 10.1016/s0165-0327(01)00395-0. [DOI] [PubMed] [Google Scholar]
- Gillessen T, Budd SL, Lipton SA. Excitatory amino acid neurotoxicity. Adv. Exp. Med. Biol. 2002;513:3–40. doi: 10.1007/978-1-4615-0123-7_1. [DOI] [PubMed] [Google Scholar]
- Gorantla S, Liu J, Sneller HM, Walters L, Dou H, Ikezu T, Volsky DJ, Poluektova L, Gendelman HE. Glatiramer acetate induces adaptive immune anti-inflammatory glial and neuroprotective responses in a murine model of HIV-1 encephalitis. J Immunol. 2007 doi: 10.4049/jimmunol.179.7.4345. In Press. [DOI] [PubMed] [Google Scholar]
- Gorantla S, Poluektova L, Klassek H, Walters L, Nelson JA, Dou H, Ikezu T, Volsky DJ, Boska MD, Gendelman HE. Copaxone Regulation of Innate and Adaptive Imminty induces Suppression of Neuroinflammation and Elicits Neuronal Protection in Murine Models of HIV-1 Encephalitis. 13th conference on retroviruses and opportunistic infections; Denver, CO, USA. 2006. [Google Scholar]
- Gordon PH, Doorish C, Montes J, Mosley RL, Diamond B, Macarthur RB, Weimer LH, Kaufmann P, Hays AP, Rowland LP, Gendelman HE, Przedborski S, Mitsumoto H. Randomized controlled phase II trial of glatiramer acetate in ALS. Neurology. 2006;66:1117–1119. doi: 10.1212/01.wnl.0000204235.81272.e2. [DOI] [PubMed] [Google Scholar]
- Hauben E, Butovsky O, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Leibowitz-Amit R, Pevsner E, Akselrod S, Neeman M, Cohen IR, Schwartz M. Passive or active immunization with myelin basic protein promotes recovery from spinal cord contusion. J Neurosci. 2000a;20:6421–6430. doi: 10.1523/JNEUROSCI.20-17-06421.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauben E, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Akselrod S, Neeman M, Cohen IR, Schwartz M. Autoimmune T cells as potential neuroprotective therapy for spinal cord injury. Lancet. 2000b;355:286–287. doi: 10.1016/s0140-6736(99)05140-5. [DOI] [PubMed] [Google Scholar]
- Heesen C, Schulz KH, Fiehler J, Von der Mark U, Otte C, Jung R, Poettgen J, Krieger T, Gold SM. Correlates of cognitive dysfunction in multiple sclerosis. Brain Behav Immun. 2010;24:1148–1155. doi: 10.1016/j.bbi.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Ho PP, Lee LY, Zhao X, Tomooka BH, Paniagua RT, Sharpe O, BenBarak MJ, Chandra PE, Hueber W, Steinman L, Robinson WH. Autoimmunity against fibrinogen mediates inflammatory arthritis in mice. J Immunol. 2010;184:379–390. doi: 10.4049/jimmunol.0901639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isingrini E, Camus V, Le Guisquet AM, Pingaud M, Devers S, Belzung C. Association between repeated unpredictable chronic mild stress (UCMS) procedures with a high fat diet: a model of fluoxetine resistance in mice. PLoS ONE. 2010;5:e10404. doi: 10.1371/journal.pone.0010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh S, Nakae S, Axtell RC, Velotta JB, Kimura N, Kajiwara N, Iwakura Y, Saito H, Adachi H, Steinman L, Robbins RC, Fischbein MP. IL-17 contributes to the development of chronic rejection in a murine heart transplant model. J Clin Immunol. 2010;30:235–240. doi: 10.1007/s10875-009-9366-9. [DOI] [PubMed] [Google Scholar]
- Jones TB, Ankeny DP, Guan Z, McGaughy V, Fisher LC, Basso DM, Popovich PG. Passive or active immunization with myelin basic protein impairs neurological function and exacerbates neuropathology after spinal cord injury in rats. J Neurosci. 2004;24:3752–3761. doi: 10.1523/JNEUROSCI.0406-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Avidan H, Markovich Y, Mizrahi T, Hauben E, Prigozhina TB, Slavin S, Schwartz M. Low-dose gamma-irradiation promotes survival of injured neurons in the central nervous system via homeostasis-driven proliferation of T cells. Eur J Neurosci. 2004;19:1191–1198. doi: 10.1111/j.1460-9568.2004.03207.x. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M. Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc Natl Acad Sci U S A. 2002;99:15620–15625. doi: 10.1073/pnas.232565399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Schwartz M. Dual action of glatiramer acetate (Cop-1) in the treatment of CNS autoimmune and neurodegenerative disorders. Trends Mol Med. 2002;8:319–323. doi: 10.1016/s1471-4914(02)02373-0. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Yoles E, Porat Z, Cohen A, Mor F, Sela M, Cohen IR, Schwartz M. T cell immunity to copolymer 1 confers neuroprotection on the damaged optic nerve: possible therapy for optic neuropathies. Proc Natl Acad Sci U S A. 2000;97:7446–7451. doi: 10.1073/pnas.97.13.7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Yoles E, Schori H, Hauben E, Shaked I, Schwartz M. Neuronal survival after CNS insult is determined by a genetically encoded autoimmune response. J Neurosci. 2001;21:4564–4571. doi: 10.1523/JNEUROSCI.21-13-04564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov-Spiegler O, Solomon AS, Schwartz M. Peripheral nerve-stimulated macrophages simulate a peripheral nerve-like regenerative response in rat transected optic nerve. Glia. 1998;24:329–337. [PubMed] [Google Scholar]
- Leonard B. Stress, depression and the activation of the immune system. World J Biol Psychiatry. 2000;1:17–25. doi: 10.3109/15622970009150562. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Molecular mechanisms of trauma-induced neuronal degeneration. Curr Opin Neurol Neurosurg. 1993;6:588–596. [PubMed] [Google Scholar]
- Liu J, Johnson TV, Lin J, Ramirez SH, Bronich TK, Caplan S, Persidsky Y, Gendelman HE, Kipnis J. T cell independent mechanism for copolymer-1-induced neuroprotection. Eur J Immunol. 2007;37:3143–3154. doi: 10.1002/eji.200737398. [DOI] [PubMed] [Google Scholar]
- Man S, Ubogu EE, Ransohoff RM. Inflammatory cell migration into the central nervous system: a few new twists on an old tale. Brain Pathol. 2007;17:243–250. doi: 10.1111/j.1750-3639.2007.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH. Depression and immunity: a role for T cells? Brain, behavior, and immunity. 2010;24:1–8. doi: 10.1016/j.bbi.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Herbert TB. Pathways linking major depression and immunity in ambulatory female patients. Psychosomatic medicine. 1999;61:850–860. doi: 10.1097/00006842-199911000-00021. [DOI] [PubMed] [Google Scholar]
- Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- Moll NM, Cossoy MB, Fisher E, Staugaitis SM, Tucky BH, Rietsch AM, Chang A, Fox RJ, Trapp BD, Ransohoff RM. Imaging correlates of leukocyte accumulation and CXCR4/CXCL12 in multiple sclerosis. Arch Neurol. 2009;66:44–53. doi: 10.1001/archneurol.2008.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley RL, Gordon PH, Hasiak CM, Van Wetering FJ, Mitsumoto H, Gendelman HE. Glatiramer acetate immunization induces specific antibody and cytokine responses in ALS patients. Amyotroph Lateral Scler. 2007;8:235–242. doi: 10.1080/17482960701374601. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Reinhard JF, Jr., Flanagan EM, Stokes BT. Elevation of the neurotoxin quinolinic acid occurs following spinal cord trauma. Brain Res. 1994;633:348–352. doi: 10.1016/0006-8993(94)91560-1. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM. Immunologic correlates of MS pathologic subtypes. Mult Scler. 2005;11:101–102. doi: 10.1177/135245850501100121. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM. EAE: pitfalls outweigh virtues of screening potential treatments for multiple sclerosis. Trends Immunol. 2006;27:167–168. doi: 10.1016/j.it.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Rapalino O, Lazarov-Spiegler O, Agranov E, Velan GJ, Yoles E, Fraidakis M, Solomon A, Gepstein R, Katz A, Belkin M, Hadani M, Schwartz M. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat Med. 1998;4:814–821. doi: 10.1038/nm0798-814. [DOI] [PubMed] [Google Scholar]
- Rudick RA, Pace A, Rani MR, Hyde R, Panzara M, Appachi S, Shrock J, Maurer SL, Calabresi PA, Confavreux C, Galetta SL, Lublin FD, Radue EW, Ransohoff RM. Effect of statins on clinical and molecular responses to intramuscular interferon beta-1a. Neurology. 2009;72:1989–1993. doi: 10.1212/WNL.0b013e3181a92b96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schori H, Kipnis J, Yoles E, WoldeMussie E, Ruiz G, Wheeler LA, Schwartz M. Vaccination for protection of retinal ganglion cells against death from glutamate cytotoxicity and ocular hypertension: implications for glaucoma. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3398–3403. doi: 10.1073/pnas.041609498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuld A, Schmid DA, Haack M, Holsboer F, Friess E, Pollmacher T. Hypothalamo-pituitary-adrenal function in patients with depressive disorders is correlated with baseline cytokine levels, but not with cytokine responses to hydrocortisone. Journal of psychiatric research. 2003;37:463–470. doi: 10.1016/s0022-3956(03)00054-2. [DOI] [PubMed] [Google Scholar]
- Schulz D, Kopp B, Kunkel A, Faiss JH. Cognition in the early stage of multiple sclerosis. J Neurol. 2006;253:1002–1010. doi: 10.1007/s00415-006-0145-8. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Kipnis J. Protective autoimmunity: regulation and prospects for vaccination after brain and spinal cord injuries. Trends in Molecular Medicine. 2001;7:252–258. doi: 10.1016/s1471-4914(01)01993-1. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Kipnis J, Yoles E. The innate neuronal response and the adaptive immune response to CNS insult: Genetic aspects and prospects for vaccination. Trends in Molecular Medicine. 2000 in press. [Google Scholar]
- Schwartz M, Shaked I, Fisher J, Mizrahi T, Schori H. Protective autoimmunity against the enemy within: fighting glutamate toxicity. Trends Neurosci. 2003;26:297–302. doi: 10.1016/S0166-2236(03)00126-7. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Yoles E. Optic nerve degeneration and potential neuroprotection: implications for glaucoma. Eur J Ophthalmol. 1999;9(Suppl 1):S9–11. doi: 10.1177/112067219900901S07. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Yoles E, Levin LA. `Axogenic' and `somagenic' neurodegenerative diseases: definitions and therapeutic implications. Mol Med Today. 1999;5:470–473. doi: 10.1016/s1357-4310(99)01592-0. [DOI] [PubMed] [Google Scholar]
- Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G, Jung S, Schwartz M. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. Astrocyte failure as a cause of CNS dysfunction. Mol Psychiatry. 2000;5:230–232. doi: 10.1038/sj.mp.4000753. [DOI] [PubMed] [Google Scholar]
- Soygur H, Palaoglu O, Akarsu ES, Cankurtaran ES, Ozalp E, Turhan L, Ayhan IH. Interleukin-6 levels and HPA axis activation in breast cancer patients with major depressive disorder. Progress in neuro-psychopharmacology & biological psychiatry. 2007;31:1242–1247. doi: 10.1016/j.pnpbp.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Teitelbaum D, Arnon R, Sela M. Copolymer 1: from basic research to clinical application. Cell Mol Life Sci. 1997a;53:24–28. doi: 10.1007/PL00000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum D, Fridkis-Hareli M, Arnon R, Sela M. Copolymer 1 inhibits chronic relapsing experimental allergic encephalomyelitis induced by proteolipid protein (PLP) peptides in mice and interferes with PLP-specific T cell responses. J Neuroimmunol. 1996;64:209–217. doi: 10.1016/0165-5728(95)00180-8. [DOI] [PubMed] [Google Scholar]
- Teitelbaum D, Sela M, Arnon R. Copolymer 1 from the laboratory to FDA. Isr J Med Sci. 1997b;33:280–284. [PubMed] [Google Scholar]
- Trapp BD, Bo L, Mork S, Chang A. Pathogenesis of tissue injury in MS lesions. J Neuroimmunol. 1999a;98:49–56. doi: 10.1016/s0165-5728(99)00081-8. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Ransohoff R, Rudick R. Axonal pathology in multiple sclerosis: relationship to neurologic disability. Curr Opin Neurol. 1999b;12:295–302. doi: 10.1097/00019052-199906000-00008. [DOI] [PubMed] [Google Scholar]
- Wujek JR, Bjartmar C, Richer E, Ransohoff RM, Yu M, Tuohy VK, Trapp BD. Axon loss in the spinal cord determines permanent neurological disability in an animal model of multiple sclerosis. J Neuropathol Exp Neurol. 2002;61:23–32. doi: 10.1093/jnen/61.1.23. [DOI] [PubMed] [Google Scholar]
- Yoles E, Hauben E, Palgi O, Agranov E, Gothilf A, Cohen A, Kuchroo V, Cohen IR, Weiner H, Schwartz M. Protective autoimmunity is a physiological response to CNS trauma. J Neurosci. 2001;21:3740–3748. doi: 10.1523/JNEUROSCI.21-11-03740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]