Abstract
Background
Infants with hyperammonemia can present with nonspecific findings so ordering an ammonia level requires a high index of suspicion. Renal replacement therapy (RRT) should be considered for ammonia concentrations >400 μmol/L since medical therapy will not rapidly clear ammonia. However, the optimal RRT prescription for neonatal hyperammonemia remains unknown. Hemodialysis (HD) and continuous renal replacement therapy (CRRT) are both effective, with differing risks and benefits.
Case-Diagnosis/Treatment
We present two neonates with hyperammonemia who were later diagnosed with ornithine transcarbamylase (OTC) deficiency and received high-dose CRRT. Using dialysis/replacement flow rates of 8000 ml/hr/1.73m2 (1000 ml/hr or four times higher than the typical rate used for acute kidney injury) the ammonia decreased to <400 μmol/L within 3 hours and <100 μmol/L within 10 hours of CRRT.
Conclusions
We propose a CRRT treatment algorithm to rapidly decrease the ammonia using collaboration between the emergency department, genetics, critical care, surgery/interventional radiology, and nephrology.
Keywords: hyperammonemia, ornithine transcarbamylase deficiency, dialysis, continuous renal replacement therapy
INTRODUCTION
Infants with hyperammonemia present with lethargy, hypotonia, and tachypnea [1]. Ammonia levels >150 μmol/L necessitate further diagnostic evaluation [2]. Worse outcome is associated with persistently elevated ammonia (>800 μmol/L) for >24 hours or prolonged coma [3–5]. Prompt treatment is critical to rapidly clear ammonia and minimize morbidity [2, 6].
Renal replacement therapy (RRT), including intermittent hemodialysis (HD) or continuous renal replacement therapy (CRRT), is considered for ammonia concentrations >400 μmol/L [1, 6, 7]. However, the optimal RRT prescription remains unknown. Intermittent HD achieves small molecule clearance with diffusion and removes ammonia more rapidly than a standard CRRT prescription used for acute kidney injury of 2000 ml/hr/1.73m2 [4]. CRRT employs diffusive and/or convective mechanisms and is traditionally favored in hemodynamically unstable, volume overloaded patients. While hyperammonemic infants are not volume overloaded, we speculated that CRRT would be preferred due to the high risks of hemodynamic instability from the underlying metabolic disorder, small patient size, and use of drugs that increase nitric oxide release (arginine) and lower systemic pressure [8]. In these patients with normal kidney function, CRRT also allows for easier management of potassium and phosphorus.
We present two cases of ornithine transcarbamylase (OTC) deficiency where the ammonia decreased rapidly using high-dose CRRT. We discuss the risks and benefits of both extracorporeal RRT modalities and present a standardized CRRT practice algorithm to improve outcomes for these rare, yet complex situations.
CASE REPORTS
Case 1
A 5 day old male (3 kg) found cyanotic and gasping was intubated for respiratory failure. Initial labs showed a bicarbonate of 12 mEq/L, creatinine 0.8 mg/dL, venous pH 7.07, pCO2 39 mm Hg, lactate 97.3 mg/dL, and ionized calcium (iCa) 1.76 mEq/L. A septic work-up was initiated and he was admitted to the neonatal intensive care unit (NICU). His ammonia level was 881 μmol/L and therapy with sodium benzoate, sodium phenylacetate, arginine, 12% dextrose with sodium acetate, and insulin was started.
After failed placement of two smaller femoral catheters, surgery inserted a single double lumen 7 French right internal jugular catheter. Because of hemodynamic instability with the need for dopamine, continuous veno-venous hemodialysis was initiated using a Prisma™ M60 filter (Gambro Renal Products, Lakewood, Colorado) with blood flow of 30 ml/min and high-dose dialysate flow of 1000 ml/hr (8650 ml/hr/1.73m2). The dialysate used was Normocarb(TM) HF solution 25 (Dialysis Solutions, Inc, Ontario), containing 35 mEq/L bicarbonate, 2 mEq/L potassium chloride, and 1 mEq/L potassium phosphate. The circuit was primed with packed red blood cells and sodium bicarbonate was used to prevent bradykinin release syndrome [9]. Citrate anticoagulation was started at 50% of the normal rate due to concern for decreased hepatic metabolism [4]. Prior to CRRT, the ammonia had peaked at 1454 μmol/L. After 2 hours of RRT, the ammonia had decreased to 367 μmol/L (Figure 1). CRRT was discontinued after 10 hours (ammonia 92 μmol/L) with a peak rebound level to 149 μmol/L. The clinical course was complicated by femoral artery thrombosis with compartment syndrome requiring fasciotomy. Although his long-term neurologic outcome is unknown, he regained spontaneous movements one hour after CRRT initiation.
Figure 1.
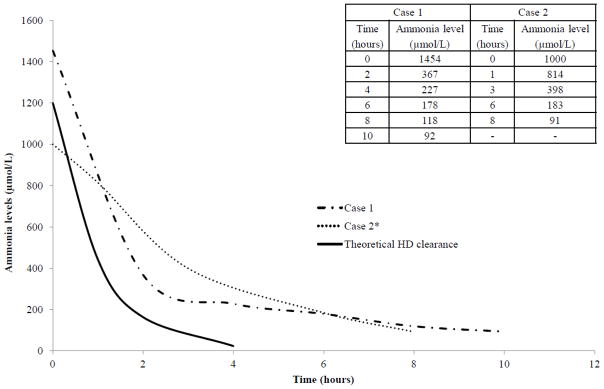
Our patients’ continuous renal replacement therapy (CRRT) prescription produced clearance comparable to HD, without significant ammonia rebound, hypotension, or electrolyte abnormalities. The actual decrease in ammonia concentration for Case 1 and Case 2 (both treated with high-dose CRRT) is compared to the theoretical clearance over time that can be achieved with intermittent hemodialysis (HD). The theoretical HD clearance was based on a blood flow of 30 ml/min, an initial ammonia level of 1200 μmol/l, a patient weight of 3 kg (total body water of 0.6 ml/kg), and an ammonia clearance = −natural log (concentration at time X/concentration at time zero). Because in Case 2, the first circuit clotted after 45 minutes due to mechanical obstruction of the RRT catheter, we illustrate the decrease in ammonia after the start of the second circuit. We assumed that the initial ammonia level was 1000 μmol/l based on the last exact value (1043 μmol/l) one hour prior to initiation of the second circuit (the ammonia level fifteen minutes before the start of the second circuit was reported as >1000 μmol/l as the lab did not dilute the sample to obtain the exact concentration). In both cases, the ammonia fell to <200 μmol/l within 6 hours of CRRT initiation.
Case 2
A 6 day old male (3.7 kg) presented with poor feeding, vomiting, and somnolence. On arrival, he was hypothermic with profound tachypnea. Labs showed a normal complete blood count, venous pH of 7.48, iCa of 2.58 mEq/L, a serum bicarbonate of 23 mEq/L, and a creatinine of 0.6 mg/dL. A septic work-up was initiated and on NICU admission, his ammonia level (drawn 7 hours after presentation) was 776 μmol/L. Intravenous arginine, sodium phenylacetate, and sodium benzoate were started.
Surgery placed a right internal jugular 7.5 French dialysis catheter and high-dose continuous veno-venous hemodiafiltration was initiated 15 hours after presentation with a Prismaflex™ M60 filter. Prismasate™ (Gambro Renal Products) dialysate contained 4 mEq/L potassium, 32 mEq/L bicarbonate, and 2 mEq/L sodium phosphate. The blood flow was 40 ml/min, dialysate flow was 900 ml/hr, and replacement flow was 100 ml/hr for a total of 1000 ml/hr (7700 ml/hr/1.73m2). The ammonia prior to CRRT was 1387 μmol/L. A red blood cell prime was used with bicarbonate to help prevent bradykinin release syndrome [9]. The first circuit with heparin anticoagulation clotted from a mechanical catheter obstruction 45 minutes after initiation and was restarted using full-dose citrate anticoagulation (ammonia level remained >1000 μmol/L prior to restart, Figure 1). Seven hours later, the ammonia level had dropped to 90 μmol/L and the second circuit was stopped due to a grade I intraventricular hemorrhage. Over the next 10 hours, without further RRT, his ammonia peaked at 402 μmol/L, but remained <100 μmol/L over the next 24 hours with continuation of medical therapy. The patient underwent liver transplantation 6 months later and currently is thriving.
DISCUSSION
Neonatal hyperammonemia requires prompt intervention to reduce neurological damage. Decreasing ammonia production by reversing catabolism is extremely important. Pharmacological management with sodium phenylacetate and sodium benzoate in conjunction with arginine and full caloric support may improve survival [6]. When the ammonia is significantly elevated (>400 μmol/L), RRT is needed to achieve rapid clearance [1]. Renal replacement therapy with HD or CRRT can achieve ammonia clearance even alone or in combination with medical therapy.
The optimal dose or preferred modality remains poorly defined. CRRT may decrease ammonia rebound compared to short duration, intermittent HD, due to the continuous nature of the therapy [2]. CRRT may offer additional advantages over HD such as electrolyte replacement that can be safely mixed in the dialysate/replacement fluid by pharmacy to maintain normal phosphorus and potassium levels.
In patients treated initially with HD for inherited metabolic disorders, dialysate rates of 500 ml/min have successfully reduced toxin levels [10], but many patients require additional CRRT for metabolite rebound [1, 11]. McBryde et al. [11] speculated that initial HD treatment may improve outcomes compared to CRRT but they noted a high risk of ammonia rebound in patients treated initially with HD. Furthermore, their CRRT clearance rates (2000 ml/hr/1.73m2) were only 25% of those used in our patients. Using CRRT as the initial therapy may avoid the risk of treatment delays from switching dialysis modalities. However, CRRT is not without risks including the bradykinin release syndrome seen especially with AN 69-membranes [9]. This risk can be lowered by normalizing the pH of the blood prime in the circuit [9].
To quickly decrease the ammonia level, collaboration between the emergency department, genetics, critical care, surgery/interventional radiology, and nephrology is essential. We recommend checking a serum ammonia level in infants presenting with unexplained lethargy, hypotonia, apnea, or seizures. An ammonia level >150 μmol/L should prompt emergent consultation with genetics/metabolism and critical care. If RRT is needed, general surgery/interventional radiology and nephrology should be consulted. The patient should be emergently transferred to an institution with the capability and expertise to perform RRT and place neonatal vascular access. The ultimate choice of RRT modality (HD versus CRRT) depends on the expertise and availability of specific resources at the treating institution. Medical management should begin immediately to prevent further increases in the ammonia level. However, since pharmacological therapy is commonly known to take more than 24 hours to effectively control ammonia, dialysis planning should begin at ammonia levels more than 400 μmol/L.
For RRT initiation, a 7 French double lumen right internal jugular dialysis catheter should be inserted. Five French catheters should be avoided [12]. We recommend a CRRT blood flow of 30–50 ml/min with citrate anticoagulation dosed at half the usual rate (0.75 ml/hr times the blood flow in ml/min) to account for decreased hepatic metabolism, precluding the need for systemic heparinization [9]. No net fluid should be removed and dialysate/replacement fluid should contain 4 mEq/L of potassium and 2 mEq/L of phosphate, adjusted to the patient’s electrolytes. During CRRT, iCa (patient and circuit), serum electrolytes, platelets, and ammonia should be monitored every 1–2 hours [1]. CRRT should continue until the ammonia level is 100–200 μmol/L [2].
To the best of our knowledge, only two studies have rigorously assessed the clearance characteristics of extracorporeal therapy in neonates, both with maple syrup urine disease [10, 13]. Our in vivo data supports the rapid ammonia clearance that can be achieved with high-dose CRRT (Figure 1). Our cases had a rapid decrease in ammonia using high-dose CRRT with dialysis/replacement flow rates of 8000 ml/hr/1.73m2 (1000 ml/hr), 4 times higher than flows used to manage acute kidney injury [9] and other inborn errors of metabolism [10]. We selected the CRRT dose based on small solute clearance calculations to match the clearance predicted with intermittent HD (Figure 1). With intermittent HD, clearance is limited by blood flow rates. In our model, assuming a blood flow of 30 ml/min (which is well tolerated by most infants) would result in a small solute clearance of 1800 ml/hr with HD. This is comparable to the clearance we observed in our patients treated with CRRT (1000 ml/hr).
Others studies have suggested CRRT clearance rates of 5 liters/hr without validating the recommendations [2, 9]. We believe CRRT clearance rates of 100–600 ml/hr [4, 14, 15] are less effective because of the time necessary to decrease ammonia [4]. Although controlled trials do not exist, a prospective study demonstrated that slower reductions in ammonia levels may worsen long-term outcomes [5]. We support the initial use of high-flow CRRT to rapidly clear ammonia, decrease rebound, safely replace electrolytes, eliminate the need for modality switching, and limit further hemodynamic instability. While our patients did have a small rebound in ammonia after stopping CRRT, neither patient required subsequent RRT. Further study is warranted to see if even higher CRRT rates may be beneficial to more closely mimic the rapid clearance that can be achieved with HD.
Acknowledgments
We thank Kimberly Windt, RN for providing outstanding care to our patient. Dr. Laskin is supported by a Career Development grant in Comparative Effectiveness Research (1KM1CA156715-01). Dr. Stuart Goldstein reports the following conflicts of interest from Gambro and Baxter. He currently is a consultant, expert panel member, and has received grant support from Gambro. He is also a consultant and has received grant support from Baxter. These sponsors played no role in the study design, collection, analysis, and interpretation of data, writing of the report, or the decision to submit the paper for publication.
References
- 1.Mathias RS, Kostiner D, Packman S. Hyperammonemia in urea cycle disorders: role of the nephrologist. Am J Kidney Dis. 2001;37:1069–1080. doi: 10.1016/s0272-6386(05)80026-5. [DOI] [PubMed] [Google Scholar]
- 2.Picca S, Bartuli A, Dionisi-Vici C. Medical management and dialysis therapy for the infant with an inborn error of metabolism. Semin Nephrol. 2008;28:477–480. doi: 10.1016/j.semnephrol.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Pela I, Seracini D, Donati MA, Lavoratti G, Pasquini E, Materassi M. Peritoneal dialysis in neonates with inborn errors of metabolism: is it really out of date? Pediatr Nephrol. 2008;23:163–168. doi: 10.1007/s00467-007-0607-y. [DOI] [PubMed] [Google Scholar]
- 4.Bunchman TE, Barletta GM, Winters JW, Gardner JJ, Crumb TL, McBryde KD. Phenylacetate and benzoate clearance in a hyperammonemic infant on sequential hemodialysis and hemofiltration. Pediatr Nephrol. 2007;22:1062–1065. doi: 10.1007/s00467-007-0436-z. [DOI] [PubMed] [Google Scholar]
- 5.Msall M, Batshaw ML, Suss R, Brusilow SW, Mellits ED. Neurologic outcome in children with inborn errors of urea synthesis. Outcome of urea-cycle enzymopathies. N Engl J Med. 1984;310:1500–1505. doi: 10.1056/NEJM198406073102304. [DOI] [PubMed] [Google Scholar]
- 6.Enns GM, Berry SA, Berry GT, Rhead WJ, Brusilow SW, Hamosh A. Survival after treatment with phenylacetate and benzoate for urea-cycle disorders. N Engl J Med. 2007;356:2282–2292. doi: 10.1056/NEJMoa066596. [DOI] [PubMed] [Google Scholar]
- 7.Schaefer F, Straube E, Oh J, Mehls O, Mayatepek E. Dialysis in neonates with inborn errors of metabolism. Nephrol Dial Transplant. 1999;14:910–918. doi: 10.1093/ndt/14.4.910. [DOI] [PubMed] [Google Scholar]
- 8.Fakler CR, Kaftan HA, Nelin LD. Two cases suggesting a role for the L-arginine nitric oxide pathway in neonatal blood pressure regulation. Acta Paediatr. 1995;84:460–462. doi: 10.1111/j.1651-2227.1995.tb13673.x. [DOI] [PubMed] [Google Scholar]
- 9.Bunchman TE, Brophy PD, Goldstein SL. Technical considerations for renal replacement therapy in children. Semin Nephrol. 2008;28:488–492. doi: 10.1016/j.semnephrol.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Phan V, Clermont MJ, Merouani A, Litalien C, Tucci M, Lambert M, Mitchell G, Jouvet P. Duration of extracorporeal therapy in acute maple syrup urine disease: a kinetic model. Pediatr Nephrol. 2006;21:698–704. doi: 10.1007/s00467-006-0044-3. [DOI] [PubMed] [Google Scholar]
- 11.McBryde KD, Kershaw DB, Bunchman TE, Maxvold NJ, Mottes TA, Kudelka TL, Brophy PD. Renal replacement therapy in the treatment of confirmed or suspected inborn errors of metabolism. J Pediatr. 2006;148:770–778. doi: 10.1016/j.jpeds.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Hackbarth R, Bunchman TE, Chua AN, Somers MJ, Baum M, Symons JM, Brophy PD, Blowey D, Fortenberry JD, Chand D, Flores FX, Alexander SR, Mahan JD, McBryde KD, Benfield MR, Goldstein SL. The effect of vascular access location and size on circuit survival in pediatric continuous renal replacement therapy: a report from the PPCRRT registry. Int J Artif Organs. 2007;30:1116–1121. doi: 10.1177/039139880703001212. [DOI] [PubMed] [Google Scholar]
- 13.Puliyanda DP, Harmon WE, Peterschmitt MJ, Irons M, Somers MJ. Utility of hemodialysis in maple syrup urine disease. Pediatr Nephrol. 2002;17:239–242. doi: 10.1007/s00467-001-0801-2. [DOI] [PubMed] [Google Scholar]
- 14.Wong KY, Wong SN, Lam SY, Tam S, Tsoi NS. Ammonia clearance by peritoneal dialysis and continuous arteriovenous hemodiafiltration. Pediatr Nephrol. 1998;12:589–591. doi: 10.1007/s004670050511. [DOI] [PubMed] [Google Scholar]
- 15.Chen CY, Tsai TC, Lee WJ, Chen HC. Aminograms during continuous hemodiafiltration in the treatment of hyperammonemia due to ornithine transcarbamylase deficiency. Ren Fail. 2007;29:661–665. doi: 10.1080/08860220701459618. [DOI] [PubMed] [Google Scholar]


