Abstract
Recurrent solid malignancies are often refractory to standard therapies. While adoptive T cell transfer may benefit select individuals, the majority of patients succumb to their disease. In order to address this important clinical dilemma, we developed a mouse melanoma model in which initial regression of advanced disease was followed by tumor recurrence. During recurrence, Foxp3+ tumor-specific CD4+ T cells became PD-1+ and represented over 60% of the tumor-specific CD4+ T cells in the host. Concomitantly, tumor-specific CD4+ T effector cells showed traits of chronic exhaustion as evidenced by their high expression of the PD-1, TIM-3, 2B4, TIGIT, and LAG-3 inhibitory molecules. While blockade of the PD-1/PD-L1 pathway with anti-PD-L1 antibodies or depletion of tumor-specific Treg cells alone failed to reverse tumor recurrence, combination of PD-L1 blockade with tumor-specific Treg cell depletion effectively mediated disease regression. Furthermore, blockade with a combination of anti-PD-L1 and anti-LAG-3 antibodies overcame the requirement to deplete tumor-specific Treg cells. In contrast, successful treatment of primary melanoma with adoptive cell therapy required only Treg depletion or antibody therapy, underscoring the differences in the characteristics of treatment between primary and relapsing cancer. These data highlight the need for preclinical development of combined immunotherapy approaches specifically targeting recurrent disease.
INTRODUCTION
Adoptive transfer of tumor-specific cytotoxic CD4+ T cells into lymphopenic hosts can eradicate large, established, vascularized tumors (1–3). Despite the efficacy of such cytotoxic CD4+ T cell transfer in the primary setting, tumor relapse remains a significant concern. While the mechanisms underlying tumor recurrence are not completely defined, they are postulated to include increases in regulatory T cells (Treg), loss of tumor antigen expression, and enhanced tumor expression of inhibitory ligands (4–7).
Foxp3+ regulatory T cells suppress immunity to cancer (8–11). Although removing Treg cells has generally enhanced the efficacy of primary therapy (12–14), depletion of these cells in more established cancers does not confer the same therapeutic benefit (15, 16). These data suggest that in the setting of disease recurrence, Treg cells work in combination and/or synergy with other mechanisms to suppress anti-tumor immunity.
One plausible mechanism for this increased tolerance observed in the setting of tumor recurrence is through the coexpression of molecules which inhibit effector T cell function(17), including Program Death-1 (PD-1) (18, 19), LAG-3 (20), TIGIT (21), and TIM-3 (22). PD-1 is part of the B7 family of molecules and regulates effector T cells. PD-1 was originally shown to be highly expressed on CD8+ T cells from chronically infected mice (19), and was later observed on CD8+ T cells in humans with chronic infections and cancer (22–26). Importantly, the ligand for PD-1, PD-L1 (B7-H1) is abundant on human carcinomas of lung, ovary, colon and melanoma (6), and functions as a “biologic shield,” protecting tumors from T cell mediated death. LAG-3 can regulate CD8+ T cells during antitumor responses (27) and is thought to play a role in Treg cell mediated suppression (28). TIGIT was recently shown to downregulate CD8+ T cells responses (21, 29) and blockade of TIM-3 has been shown to enhance therapy of primary tumors when combined with anti-PD-1 antibodies (22, 26). The role of each of these inhibitory receptors on cytotoxic CD4+ T effector cells is currently unknown.
From a functional perspective, blockade of PD1/PD-L1 interactions can restore anti-tumor immunity in mice (30). These observations have now been translated into humans, with phase I data clearly demonstrating that either PD-L1 (B7-H1) or PD-1 blockade, can lead to meaningful disease regression and survival improvements in patients with large tumor burdens (18, 31, 32). Unfortunately, in the setting of widely metastatic disease, anti-PD-1 treatment, like other single agent mAbs, is seldom curative (33).
Based on these collective data showing the potential import of CD4+ T cells combined with lymphopenia and PD-1/PD-L1 interactions in tumor recurrence, in this study, we investigated how these diverse mechanisms interact to dictate anti-tumor function in this setting. To accomplish this goal, we built upon a model system in which adoptive cell transfer of naïve tumor-specific CD4+ T cells into tumor bearing lymphopenic mice differentiate into Th1 cytotoxic T cells(1), capable of mediating the regression of primary melanomas through class II recognition and subsequent eradication through perforin and granzyme (1, 2, 34–36). Despite such initial efficacy, approximately 50% of mice ultimately relapse. Using this model, we now demonstrate that during recurrence, tumor-specific regulatory T cells increase concomitantly with chronically exhausted tumor-specific CD4+ TE cells. Although Foxp3 Treg cells increased during recurrence, their removal by targeted cell-specific ablation was not sufficient to initiate tumor regression. Instead, removal of tumor-specific Treg cells in combination with anti-PD-L1 (B7-H1) antibodies was necessary to restore immune function of tumor-specific CD4+ TE cells during cancer recurrence. In addition, combination immunotherapy against two inhibitory receptors with anti-PD-L1 and anti-LAG-3 antibodies overcame the necessity to deplete tumor-specific Treg cells and restored antitumor immunity in a host which had already received adoptive transfer of T cells before relapsed occurred, providing a clinically relevant alternative to Treg cell depletion for the treatment of recurrent disease (37). These data suggest that the immunobiology of T cell suppression differs in the setting of primary and recurrent malignancies and defines a combinatorial treatment strategy for clinical study.
METHODS
Mice
Tyrp1B-wRAG−/− TRP-1-specific CD4+ TCR transgenic mice (B6.Cg-Rag1tm1MomTyrp1B-w Tg(Tcra,Tcrb)9Rest/J) were previously described (1). Foxp3-DTR mice were provided by A. Rudensky (MSKCC) (38). Foxp3-DTR mice were crossed with transgenic TRP-1 mice to create tyrp1B-wRAG−/− Foxp3-DTR TRP-1-specific CD4+ TCR transgenic mice. Recombination-activating gene 1−/− (Rag1tm1Mom) mice were purchased from Jackson Labs. All mice were used in accordance with guidelines from the University of Maryland Institutional Animal Care Committee.
Tumor lines and measurement
B16.F10 (H-2b), hereafter called B16, is a TRP-1+ spontaneous murine melanoma that was obtained from ATCC and maintained in culture media (CM) as previously described (1). Tumors were injected subcutaneously at 2 × 105 cells/mouse. Tumors are measured blindly with digital calipers. The perpendicular diameters are determined and multiplied to generate the area in mm2 as previously described (1).
Sorting and adoptive cell transfer
TRP-1 Foxp3-DTR CD4+ T cells were sorted from spleens of donor Tyrp1B-wRAG-1−/− Foxp3-DTR TRP-1-specific transgenic male mice. Non-DTR+ TRP-1 CD4+ T cells were sorted from spleens of donor Tyrp1B-wRAG-1−/− TRP-1-specific transgenic male mice. Spleens were harvested and made into single-cell suspensions. Cells were made devoid of red blood cells by ACK lysis. Subsequently, cells were counted and enriched for CD4+ T cells by magnetic bead sorting using a CD4+ T cell enrichment kit from Miltenyi Biotech. Enriched CD4+ T cells were counted and resuspended in PBS and used in adoptive transfer studies (2 × 105 cells/mouse). T cells were injected intravenously through the tail vein. For tumor sensitized TRP-1 Foxp3-DTR CD4+ T cells, tyrp1B-wRAG−/− Foxp3-DTR TRP-1-specific CD4+ TCR transgenic mice were given B16.F10 tumor at 1 × 106 cells and T cells were harvested from spleen as described above when tumors reached ~400 mm2.
Flow cytometry
Anti-CD4 (RM4–5), anti-IFN-γ (XMG1.2), anti-TNF-α (MP6-XT22) and anti-CD244.2 (2B4) were obtained from BD Biosciences. Anti-IL-7Rα (SB/199), anti-CXCR3 (CXCR3-173), anti-Foxp3 (FJK-16s), anti-PD-1 (RMP1–30), anti-PD-L1 (MIH5), anti-TIM3 (RMT3–23), and anti-CD160 (BY55) were obtained from eBiosciences. Anti-LAG-3 (C9B7W) and anti-TIGIT (1G9) were obtained from Biolegend. All flow cytometry scales are log scales, if not otherwise specified. Intracellular staining for cytokines was done with the BD Biosciences cytofix/cytoperm intracellular staining kit. Foxp3 staining where indicated was done with the Foxp3 staining kit. All samples were run on a BD FACalibur (Department of Surgery, University of Maryland School of Medicine) and analyzed by Flowjo Software (Treestar).
Antibody and Treg depletion therapy
Anti-PD-L1 (10F.9G2), anti-CTLA-4 (9H10), and anti-LAG-3 (C9B7W) were purchased from BioXcell (West Lebanon, NH). Diphtheria toxin (DT) was purchased from Sigma and was reconstituted according to the manufacturer's protocol. Frozen diphtheria toxin stocks were thawed once and 50 μg/kg of diphtheria toxin was injected intraperitoneally as previously described (38).
Measurement of Serum Cytokines and Chemokines
Serum was collected via tail vein using serum collection tubes (B.D. Biosciences). Serum was analyzed by MILLIPLEX 32-Plex assay (University of Maryland Baltimore, Cytokine Core Lab).
Statistics
A student's unpaired t test was used to compare the differences between cytokines and chemokines as indicated. Tumor curves were compared using a two-way ANOVA, non-repeated measures. p values of 0.05 or less were considered significant. PRISM 5.0d software was used to analyze the data (Graphpad).
Support
This paper was supported by a K22 NCI Award (NIH), DOD Cancer Idea Award, and the Harry Lloyd Charitable Trust Fund.
Dedication
This paper is dedicated to Bernadette A. Estrada who fearlessly battled cervical cancer and dedicated her life to cancer awareness. She was one of the first to receive anti-PD-L1 (B7-H1) therapy. She will be missed dearly.
RESULTS
Tumor-specific Foxp3+ T regulatory cells expand during recurrence
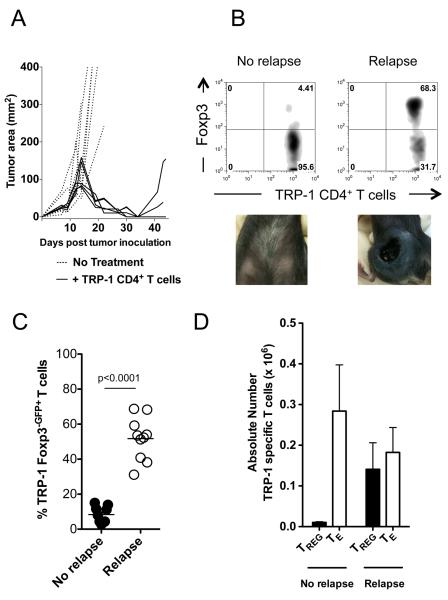
We have shown that tumor antigen-specific TRP-1 CD4+ T cells can eradicate large established melanoma in lymphopenic RAG−/− mice by direct killing of melanoma cells without the aid of CD8+ T cells (1). This therapy is enhanced by blockade of negative costimulation through anti-CTLA-4 (2). Preliminary evidence suggests that the enhanced immunity associated with CTLA-4 blockade, is related to inhibition of tumor-specific TRP-1 Treg cell expansion (2). Based on these findings in primary tumors, in this study, we first investigated whether tumor-specific Treg cells are expanded during tumor recurrence. Using our previously published model (1), we found that select tumors recurred, even after successful initial treatment (Fig. 1A). Recurrence was variable, with events occurring from 30–120 days. Analysis of TRP-1 CD4+ T cells from mice with recurrent disease and paired animals without relapse, demonstrated that relapsing mice had consistently higher levels of tumor-specific CD4+Foxp3+TRP-1 Treg cells, with levels varying from about 25% to 80% (Fig. 1B, C, and D). Importantly, the number of T effector cells (TE) was approximately the same in each group indicating that TE cells were not deleted (Fig. 1D). These data demonstrate that, in this model system, tumor recurrence is associated with a dramatic increase the tumor specific inhibitory:effector T cell ratio.
Figure 1. Foxp3+ tumor-specific Treg cells increase during melanoma recurrence.
(A) After successful tumor-specific CD4+ T cell adoptive cell immunotherapy melanoma recurs. C57BL/6 lymphopenic RAG−/− mice (5 mice/group) were inoculated with B16.F10 melanoma (2 × 105 cells). Tumor-bearing mice were treated with 2 × 105 naïve TRP-1 CD4+ T cells by intravenous tail vein injection on day 10 after tumor inoculation. Tumors were followed until recurrence of melanoma. Experiments repeated at least 10 times. (B) Foxp3+ tumor-specific CD4+ T cells increase during relapsing melanoma. TRP-1 CD4+ T cells were analyzed for Foxp3 expression by flow cytometry from relapsing mice (68.3%) and compared to cells from mice with no tumors (4.4%). Data represent ten independent experiments. (C) Percent Foxp3-eGFP+ cells from ten experiments. Using Foxp3-DTR TRP-1 T cells, which fluoresce with eGFP when expressing Foxp3, similar results were obtained in relapsing and non-relapsing mice (No recurrence, ~5%; Recurrence ~50%). P < 0.0001 for Foxp3 expression between non-relapsing versus relapsing mice. (D) Absolute numbers of tumor-specific CD4+ Treg and Foxp3− CD4+ TE cells during recurrence compared to nonrelapsing mice. Experiments repeated ten times.
Depletion of tumor-specific Foxp3+T cells does not treat recurrence
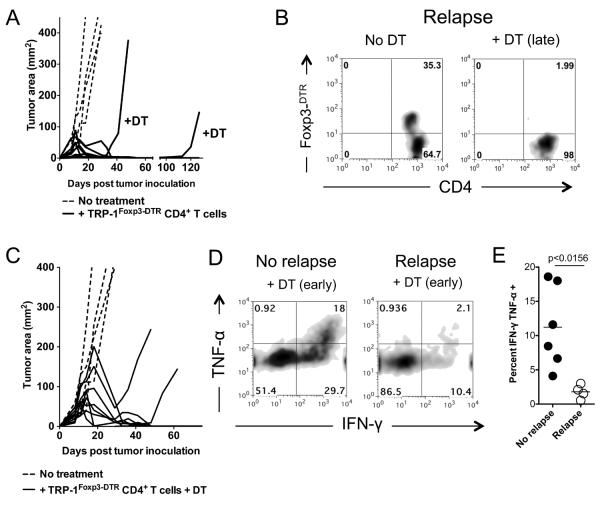
In order to determine if TRP-1 Foxp3+ tumor-specific CD4+ T cells were causing relapse, we crossed TRP-1 transgenic mice to the Foxp3-DTR transgenic reporter mouse which allows both unequivocal cell-specific ablation of Foxp3+ Treg cells (38) and cell-specific tracking (38). Similar to our initial studies, transfer of TRP-1Foxp3-DTR CD4+ tumor-specific T cells resulted in analogous rates of tumor relapse, indicating no difference in anti-tumor T cell function associated with DTR expression (Fig. 2A).
Figure 2. Depletion of tumor-specific Treg cells does not prevent or treat relapsing melanoma.
(A) Depletion of tumor-specific Foxp3+ Treg cells during recurrence (late) does not retreat melanoma. C57BL/6 lymphopenic RAG−/− mice (5 mice/group) were inoculated with B16.F10 melanoma (2 × 105 cells). Tumor-bearing mice were treated with 2 × 105 naïve TRP-1 Foxp3-DTR CD4+ T cells by intravenous tail vein injection on day 10 after tumor inoculation. Tumors were followed until recurrence of melanoma. DT (diphtheria toxin) was injected i.p. at the prescribed concentration of 50 μg/Kg every other day for three doses total. PBS controls had no effect on Treg cells (not shown). Experiments repeated 5 times. (B) Flow cytometry of Foxp3 eGFP expression in TRP-1 Foxp3-DTR CD4+ T cells from relapsing mice treated with or without DT at time of sacrifice. (No DT, 35.3%; +DT (late), ~2%). Experiments repeated at least five times. (C) Depletion of tumor-specific Foxp3+ Treg cells before recurrence (early) does not prevent melanoma recurrence. C57BL/6 lymphopenic RAG−/− mice (5 mice/group) were inoculated with B16.F10 melanoma (2 × 105 cells). Tumor-bearing mice were treated with 2 × 105 naïve TRP-1 Foxp3-DTR CD4+ T cells by intravenous tail vein injection on day 10 after tumor inoculation. Tumors were followed until recurrence of melanoma. At injection of T cells (day 10), DT (diphtheria toxin) was injected i.p. at the prescribed concentration of 50 μg/Kg every other day for three doses total. PBS controls had no effect on Treg cells (not shown). Experiments repeated five times. (D) Functional characteristics of tumor-specific CD4+ T cells during recurrence with Treg cells depleted early. Flow cytometry of IFN-γ and TNF-α expression in TRP-1 Foxp3-DTR CD4+ T cells from non-relapsing and relapsing mice. Data represent at least three experiments. (E) Percent of TRP-1 CD4+ T cells that are dual producers of IFN-γ and TNF-α during recurrence. P < 0.0156 for non-relapsing and relapsing groups. Experiments repeated four times.
Interestingly, in animals undergoing tumor recurrence, depletion of tumor-specific Treg cells failed to prevent tumor regrowth (Fig. 2A). Importantly, tumor relapses were not secondary to loss of tumor-specific TE cells, as flow cytometric analysis revealed that TE cells remained present during depletion and that GFP expression faithfully marked Foxp3-DTR TRP-1 Treg cells that had expanded (Fig 2B). Furthermore, depletion of tumor-specific Treg cells (i.e. TRP-1 specific Treg cells) at the time of primary treatment failed to prevent tumor recurrence, suggesting that Treg cells present at the time of adoptive transfer cannot mediate relapse (Fig 2C). In order to rule out the possibility that our findings were secondary to the induction of Treg cells from TE after initial depletion, we depleted tumor-specific Treg cells continuously for about 100 days. Even under these stringent conditions, tumors still recurred in select animals (Supplemental Figure 1).
Because depletion of tumor-specific Treg cells alone failed to mediate the regression of recurrent tumors, we postulated that functional differences in effector T cell populations might exist in the setting of tumor relapse. In order to test this supposition, we tested T cell functional capabilities by IFN-γ and TNF-α release in vitro. Strikingly, while TRP-1Foxp3-DTR CD4+ T cells from relapsing mice depleted of tumor-specific Treg cells produced only low levels of IFN-γ and TNF-α, cells from non-relapsing mice generated high levels of both cytokines (Fig. 2D and E). These data indicate an intrinsic dysfunction of effector CD4+ T cells in relapsing mice.
Tumor-specific CD4+ T cells become exhausted during cancer recurrence
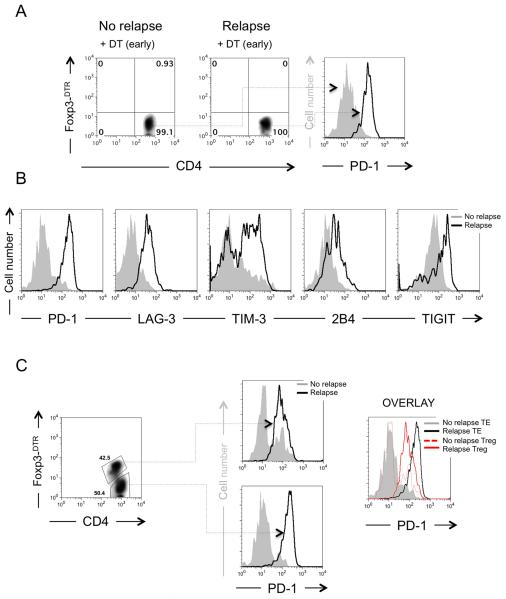
In the setting of recurrent disease, our data suggest that tumor-specific CD4+ T cells are functionally crippled, even in the absence of cell-extrinsic suppression. In order to understand the mechanisms underlying these observations, we compared the expression of inhibitory receptors on CD4+ T cells during recurrence and successful primary therapy. We first screened a broad panel of markers including LAG3, the T cell inhibitory molecule (TIGIT), TIM3, 2B4, CD160, Klrg1 and PD-1(17). Consistent with previous reports, T cells from animals with recurrent disease evidenced higher expression of LAG3, TIGIT and TIM3, slightly enhanced expression of 2B4, and similar expression of CD160 (not shown) (Fig. 3B). Interestingly, Klrg1 was shown to be down-regulated on exhausted cells, suggesting loss of cytotoxic function (Supplemental Figure 2). Taken in concert, these phenotypic and functional observations suggest that CD4+ TE cells become chronically exhausted during melanoma recurrence.
Figure 3. Tumor-specific CD4+ T cells express multiple inhibitory receptors during recurrence of melanoma.
(A) Depletion of tumor-specific Treg cells with DT before recurrence does not prevent exhaustion of TRP-1 Foxp3-DTR CD4+ T cells. Flow cytometry of TRP-1 Foxp3-DTR CD4+ T cells showing complete depletion of Treg cells during recurrence compared to non recurring (left two panels) and expression of PD-1 on TRP-1 Foxp3-DTR CD4+ TE cells (right panel) from either group. Experiment repeated five times. (B) Tumor-specific TRP-1 CD4+ TE cells express multiple inhibitory receptors during relapsing melanoma. Foxp3− tumor-specific CD4+ TE cells were analyzed by flow cytometry for expression of inhibitory receptors as indicated. Gray filled histograms represent non-relapsing groups. Open histograms with a black line represent relapsing groups. Representative of three to ten experiments. (C) Foxp3+ tumor-specific Treg cells express PD-1 during relapsing melanoma although at lower levels than tumor-specific TRP-1 CD4+ TE cells. Foxp3+ and Foxp3− tumor-specific CD4+ T cells were analyzed by flow cytometry for expression of the inhibitory receptor PD-1 (left panel). PD-1 is expressed on both CD4+ Treg and TE cells (middle panel). Overlay of flow cytometry shows that PD-1 expression is higher on CD4+ TE cells than on Foxp3+ Treg cells (right panel). Representative of three experiments.
Next, given the knowledge that the inhibitory receptor, PD-1, is highly expressed on exhausted T cells from chronically infected mice (19), we compared its expression on T cells from non-relapsing and recurring mice. Even in the absence of Treg cells, PD-1 expression on T cells was vastly enhanced in animals with recurrent disease (Fig. 3A). Because ligation of PD-1 mediates diverse functional properties, suppressing TE cells and stimulating Treg cells (39, 40), we subsequently analyzed PD-1 expression on both Foxp3− TRP-1Foxp3-DTR CD4+ TE and Foxp3+ TRP-1Foxp3-DTR Treg cells (Fig. 3C). During recurrence, both Treg cells and TE cells expressed PD-1 at high levels, independent of Treg depletion (Fig. 3C). However, when compared to each other, Treg cells always expressed lower levels of PD-1 than TE and always had higher levels of PD-1 than Treg cells from non-relapsing mice (Fig. 3C). Like TE cells, tumor-specific Treg cells from relapsing mice also expressed higher levels of TIM-3, TIGIT, and LAG-3 (not shown). These findings suggest that during recurrence Treg cells acquire distinctive functional properties that may interfere with anti-tumor immunity and that blockade of PD-L1 might restore anti-tumor immune function of tumor-specific CD4+ T cells in vivo by simultaneously blocking exhaustion and Treg cell mediated suppression.
PD-L1 specific blockade restores anti-tumor immunity and treats recurrence only in combination with tumor-specific Treg cell ablation
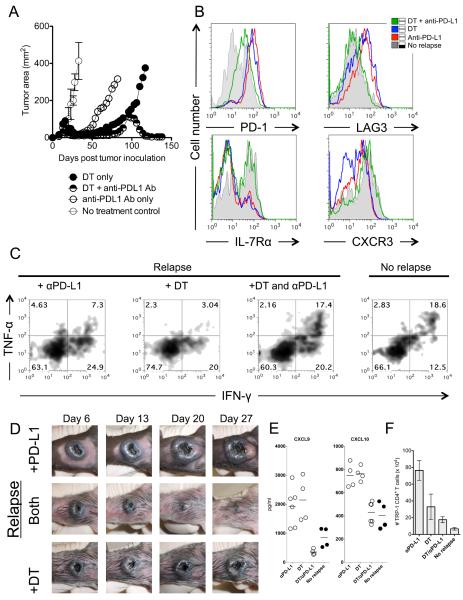
Because PD-1 increased during recurrence on both TRP-1 specific Treg and TE CD4+ T cells, we hypothesized that blocking PD-1 on Treg cells would prevent their suppression and/or expansion and blocking PD-1 on TE cells would restore their function (6, 17, 19, 22–26, 41–43). In order to avoid any confounding effects associated with potential stimulatory effects associated with anti-PD-1 administration on Treg cells, we opted to block its ligand, PD-L1. Treatment with anti-PD-L1 antibodies alone afforded no benefit (Fig. 4A). This lack of response was not due to timing, as later treatment of recurrences was met with similar results (not shown).
Figure 4. Treatment of relapsing melanoma requires both Treg depletion and blockade of PD-L1.
(A) Treatment of relapsing melanoma requires combination Treg depletion and anti-PD-L1 therapy. C57BL/6 lymphopenic RAG−/− mice (5–10/group) were inoculated with B16.F10 melanoma (2 × 105 cells). Tumor-bearing mice were treated with 2 × 105 naïve TRP-1 Foxp3-DTR CD4+ T cells by intravenous tail vein injection on day 7–10 after tumor inoculation. Tumors were followed until recurrence of melanoma. At recurrence, DT (diphtheria toxin) was injected i.p. at the prescribed concentration of 50 mg/Kg every other day for three doses total. At recurrence, anti-PD-L1 was given as a bolus injection i.p. at 500 μg for the first dose and subsequently given every three days thereafter at 200 μg/injection for 5 doses. For combination therapy, both were given at the same time as described above. PBS controls had no effect (not shown). Data shown is representative of more than 5 experiments. (B) Blockade of PD-L1 and depletion of tumor-specific Treg cells reinvigorates tumor-specific CD4+ T cells by reducing inhibitory receptor expression of PD-1 and LAG-3, and increases IL-7R and CXCR3. Flow cytometry shows four groups (No recurrence, DT only, DT plus anti-PD-L1 antibody, and anti-PD-L1 antibody only). Gray filled histograms represent no recurrence, blue solid line represents DT therapy only, red solid line represents anti-PD-L1 only, and green solid line represents therapy with DT and anti-PD-L1 together. All therapies were given at time of recurrence. Flow cytometry is performed on each group 3–5 days after the last dose of therapy. Shown is representative of 5 experiments. (C) Tumor-specific CD4+ T cells regain effector function with dual therapy as defined by re-expression of IFN-γ and TNF-α when compared to relapsing groups with single therapies (anti-PD-L1 (7.3%), DT (3.04%), +DT and anti-PD-L1 (17.4%), no recurrence (18.6%)). Experiment repeated three times. (D) Gross depiction of tumor regression after combination therapy. Days indicate day after therapy was given. All tumors depicted are relapsing tumors that were previously treated as a primary tumor. (E) IFN-γ inducible chemokines (CXCL9 and CXCL10) are highly increased during recurrence and return to treatment levels with combination therapy. Each dot represents and individual mouse. (F) Total number of tumor-specific CD4+ T cells in the spleen stabilizes to non-relapsing levels with combination therapy. Absolute number of TRP-1 CD4+ T cells during different treatments. Each bar graph represents 5–10 individual mice.
Because DT mediated Treg depletion alone also failed to treat recurrence as shown in Figure 2, we evaluated a combinatorial approach. Depletion of tumor-specific Treg cells and concomitant PD-L1 blockade, with anti-PD-L1 antibodies, effectively mediated regression of recurrent B16 melanoma. Importantly, these effects were observed in the absence of tumor-specific T cell retransfer, a vaccine, or induction of lymphopenia. These data indicate that tumor-specific CD4+ T cells were present and capable of being reinvigorated by PD-L1 blockade and Treg cell depletion.
Next, we examined the changes that occurred to the T cells and the serum during a successful treatment with combination therapy. We looked for expression of markers of exhaustion, IL-7Rα, and CXCR3. Reexpression of IL-7Rα indicates proper memory cell transitioning from naïve T cells and CXCR3 expression indicates a functional TH1 phenotype that allows TH1 CD4+ T cells to become properly differentiated (44–46). Most notably, PD-1 and LAG-3 expression went down when Treg cells were depleted and anti-PD-L1 therapy was given (Fig. 4B). IL-7Rα and CXCR3 expression was restored to levels seen on functional CD4+ T cells. However, either therapy alone induced no changes in PD-1 or LAG-3 expression when compared to non-recurring mice. IL-7Rα and CXCR3 also stayed low. Changes in phenotype predicted changes in function as CD4+ T cells were reinvigorated as evident by their re-expression of IFN-γ and TNF-α, similar to non-recurring mice (Fig 4C) and their ability to retreat recurring tumors (Fig 4D). Again, Treg cell depletion alone or anti-PD-L1 therapy alone had little to no affect on T cell function or their treatment ability (Fig. 4C and D) as evidenced by low co-expression of IFN-γ and TNF-α. Interestingly, IFN-γ was still expressed at low levels in the chronically exhausted cells suggesting that possibly low levels of IFN-γ in vivo may be sustaining PD-L1 expression through an adaptive resistance mechanism (7).
Previously we showed that specific chemokines and cytokines changed their expression during treatment of primary melanoma when compared to no treatment groups (1). Therefore, we wanted to determine if similar changes took place during recurrence and how they compared with non-relapsing mice and the original primary treatment. We found that CXCL9 and CXCL10 could predict treatment success (Fig. 4E). Interestingly, in contrast to our prior observations in the setting of primary disease, chemokines decreased during resolution of tumor and increased during recurrence (1). Chemokine levels tended toward levels in non-recurring mice during treatment with anti-PD-L1 and DT and correlated with increased CXCR3 expression on tumor-specific T cells (Fig. 4B). CD4+ T cell numbers in the spleen in mice being treated with both therapies tended toward levels in non-recurring mice (Fig. 4F). These results clearly indicate that blockade of PD-L1 and loss of Treg cells allows tumor-specific CD4+ T cells to become reinvigorated in vivo without retransfer of additional T cells or administration of a vaccine and no reinstatement of lymphopenia with cytotoxic drugs or radiation.
Disparate treatment requirements between primary and recurrent cancer
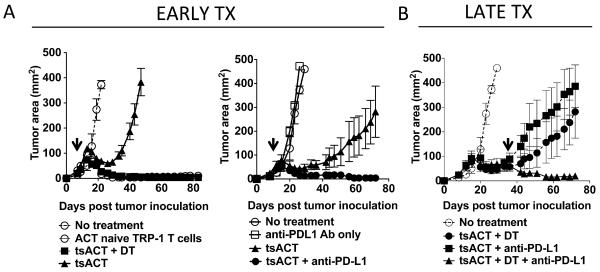
Surprisingly, treatment of recurring tumors was not predicted from treatment of primary tumors. CD4+ T cells from tumor-bearing transgenic mice (where tumors were growing unimpeded) were sorted and retransferred into tumor bearing animals that were treated with a combination of DT or anti-PD-L1 antibody (early treatment; Fig. 5A). CD4+ T cells from tumor-bearing tyrp1BwRAG−/− TRP-1 transgenic mice, termed tumor sensitized (ts), predictably expressed PD-1 and had increased levels of Foxp3 (Supplemental Figure S3A). Because of this, tumor recurred faster and at the same time in individual mice. Like mice, which recurred naturally, tsACT cells expressed higher levels of Foxp3 and PD-1 during recurrence (Supplement Figure S3B). However, early treatment of primary tumors with anti-PD-L1 or DT therapy worked alone when given with tsACT (Fig. 5A). When mice recurred in the same experiment, only the combination of anti-PD-L1 antibody and Treg depletion was therapeutic (late treatment; Fig. 5B). These findings underscore the differences between primary tumor and recurring tumor microenvironments.
Figure 5. Disparate treatment requirements between primary and relapsing melanoma.
(A) Early treatment of melanoma with tumor sensitized TRP-1 CD4+ T cells (tsACT) from mice with progressively growing tumors fails in all mice when compared to naïve TRP-1 CD4+ T cell transfer. TRP-1 Foxp3-DTR tyrp1BwRAG−/− transgenic mice (5–10 mice/group) were inoculated with B16.F10 melanoma (1 × 106 cells). When tumors had reached ~400 mm2, tumor sensitized (ts) CD4+ T cells were harvested and sorted and 2 × 105 cells were transferred i.v. into 7 day tumor-bearing lymphopenic mice. tsTRP-1 CD4+ T cells (tsACT) alone fail to control tumor but when depleted of Treg cells (+DT) treated primary tumor (right panel). Anti-PD-L1 alone without T cell transfer fails to treat primary tumors. Anti-PD-L1 with tsTRP-1 CD4+ T cells (tsACT) treated primary tumor (middle panel). (B) Single therapies given late during relapsing tumor fail to treat tumor as in figure (A). Combination therapy with anti-PD-L1 and Treg depletion was still required (right panel). Arrows indicate beginning of therapy with either DT, anti-PD-L1, or both. Experiments repeated three times.
Simultaneous blockade of PD-L1 and LAG-3 in vivo treats recurring tumors overcoming the necessity to deplete tumor-specific Treg cells
Although combination Treg depletion with anti-PD-L1 therapy can treat relapsing poorly-immunogenic B16 melanoma, this type of therapy in humans is not practical (47, 48). Depletion of Treg cells is also potentially dangerous in humans because of the autoimmune diseases that could follow. Therefore, we looked for another means to overcome Treg mediated suppression that was controllable without having to deplete Treg cells. First, we blocked CTLA-4, an antibody that has been recently approved for melanoma (known clinically as ipilimumab or Yervoy). We surmised that it could block the generation of iTreg cells or enhance therapy like shown before in many models (2, 49). It was given in combination with tumor-specific Treg depletion therapy and there was no affect on relapsing cancer (Supplemental Figure 4A), thus showing the dominance of the PD-1 pathway in tumor recurrence.
We noticed that CD4+ T cells from relapsing mice expressed both PD-1 and LAG-3 (Fig. 6A). We also noticed that during successful therapy with anti-PD-L1 and tumor-specific Treg depletion that both PD-1 and LAG-3 decreased in expression on tumor-specific CD4+ T cells reaching levels similar to nonrelapsing mice (50% to 13%, Fig. 6A. We reasoned that if we could block both PD-1 and LAG-3 together that this would reproduce the same therapy effect with anti-PD-L1 in combination with Treg depletion.
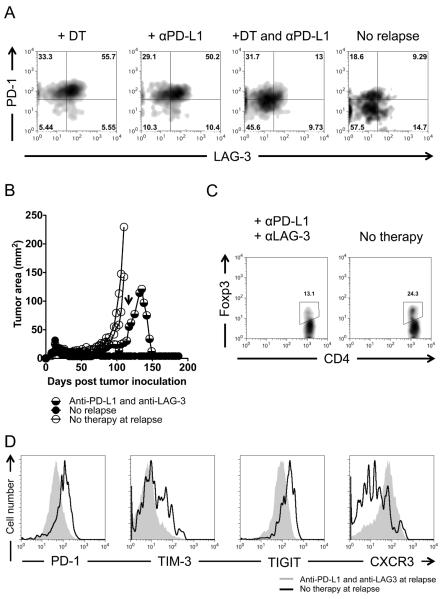
Figure 6. Simultaneous blockade with combination therapy to PD-L1 and LAG-3 inhibitory receptors treats recurring melanoma.
(A) PD-1 and LAG-3 are coexpressed on chronically exhausted tumor-specific CD4+ T cells and decrease during anti-PD-L1 and Treg cell depletion therapy or during a potential cure. C57BL/6 lymphopenic RAG−/− mice (5–10 mice/group) were inoculated with B16.F10 melanoma (2 × 105 cells). Tumor-bearing mice were treated with 2 × 105 naïve TRP-1 Foxp3-DTR CD4+ T cells by intravenous tail vein injection on day 7–10 after tumor inoculation. Tumors were followed until recurrence of melanoma. At recurrence, DT (diphtheria toxin) was injected i.p. at the prescribed concentration of 50 mg/Kg every other day for three doses total. At recurrence, Anti-PD-L1 was given as a bolus injection i.p. at 500 μg for the first dose and subsequently given every three days thereafter at 200 μg/injection for 5 doses. For combination therapy, both were given at the same time as described above. Experiment repeated at least five times. (B) Anti-PD-L1 and anti-LAG-3 antibodies given during relapse treat recurring tumor. Arrow indicates the time in which the antibodies were given. Treatment was similar as in (A). However, a 500 μg loading dose of each antibody (anti-PD-L1 and anti-LAG-3) was given initially with 200 μg doses given every 3 days for 5 doses total. (C) Treg cell frequency during treatment with dual antibody therapy in (B). (D) Inhibitory receptor expression on tumor-specific T effector cells during dual antibody therapy. CD4+ T cells were isolated from mice undergoing regression after dual antibody therapy approximately 3–7 days after the last dose of antibody and analyzed by flow cytometry.
Anti-PD-L1 and anti-LAG-3 antibodies were given in combination at time of relapse without Treg cell depletion. As with anti-PD-L1 and Treg cell depletion, tumors regressed (Fig. 6B). Analysis of mice regressing showed lower amounts of tumor-specific Treg cells when compared to relapsing mice (Fig. 6C) and decreases in inhibitory receptors on tumor-specific CD4+ T cells, as well as an increase in CXCR3 similar to anti-PD-L1 therapy combined with Treg cell depletion (Fig. 6D). LAG-3 was blocked with unlabeled anti-LAG-3 and therefore could not be assessed by flow cytometry (not shown). Anti-LAG-3 given with DT therapy also had no affect on relapsing cancer (Supplemental Data 4B). Thus, tumor-specific Treg mediated suppression and chronic exhaustion could be simultaneously overcome with dual antibody therapy to PD-L1 and LAG-3 inhibitory receptors.
DISCUSSION
We demonstrate that Treg mediated suppression and chronic exhaustion of CD4+ T cells are intertwined during cancer recurrence. While blockade of either pathway alone can successfully treat primary tumors, these monotherapies cannot reactivate tumor-specific CD4+ T cells in vivo for the treatment of recurrent disease. In contrast, combinational blockade of the inhibitory molecule PD-L1 and depletion of tumor-specific Treg cells or more practically combination blockade of the inhibitory molecules PD-L1 and LAG-3 effectively treated recurrent melanoma, and therapeutic responses were predicted by changes in well-defined serum biomarkers. Taken in concert, our data delineate a new potential approach for the treatment of recurrent solid tumors and define biomarkers that may be useful for predicting treatment response.
CD4+ T cells can effectively enhance immunotherapy of cancer (3, 50–53), and in certain cases, are superior to traditional CD8+ T cell based approaches for the treatment of melanoma (34) (54). For example, the combination of tumor-specific CD4+ T helper cells, anti-PD-1 and cytoxan enhanced anti-tumor immunity by reactivating tumor-specific CD8+ T cells (55). Despite there therapeutic potential, recent observations suggest that antigen specific CD4+ T cells are a “double edged sword”, capable of both directly targeting class II expressing tumors and suppressing anti-tumor immunity. Based on these data, we first asked whether depletion Treg cells could improve anti-tumor immunity against recurrent melanoma. Unlike the primary tumor setting, where Treg cell depletion enhances the efficacy of adoptive transfer (14, 15, 56); elimination of tumor-specific Treg cells was ineffective in mediating regression of recurrent disease. Importantly, this lack of therapeutic utility was not secondary to loss of tumor specific TE cells. Furthermore, our findings cannot be explained by incomplete Treg depletion as we demonstrate unequivocal elimination with targeted cell-specific ablation as previously shown(38). These data indicate that in our model system TE cells cannot mediate regression of recurrent tumors, even in the setting of Treg depletion.
In order to understand why antigen specific CD4+ T cells are permissive to disease recurrence, we evaluated them for markers of exhaustion. Interestingly, tumor-specific CD4+ TE cells expressed the hallmark exhaustion markers PD-1, LAG-3, TIM-3, 2B4 and newly described TIGIT, lost expression of IL-7Rα+ and CXCR3, and lost co-expression of INF-γ and TNF-α. Furthermore, the quintessential chronic inhibitory receptor, PD-1, was highly expressed on both tumor-specific CD4+ Treg and TE cells. This expression of PD-1 on antigen specific CD4+ T cells was limited to mice with recurrent disease and was not observed in the primary setting.
Interestingly, despite the increased level of PD-1 expression on CD4+ T cells from mice with recurrent tumors, anti-PD-L1 alone could not treat relapsing cancer. Based on this surprising observation, we evaluated the possibility that anti-PD-L1 might have both positive and adverse effects on CD4+ T cells in the setting of disease recurrence – stimulating CD4+ PD-1+ Treg cells and suppressing TE. Our data indicate that following CD4+ tumor-specific Treg depletion, anti-PD-L1 administration can effectively mediate the regression of recurrent tumor in vivo. Although these findings appear analogous to previous studies, using anti-PD-L1 and Treg depletion with IL-2DT (IL-2 conjugated to diphtheria toxin) to treat a murine model of acute myelogenous leukemia (AML), close scrutiny of this report reveals that the treatment regimen was administered 4 to 10 days after tumor inoculation, indicating a therapy of primary tumors. In addition, IL-2DT therapy can target non-Treg cells especially TE cells expressing CD25.
In order to begin to understand the mechanisms through which anti-PD-L1 restores TE function following tumor-specific Treg depletion, we analyzed phenotypic markers of T cell function. In this context, the inhibitory markers PD-1, LAG-3, TIGIT, TIM-3 decrease and IFN-γ and TNF-α increase. Furthermore, IL-7Rα+ a marker of memory cell differentiation and CXCR3, which allows cell to traffic to peripheral sites, were higher. Klrg1 is upregulated on CD4+ T cells from nonrecurring mice, but down regulated during recurrence. This phenotype is associated with terminal differentiation and cytolytic potential (57). Hence Klrg1 loss and PD-1 expression was associated with loss of effector function. These data indicate that the combination of tumor-specific Treg depletion and anti-PD-L1 rejuvenates TE function, enabling them to mediate the regression of recurrent disease. In addition, these observations delineate phenotypic changes on TE cells, which may be useful in monitoring clinical response to therapy.
These phenotypical changes pointed to a possible mechanism that could be exploited by antibody therapies. PD-1 and LAG-3 both decreased together on TE cells during a potential cure or treatment with anti-PD-L1 and tumor-specific Treg cell depletion. Therefore, we reasoned that blockade of these two negative inhibitory pathways together might restore effector function in the absence of Treg cell depletion. Administration of anti-PD-L1 and LAG-3 mirrored the therapy with anti-PD-L1 and tumor-specific Treg depletion, with similar phenotypical changes occurring on TE cells. This therapy overcomes a major translation hurdle in clinical medicine, being the depletion of Treg cells. This combination used as a therapy has also benefited chronic infections such as malaria and LCMV in mice (17, 58).
So what could cause melanoma recurrence? Whether or not exhaustion is caused directly by Treg cells in general is not answerable in this report, as the role of endogenous Treg cells was not studied. Endogenous Treg cells may contribute to tumor recurrence given their wide antigen specific repertoire and their ability to suppress antigen specific transgenic pmel CD8+ T cells (12, 13). Our data suggest that tumor-specific Treg cells may have some contribution to exhaustion as continuous depletion of TRP-1 Treg cells appeared to attenuate relapsing cancer but did not solely prevent it. These same tumor-specific Treg cells also became PD-1+ during recurrence and expanded to high levels, suggesting that they may be acting as a secondary layer of adaptive tolerance similar to expansion of PD-1+ Treg cells during chronic HCV infection (59). Since tumor may be like a chronic infection, analogous mechanisms may be employed where Treg cells become simultaneously exhausted like TE cells in order to control tissues homeostasis (60). Possibly the opposite occurs during spontaneous remissions similarly to severe flares in HCV (60). Whether the expansion of TRP-1 Treg cells in our model are induced Treg cells or an expansion of Treg cells already present upon transfer is not known, but we are currently exploring these possibilities.
Relapsing melanoma may also be caused by inflammatory mediators themselves. In a recent study, TNF-α caused melanoma cells to dedifferentiate and express lower levels of class I and tumor-associated melanocytic antigens including TRP-1 (4). Another group showed that MHC class I and another melanocytic antigen, gp100, were decreased during recurrence (5). Class II expression in the tumor microenvironment is dependent on IFN-γ (1, 2), therefore, class II might not be susceptible to loss like MHC class I. Although both studies showed loss of antigen as a cause of recurrence, recurring tumors in the current report were still amenable to treatment. CD4+ T cells also reacquired TNF-α during reinvigoration with combination therapy. Thus, treatment of recurrent cancer seems to require TNF-α in our studies, as tumor-specific CD4+ T cells from non-recurring mice express it and reinvigorated cells reexpress it after Treg depletion and anti-PD-L1 blockade. Conversely, more in line with our results here, a recent study showed that Th1 cytokines, IFN-γ and TNF-α, induced tumor cell senescence and arrested cancer progression (61). Thus, both cytokines in our model may be helping to prevent recurrence, because during cancer relapse TNF-α expression is lost by tumor-specific Th1 cytotoxic TRP-1 CD4+ T cells.
Another possible underappreciated mechanism of recurrence and cancer metastasis is the expression of high levels of IFN-γ inducible chemokines CXCL9 and CXCL10. Although these chemokines are known to attract T cells to sites of inflammation, they are also known to be highly expressed during melanoma and other cancer burdens, allowing malignant cancer cells to migrate to distant nodes (45, 62). CXCR3+ T cells in the presence of these high chemokines also can lose CXCR3 expression preventing the cells from migrating into the cancerous tissue (63). In fact, we see high levels of these chemokines during recurrence and loss of CXCR3 on the tumor-specific CD4+ T cells. With resolution of tumor during combination therapy, CXCL9 and CXCL10 decrease in the serum and CXCR3 is reexpressed on tumor-specific CD4+ T cells. As CXCL9 and CXCL10 are important for Th1 priming (46), we suspect that they may also be important in the initiation of exhaustion and suppression.
More and more, CD4+ T cells in many different contexts are being shown to enhance immunotherapy of cancer. Recently a group reported in vivo modulation of cytotoxic TRP-1 CD4+ T cells through the antibody OX40, which enhanced the cytotoxic abilities of TRP-1 CD4+ T cells through expression of Eomes (34). TRP-1 CD4+ T cells differentiated into Th17 cells in vitro appeared to resist exhaustion and treat better than Th1 CD4+ T cells through acquiring a stem-like signature (3), CD4+ T cells differentiated into TH9 cells also were beneficial in preventing melanoma (53), and lastly, CD4+ T cells differentiated into Th17 T helper cells can assist CD8+ T cells to clear tumor better than Th1 CD4+ T helper cells (50). Although treatment of recurrences themselves were not the focus of these papers, these works in combination with our previous work (1) and our results presented here show how the uses of CD4+ T helper (whether Th1, TH9, or TH17) and cytotoxic CD4+ T cells themselves can be harnessed and combined with unique antibody therapies to fight cancer at different stages of disease.
PD-L1 is, in many respects, an ideal system for tumor mediated immune evasion. Specifically, its expression is enhanced by IFNγ, one of the most potent weapons T cells employ to mediate tumor cell death. Our findings now suggest that unlike in the setting of primary disease, where blockade of this molecule can restore T cell function, recurrent tumors have built a second line of biologic defense. Specifically, in this setting, Treg cells showed enhanced expression of PD-1, which may become activated upon PD-L1 binding (59) or contradictorily by anti-PD-L1 blockade (59, 60). This inhibition can be effectively overcome by a “double pronged” therapeutic approach in which Treg depletion and systemic anti-PD-L1 or anti-PD-L1 and anti-LAG-3 antibodies can restore the ability of resident TE to mediate tumor regression. Furthermore the efficacy of this response can be predicted by the loss of typical markers of exhaustion on TE. Monotherapy may be an Achilles' heel of cancer immunotherapeutics. Therefore, these data offer a testable model to define the role of PD-L1 in mediating disease recurrence and suggest a new paradigm for the use of combinatorial approaches such as PD-L1 and LAG-3 blockade in the clinical setting.
One caveat to the current report is that they are performed in lymphopenic RAG−/− mice, which lack endogenous Treg cells, B cells, and CD8+ T cells. Although we were strictly interested in the role of cytotoxic CD4+ T cells in this study as in our last report (1), we cannot rule out whether the roles of the other missing cells are important during recurrence. Based on evidence from the literature, we would expect endogenous Treg cells to hinder tumor immunity (12, 13), B cells to produce antibodies to TRP-1 (64), and CD8+ T cells to become cytotoxic T cells (50, 55, 65). These may or may not affect cancer recurrence. Currently, we are exploring the role of endogenous Treg cells on recurrence and whether in vivo modulation in irradiated and non-irradiated settings can overcome recurrence. Our current report is the first step in determining the basic mechanisms of cancer recurrence.
In vivo modulation is an attractive form of immunotherapy long after adoptive cell therapy has been given. Our results have a long reaching impact on not just CD4+ T cells, but any adoptive cell therapy regimen, including cytotoxic CD8+ T cells, and chimeric antigen receptor (CAR) T cells (66–68), which may be susceptible to the same tolerogenic mechanisms. Understanding the mechanism of recurrence and knowing the right combinations of antibodies may improve clinical care of patients with cancer.
Supplementary Material
REFERENCES
- 1.Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med. 2010;207:651–667. doi: 10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, Restifo NP, Allison JP. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ, Roychoudhuri R, Ferreyra GA, Shen W, Durum SK, Feigenbaum L, Palmer DC, Antony PA, Chan CC, Laurence A, Danner RL, Gattinoni L, Restifo NP. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landsberg J, Kohlmeyer J, Renn M, Bald T, Rogava M, Cron M, Fatho M, Lennerz V, Wolfel T, Holzel M, Tuting T. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature. 2012 doi: 10.1038/nature11538. [DOI] [PubMed] [Google Scholar]
- 5.Jensen SM, Twitty CG, Maston LD, Antony PA, Lim M, Hu HM, Petrausch U, Restifo NP, Fox BA. Increased frequency of suppressive regulatory T cells and T cell-mediated antigen loss results in murine melanoma recurrence. J Immunol. 2012;189:767–776. doi: 10.4049/jimmunol.1103822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 7.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127–137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 9.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Kim JH, Falo LD, Jr., You Z. Tumor regulatory T cells potently abrogate antitumor immunity. J Immunol. 2009;182:6160–6167. doi: 10.4049/jimmunol.0802664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antony PA, Paulos CM, Ahmadzadeh M, Akpinarli A, Palmer DC, Sato N, Kaiser A, Hinrichs CS, Klebanoff CA, Tagaya Y, Restifo NP. Interleukin-2-dependent mechanisms of tolerance and immunity in vivo. Journal of immunology. 2006;176:5255–5266. doi: 10.4049/jimmunol.176.9.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, Rosenberg SA, Restifo NP. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR, Allison JP. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med. 2008;205:2125–2138. doi: 10.1084/jem.20080099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Q, Munger ME, Highfill SL, Tolar J, Weigel BJ, Riddle M, Sharpe AH, Vallera DA, Azuma M, Levine BL, June CH, Murphy WJ, Munn DH, Blazar BR. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood. 2010;116:2484–2493. doi: 10.1182/blood-2010-03-275446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Current opinion in immunology. 2012 doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 20.Liang B, Workman C, Lee J, Chew C, Dale BM, Colonna L, Flores M, Li N, Schweighoffer E, Greenberg S, Tybulewicz V, Vignali D, Clynes R. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol. 2008;180:5916–5926. doi: 10.4049/jimmunol.180.9.5916. [DOI] [PubMed] [Google Scholar]
- 21.Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, Eaton D, Grogan JL. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 22.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK, Blazar BR. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC, Goldberg MV, Pardoll DM, Drake CG. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. The Journal of clinical investigation. 2007;117:3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, Powell JD, Pardoll DM, Drake CG, Vignali DA. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD, Sharpe AH, Kuchroo VK. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011;186:1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, Kasperbauer JL, Ballman KV, Chen L. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 31.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, Xu H, Yao S, Pons A, Chen L, Pardoll DM, Brahmer JR, Topalian SL. Durable Cancer Regression Off-treatment and Effective Re-induction Therapy with an Anti-PD-1 Antibody. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirschhorn-Cymerman D, Budhu S, Kitano S, Liu C, Zhao F, Zhong H, Lesokhin AM, Avogadri-Connors F, Yuan J, Li Y, Houghton AN, Merghoub T, Wolchok JD. Induction of tumoricidal function in CD4+ T cells is associated with concomitant memory and terminally differentiated phenotype. J Exp Med. 2012 doi: 10.1084/jem.20120532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown DM. Cytolytic CD4 cells: Direct mediators in infectious disease and malignancy. Cell Immunol. 2010;262:89–95. doi: 10.1016/j.cellimm.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown DM, Kamperschroer C, Dilzer AM, Roberts DM, Swain SL. IL-2 and antigen dose differentially regulate perforin- and FasL-mediated cytolytic activity in antigen specific CD4+ T cells. Cell Immunol. 2009;257:69–79. doi: 10.1016/j.cellimm.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rech AJ, Mick R, Martin S, Recio A, Aqui NA, Powell DJ, Jr., Colligon TA, Trosko JA, Leinbach LI, Pletcher CH, Tweed CK, DeMichele A, Fox KR, Domchek SM, Riley JL, Vonderheide RH. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci Transl Med. 2012;4:134–162. doi: 10.1126/scitranslmed.3003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 39.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amarnath S, Mangus CW, Wang JC, Wei F, He A, Kapoor V, Foley JE, Massey PR, Felizardo TC, Riley JL, Levine BL, June CH, Medin JA, Fowler DH. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3:111–120. doi: 10.1126/scitranslmed.3003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, Tangsombatvisit S, Grosso JF, Netto G, Smeltzer MP, Chaux A, Utz PJ, Workman CJ, Pardoll DM, Korman AJ, Drake CG, Vignali DA. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grakoui A, John Wherry E, Hanson HL, Walker C, Ahmed R. Turning on the off switch: regulation of anti-viral T cell responses in the liver by the PD-1/PD-L1 pathway. J Hepatol. 2006;45:468–472. doi: 10.1016/j.jhep.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Lundstrom W, Fewkes NM, Mackall CL. IL-7 in human health and disease. Semin Immunol. 2012;24:218–224. doi: 10.1016/j.smim.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fulton AM. The chemokine receptors CXCR4 and CXCR3 in cancer. Curr Oncol Rep. 2009;11:125–131. doi: 10.1007/s11912-009-0019-1. [DOI] [PubMed] [Google Scholar]
- 46.Groom JR, Richmond J, Murooka TT, Sorensen EW, Sung JH, Bankert K, von Andrian UH, Moon JJ, Mempel TR, Luster AD. CXCR3 Chemokine Receptor-Ligand Interactions in the Lymph Node Optimize CD4(+) T Helper 1 Cell Differentiation. Immunity. 2012 doi: 10.1016/j.immuni.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 48.Barnett B, Kryczek I, Cheng P, Zou W, Curiel TJ. Regulatory T cells in ovarian cancer: biology and therapeutic potential. Am J Reprod Immunol. 2005;54:369–377. doi: 10.1111/j.1600-0897.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 49.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4(+) T cells. Current opinion in immunology. 2009;21:200–208. doi: 10.1016/j.coi.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, Jetten AM, Khoury SJ, Fuhlbrigge RC, Kuchroo VK, Clark RA, Kupper TS. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med. 2012 doi: 10.1038/nm.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O, Matzinger P. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007;109:5346–5354. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding ZC, Huang L, Blazar BR, Yagita H, Mellor AL, Munn DH, Zhou G. Polyfunctional CD4+ T cells are essential for eradicating advanced B-cell lymphoma after chemotherapy. Blood. 2012;120:2229–2239. doi: 10.1182/blood-2011-12-398321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belz GT, Kallies A. Effector and memory CD8+ T cell differentiation: toward a molecular understanding of fate determination. Current opinion in immunology. 2010;22:279–285. doi: 10.1016/j.coi.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 58.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franceschini D, Paroli M, Francavilla V, Videtta M, Morrone S, Labbadia G, Cerino A, Mondelli MU, Barnaba V. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. The Journal of clinical investigation. 2009;119:551–564. doi: 10.1172/JCI36604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Radziewicz H, Dunham RM, Grakoui A. PD-1 tempers Tregs in chronic HCV infection. The Journal of clinical investigation. 2009;119:450–453. doi: 10.1172/JCI38661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Braumuller H, Wieder T, Brenner E, Assmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C, Ranta F, Ullrich S, Mocikat R, Braungart K, Mehra T, Fehrenbacher B, Berdel J, Niessner H, Meier F, van den Broek M, Haring HU, Handgretinger R, Quintanilla-Martinez L, Fend F, Pesic M, Bauer J, Zender L, Schaller M, Schulze-Osthoff K, Rocken M. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–365. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 62.Ma X, Norsworthy K, Kundu N, Rodgers WH, Gimotty PA, Goloubeva O, Lipsky M, Li Y, Holt D, Fulton A. CXCR3 expression is associated with poor survival in breast cancer and promotes metastasis in a murine model. Mol Cancer Ther. 2009;8:490–498. doi: 10.1158/1535-7163.MCT-08-0485. [DOI] [PubMed] [Google Scholar]
- 63.Winter D, Moser J, Kriehuber E, Wiesner C, Knobler R, Trautinger F, Bombosi P, Stingl G, Petzelbauer P, Rot A, Maurer D. Down-modulation of CXCR3 surface expression and function in CD8+ T cells from cutaneous T cell lymphoma patients. J Immunol. 2007;179:4272–4282. doi: 10.4049/jimmunol.179.6.4272. [DOI] [PubMed] [Google Scholar]
- 64.Overwijk WW, Lee DS, Surman DR, Irvine KR, Touloukian CE, Chan CC, Carroll MW, Moss B, Rosenberg SA, Restifo NP. Vaccination with a recombinant vaccinia virus encoding a “self” antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4(+) T lymphocytes. Proc Natl Acad Sci U S A. 1999;96:2982–2987. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ding ZC, Blazar BR, Mellor AL, Munn DH, Zhou G. Chemotherapy rescues tumor-driven aberrant CD4+ T-cell differentiation and restores an activated polyfunctional helper phenotype. Blood. 2010;115:2397–2406. doi: 10.1182/blood-2009-11-253336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scholler J, Brady TL, Binder-Scholl G, Hwang WT, Plesa G, Hege KM, Vogel AN, Kalos M, Riley JL, Deeks SG, Mitsuyasu RT, Bernstein WB, Aronson NE, Levine BL, Bushman FD, June CH. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med. 2012;4:132–153. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liddy N, Bossi G, Adams KJ, Lissina A, Mahon TM, Hassan NJ, Gavarret J, Bianchi FC, Pumphrey NJ, Ladell K, Gostick E, Sewell AK, Lissin NM, Harwood NE, Molloy PE, Li Y, Cameron BJ, Sami M, Baston EE, Todorov PT, Paston SJ, Dennis RE, Harper JV, Dunn SM, Ashfield R, Johnson A, McGrath Y, Plesa G, June CH, Kalos M, Price DA, Vuidepot A, Williams DD, Sutton DH, Jakobsen BK. Monoclonal TCR-redirected tumor cell killing. Nat Med. 2012;18:980–987. doi: 10.1038/nm.2764. [DOI] [PubMed] [Google Scholar]
- 68.June C, Rosenberg SA, Sadelain M, Weber JS. T-cell therapy at the threshold. Nat Biotechnol. 2012;30:611–614. doi: 10.1038/nbt.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.