Abstract
Glucocorticoids (GC) are powerful anti-inflammatory agents frequently used to protect the auditory organ against damage associated with a variety of conditions, including noise exposure and ototoxic drugs as well as bacterial and viral infections. In addition to glucocorticoid receptors (GC-R), natural and synthetic GC are known to bind mineralocorticoid receptors (MC-R) with great affinity. We used light and laser scanning confocal microscopy to investigate the expression of GC-R and MC-R in different cell populations of the guinea pig cochlea, and their translocation to different cell compartments after treatment with the synthetic GC dexamethasone. We found expression of both types of receptors in the cytoplasm and nucleus of sensory inner and outer hair cells as well as pillar, Hensen and Deiters cells in the organ of Corti, inner and outer sulcus cells, spiral ganglion neurons and several types of spiral ligament and spiral limbus cells; stria vascularis cells expressed mostly MC-R whereas fibrocytes type IV were positive for GC-R only. GC-R and MC-R were also localized at or near the plasma membrane of pillar cells and outer hair cells, whereas GC-R were found at or near the plasma membrane of Hensen cells only. We investigated the relative levels of receptor expression in the cytoplasm and the nucleus of Hensen cells treated with dexamethasone, and found they varied in a way suggestive of dose-induced translocation. These results suggest that the oto-protective effects of GC could be associated with the concerted activation of genomic and non-genomic, GC-R and MC-R mediated signaling pathways in different regions of the cochlea.
Keywords: glucocorticoids, glucocorticoid receptors, mineralocorticoid receptors, dexamethasone, cochlea, guinea pig
1. Introduction
The current clinical strategy to protect the auditory organ against damage associated with a variety of conditions, including noise exposure, ototoxic drugs as well as bacterial and viral infections, is the local delivery of glucocorticoids (GC). Although there is considerable support to the idea that GC-mediated inner ear protection would involve regulating immune suppression and anti-inflammation (Meltser et al., 2011; Ruckenstein et al., 1999b; Tahera et al., 2007; Tahera, 2006a; Tahera, 2006b), the precise mechanisms activated by GC in the cochlea are still unknown.
GCs are able to bind the two classic steroid hormone receptors, glucocorticoid receptors (GC-R) and mineralocorticoid receptors (MC-R) (Arriza et al., 1987; Claire et al., 1993; Rupprecht et al., 1993). One of the major activators of MC-R is the mineralocorticoid (MC) aldosterone, but MC-R also respond strongly to the GC cortisone (Krozowski et al., 1983; Myles et al., 1994; Reichardt et al., 2000) and show high degree colocalization with GC-R in different organs (Arriza et al., 1987; Beavan et al., 2001; van Steensel et al., 1996). Moreover, cortisone has been shown to regulate two distinct signaling pathways via MC-R and GC-R, respectively (Kawata, 1995; Pearce et al., 1993; Reul et al., 1985), and both GC-R and MC-R have been implicated in mediating GC effects in the inner ear. (Pondugula et al., 2004; Trune et al., 2006; Trune et al., 2001). Because aldosterone regulates homeostasis of Na+, K+, and other electrolytes in the body, it is probable that MC and MC-R are important for regulation of ion balance in the inner ear. Similarly, since experimental evidence suggests that GC stimulate Na+ absorption in the semicircular canals (Pondugula et al., 2004), GC also might have an important role in maintaining ionic homeostasis in the cochlea. Studies on cochlear dysfunction in autoimmune mice, for example, suggested it is controlled with the GC prednisolone as effectively as with aldosterone (Trune et al., 2006; Trune et al., 2001). Altogether, these studies suggests that GC may be controlling hearing recovery in part through their binding to MC-R to maintain endolymph homeostasis in addition to their GC-R-mediated anti-inflammatory and immunosuppressive functions.
GC-R and MC-R reside normally in the cytoplasm, but after binding to their ligands they usually heterodimerize and translocate to the nucleus where they transactivate or transrepress target genes. The full process normally takes place over a course of hours (Lowenberg et al., 2008). In addition to gene-mediated effects, steroid hormone receptors are known to activate non-genomic signaling pathways that proceed within minutes or even seconds of ligand binding (Boldyreff et al., 2003; Cato et al., 2002; Haller et al., 2008; Losel et al., 2003). The involvement of classical MR and GR in nongenomic corticosteroid signaling in neurons is supported by studies using both antagonists and knockout mice for the MC-R and GC-R (Karst, 2005), and it is believed that at least part of the nongenomic effects are mediated either by binding of GC to receptors located in or at the plasma membrane (Boldyreff et al., 2003; Gametchu et al., 1993; Guo et al., 1995; Losel et al., 2003).
A number of studies have demonstrated GC-R and MC-R expression in the inner ear of mice and rats with techniques like ELISA (Furuta et al., 1994; Pitovski et al., 1993; Pitovski et al., 1994; Rarey et al., 1989; Rarey et al., 1996; ten Cate et al., 1992), immunohistochemistry, in situ hybridization and PCR (Erichsen et al., 1996; Shimazaki et al., 2002; Sinha et al., 1995; ten Cate et al., 1992; Yao et al., 1996; Zuo et al., 1995). GC-R expression was reported in spiral ligament fibrocytes, stria vascularis and organ of Corti cells (Shimazaki et al., 2002; Zuo et al., 1995). In the organ of Corti, expression of GC-R in outer and inner hair cells (OHCs and IHCs) was detected in rats but not in mice. Supporting cells displayed GC-R expression in both mice and rats, but specific supporting cell types expressing GC-R immunoreactivity were not identified in these studies (Shimazaki et al., 2002; Zuo et al., 1995). In rats, MC-R mRNA expression was found in the marginal cells of stria vascularis (Furuta et al., 1994) and protein expression was found in IHCs and OHCs, spiral limbus and spiral ganglion cells (Yao et al., 1996). In mouse, protein expression was detected in IHCs and OHCs, spiral limbus, spiral ligament and spiral ganglion cells (Erichsen et al., 2001). In guinea pigs the cellular distribution of GC-R and MC-R has not been explored, although binding assays demonstrated their presence in cochlear and vestibular tissues (Pitovski et al., 1994) and GC-R mRNA expression was reported in spiral ligament, stria vascularis, spiral limbus and spiral ganglion using in situ hybridization techniques (Terunuma et al., 2003).
To understand the complex mechanisms involved in the GC-mediated otoprotection it is vital to identify potential target cells and the receptors mediating these effects in the cochlea. In this study we describe in detail the cellular distribution of both GC-R and MC-R in the guinea pig cochlea, providing evidence that these receptors show a high degree of co-localization and that dexamethasone can induce their translocation to the nucleus in both Hensen and HEI-OC1 cells. These results indicate that cochlear effects of GC could be associated with the activation of both GC-R and MC-R mediated signaling pathways.
2. Material Methods
2.1. Samples’ preparation
A total of 20 young albino guinea pigs (Cavias porcellus), 2-8 week-old, were euthanized with CO2 following procedures approved by the House Research Institute’s IACUC and used in the described experiments. a) Organ of Corti cultures: Temporal bones (n=20) were isolated and placed in phosphate-buffered saline without calcium and magnesium (PBS, 0.1 M, pH 7.6; Sigma, St. Louis, MO). The cochlear spiral was removed in PBS and organ of Corti tissues detached from modiolus with 30-gauge needles and forceps. Explants were then placed on polycarbonate track membrane filters with 1 μm pores (SPI, West Chester, PA) and immersed overnight in Dulbecco’s Modified Eagle Medium (DMEM, Life Technologies, Grand Island, NY) with 10% fetal bovine serum without phenol red (Life Technologies) at 37°C. b) Dexamethasone treatment: DMEM + 10% FBS was removed and replaced by DMEM w/o phenol red. Explants in DMEM alone were treated with 10 pM, 100 nM, and 10 μM dexamethasone (Sigma) for 2 hrs at 37°C followed by 1 hr at 33°C, fixed with 4% PFA and probed with antibodies against GC-R and MC-R as described in the next section. c) Cochlea preparations: Cochleae were quickly removed from the temporal bone, decalcified and treated for cryostat sectioning as previously described (Coleman et al., 2009). Briefly, cochleae were fixed in 4 % paraformaldehyde (PFA; EMS, Hatfield, PA) overnight at 4°C. After fixation, cochleae were washed three times for 5 min in PBS and transferred into 125 mM EDTA + 2% PFA in 200 mM HEPES buffer at room temperature for decalcification for 2 weeks. After being removed from decalcification solution, cochleae were first washed three times in PBS for 5 min, next once in 10% sucrose solution for 30 min, and then placed in 15% sucrose solution and kept on rotation for a further 30 min at room temperature or overnight at 4°C. After replacing the 15% sucrose solution with 1:1 solution of 15% sucrose and Tissue-Tek Optimal Cutting Temperature (OCT; Sakura Finetek, Torrance, CA), cochleae were transferred into a cryo-mold, frozen in slurry of solid CO2 and 100% ethanol, and sectioned at 10 μm thickness with a cryostat (Leica Microsystems, Buffalo Grove, IL). d) HEI-OC1 cells: Cells were grown at 33°C in 100×20 mm plastic cell culture dishes with DMEM plus 10 % FBS (Life Technologies). For dexamethasone treatment, DMEM + 10% FBS was removed and replaced by DMEM w/o phenol red alone. Cells in DMEM alone were treated with 10 pM, 100 nM, and 10 μM dexamethasone (Sigma) for 3 hrs at 33°C, and processed for immunofluorescence as described below.
2.2. Immunolocalization
a) Immunohistochemistry: Cryosections of guinea pig cochleae (n=8) were air-dried for 2 hour-overnight, then fixed for 30 min with 1.5 % PFA and washed with 1X PBS for 5 min. Sections were then incubated with Vectastain ABC peroxidase blocking buffer (Life Technologies) for 30 min, and next with primary antibodies against anti-glucocorticoid receptor (Pierce, Rockford, IL) or anti-mineralocorticoid receptor (Developmental Hybridoma Bank, Iowa City, Iowa) overnight at 4° C, at 1:100-1:200 dilution in blocking solution. Sections were then washed with 1X PBS for 5 min. and incubated with biotinylated anti-mouse IgG antibodies (Vectastain Kit, Life Technologies) for 30 min, washed with 1X PBS for 5 min, followed by 30 min incubation with ABC reagent (Vectastain kit, Life Technologies). DAB substrate was applied to the sections for 30 min.-2 hrs, mounted and samples were observed with Axiovert 135 TV microscope (Zeiss, Germany) with 10X (NA = 0.3) and 40X (NA = 1.2) objectives. b) Immunofluorescence: Organ of Corti tissue and explants from guinea pig cochleae (n=12) were fixed with 4% PFA overnight at 4°C. HEI-OC1 cells were fixed with 4% PFA for 30 min at room temperature. After fixation, organ of Corti explants and HEI-OC1 cells were washed 3 times with 1X PBS with 0.1 % Triton X-100 (BioRad, Hercules, CA) for 10 min each, blocked with 10 % fish serum (Norland Inc., Cranbury, NJ) plus 1 % Bovine Serum Albumin (BSA) in 1X PBS with 0.1 % triton for 2 hours, and then incubated with primary antibodies anti-glucocorticoid receptor (Pierce), or anti-mineralocorticoid receptor (Developmental Hybridoma Bank) overnight in 1:100-1:200 blocking solution at 4°C. Tissues were then washed 3 times with 1X PBS with 0.1 % Triton and incubated with either Alexa 488 or 555 anti-mouse IgG antibodies (Molecular Probes-Invitrogen, Eugene, Oregon) for 2 hours, washed 3 times with 1X PBS with 0.1% Triton, and mounted in ProLong Gold slow antifade reagent with DAPI (Life Technologies). Either Alexa 542 or Alexa 633 phalloidin (Molecular Probes-Invitrogen) were used to stain actin. Blocking peptides were used in pre-adsorption experiments with primary anti-glucocorticoid receptor antibodies, whereas omission of primary antibody was used as negative control for experiments involving anti-mineralocorticoid receptor antibodies. Some organ of Corti tissues and explants were labeled with CellMask Deep Red Plasma Membrane Stain (Life Technologies) at 1:100 for 2 hours. Samples were observed with a TCS-SP5 Broadband Spectra laser scanning confocal microscope with a 63X (NA = 1.2) objective (Leica Microsystems, Deerfield, IL, USA). Images were cropped, resized, and brightness and contrast over the whole image adjusted where necessary, using Photoshop (Adobe Software).
2.3. Nuclear labeling quantification and statistical analysis
Relative quantification of MC-R and GC-R labeling in nuclei of HEI-OC1 and Hensen cells (at least 100 cells per condition) was performed using the Analysis feature in Photoshop CS5 Extended software (Adobe). A circular area, with the approximate diameter of the nuclei under study, was selected in the elliptical marquee tool and used in all the conditions being compared. The feature “integrated density” (ID) in this circular region, when centered on the nuclei being evaluated, was used as an estimation of the receptor-associated nuclear labeling in all the experimental conditions. Statistical analysis of the data, including One-way and Two-way ANOVA, was performed using JMP 9 software (SAS Institute, Cary, NC) and p ≤ 0.05 as the criterion for statistical significance.
3. Results
3.1. MC-R and GC-R are expressed in several cochlear cell populations
MC-R expression was observed all along the cochlea, although with regional differences. Whereas labeling of spiral limbus, inner sulcus, spiral ganglion neurons and organ of Corti cells was consistently similar in all the cochlear turns, staining of outer sulcus cells, stria vascularis cells and spiral ligament fibrocytes types I, II and V increased, and staining of spiral ligament fibrocytes type III decreased, from the base to the apex (Fig. 1 A, B). Not evident expression of MC-R in fibrocytes type IV in the spiral ligament was detected. Spiral limbus and inner sulcus cells labeling was generally strong, whereas spiral ganglion neurons showed only moderate reactivity (Fig. 1 D). In the organ of Corti, MC-R expression was observed in the cytoplasm and nucleus of OHCs (Fig. 2, arrowheads), Deiter cells (Fig. 2, small arrows, and Fig. 5 D & E) and Hensen cells (Fig. 2, arrows), as well as cytoplasm, nucleus and cell boundaries of inner and outer pillar cells (Fig. 5 D-F). MC-R immunoreactivity was stronger in the IHC cytoplasm than in IHCs’ nuclei (Fig. 5 B).
Figure 1. Immunohistochemical detection of MC-R in cryo-sections of guinea pig cochlea.
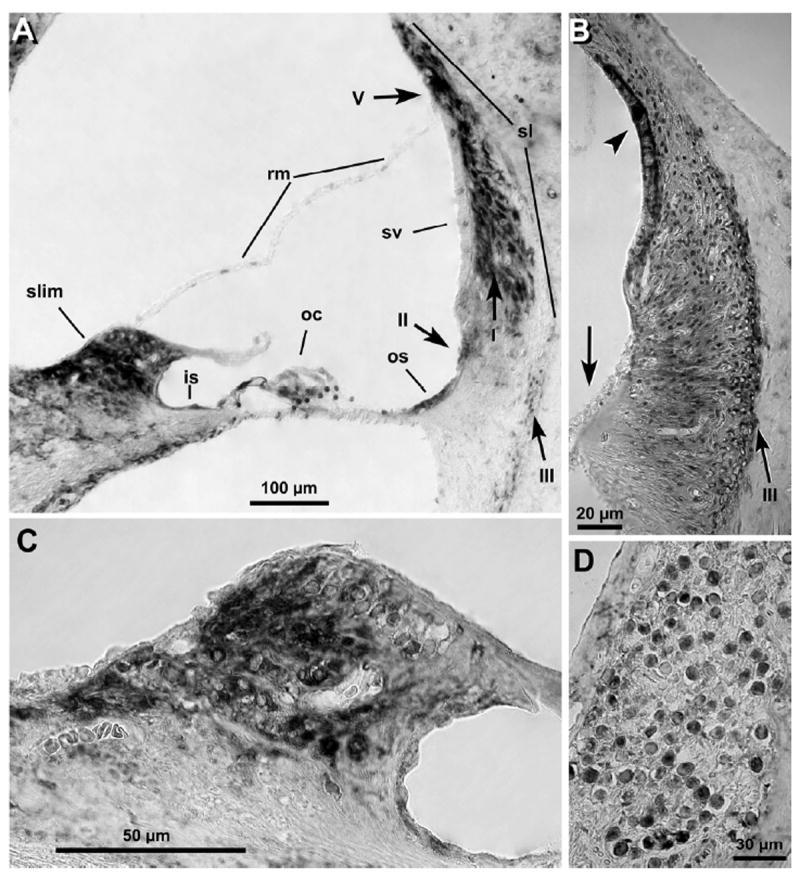
MC-R were found expressed in spiral ligament (A and B), spiral limbus (A and C), outer and inner sulcus cells (A-C), spiral ganglion neurons (D), and the organ of Corti (A). Interestingly, MC-R expression in stria vascularis and fibrocytes type III was higher in the basal region (arrowheads in B, cochlea 1st turn), and lower in outer sulcus cells (arrow in B), than in upper turns of the cochlea (compare with A, cochlea 3rd turn). In contrast, stria vascularis labeling was near absent in upper turns, whereas strong reactivity was observed in fibrocytes type I, II and V (arrows in A). No labeling was evident in Claudius and Boetcher cells (A), as well as in fibrocytes type IV (A and B) in any cochlear region. sv: stria vascularis, rm: Reisner membrane, sl: spiral ligament, slim: spiral limbus, oc: organ of Corti, os: outer sulcus cells, is: inner sulcus cells, I: fibrocytes type I, II: fibrocytes type II, III: fibrocytes type III, V: fibrocytes type V.
Figure 2. OHCs, Deiters and Hensen cells express MC-R.
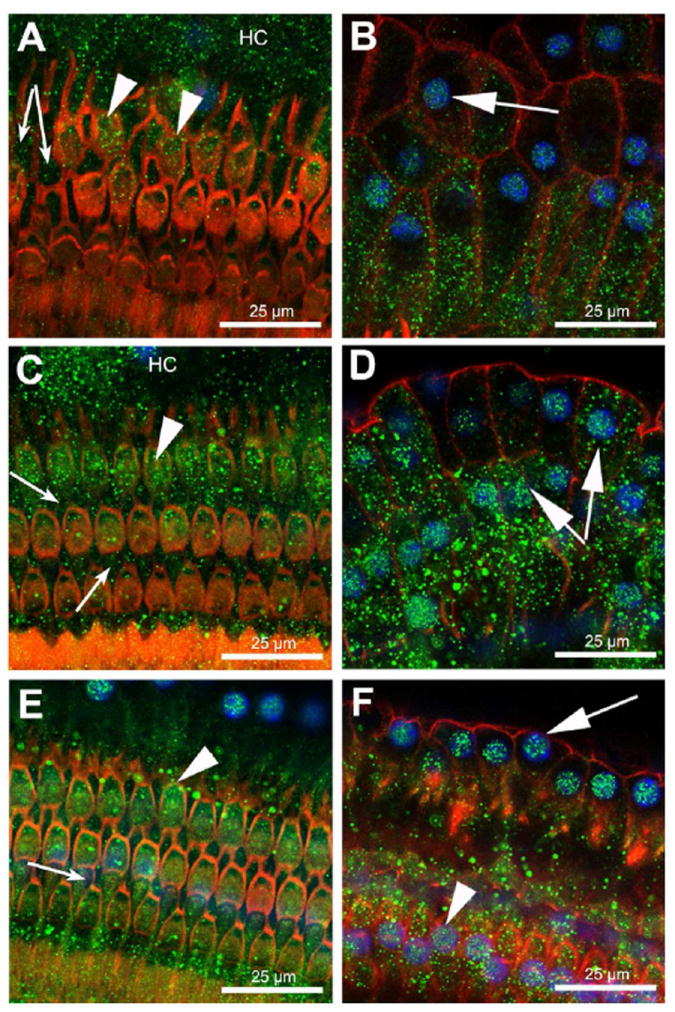
Immunofluorescent detection of MC-R in the apical (A, B), middle (C, D), and basal (E, F) turns of the guinea pig cochlea. MC-R were found expressed in the cytoplasm and nucleus of OHCs (arrowheads), Hensen (arrows) and Deiters cells (small arrows). Note strong nuclear labeling in Hensen cells. MC-R expression is shown in green, nuclei in blue and actin in red.
Figure 5. GC-R and MC-R are also expressed in IHCs and pillar cells.
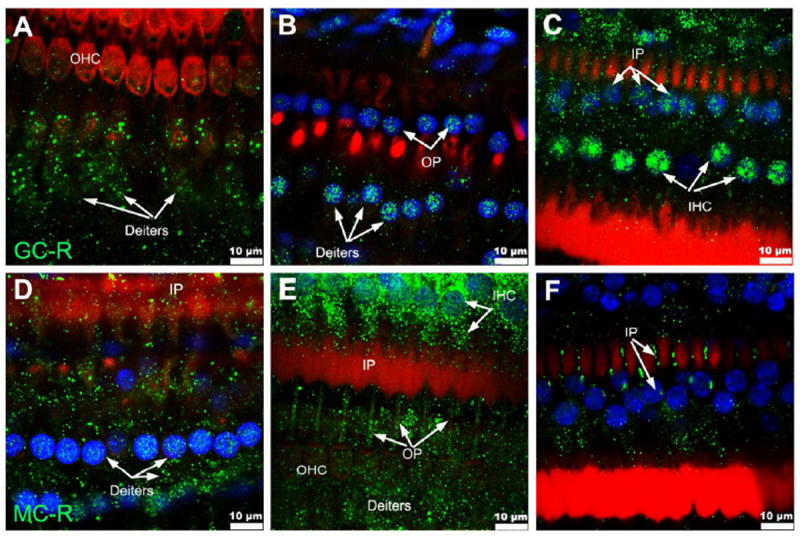
GC-R expression is shown in (A-C), and MC-R in (D-F). GC-R was found strongly expressed in nuclei and cytoplasm of IHCs (C). GC-R was also detected in the nuclei of inner (C) and outer (B) pillar cells as well as nuclei (B) and cytoplasm (A) of Deiters cells. MC-R labeling was strong at the cytoplasm and nucleus of IHCs (E). In inner and outer pillar cells, expression of MC-R was evident at the cells’ boundaries (E-F) and cytoplasm (D), whereas lower levels of MC-R expression were apparent in their nuclei (D and F). Deiters cells also showed cytoplasmic and nuclear MC-R expression (D, E). GC-R and MC-R are shown in green, nuclei in blue and actin in red.
GC-R showed small differences in localization with MC-R (Fig. 3). In addition to spiral limbus, inner sulcus, spiral ganglion neurons and organ of Corti cells, labeling of outer sulcus cells was also consistently similar in all the cochlear turns. GC-R labeling, however, was generally weaker than MC-R labeling at the spiral limbus but stronger at the inner and outer sulcus cells as well as in fibrocytes type IV in the spiral ligament (compare Figs. 1 A and 3 A). As in the case of MC-R, within the spiral ligament expression of GC-R increased from the base to the apex in fibrocytes types I, II and V, and decreased in fibrocytes type III and IV. In stria vascularis, in contrast, GC-R labeling was generally absent (Fig. 3 A, B). In the organ of Corti (Fig. 4), they were localized in the nucleus and cytoplasm of Hensen cells (Fig. 4, arrows), OHCs (Fig. 4, arrowheads), IHCs (Fig. 5 C), Deiters cells (small arrows in Fig. 4 C, E and Fig. 5 A, B), as well as in inner and outer pillar cells (Fig. 5 B, C). In the IHC cytoplasm GC-R immunoreactivity was weaker than MC-R immunoreactivity, whereas a less intense expression of GC-R was observed in IHCs’ nuclei (Fig. 5 B).
Figure 3. Immunohistochemical detection of GC-R in cryo-sections of guinea pig cochlea.
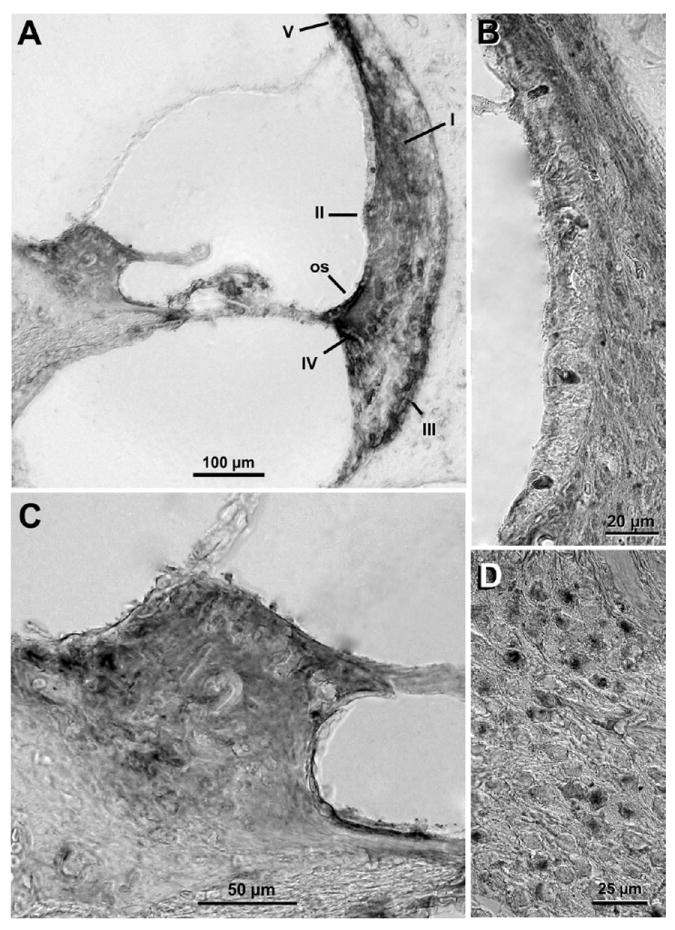
Labeling of GC-R was evident in spiral ligament fibrocytes type I and IV (A and B), spiral limbus (A and C), outer and inner sulcus cells (B and C), spiral ganglion neurons (D), and cells of organ of Corti (A). No labeling was evident in Claudius and Boetcher cells (A). GC-R expression at the stria vascularis was near absent (A and B), and lower than MC-R expression at the spiral limbus (A and C. Compare with Fig. 1 A and C) and spiral ganglion neurons (D. Compare with Fig. 1 D). In contrast, inner sulcus and outer sulcus cells as well as fibrocytes type III and IV showed generally more intense labeling of GC-R than MC-R. os: outer sulcus cells, I: fibrocytes type I, II: fibrocytes type II, III: fibrocytes type III, IV: fibrocytes type IV, V: fibrocytes type V.
Figure 4. OHCs, Deiters and Hensen cells express GC-R.
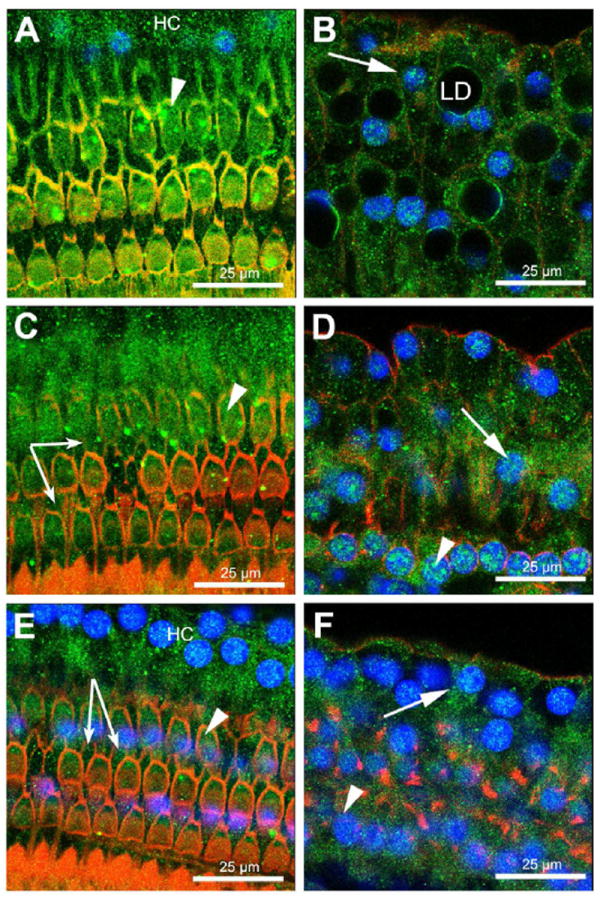
Immunofluorescence techniques showed expression of GC-R in the organ of Corti at the apical (A, B), middle (C, D), and basal (E, F) turns of the cochlea. In all these regions GC-R expression was detected in the nucleus and cytoplasm of OHCs, Deiters and Hensen cells. Arrowheads indicate OHCs, arrows Hensen cells, and small arrows Deiters cells. GC-R expression is shown in green, nuclei in blue and actin in red.
To investigate the presence of GC-R and MC-R in or near the plasma membrane of different organ of Corti cell population we used, in addition to anti-GC-R and anti-MC-R antibodies, plasma membrane stain CellMask and antibodies against the membrane associated protein β-catenin (Fig. 6). We observed expression of GC-R in or near the plasma membrane of OHCs (Fig. 6B), Hensen cells (Fig. 6 C) and pillar cells (Fig. 6D). MC-R, in turn, colocalized with β-catenin and CellMask in the cuticular plate of OHCs (Figs. 6 G and F) and the plasma membrane of pillar cells (Fig. 6H).
Figure 6. GC-R and MC-R localize in or at the plasma membrane of organ of Corti cells.
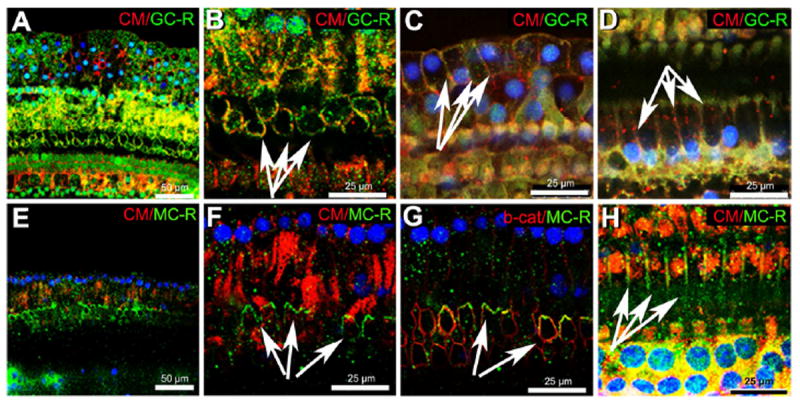
GC-R labeling (A-D) was observed in or at the plasma membrane of OHCs (B, arrows), Hensen cells (C, arrows) and pillar cells (D, arrows). MC-R labeling (E-H) in or near the plasma membrane of OHCs (F and G, arrows) and pillar cells (H, arrows). Both receptors colocalized with the membrane dye CellMask (CM), shown in red in panels A-D, E, F and H. Panel G shows colocalization of MC-R with the plasma membrane marker β-catenin (red).
3.2. Dexamethasone induces nuclear translocation of GC-R and MC-R in Hensen cells
We investigated nuclear translocation of GC-R and MC-R in Hensen cells on organ of Corti explants treated with 10 pM, 100 nM, and 10 μM dexamethasone (Figs. 7 and 8). GC-R were found in the cytoplasm and nucleus of Hensen cells in all conditions tested, including untreated cells. However, nuclear expression of GC-R was significantly stronger in Hensen cells treated with 10 μM dexamethasone (Figs. 7 D, H, L, P), and significantly weaker in those treated with 100 nM dexamethasone (Figs. 7 C, G, K, O), than in untreated Hensen cells (Figs. 7A, E, I, M). We detected no differences between Hensen cells untreated and treated with 10 pM dexamethasone (Figs. 7 B, F, J, N). MC-R expression was also observed in the cytoplasm and the nucleus of Hensen cells in all conditions tested (Fig. 8). Interestingly, a clear increase in nuclear immunoreactivity respect to untreated cells was observed in Hensen cells treated with 10 pM and 100 nM dexamethasone, whereas no differences were observed with those treated with 10 μM dexamethasone (Fig. 8).
Figure 7. Dexamethasone induced nuclear translocation of GC-R in Hensen cells.
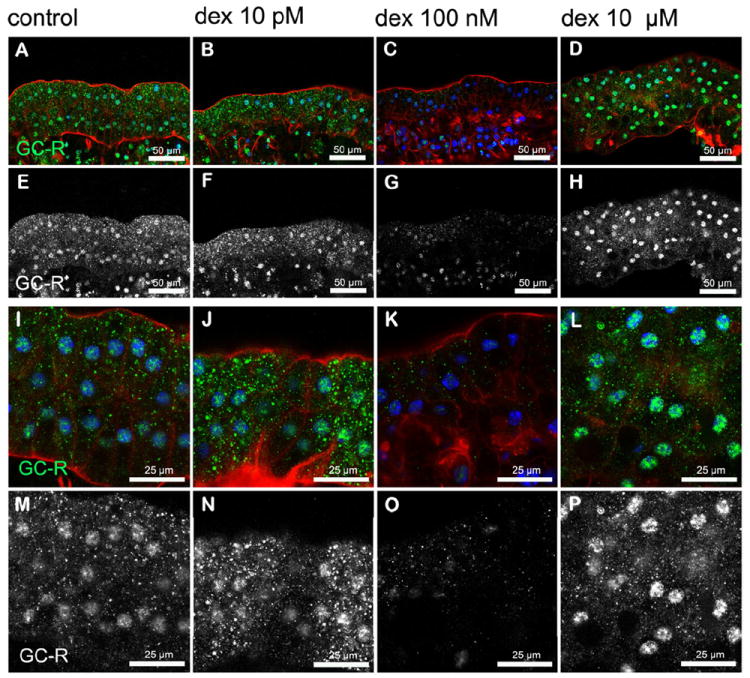
A significant (p≤0.05) increase in GC-R reactivity was observed in nuclei of Hensen cells treated with 10 μM (D, L, H, P) dexamethasone, when compared to untreated explants (A, E, I, M). No differences in nuclear expression of GC-R were observed in Hensen cell treated with 100 nM dexamethasone (C, G, K, O) respect to untreated ones. Although it was a perceptible increase in immunolabeling in Hensen cells treated with 10 pm (B, F, J, N), differences with the control condition were not statistically significant. Images of Hensen cell are shown at low magnification in A-H and higher magnification in I-P. Panels E-H and M-P are identical to panels A-D and I-L, respectively, except that the blue and red channels were turned off and GC R expression is shown in white to facilitate comparisons. Actin is shown in red and nuclei in blue in panels A-D and I-L whereas MC-R is shown in green in panels A-D and I-L or white in panels E-H and M-P.
Figure 8. Dexamethasone induced nuclear translocation of MC-R in Hensen cells.
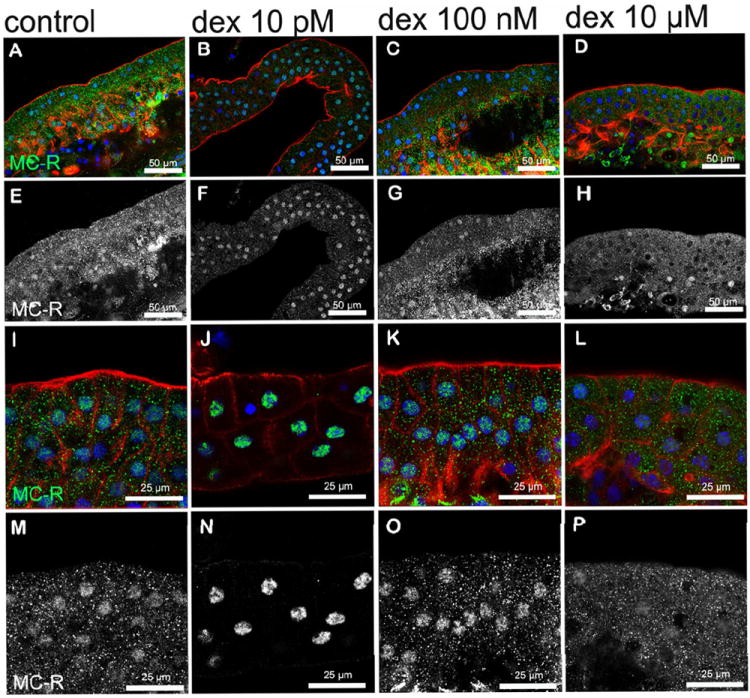
A reduction in cytoplasmic labeling (p≤0.05) and a moderated increase in nuclear labeling (p≤0.05) of MC-R was observed in Hensen cells treated with 10 pM dexamethasone (B, F, J, N) when compared with those untreated (A, E, I, M) or treated with 100 nM (C, G, K, O) or 10 μM (D, H, L, P) dexamethasone. Images of Hensen cells are shown at low magnification from A-H and higher magnification is shown in I-P. Panels E-H and M-P shows only MC- R labeling (in white). Actin is shown in red and nuclei in blue in panels A-D and I-L, whereas MC-R is shown in green in panels A-D and I-L or white in panels E-H and M-P.
Fig. 9 summarizes the measurements of GC-R and MC-R nuclear labeling in Hensen cells either untreated or exposed to different doses of dexamethasone. Note that the dose-response is U-shaped rather than lineal for GC-R, and has an inverted U-shape for MC-R.
Figure 9. MC-R and GC-R relative nuclear labeling in Hensen cells.
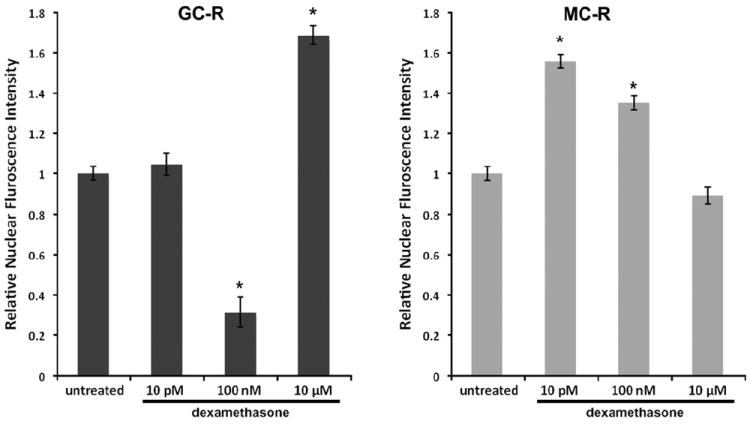
Relative nuclear labeling of GC-R and MC-R in Hensen cells was evaluated in guinea pig’s organ of Corti explants (3 explants/condition, n=12) using Two-Way ANOVA techniques. A – Whereas 10 pM dexamethasone induced no changes in GC-R nuclear labeling in Hensen cells (1.04±0.06, N.S.), 100 nM result in a significant decrease (0.31±0.08), and 10 μM in a significant increase (1.69±0.05), in GC-R translocation respect to untreated cells (1.00±0.04, p≤0.0001). B – Exposure to 10 pM or 100 nM dexamethasone induced a significant increase (1.57±0.04 and 1.35±0.04, respectively) in MC-R nuclear labeling respect to untreated cells (1.00±0.04, p≤0.0001), suggesting increased receptor translocation from the cytoplasm. In contrast, no significant changes were observed with a dose of 10 μM dexamethasone (0.89±0.05, N.S.). Note that the dose-response is inverted U-shaped rather than lineal for MC-R, and has an U-shape for GC-R.
3.3. Dexamethasone also induces nuclear translocation of GC-R and MC-R in HEI-OC1 cells
We used HEI-OC1 cells to investigate whether dexamethasone also induced nuclear translocation of MC-R and GC-R in auditory cells capable of proliferating in vitro (Fig. 10). HEI-OC1, mouse organ of Corti-derived cells (Kalinec et al., 2003) already used as a model in a wide variety of studies, provide the possibility of performing experiments impossible to achieve in vivo or with isolated organ of Corti cells. HEI-OC1 cells treated with 10 μM (Figs. 10 D, H) showed stronger nuclear GC-R expression than untreated cells (Figs. 10 A, E), whereas no differences with the control were evident in cells treated with 10 pM (Figs. 10 B, F) and 100 nM (Figs. 10 C, G) dexamethasone. In contrast, increased nuclear expression of MC-R was observed in cells treated with 100 nM dexamethasone (Figs. 10 K, O) compared to untreated cells (Figs. 10 I, M) and cells treated with other doses of dexamethasone (Figs. 10 J, N and L, P). The measurements of GC-R and MC-R nuclear labeling in HEI-OC1 cells are summarized in Fig. 11. As in the case of Hensen cells, the dose-response is U-shaped for GC-R and inverted U-shaped for MC-R, although the extreme values occurred at different doses of dexamethasone.
Figure 10. Dexamethasone induced nuclear translocation of GC-R and MC-R in HEI-OC1 cells.
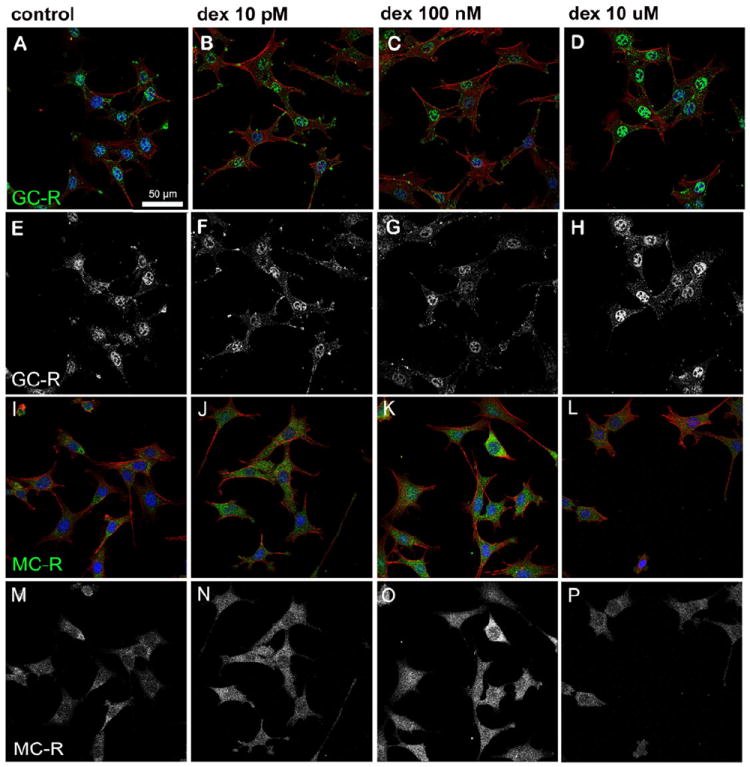
A significant (p≤0.05) increase in nuclear GC-R expression was observed in cells treated with 10 μM dexamethasone (D, H) when compared to untreated cells (A, E) or cells exposed to 10 pM (B, F) or 100 nM (C, G) dexamethasone. A significantly higher (p≤0.0016) nuclear expression of MC-R was observed in cells treated with 100 nM dexamethasone (K, O) when compared to untreated cells (I, M) and cells treated with 10 pM (J, N) or 10 μM (L, P) dexamethasone. Actin is shown in red and nuclei in blue in panels A-D and I-L, whereas GC-R and MC-R are shown in green in panels A-D and I-L, respectively, or white in panels E-H and M-P.
Figure 11. MC-R and GC-R nuclear labeling in HEI-OC1 cells.
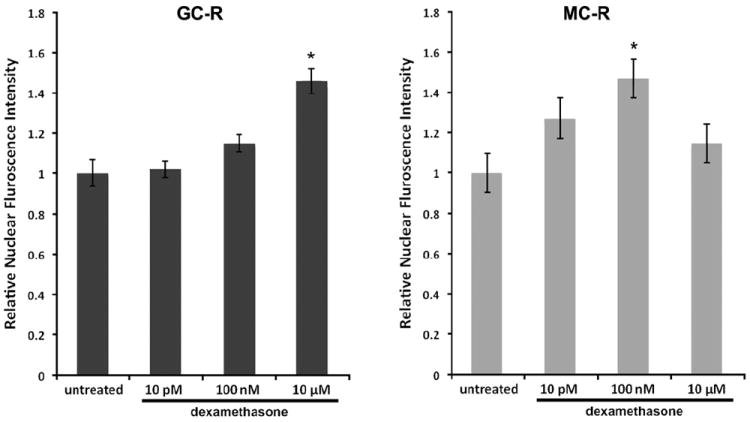
Nuclear labeling of GC-R and MC-R in HEI-OC1 cells was evaluated in triplicate using Two-Way ANOVA techniques. A – Whereas 10 pM and 100 nM dexamethasone induced no changes in GC-R nuclear labeling in HEI-OC1 cells (1.02±0.05 and 1.15±0.06, respectively), 10 μM result in a significant increase (1.46±0.06) in GC-R translocation respect to untreated cells (1.00±0.06, p≤0.0001). B – Exposure to 100 nM dexamethasone induced a significant increase (1.48±0.12) in MC-R nuclear labeling respect to untreated cells (1.00±0.11, p≤0.001), suggesting increased receptor translocation from the cytoplasm. In contrast, no significant changes were observed with doses of 10 pM (1.27±0.10, N.S.) and 10 μM dexamethasone (1.15±0.11, N.S.). Note that, just as in Hensen cells (Fig. 9), the dose-response is inverted U-shaped rather than lineal for MC-R, and has an U-shape for GC-R.
4. Discussion
We have investigated the localization of MC-R and GC-R in the guinea pig cochlea as a necessary step to elucidate the cells and processes potentially responsible for the oto-protective properties of GC. Our results indicate that sensory IHCs and OHCs as well as pillar, Hensen and Deiters cells in the organ of Corti, inner and outer sulcus cells, spiral ganglion neurons and several types of fibroblastic cells in the spiral ligament express significant amounts of both types of receptors; in contrast, MC-R were relatively abundant in stria vascularis and near absent in fibrocytes type IV in the spiral ligament, with the opposite being true for GC-R (Table 1 and Fig. 12). In addition, we report that these receptors show a high degree of co-localization, and dexamethasone can induce translocation to the nucleus of both receptors in Hensen and HEI-OC1 cells. Interestingly, GC-R and MC-R were found not only in the cytoplasm and nucleus but also at the plasma membrane of several of these cell populations, with the relative levels of receptor expression in these cellular compartments varying with the dose of dexamethasone assayed. Altogether, our results suggest that oto-protective effects of GC could be associated with the concerted activation of genomic and non-genomic, GC-R and MC-R mediated signaling pathways in different regions of the cochlea.
Table 1.
| A: Expression of GC-R in cochlear cells | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | OHC | IHC | DC | IP | OP | HC | SV | SL | SLi | ISC | OSC | SGN | SP | References |
| Guinea pig | + | + | + | + | + | + | - | + | + | + | + | + | + | This work Terunuma et al., 2003 |
| Mouse | - | - | + | + | + | + | + | Erichsen et al., 1996, 2001 Shimazaki et al., 2002 | ||||||
| Rat | + | + | + | + | + | + | ten Cate et al., 1993 Zuo et al., 1995 | |||||||
| Human | + | + | Rarey et al., 1996 | |||||||||||
|
| ||||||||||||||
| B: Expression of MC-R in cochlear cells | ||||||||||||||
| Species | OHC | IHC | DC | IP | OP | HC | SV | SL | SLi | ISC | OSC | SGN | SP | References |
|
| ||||||||||||||
| Guinea pig | + | + | + | + | + | + | + | + | + | + | + | + | + | This work Sinha et al., 1995 |
| Mouse | + | + | + | + | + | + | Erichsen et al., 2001 | |||||||
| Rat | + | + | + | + | + | + | Furuta et al., 1994 Yao et al., 1996 | |||||||
| Human | ||||||||||||||
OHC=outer hair cells, IHC=inner hair cells, DC=Deiters cells, IP=inner pillar cells, OP=outer pillar cells, HC=Hensen cells, SV=stria vascularis, SL=spiral ligament, SLi= spiral limbus, ISC=inner sulcus cells, OSC=outer sulcus cells, SGN=spiral ganglion neurons, SP=spiral prominence.
Grey shading denotes positive staining but specific cell types were not indentified in the references.
Black shading denotes no data.
Figure 12. Diagram of the guinea pig cochlear partition depicting the distribution of MC-R and GC-R suggested by the results reported in this study.
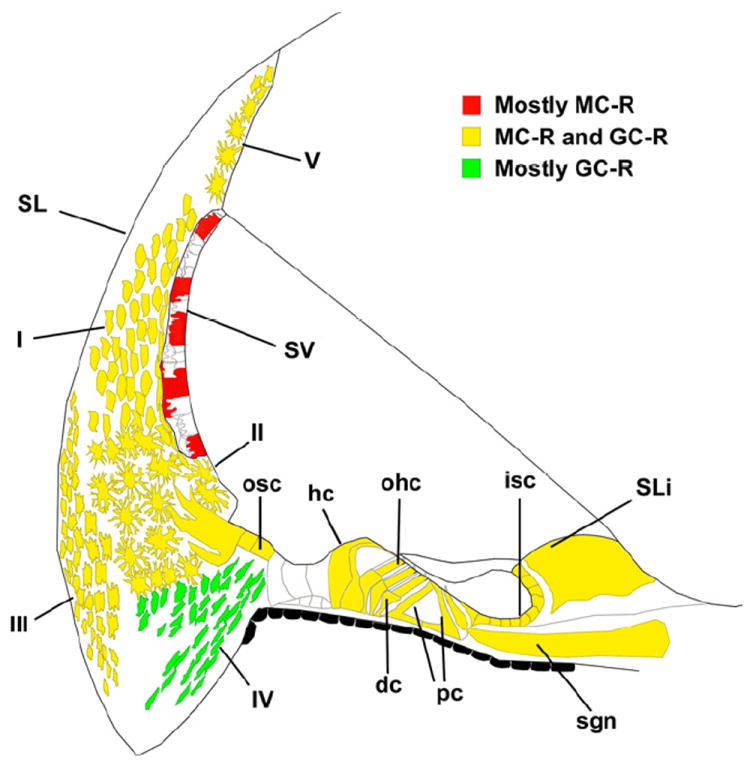
Red color indicate mostly MC-R protein expression, yellow color indicates both MC-R and GC-R expression and green indicate GC-R expression. Modified from (Imamura et al., 2003)
4.1. Expression of GC-R and MC-R in the guinea pig cochlea
4.1.1. Spiral limbus, organ of Corti and outer sulcus cells
Previous reports from different laboratories (Table 1) indicated that OHCs and IHCs from rat (Zuo et al., 1995), but not mouse (Shimazaki et al., 2002), expressed GC-R. These studies also suggested that GC-R were present in supporting cells in the organ of Corti of both mice and rats, but specific supporting cell types as well as cellular compartments were not identified in these studies. In addition, MC-R mRNA expression was reported in the marginal cells of stria vascularis (Furuta et al., 1994) and protein expression in IHCs, OHCs, spiral limbus and spiral ganglion cells of rats (Yao et al., 1996).
We immunolocalized GC-R and MC-R in the cytoplasm and the nucleus of several cell populations of the organ of Corti and neighboring regions of the guinea pig cochlea, including epithelial cells as well as fibrocytes in the spiral limbus, inner sulcus cells, OHCs, IHCs, outer and inner pillar cells, Deiters, Hensen and outer sulcus cells. Intriguingly, no labeling was evident in Boettcher and Claudius cells (Figs. 1A and 3A). Interdental cells in the spiral limbus are connected between them and with inner sulcus cells by gap junctions, forming part of the so called “epithelial cell gap junction system” also composed by all organ of Corti supporting cells and outer sulcus cells (Kikuchi et al., 2000). Spiral limbus fibrocytes, on the other hand, are also interconnected by gap junctions, and form part of a second group, the “connective tissue cell gap junction system”, together with strial intermediate cells, strial basal cells, fibrocytes in the spiral ligament, mesenchymal cells lining the bony otic capsule facing the scala vestibuli, and mesenchymal dark cells in the supralimbal zone (Kikuchi et al., 2000). Importantly, these two gap junction systems have contiguity at the level of outer sulcus cells, which express both GC-R and MC-R. Since it is widely accepted that these two systems work in concert to keep cochlear ion homeostasis, these results support the idea that GC may have a role in the regulation of this process. We also speculate that the regional differences observed in the staining of outer sulcus cells, stria vascularis cells and spiral ligament fibrocytes could be associated with different requirements for ion homeostasis at the low and high frequency regions of the cochlea.
4.1.2. Stria vascularis
GC-R were previously found expressed in mouse stria vascularis (Erichsen et al., 1996), while in the rat one study reported strong expression (ten Cate et al., 1993) and a different one suggested a very low level of expression (Zuo et al., 1995). In our study, we did not detect significant GC-R expression in guinea pig stria vascularis except for a weak labeling in a single sample, but differences between our results and those in the literature may reflect species differences. On the other hand, one research group detected MC-R mRNA only in marginal cells of rat stria vascularis, whereas other laboratories reported expression in all strial cell types (Yao et al., 1996). Using binding assay with radioactively labeled aldosterone, Sinha & Pitovski (Sinha et al., 1995) found MC-R expression in guinea pig strial marginal cells. Our results with anti-MC-R antibodies, in contrast, suggest MC-R expression in all three strial cell types but in a “patchy” pattern, with labeled cells surrounded by others showing no reactivity (Fig. 1 B). Although marginal cells contain ion transporters that ultimately form the endolymph potential, intermediate cells —which form a virtual syncytium with basal cells— are crucial for generating the high K+ intrastrial fluid that is thought to be drive K+ transport from the stria vascularis into the endolymph (Nin et al., 2012; Patuzzi, 2011). An abnormal stria vascularis (Trune et al., 1997), as well as a depressed endocochlear resting potential (Ruckenstein et al., 1999a; Ruckenstein et al., 1999b) and an elevated auditory threshold (Ruckenstein et al., 1993; Rukckenstein et al. 1999a), were reported in the MRL/MpJ-Faslpr mouse, a model for autoimmune disease. Importantly, hearing loss in these autoimmune mice is reversed with GC and aldosterone (Trune, 1999a; Trune, 1999b) and mediated by MC-R (Trune et al., 2006). Therefore, expression of MC-R either in marginal or intermediate cells of the guinea pig stria vascularis strongly supports a role for GC in the regulation of cochlear ion homeostasis.
4.1.3. Spiral ligament
Otic fibrocytes are a heterogeneous population of cells with unique functions based on their location and physiological properties (Spicer et al., 1991). There are five types of fibrocytes in the spiral ligament of the cochlea. Fibrocytes subtypes I, II and V are highly interconnected via gap junctions, and form a virtual syncytium with outer sulcus cells and basal and intermediate cells of the stria vascularis (Patuzzi, 2011). This syncytium is believed to play a central role in endolymph homeostasis, with recycling of K+ ions through this network and stria vascularis being a critical process for cochlear physiology. Interestingly, we found that receptors’ staining in outer sulcus cells, stria vascularis cells and spiral ligament fibrocytes types I, II and V, increased from the base to the apex of the guinea pig cochlea. Type III fibrocytes, on the other hand, border the otic capsule opposite to the basilar crest, and type IV fibrocytes are present in the basal part of the spiral ligament, inferior to the basilar crest. Spiral ligament fibrocytes have been shown to release proinflammatory cytokines in culture (Yoshida et al., 1999), in noised-stimulated cochleae (Fujioka et al., 2006) and in response to otitis media pathogens (Moon et al., 2006). Furthermore, they are the initial target of ototoxic drugs such as cisplatin (García-Berrocal et al., 2007). Other causes of cochleo-vestibular dysfunction such as DFNA9, some variants of Ménière’s disease and presbyacusis, may be due to the aggression suffered by the fibrocytes of the inner ear (García-Berrocal et al., 2008). In our study, we found expression of GC-R in all five fibrocyte subtypes, but MC-R were detected only in fibrocytes type I and V. Based on this expression pattern, spiral ligament fibrocytes are clearly a target cell population of GC in the inner ear, and it is possible that MC-R activated by glucocorticoids and aldosterone may also play an important role in endolymph homeostasis (Trune et al., 2006).
4.2 GC-R and MC-R were localized at the plasma membrane of organ of Corti cells
Whereas cytoplasmic and nuclear localization of GC-R and MC-R is indisputable, their membrane localization is still under discussion although multiple observations collectively support this possibility (Bartholome et al., 2004; Johnson et al., 2005; Liposits et al., 1993; Pedram et al., 2007; Prager et al., 2010). For instance, when applied intracellularly, corticosterone cannot induce rapid nongenomic effects making it unlikely that the receptors are located inside the cells. Moreover, the receptors would be accessible only from outside of the plasma membrane. This assertion is further supported by experiments with membrane impermeable corticosterone-BSA and dexamethasone-BSA conjugates, which induce the same rapid effects as free corticosterone or dexamethasone (Chen et al., 1991; Hua et al., 1989; Lou et al., 1998). Most convincingly, electron microscopy studies showed association of MC-R and GC-R with the plasma membrane in neurons, synaptosome extracts and presynaptic membranes (Komatsuzaki et al., 2005; Qiu et al., 2010; Wang et al., 2009).
Membrane localization may be critical in mediating non-genomic effects in different organs and tissues, including the inner ear. Since our results indicate that GC-R and MC-R localize at the plasma membrane in OHCs, Hensen and pillar cells of the guinea pig cochlea, perhaps the membrane fraction could be responsible for the non-genomic effects recently reported by our laboratory in guinea pig Hensen cells in vitro (Kalinec et al., 2009). Further experiments will determine whether GC binding to GC-R, MC-R or both mediate these non-genomic effects in Hensen cells.
4.3 Dexamethasone induces nuclear translocation of GC-R and MC-R
Many studies have suggested that stress conditions result in progressive activation of MC-R at a low cortisone concentration followed by activation of GC-R when cortisone levels increase, and that this effect can modulate neuronal integrity in response to stress (Magariños et al., 1996) and neuronal excitability associated with changes in neuroendocrine regulation and behavior (Joëls et al., 1992). As already mentioned, hearing loss associated with MRL/MpJ-Faslpr autoimmune responses can be reduced through GC and aldosterone treatment. Moreover, preservation of hearing loss associated with GC treatment is eliminated with addition of MR-C antagonist spironolactone, whereas GC-R mediated anti-inflammatory and immunosupressive effect were not diminished (Trune et al., 2006). These results strongly suggest that activation of MC-R- and GC-R-mediated signaling pathways might be necessary for GC-mediated otoprotection. Our results showing nuclear translocation of both GC-R and MC-R by dexamethasone in Hensen cells of the guinea pig cochlea and the auditory cell line HEI-OC1 are consistent with this hypothesis.
Interestingly, GC-R and MC-R nuclear translocation induced by dexamethasone showed a U-shaped (or inverted U-shaped) dose-response both in Hensen and HEI-OC1-cells. These results represent a quite typical case of hormesis (Calabrese et al., 2003). In fact, one of the earliest studies in which hormesis was observed was associated to GC effect on learning performance in rodents. Experiments conducted by Yerkes and Dodson more than one hundred years ago showed that this response was optimized by modest amounts of stress but diminished with either too little or excessive stress (Lupien et al., 1997; Lupien et al., 2005). Hormesis is not unexpected for ligands that function through more than one receptor subtypes with specific, and sometimes antagonistic, characteristics. For example, in the hippocampus and other brain regions activation of MC-R led to an increase in long-term potentiation (LTP) while the activation of GC-R led to a decrease in LTP, a frequently mentioned example of antagonistic effects of MC-R and GC-R activation (Joels, 2008). Currently the effect of GC in the hippocampus is one of the best-documented examples of a U-shaped dose–response relationship, and complex dose-responses are generally associated with the existence of different MC-R and GC-R variants that could be differentially distributed among distinct cell populations (Diamond et al., 1992; Joels, 2006; Joels, 2008; Pascual-Le Tallec et al., 2005; Zhou et al., 2005). Thus, the different U-shaped dose–response curves observed in Hensen and HEI-OC1 cells could be indicating the presence of different subtypes of MC-R and GC-R in these populations.
4.4. GC and inflammatory responses
Leukocyte migration to sites of injury or infection is a defining step of inflammatory responses. However, migrating leukocytes produce and release enzymes and radicals that can damage normal tissue (Perretti et al., 2003). In the mammalian cochlea leukocyte migration into the auditory organ must be prevented because it may abolish the endocochlear potential by disrupting the tight-junction barrier at the organ of Corti luminal border, leading to apoptosis of OHCs. Accordingly, leukocytes are never found in the organ of Corti except in cases of extreme, irreversible cochlear damage (Hirose et al., 2003; Tornabene et al., 2006). Thus, in the scala media, inflammatory responses must be reduced to the resolution phase, and results from our laboratory suggested a role for the anti-inflammatory mediator Annexin A1 (ANXA1) released by Hensen cells in this process (Kalinec et al., 2009). ANXA1 is known to inhibit leukocyte migration, and help to the resolution of the inflammatory response by promoting phagocytosis of dying cells. In addition, ANXA1 is a downstream effector of GC and it is believed to be the ultimate responsible for the anti-inflammatory effects of GCs in the host defense system (Flower, 1988). ANXA 1 and its receptor ALX (Walther et al., 2000) are expressed by Hensen cells in the organ of Corti, fibrocytes II and III, IV and V of spiral ligament, and marginal cells of the stria vascularis (Kalinec et al., 2009). Thus, in addition to regulation of ion homeostasis, GC could have multiple functions in the inner ear including release of ANXA1, inhibition of leukocyte migration and even promotion of apoptotic cells’ clearance by phagocytosis.
5. Conclusions
The current clinical strategy to protect the auditory organ against damage associated with a variety of conditions, including noise exposure, ototoxic drugs hearing loss, tinnitus, Meniére’s disease, and autoimmune diseases as well as bacterial and viral infections, is the local delivery of GC. However, the mechanisms activated by GC in the cochlea are still unknown. Our results suggest the effects of GC would be tissue-and concentration specific and under tight local control by presence and availability of GC-R and MC-R. Through GC-R- and MC-R-mediated pathways GC would influence at least two different mechanisms in the guinea pig cochlea, ion homeostasis and the resolution of inflammatory responses. By pinpointing target effectors and molecular responses of steroid and their receptors it might be possible in the near future to select specific therapies to prevent or ameliorate hearing loss associated with a variety of clinical conditions.
Highlights.
-
>
GC-R and MC-R are expressed in epithelial and connective cells of the guinea pig cochlea
-
>
GC-R and MC-R were found in different compartments of guinea pig cochlear cells
-
>
GC effects could be associated with activation of GC-R and MC-R mediated pathways
Acknowledgments
This work was supported by National Institutes of Health grant DC010397 and House Research Institute. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the House Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237:268–75. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- Bartholome B, Spies CM, Gaber T, Schuchmann S, Berki T, Kunkel D, Bienert M, Radbruch A, Burmester GR, Lauster R, Scheffold A, Buttgereit F. Membrane glucocorticoid receptors (mGCR) are expressed in normal human peripheral blood mononuclear cells and up-regulated after in vitro stimulation and in patients with rheumatoid arthritis. FASEB J. 2004;18:70–80. doi: 10.1096/fj.03-0328com. [DOI] [PubMed] [Google Scholar]
- Beavan S, Horner A, Bord S, Ireland D, Compston J. Colocalization of glucocorticoid and mineralocorticoid receptors in human bone. J Bone Miner Res. 2001;16:1496–504. doi: 10.1359/jbmr.2001.16.8.1496. [DOI] [PubMed] [Google Scholar]
- Boldyreff B, Wehling M. Rapid aldosterone actions: from the membrane to signaling cascades to gene transcription and physiological effects. J Steroid Biochem Mol Biol. 2003;85:375–81. doi: 10.1016/s0960-0760(03)00202-4. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: the dose-response revolution. Annu Rev Pharmacol Toxicol. 2003;43:175–97. doi: 10.1146/annurev.pharmtox.43.100901.140223. [DOI] [PubMed] [Google Scholar]
- Cato AC, Nestl A, Mink S. Rapid actions of steroid receptors in cellular signaling pathways. Sci STKE. 2002;2002:re9. doi: 10.1126/stke.2002.138.re9. [DOI] [PubMed] [Google Scholar]
- Chen YZ, Hua SY, Wang CA, Wu LG, Gu Q, Xing BR. An electrophysiological study on the membranne receptor mediated action of glucocorticoids in mammalian neurons. Neuroendocrinology. 1991;53:25–30. doi: 10.1159/000125791. [DOI] [PubMed] [Google Scholar]
- Claire M, Faraj H, Grassy G, Aumelas A, Rondot A, Auzou G. Synthesis of new 11 beta-substituted spirolactone derivatives. Relationship with affinity for mineralocorticoid and glucocorticoid receptors. J Med Chem. 1993;36:2404–7. doi: 10.1021/jm00068a018. [DOI] [PubMed] [Google Scholar]
- Coleman B, Hardie NA, de Silva MG, Shepherd RK. A Protocol for cryoembedding the adult guinea pig cochlea fro fluorescence immunhistology. J Neurosci Methods. 2009;176:144–151. doi: 10.1016/j.jneumeth.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–30. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Erichsen S, Mikkola M, Sahlin L, Hultcrantz M. Cochlear distribution of Na, K-ATPase and corticosteroid receptors in two mouse strains with congenital hearing disorders. Acta Otolaryngol. 2001;121:794–802. doi: 10.1080/00016480152602221. [DOI] [PubMed] [Google Scholar]
- Erichsen S, Bagger-Sjöbäck D, Curtis L, Zuo J, Rarey KE, Hultcrantz M. Appearance of glucocorticoid receptors in the inner ear of the mouse during development. Acta Otolaryngol. 1996;116:721–5. doi: 10.3109/00016489609137913. [DOI] [PubMed] [Google Scholar]
- Flower RJ. Lipocortin and the mechanism of action of the glucocorticoids. British Journal Pharmacology. 1988;94:987–1015. doi: 10.1111/j.1476-5381.1988.tb11614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka M, Kanzake S, Okano HJ, Masuda M, Ogawa K, Okano H. Proinflammatory cytokines expression in noise-induced damaged cochlea. J Neurosci Res. 2006;83:575–83. doi: 10.1002/jnr.20764. [DOI] [PubMed] [Google Scholar]
- Furuta H, Mori N, Sato C, Hoshikawa H, Sakai S, Iwakura S, Doi K. Mineralocorticoid type I receptor in the rat cochlea: mRNA identification by polymerase chain reaction (PCR) and in situ hybridization. Hearing Research. 1994;78:175–180. doi: 10.1016/0378-5955(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Gametchu B, Watson CS, Wu S. Use of receptor antiboides to demonstrate membrane glucocorticoid receptor in cells from human leukemic patients. FASEB J. 1993;7:1283–1292. doi: 10.1096/fasebj.7.13.8405814. [DOI] [PubMed] [Google Scholar]
- García-Berrocal JR, Méndez-Benegassi I, Martí C, Ramírez Camacho R. Intervention of spiral ligament fibrocytes in the metabolic regulation of the inner ear. Acta Otolaryngol. 2008;59:494–9. [PubMed] [Google Scholar]
- García-Berrocal JR, Nevado J, Ramírez-Camacho R, Sanz R, González-García JA, Sánchez-Rodríguez C, Cantos B, España P, Verdaguer JM, Trinidad Cabezas A. The anticancer drug cisplatin induces an intrinsic apoptotic pathway inside the inner ear. Br J Pharmacol. 2007;152:1012–20. doi: 10.1038/sj.bjp.0707405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Z CY, Su RB, Fu H. Binding characteristics of glucocorticoid receptor in synaptic plasma membrane from rat brain. Funct Neurol. 1995;10:183–194. [PubMed] [Google Scholar]
- Haller J, Mikics É, Makara GB. The effects of non-genomic glucocorticoid mechanisms on bodily functions and the central neural system. A critical evaluation of findings. Frontiers in Neuroendocrinology. 2008;29:273–291. doi: 10.1016/j.yfrne.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Hirose K, Liberman MC. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J Assoc Res Otolaryngol. 2003;4:339–52. doi: 10.1007/s10162-002-3036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua SY, Chen YZ. Membrane receptor-mediated electrophysiological effects of glucocorticoid on mammalian neurons. Endocrinology. 1989;124:687–691. doi: 10.1210/endo-124-2-687. [DOI] [PubMed] [Google Scholar]
- Imamura S, Adams JC. Distribution of gentamicin in the guinea pig inner ear after local or systemic application. J Assoc Res Otolaryngol. 2003;4:176–95. doi: 10.1007/s10162-002-2036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci. 2006;27:244–50. doi: 10.1016/j.tips.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Joels M. Functional actions of corticosteroids in the hippocampus. Eur J Pharmacol. 2008;583:312–21. doi: 10.1016/j.ejphar.2007.11.064. [DOI] [PubMed] [Google Scholar]
- Joëls M, De Kloet ER. Coordinative mineralocorticoid and glucocorticoid receptor mediated control of responses to serotonin in rat hippocampus. Neuroendocrinology. 1992;55:344–50. doi: 10.1159/000126135. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Farb C, Morrison JH, McEwen BS, LeDoux JE. Localization of glucocorticoid receptors at postsynaptic membranes in the lateral amygdala. Neuroscience. 2005;136:289–99. doi: 10.1016/j.neuroscience.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Kalinec F, Webster P, Lim DJ, Kalinec F. A cochlear cell line as an in vitro system for drug ototoxicity screening. Audiol Neurootol. 2003;8:177–189. doi: 10.1159/000071059. [DOI] [PubMed] [Google Scholar]
- Kalinec GM, Webster P, Maricle A, Guerrero D, Chakravarti DN, Chakravarti B, Gellibolian R, Kalinec G. Glucocorticoid-stimulated, transcription-independent release of annexin A1 by cochlear Hensen cells. Br J Pharmacol. 2009;158:1820–34. doi: 10.1111/j.1476-5381.2009.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci U S A. 2005;102:19204–7. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata M. Roles of steroid hormones and their receptors in structural organization in the nervous system. Neurosci Res. 1995;24:1–46. doi: 10.1016/0168-0102(96)81278-8. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Kimura RS, Paul DL, Takasaka T, Adams JC. Gap junction systems in the mammalian cochlea. Brain Res Brain Res Rev. 2000;32:163–6. doi: 10.1016/s0165-0173(99)00076-4. [DOI] [PubMed] [Google Scholar]
- Komatsuzaki Y, Murakami G, Tsurugizawa T, Mukai H, Tanabe N, Mitsuhashi K, Kawata M, Kimoto T, Ooishi Y, Kawato S. Rapid spinogenesis of pyramidal neurons induced by activation of glucocorticoid receptors in adult male rat hippocampus. Biochem Biophys Res Commun. 2005;335:1002–7. doi: 10.1016/j.bbrc.2005.07.173. [DOI] [PubMed] [Google Scholar]
- Krozowski ZS, Funder JW. Renal mineralocorticoid receptors and hippocampal corticosterone-binding species have identical intrinsic steroid specificity. Proc Natl Acad Sci U S A. 1983;80:6056–60. doi: 10.1073/pnas.80.19.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liposits Z, Bohn MC. Association of glucocorticoid receptor immunoreactivity with cell membrane and transport vesicles in hippocampla and hypothalamic neurons of the rat. J Neurosci Res. 1993;35:14–19. doi: 10.1002/jnr.490350103. [DOI] [PubMed] [Google Scholar]
- Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4:46–56. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- Lou SJ, Chen YZ. The rapid inhibitory effect of glucocorticoid on cytosolic free Ca2+ increment induced by high extracellular K+ and its underlying mechanism in PC12 cells. Biochem Biophys Res Commun. 1998;244:403–7. doi: 10.1006/bbrc.1998.8280. [DOI] [PubMed] [Google Scholar]
- Lowenberg M, Stahn C, Hommes DW, Buttgereit F. Novel insights into mechanisms of glucocorticoid action and the development of new glucocorticoid receptor ligands. Steroids. 2008;73:1025–9. doi: 10.1016/j.steroids.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Brain Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Buss C, Schramek TE, Maheu F, Pruessner J. Hormetic influence of glucocorticoids on human memory. Nonlinearity Biol Toxicol Med. 2005;3:23–56. doi: 10.2201/nonlin.003.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS, Flügge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neuroscience. 1996;16:3534–40. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltser I, Canlon B. Protecting the auditory system with glucocorticoids. Hearing Research. 2011;281:47–55. doi: 10.1016/j.heares.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Moon SK, Park R, Lee HY, Nam GJ, Cha K, Andalibi A, Lim DJ. Spiral ligament fibrocytes may be involved in the innate immune response of the inner ear by producing chemoattaractants for recruiting inflammaotry cells such as neutrophils and monocytes. Acta Otolaryngol. 2006;126:564–9. [Google Scholar]
- Myles K, Funder JW. Type I (mineralocorticoid) receptors in the guinea pig. Am J Physiol Endocrinol Metab. 1994;267:E268–72. doi: 10.1152/ajpendo.1994.267.2.E268. [DOI] [PubMed] [Google Scholar]
- Nin F, Hibino H, Doi K, Suzuki T, Hisa Y, Kurachi Y. Computational model of a circulation current that controls electrochemical properties in the mammalian cochlea. Proc Natl Acad Sci U S A. 2012;109:9191–6. doi: 10.1073/pnas.1120067109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Le Tallec L, Lombes M. The mineralocorticoid receptor: a journey exploring its diversity and specificity of action. Mol Endocrinol. 2005;19:2211–21. doi: 10.1210/me.2005-0089. [DOI] [PubMed] [Google Scholar]
- Patuzzi RB. Ion flow in stria vascularis and the production and regulatiion of cochlear endolymph and the endolymphtic potential. Hear Res. 2011;125:1–16. doi: 10.1016/j.heares.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Pearce D, Yamamota KR. Mineralocorticoid and glucocorticoid receptor activities distinguished by nonreceptor factors at a composite response element. Science. 1993;259:1161–5. doi: 10.1126/science.8382376. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282:22278–88. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- Perretti M, Gavins FN. Annexin 1: an endogenous anti-inflammatory protein. News Physiol Sci. 2003;18:60–4. doi: 10.1152/nips.01424.2002. [DOI] [PubMed] [Google Scholar]
- Pitovski DZ, Drescher MJ, Drescher DG. High affinity aldosterone binding sites (type I receptors) in the mammalian inner ear. Hear Res. 1993;69:10–4. doi: 10.1016/0378-5955(93)90088-i. [DOI] [PubMed] [Google Scholar]
- Pitovski DZ, Drescher MJ, Drescher DG. Glucocorticoid receptors in the mammalian inner ear: RU 28362 binding sites. Hear Res. 1994;77:216–20. doi: 10.1016/0378-5955(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Pondugula SR, Sanneman JD, Wangemann P, Milhaud PG, Marcus DC. Glucocorticoids stimulate cation absorption by semicircular canal duct epithelium via epithelial sodium channel. Am J Physiol Renal Physiol. 2004;286:F1127–35. doi: 10.1152/ajprenal.00387.2003. [DOI] [PubMed] [Google Scholar]
- Prager EM, Brielmaier J, Bergstrom HC, McGuire J, Johnson LR. Localization of mineralocorticoid receptors at mammalian synapses. PLoS ONE. 2010;5:e14344. doi: 10.1371/journal.pone.0014344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Champagne DL, Peters M, Catania EH, Weeber EJ, Levitt P, Pimenta AF. Loss of limbic system-associated membrane protein leads to reduced hippocampal mineralocorticoid receptor expression, impaired synaptic plasticity, and spatial memory deficit. Biol Psychiatry. 2010;68:197–204. doi: 10.1016/j.biopsych.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rarey KE, Luttge WG. Presence of type I and type II/IB receptors for adrenocorticosteroid hormones in the inner ear. Hearing Research. 1989;41:217–21. doi: 10.1016/0378-5955(89)90013-0. [DOI] [PubMed] [Google Scholar]
- Rarey KE, Curtis LM. Receptors for glucocorticoids in the human inner ear. Otolaryngol Head Neck Surg. 1996;115:38–41. doi: 10.1016/S0194-5998(96)70133-X. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Tronche F, Berger S, Kellendonk C, Schutz G. New insights into glucocorticoid and mineralocorticoid signaling: lessons from gene targeting. Adv Pharmacol. 2000;47:1–21. doi: 10.1016/s1054-3589(08)60108-8. [DOI] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–11. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Ruckenstein MJ, Mount RJ, Harrison RV. The MRL-lpr/lpr mouse: a potential model of autoimmune inner ear disease. Acta Otolaryngol Head Neck Surg. 1993;113:160–165. doi: 10.3109/00016489309135785. [DOI] [PubMed] [Google Scholar]
- Ruckenstein MJ, Milburn M, Hu L. Strial dysfunction in the MRL-Faslpr autoimmune mouse. Otolaryngol Head Neck Surg. 1999a;121:452–456. doi: 10.1016/S0194-5998(99)70236-6. [DOI] [PubMed] [Google Scholar]
- Ruckenstein MJ, Sarwar A, Hu L, Shami H, Marion TN. Effects of immunosuppression on the development of cochlear disease in the MRL-Fas(lpr) mouse. Laryngoscope. 1999b;109:626–30. doi: 10.1097/00005537-199904000-00020. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Reul JM, van Steensel B, Spengler D, Söder M, Berning B, Holsboer F, Damm K. Pharmacological and functional characterization of human mineralocorticoid and glucocorticoid receptor ligands. Eur J Pharmacol. 1993;247:145–54. doi: 10.1016/0922-4106(93)90072-h. [DOI] [PubMed] [Google Scholar]
- Shimazaki T, Ichimiya I, Suzuki M, Mogi G. Localization of glucocortiocid receptors in the murine inner ear. Ann Otol Rhinol Laryngol. 2002;111:1133–8. doi: 10.1177/000348940211101213. [DOI] [PubMed] [Google Scholar]
- Sinha PK, Pitovski DZ. [3H]-aldosterone binding sites (type I receptors) in the lateral wall of the cochlea: distribution assessment by quantitative autoradiography. Acta Otolaryngol. 1995;115:643–47. doi: 10.3109/00016489509139380. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Schulte BA. Differentiation of inner ear fibrocytes according to their ion transport related activity. Hearing Research. 1991;56:53–64. doi: 10.1016/0378-5955(91)90153-z. [DOI] [PubMed] [Google Scholar]
- Tahera Y, Meltser I, Johansson P, Salman H, Canlon B. Sound conditioning protects hearing by activating the hypothalamic–pituitary–adrenal axis. Neurobiology of Disease. 2007;25:189–197. doi: 10.1016/j.nbd.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Tahera Y, Meltser I, Johansson P, Bian Z, Stierna P, Hansson AC, Canlon B. NF-kappaB mediated glucocorticoid response in the inner ear after acoustic trauma. J Neurosci Res. 2006a;83:1066–1076. doi: 10.1002/jnr.20795. [DOI] [PubMed] [Google Scholar]
- Tahera Y, Meltser I, Johansson P, Canlon B. Restraint stress modulates glucocorticoid receptors and nuclear factor kappa B in the cochlea. Neuroreport. 2006b;17:879–882. doi: 10.1097/01.wnr.0000220131.24468.e7. [DOI] [PubMed] [Google Scholar]
- ten Cate WJ, Curtis LM, Rarey KE. Immunochemical detection of glucocorticoid receptors within rat cochlear and vestibular tissues. Hear Res. 1992;60:199–204. doi: 10.1016/0378-5955(92)90021-e. [DOI] [PubMed] [Google Scholar]
- ten Cate WJ, Curtis LM, Small GM, Rarey KE. Localization of glucocorticoid receptors and glucocorticoid receptor mRNAs in the rat cochlea. Laryngoscope. 1993;103:865–71. doi: 10.1288/00005537-199308000-00007. [DOI] [PubMed] [Google Scholar]
- Terunuma T, Kawauchi S, Kajihara M, Takahashi S, Hara A. Effect of acoustic stress on glucocorticoid receptor mRNA in the cochlea of the guinea pig. Mol Brain Res. 2003;120:65–72. doi: 10.1016/j.molbrainres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Tornabene SV, Sato K, Pham L, Billings P, Keithley EM. Immune cell recruitment following acoustic trauma. Hear Res. 2006;222:115–24. doi: 10.1016/j.heares.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Trune DR, Kempton JB, Gross ND. Mineralocorticoid receptor mediates glucocorticoid treatment effects in the autoimmune mouse ear. Hear Res. 2006;212:22–32. doi: 10.1016/j.heares.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Trune DR, Kempton JB, Hefeneider SH, Bennett RM. Inner ear DNA receptors in MRL/lpr autoimmune mice: potential 30 and 70 kilodalton link between autoimmune disease and hearing loss. Hear Res. 1997;105:57–64. doi: 10.1016/s0378-5955(96)00191-8. [DOI] [PubMed] [Google Scholar]
- Trune DR, Kempton JB, Hefeneider SH, Bennett RM. Aldosterone and prednisolone control of cochlear function in MRL/MpJ-Faslpr autoimmune mice. Hear Res. 2001;155:9–20. doi: 10.1016/s0378-5955(01)00240-4. [DOI] [PubMed] [Google Scholar]
- Trune DR, Wobig RJ, Kempton JB, Hefeneider SH. Steroid treatment in young MRL.MpJ-Faslpr autoimmune mice prevents cochlear dysfunction. Hear Res. 1999a;137:167–173. doi: 10.1016/s0378-5955(99)00148-3. [DOI] [PubMed] [Google Scholar]
- Trune DR, Wobig RJ, Kempton JB, Hefeneider SH. Steroid therapy improves cochlear function in the MRL.MpJ-Faslpr autoimmune mouse. Hear Res. 1999b;137:167–173. doi: 10.1016/s0378-5955(99)00147-1. [DOI] [PubMed] [Google Scholar]
- van Steensel B, van Binnendijk EP, Hornsby CD, van der Voort HT, Krozowski ZS, de Kloet ER, van Driel R. Partial colocalization of glucocorticoid and mineralocorticoid receptors in discrete compartments in nuclei of rat hippocampus neurons. J Cell Sci. 1996;109(Pt 4):787–92. doi: 10.1242/jcs.109.4.787. [DOI] [PubMed] [Google Scholar]
- Walther A, Riehemann K, Gerke V. A novel ligand of the formyl peptide receptor: annexin I regulates neutrophil extravasation by interacting with the FPR. Mol Cell. 2000;5:831–40. doi: 10.1016/s1097-2765(00)80323-8. [DOI] [PubMed] [Google Scholar]
- Wang CC, Wang SJ. Modulation of presynaptic glucocorticoid receptors on glutamate release from rat hippocampal nerve terminals. Synapse. 2009;2009:745–51. doi: 10.1002/syn.20654. [DOI] [PubMed] [Google Scholar]
- Yao X, Rarey KE. Localization of the mineralocorticoid receptor in rat cochlear tissue. Acta Otolaryngol. 1996;116:493–6. doi: 10.3109/00016489609137879. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Ichimiya I, Suzuki M, Mogi G. Effects of proinflammatory cytokines on cultured spiral ligament fibrocytes. Hearing Research. 1999;137:155–9. doi: 10.1016/s0378-5955(99)00134-3. [DOI] [PubMed] [Google Scholar]
- Zhou J, Cidlowski JA. The human glucocorticoid receptor: one gene, multiple proteins and diverse responses. Steroids. 2005;70:407–17. doi: 10.1016/j.steroids.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Zuo J, Curtis LM, Yao X, ten Cate WJ, Bagger-Sjoback D, Hultcrantz M, Rarey KE. Glucocorticoid receptor expression in the postnatal rat cochlea. Hear Res. 1995;87:220–7. doi: 10.1016/0378-5955(95)00092-i. [DOI] [PubMed] [Google Scholar]


