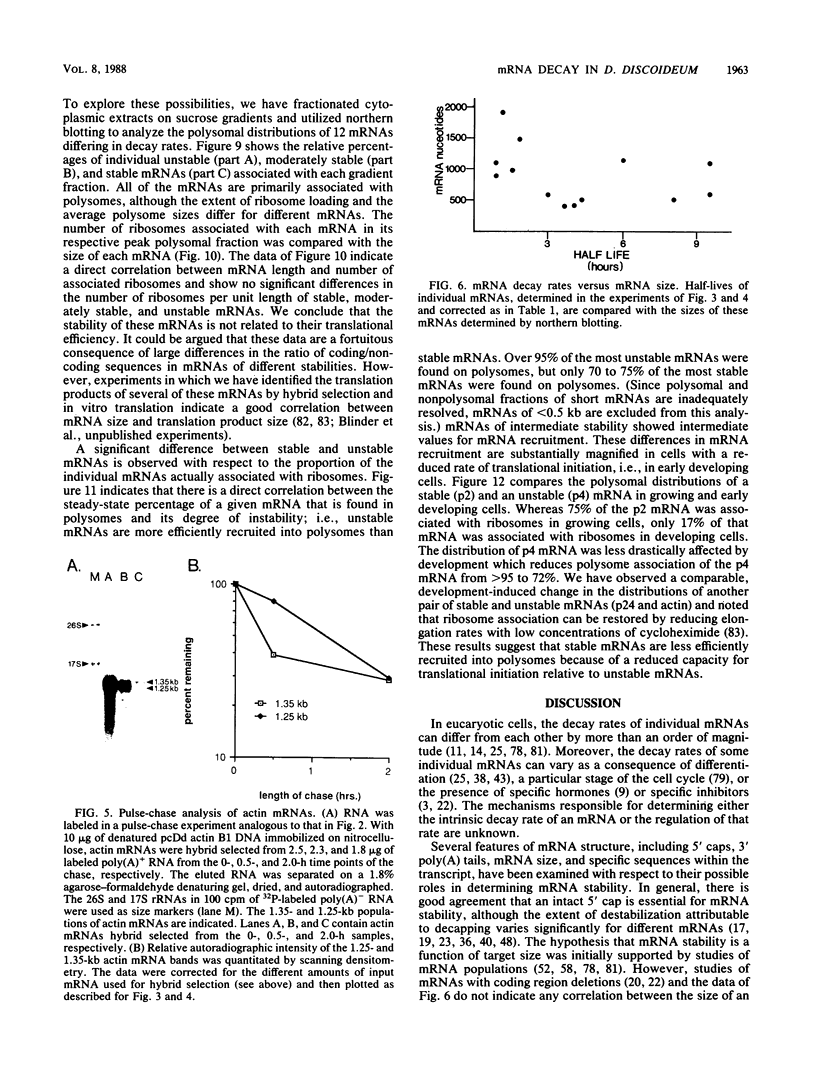
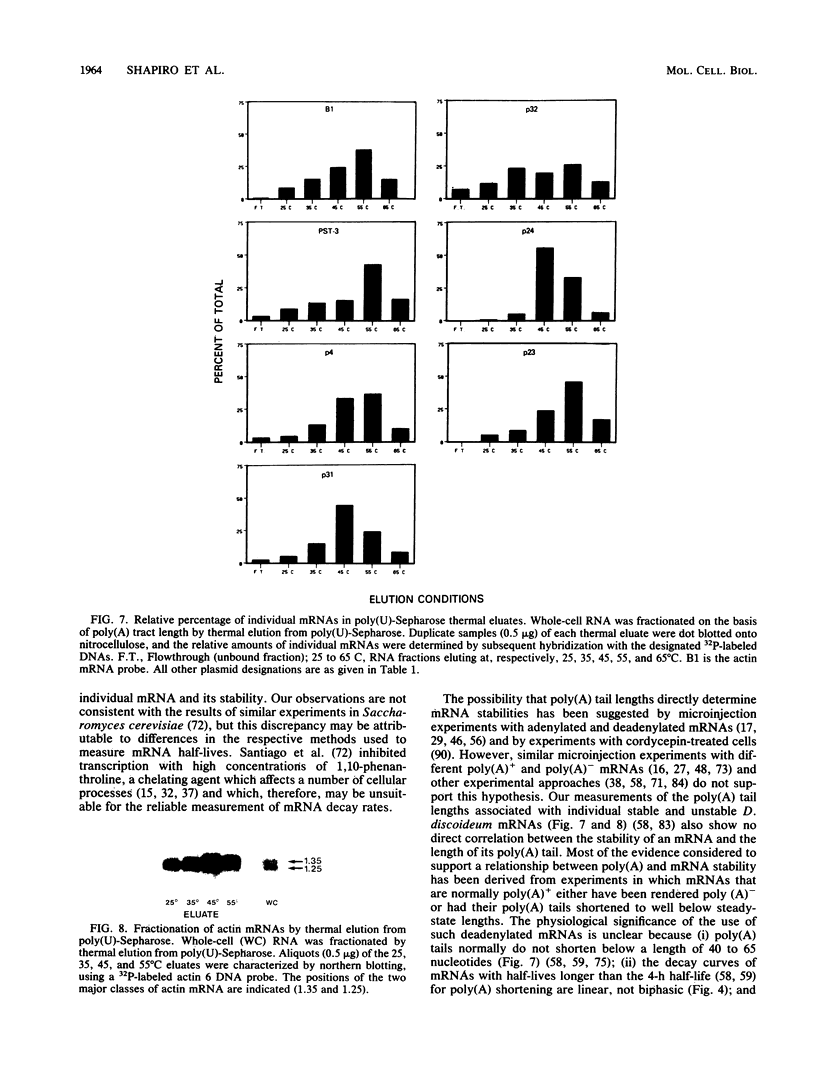
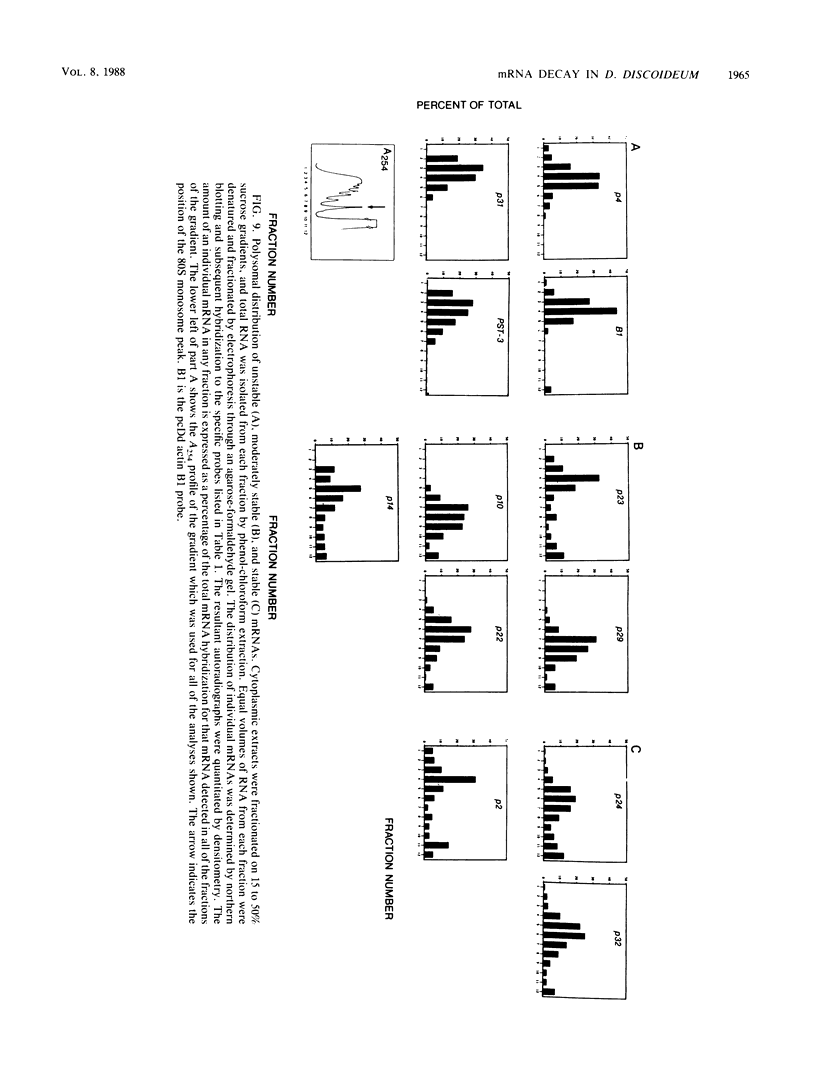
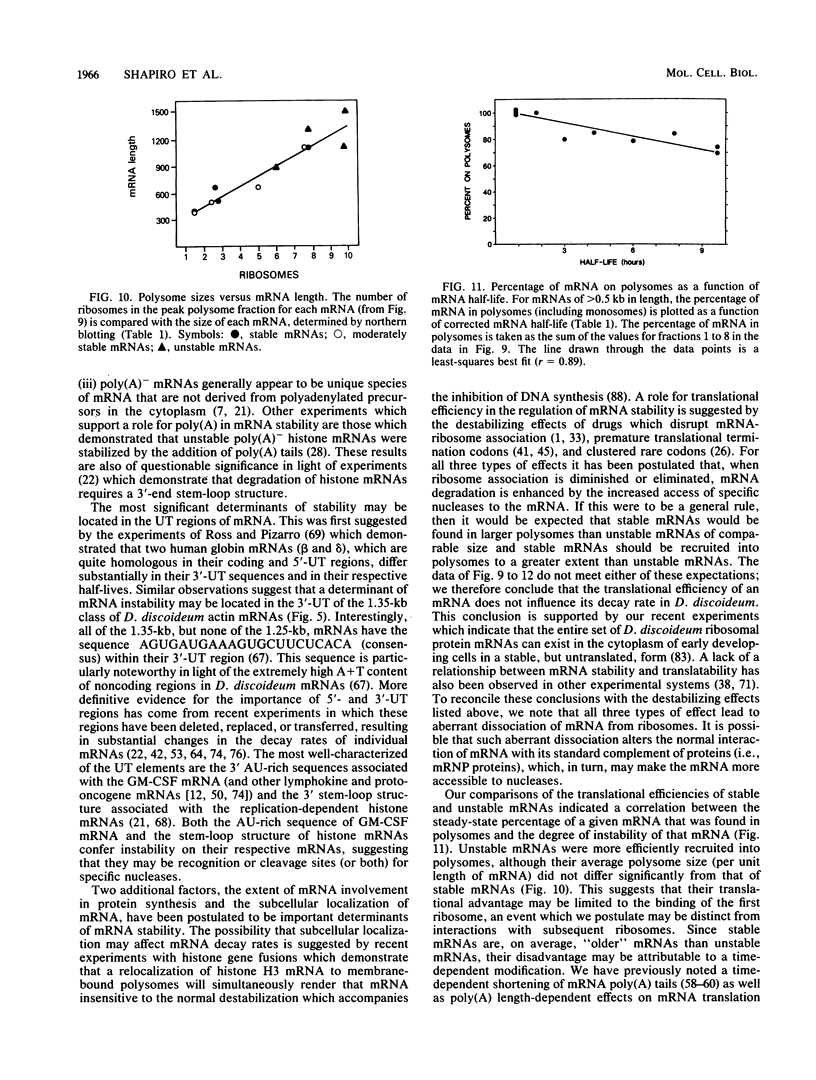
Abstract
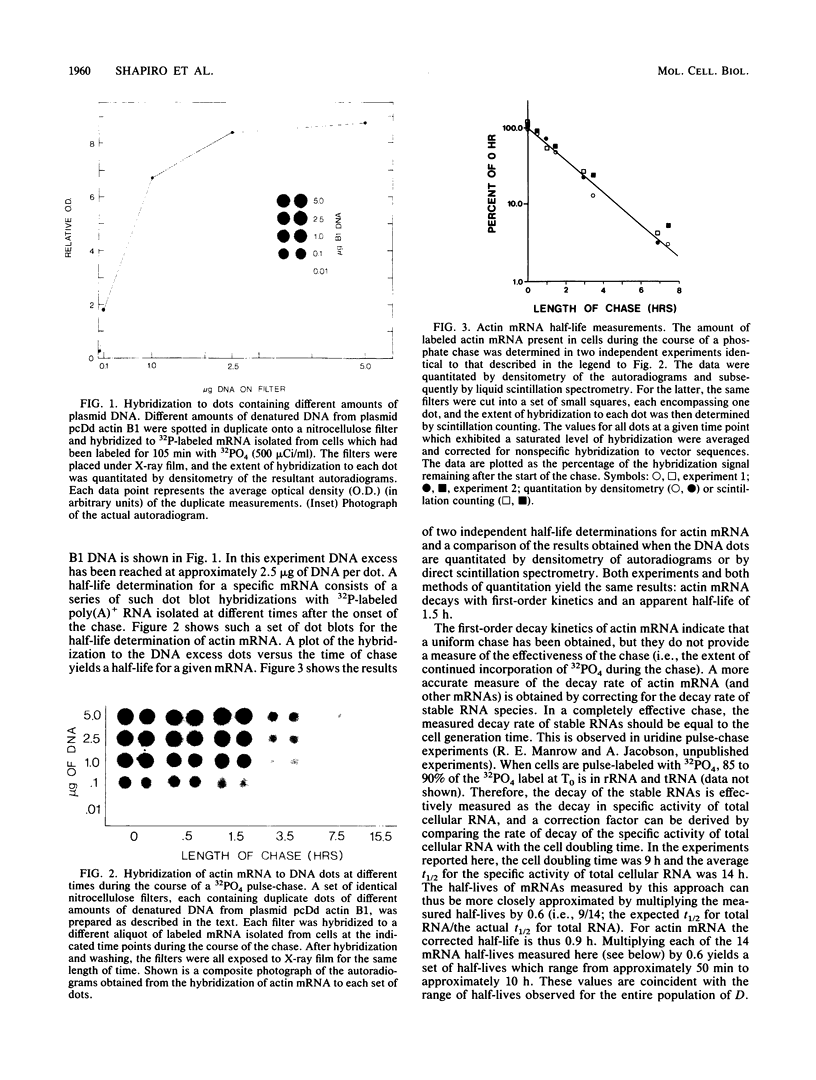
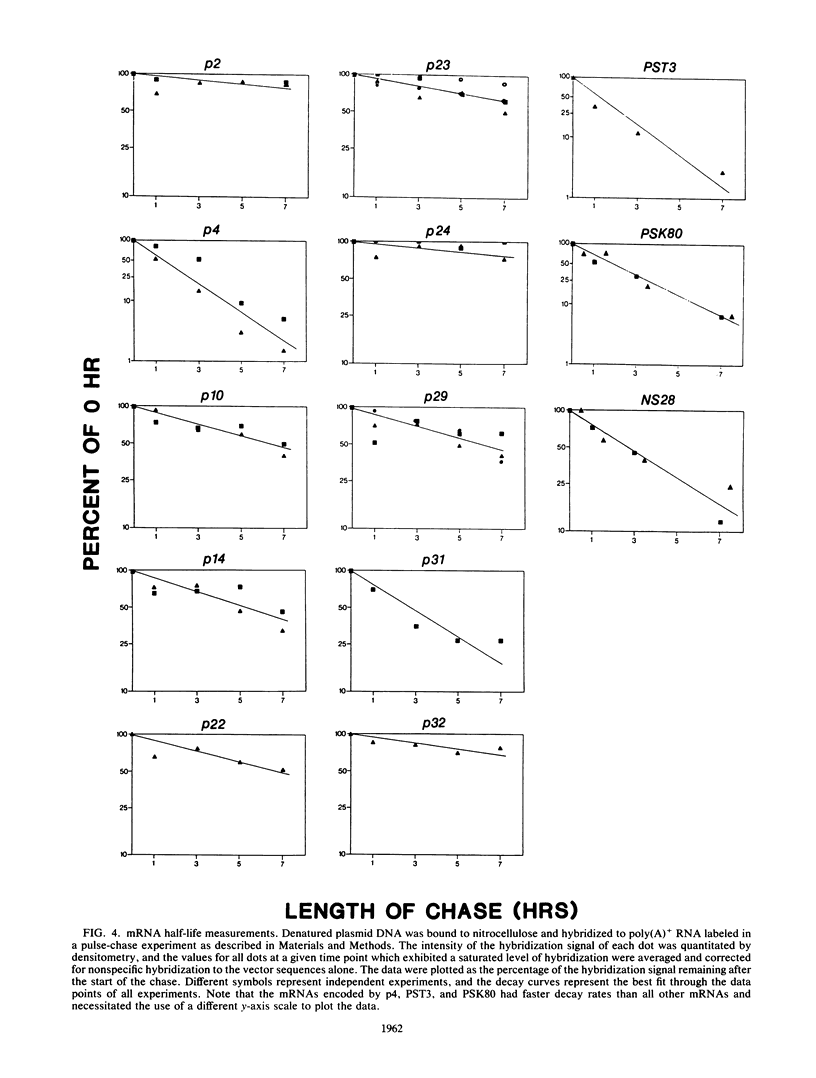
As an approach to understanding the structures and mechanisms which determine mRNA decay rates, we have cloned and begun to characterize cDNAs which encode mRNAs representative of the stability extremes in the poly(A)+ RNA population of Dictyostelium discoideum amoebae. The cDNA clones were identified in a screening procedure which was based on the occurrence of poly(A) shortening during mRNA aging. mRNA half-lives were determined by hybridization of poly(A)+ RNA, isolated from cells labeled in a 32PO4 pulse-chase, to dots of excess cloned DNA. Individual mRNAs decayed with unique first-order decay rates ranging from 0.9 to 9.6 h, indicating that the complex decay kinetics of total poly(A)+ RNA in D. discoideum amoebae reflect the sum of the decay rates of individual mRNAs. Using specific probes derived from these cDNA clones, we have compared the sizes, extents of ribosome loading, and poly(A) tail lengths of stable, moderately stable, and unstable mRNAs. We found (i) no correlation between mRNA size and decay rate; (ii) no significant difference in the number of ribosomes per unit length of stable versus unstable mRNAs, and (iii) a general inverse relationship between mRNA decay rates and poly(A) tail lengths. Collectively, these observations indicate that mRNA decay in D. discoideum amoebae cannot be explained in terms of random nucleolytic events. The possibility that specific 3'-structural determinants can confer mRNA instability is suggested by a comparison of the labeling and turnover kinetics of different actin mRNAs. A correlation was observed between the steady-state percentage of a given mRNA found in polysomes and its degree of instability; i.e., unstable mRNAs were more efficiently recruited into polysomes than stable mRNAs. Since stable mRNAs are, on average, "older" than unstable mRNAs, this correlation may reflect a translational role for mRNA modifications that change in a time-dependent manner. Our previous studies have demonstrated both a time-dependent shortening and a possible translational role for the 3' poly(A) tracts of mRNA. We suggest, therefore, that the observed differences in the translational efficiency of stable and unstable mRNAs may, in part, be attributable to differences in steady-state poly(A) tail lengths.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allende C. C., Allende J. E., Firtel R. A. The degradation of ribonucleic acids injected into Xenopus laevis oocytes. Cell. 1974 Jul;2(3):189–196. doi: 10.1016/0092-8674(74)90093-2. [DOI] [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Baker E. J., Keller L. R., Schloss J. A., Rosenbaum J. L. Protein synthesis is required for rapid degradation of tubulin mRNA and other deflagellation-induced RNAs in Chlamydomonas reinhardi. Mol Cell Biol. 1986 Jan;6(1):54–61. doi: 10.1128/mcb.6.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender W., Davidson N., Kindle K. L., Taylor W. C., Silverman M., Firtel R. A. The structure of M6, a recombinant plasmid containing Dictyostelium DNA homologous to actin messenger RNA. Cell. 1978 Nov;15(3):779–788. doi: 10.1016/0092-8674(78)90263-5. [DOI] [PubMed] [Google Scholar]
- Berger S. L., Cooper H. L. Very short-lived and stable mRNAs from resting human lymphocytes. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3873–3877. doi: 10.1073/pnas.72.10.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G. The Role of the poly(A) sequence in mammalian messenger RNA. CRC Crit Rev Biochem. 1981;10(1):1–38. doi: 10.3109/10409238109114634. [DOI] [PubMed] [Google Scholar]
- Brock M. L., Shapiro D. J. Estrogen stabilizes vitellogenin mRNA against cytoplasmic degradation. Cell. 1983 Aug;34(1):207–214. doi: 10.1016/0092-8674(83)90151-4. [DOI] [PubMed] [Google Scholar]
- Buell G. N., Wickens M. P., Payvar F., Schimke R. T. Synthesis of full length cDNAs from four partially purified oviduct mRNAs. J Biol Chem. 1978 Apr 10;253(7):2471–2482. [PubMed] [Google Scholar]
- Cabrera C. V., Lee J. J., Ellison J. W., Britten R. J., Davidson E. H. Regulation of cytoplasmic mRNA prevalence in sea urchin embryos. Rates of appearance and turnover for specific sequences. J Mol Biol. 1984 Mar 25;174(1):85–111. doi: 10.1016/0022-2836(84)90366-8. [DOI] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro M., Schibler U. Accumulation of rare and moderately abundant mRNAs in mouse L-cells is mainly post-transcriptionally regulated. J Mol Biol. 1984 Oct 5;178(4):869–880. doi: 10.1016/0022-2836(84)90316-4. [DOI] [PubMed] [Google Scholar]
- Casey L., Palatnik C. M., Jacobson A. Messenger RNA half-life in Dictyostelium discoideum. Dev Biol. 1983 Jan;95(1):239–243. doi: 10.1016/0012-1606(83)90023-4. [DOI] [PubMed] [Google Scholar]
- Chang C. H., Yarbro J. W., Mann D. E., Jr, Gautieri R. F. Effects of 1,10-phenanthroline and a zinc complex of 1,10-phenanthroline on nucleic acid synthesis in mouse liver and spleen. J Pharmacol Exp Ther. 1978 Apr;205(1):27–32. [PubMed] [Google Scholar]
- Deshpande A. K., Chatterjee B., Roy A. K. Translation and stability of rat liver messenger RNA for alpha 2 mu-globulin in Xenopus oocyte. The role of terminal poly(A). J Biol Chem. 1979 Sep 25;254(18):8937–8942. [PubMed] [Google Scholar]
- Drummond D. R., Armstrong J., Colman A. The effect of capping and polyadenylation on the stability, movement and translation of synthetic messenger RNAs in Xenopus oocytes. Nucleic Acids Res. 1985 Oct 25;13(20):7375–7394. doi: 10.1093/nar/13.20.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis H. L. Nogalamycin inhibits ribonucleic acid synthesis in growing and developing cells of the slime mold Dictyostelium discoideum. Antimicrob Agents Chemother. 1981 Apr;19(4):657–665. doi: 10.1128/aac.19.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., LaFiandra A., Shatkin A. J. 5'-Terminal structure and mRNA stability. Nature. 1977 Mar 17;266(5599):235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- Gay D. A., Yen T. J., Lau J. T., Cleveland D. W. Sequences that confer beta-tubulin autoregulation through modulated mRNA stability reside within exon 1 of a beta-tubulin mRNA. Cell. 1987 Aug 28;50(5):671–679. doi: 10.1016/0092-8674(87)90325-4. [DOI] [PubMed] [Google Scholar]
- Geoghegan T. E., McCoy L. Biogenesis and cell cycle relationship of poly(A)- actin mRNA in mouse ascites cells. Exp Cell Res. 1986 Jan;162(1):175–182. doi: 10.1016/0014-4827(86)90436-2. [DOI] [PubMed] [Google Scholar]
- Graves R. A., Pandey N. B., Chodchoy N., Marzluff W. F. Translation is required for regulation of histone mRNA degradation. Cell. 1987 Feb 27;48(4):615–626. doi: 10.1016/0092-8674(87)90240-6. [DOI] [PubMed] [Google Scholar]
- Green M. R., Maniatis T., Melton D. A. Human beta-globin pre-mRNA synthesized in vitro is accurately spliced in Xenopus oocyte nuclei. Cell. 1983 Mar;32(3):681–694. doi: 10.1016/0092-8674(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Hoekema A., Kastelein R. A., Vasser M., de Boer H. A. Codon replacement in the PGK1 gene of Saccharomyces cerevisiae: experimental approach to study the role of biased codon usage in gene expression. Mol Cell Biol. 1987 Aug;7(8):2914–2924. doi: 10.1128/mcb.7.8.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huez G., Cleuter Y., Bruck C., Van Vloten-Doting L., Goldbach R., Verduin B. Translational stability of plant viral RNAs microinjected into living cells. Influence of a 3'-poly(A) segment. Eur J Biochem. 1983 Jan 17;130(1):205–209. doi: 10.1111/j.1432-1033.1983.tb07137.x. [DOI] [PubMed] [Google Scholar]
- Huez G., Marbaix G., Gallwitz D., Weinberg E., Devos R., Hubert E., Cleuter Y. Functional stabilisation of HeLa cell histone messenger RNAs injected into Xenopus oocytes by 3'-OH polyadenylation. Nature. 1978 Feb 9;271(5645):572–573. doi: 10.1038/271572a0. [DOI] [PubMed] [Google Scholar]
- Huez G., Marbaix G., Hubert E., Leclercq M., Nudel U., Soreq H., Salomon R., Lebleu B., Revel M., Littauer U. Z. Role of the polyadenylate segment in the translation of globin messenger RNA in Xenopus oocytes. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3143–3146. doi: 10.1073/pnas.71.8.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A., Favreau M. Possible involvement of poly(A) in protein synthesis. Nucleic Acids Res. 1983 Sep 24;11(18):6353–6368. doi: 10.1093/nar/11.18.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Singer R. A. RNA synthesis and control of cell division in the yeast S. cerevisiae. Cell. 1978 Aug;14(4):951–958. doi: 10.1016/0092-8674(78)90349-5. [DOI] [PubMed] [Google Scholar]
- Kelly R., Shaw D. R., Ennis H. L. Role of protein synthesis in decay and accumulation of mRNA during spore germination in the cellular slime mold Dictyostelium discoideum. Mol Cell Biol. 1987 Feb;7(2):799–805. doi: 10.1128/mcb.7.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel A. R., Firtel R. A. A family of short, interspersed repeat sequences at the 5' end of a set of Dictyostelium single-copy mRNAs. Cell. 1979 Apr;16(4):787–796. doi: 10.1016/0092-8674(79)90094-1. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Cohen S. M., Loomis W. F., Lodish H. F. Developmental regulation of Dictyostelium discoideum actin gene fusions carried on low-copy and high-copy transformation vectors. Mol Cell Biol. 1986 Nov;6(11):3973–3983. doi: 10.1128/mcb.6.11.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurti C., Saryan L. A., Petering D. H. Effects of ethylenediaminetetraacetic acid and 1,10-phenanthroline on cell proliferation and DNA synthesis of Ehrlich ascites cells. Cancer Res. 1980 Nov;40(11):4092–4099. [PubMed] [Google Scholar]
- Krowczynska A., Yenofsky R., Brawerman G. Regulation of messenger RNA stability in mouse erythroleukemia cells. J Mol Biol. 1985 Jan 20;181(2):231–239. doi: 10.1016/0022-2836(85)90087-7. [DOI] [PubMed] [Google Scholar]
- Lengyel J. A., Penman S. Differential stability of cytoplasmic RNA in a Drosophila cell line. Dev Biol. 1977 Jun;57(2):243–253. doi: 10.1016/0012-1606(77)90212-3. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Lane C. Requirement for 7-methylguanosine in translation of globin mRNA in vivo. Nucleic Acids Res. 1978 Sep;5(9):3237–3247. doi: 10.1093/nar/5.9.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losson R., Lacroute F. Interference of nonsense mutations with eukaryotic messenger RNA stability. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5134–5137. doi: 10.1073/pnas.76.10.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher B., Stauber C., Schindler R., Schümperli D. Faithful cell-cycle regulation of a recombinant mouse histone H4 gene is controlled by sequences in the 3'-terminal part of the gene. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4389–4393. doi: 10.1073/pnas.82.13.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarotti G., Lefebvre P., Lodish H. F. Differences in the stability of developmentally regulated mRNAs in aggregated and disaggregated Dictyostelium discoideum cells. Dev Biol. 1982 Jan;89(1):82–91. doi: 10.1016/0012-1606(82)90296-2. [DOI] [PubMed] [Google Scholar]
- Maquat L. E., Kinniburgh A. J., Rachmilewitz E. A., Ross J. Unstable beta-globin mRNA in mRNA-deficient beta o thalassemia. Cell. 1981 Dec;27(3 Pt 2):543–553. doi: 10.1016/0092-8674(81)90396-2. [DOI] [PubMed] [Google Scholar]
- Marbaix G., Huez G., Burny A., Cleuter Y., Hubert E., Leclercq M., Chantrenne H., Soreq H., Nudel U., Littauer U. Z. Absence of polyadenylate segment in globin messenger RNA accelerates its degradation in Xenopus oocytes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3065–3067. doi: 10.1073/pnas.72.8.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolskee J. P., Lodish H. F. Half-lives of messenger RNA species during growth and differentiation of Dictyostelium discoideum. Dev Biol. 1980 Jan;74(1):37–49. doi: 10.1016/0012-1606(80)90051-2. [DOI] [PubMed] [Google Scholar]
- McCrae M. A., Woodland H. R. Stability of non-polyadenylated viral mRNAs injected into frog oocytes. Eur J Biochem. 1981 Jun 1;116(3):467–470. doi: 10.1111/j.1432-1033.1981.tb05359.x. [DOI] [PubMed] [Google Scholar]
- Meijlink F., Curran T., Miller A. D., Verma I. M. Removal of a 67-base-pair sequence in the noncoding region of protooncogene fos converts it to a transforming gene. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4987–4991. doi: 10.1073/pnas.82.15.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J. F., Wake S. A. An analysis of the rate of metallothionein mRNA poly(A)-shortening using RNA blot hybridization. Nucleic Acids Res. 1985 Nov 25;13(22):7929–7943. doi: 10.1093/nar/13.22.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Meyuhas O., Perry R. P. Relationship between size, stability and abundance of the messenger RNA of mouse L cells. Cell. 1979 Jan;16(1):139–148. doi: 10.1016/0092-8674(79)90195-8. [DOI] [PubMed] [Google Scholar]
- Morris T., Marashi F., Weber L., Hickey E., Greenspan D., Bonner J., Stein J., Stein G. Involvement of the 5'-leader sequence in coupling the stability of a human H3 histone mRNA with DNA replication. Proc Natl Acad Sci U S A. 1986 Feb;83(4):981–985. doi: 10.1073/pnas.83.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson G., Belasco J. G., Cohen S. N., von Gabain A. Growth-rate dependent regulation of mRNA stability in Escherichia coli. Nature. 1984 Nov 1;312(5989):75–77. doi: 10.1038/312075a0. [DOI] [PubMed] [Google Scholar]
- Nordstrom J. L., Roop D. R., Tsai M. J., O'Malley B. W. Identification of potential ovomucoid mRNA precursors in chick oviduct nuclei. Nature. 1979 Mar 22;278(5702):328–331. doi: 10.1038/278328a0. [DOI] [PubMed] [Google Scholar]
- Nudel U., Soreq H., Littauer U. Z. Globin mRNA species containing poly(A) segments of different lengths. Their functional stability in Xenopus oocytes. Eur J Biochem. 1976 Apr 15;64(1):115–121. doi: 10.1111/j.1432-1033.1976.tb10279.x. [DOI] [PubMed] [Google Scholar]
- Ouellett A. J., Malt R. A. Accumulation and decay of messenger ribonucleic acid in mouse kidney. Biochemistry. 1976 Jul 27;15(15):3358–3361. doi: 10.1021/bi00660a029. [DOI] [PubMed] [Google Scholar]
- Palatnik C. M., Storti R. V., Capone A. K., Jacobson A. Messenger RNA stability in Dictyostelium discoideum: does poly(A) have a regulatory role? J Mol Biol. 1980 Aug 5;141(2):99–118. doi: 10.1016/0022-2836(80)90379-4. [DOI] [PubMed] [Google Scholar]
- Palatnik C. M., Storti R. V., Jacobson A. Fractionation and functional analysis of newly synthesized and decaying messenger RNAs from vegetative cells of Dictyostelium discoideum. J Mol Biol. 1979 Mar 5;128(3):371–395. doi: 10.1016/0022-2836(79)90093-7. [DOI] [PubMed] [Google Scholar]
- Palatnik C. M., Storti R. V., Jacobson A. Partial purification of a developmentally regulated messenger RNA from Dictyostelium discoideum by thermal elution from poly(U)-sepharose. J Mol Biol. 1981 Aug 15;150(3):389–398. doi: 10.1016/0022-2836(81)90554-4. [DOI] [PubMed] [Google Scholar]
- Palatnik C. M., Wilkins C., Jacobson A. Translational control during early Dictyostelium development: possible involvement of poly(A) sequences. Cell. 1984 Apr;36(4):1017–1025. doi: 10.1016/0092-8674(84)90051-5. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA turnover in mouse L cells. J Mol Biol. 1973 Oct 5;79(4):681–696. doi: 10.1016/0022-2836(73)90071-5. [DOI] [PubMed] [Google Scholar]
- Rabbitts P. H., Forster A., Stinson M. A., Rabbitts T. H. Truncation of exon 1 from the c-myc gene results in prolonged c-myc mRNa stability. EMBO J. 1985 Dec 30;4(13B):3727–3733. doi: 10.1002/j.1460-2075.1985.tb04141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restifo L. L., Guild G. M. Poly(A) shortening of coregulated transcripts in Drosophila. Dev Biol. 1986 Jun;115(2):507–510. doi: 10.1016/0012-1606(86)90271-x. [DOI] [PubMed] [Google Scholar]
- Romans P., Firtel R. A. Organization of the Dictyostelium discoideum actin multigene family. Flanking sequences show subfamily homologies and unusual dyad symmetries. J Mol Biol. 1985 Jun 5;183(3):311–326. doi: 10.1016/0022-2836(85)90003-8. [DOI] [PubMed] [Google Scholar]
- Ross J., Peltz S. W., Kobs G., Brewer G. Histone mRNA degradation in vivo: the first detectable step occurs at or near the 3' terminus. Mol Cell Biol. 1986 Dec;6(12):4362–4371. doi: 10.1128/mcb.6.12.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Pizarro A. Human beta and delta globin messenger RNAs turn over at different rates. J Mol Biol. 1983 Jul 5;167(3):607–617. doi: 10.1016/s0022-2836(83)80101-6. [DOI] [PubMed] [Google Scholar]
- Rowekamp W., Firtel R. A. Isolation of developmentally regulated genes from Dictyostelium. Dev Biol. 1980 Oct;79(2):409–418. doi: 10.1016/0012-1606(80)90126-8. [DOI] [PubMed] [Google Scholar]
- Santiago T. C., Bettany A. J., Purvis I. J., Brown A. J. Messenger RNA stability in Saccharomyces cerevisiae: the influence of translation and poly(A) tail length. Nucleic Acids Res. 1987 Mar 25;15(6):2417–2429. doi: 10.1093/nar/15.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago T. C., Purvis I. J., Bettany A. J., Brown A. J. The relationship between mRNA stability and length in Saccharomyces cerevisiae. Nucleic Acids Res. 1986 Nov 11;14(21):8347–8360. doi: 10.1093/nar/14.21.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B., Soreq H., Tamm I. Does 3'-terminal poly(A) stabilize human fibroblast interferon mRNA in oocytes of Xenopus laevis? Proc Natl Acad Sci U S A. 1978 Oct;75(10):5030–5033. doi: 10.1073/pnas.75.10.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Sheiness D., Darnell J. E. Polyadenylic acid segment in mRNA becomes shorter with age. Nat New Biol. 1973 Feb 28;241(113):265–268. doi: 10.1038/newbio241265a0. [DOI] [PubMed] [Google Scholar]
- Simcox A. A., Cheney C. M., Hoffman E. P., Shearn A. A deletion of the 3' end of the Drosophila melanogaster hsp70 gene increases stability of mutant mRNA during recovery from heat shock. Mol Cell Biol. 1985 Dec;5(12):3397–3402. doi: 10.1128/mcb.5.12.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. H., Kessler-Icekson G. Stability of polyadenylated RNA in differentiating myogenic cells. Eur J Biochem. 1978 Aug 1;88(2):395–402. doi: 10.1111/j.1432-1033.1978.tb12461.x. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Messenger RNA in HeLa cells: kinetics of formation and decay. J Mol Biol. 1973 Aug 5;78(2):321–334. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Sive H. L., Heintz N., Roeder R. G. Regulation of human histone gene expression during the HeLa cell cycle requires protein synthesis. Mol Cell Biol. 1984 Dec;4(12):2723–2734. doi: 10.1128/mcb.4.12.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spradling A., Hui H., Penman S. Two very different components of messenger RNA in an insect cell line. Cell. 1975 Feb;4(2):131–137. doi: 10.1016/0092-8674(75)90119-1. [DOI] [PubMed] [Google Scholar]
- Steel L. F., Jacobson A. Ribosomal proteins are encoded by single copy genes in Dictyostelium discoideum. Gene. 1986;41(2-3):165–172. doi: 10.1016/0378-1119(86)90095-8. [DOI] [PubMed] [Google Scholar]
- Steel L. F., Jacobson A. Translational control of ribosomal protein synthesis during early Dictyostelium discoideum development. Mol Cell Biol. 1987 Mar;7(3):965–972. doi: 10.1128/mcb.7.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartwout S. G., Preisler H., Guan W. D., Kinniburgh A. J. Relatively stable population of c-myc RNA that lacks long poly(A). Mol Cell Biol. 1987 Jun;7(6):2052–2058. doi: 10.1128/mcb.7.6.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Zambetti G., Stein J., Stein G. Targeting of a chimeric human histone fusion mRNA to membrane-bound polysomes in HeLa cells. Proc Natl Acad Sci U S A. 1987 May;84(9):2683–2687. doi: 10.1073/pnas.84.9.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. Mutationally altered 3' ends of yeast CYC1 mRNA affect transcript stability and translational efficiency. J Mol Biol. 1984 Jul 25;177(1):107–135. doi: 10.1016/0022-2836(84)90060-3. [DOI] [PubMed] [Google Scholar]
- Zeevi M., Nevins J. R., Darnell J. E., Jr Newly formed mRNA lacking polyadenylic acid enters the cytoplasm and the polyribosomes but has a shorter half-life in the absence of polyadenylic acid. Mol Cell Biol. 1982 May;2(5):517–525. doi: 10.1128/mcb.2.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker C., Lodish H. F. Repetitive DNA sequences cotranscribed with developmentally regulated Dictyostelium discoideum mRNAs. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5386–5390. doi: 10.1073/pnas.78.9.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]