Abstract
A third signal is required for maturation of effector CD8 CTL in addition to TCR and CD28 engagement. Inflammatory cytokines can provide a third signal however in non-pathogen settings (i.e. anti-tumor responses), the identity of the third signal is not clear. A useful model for in vivo CD8 CTL in the absence of exogenous pathogens is the alloantigen driven parent-into F1 model of acute graft-vs.-host disease (GVHD) characterized by a strong TNF-dependent donor anti-host CD8 CTL T cell response. To determine whether TNF acts directly on donor T cells in a signal 3 manner, F1 mice received TNF receptor p55 knock out (KO) and/or p55 KO donor T cells. Donor p75 K but not p55 KO donor T cells failed to induce acute GVHD phenotype and instead induced a lupus-like chronic GVHD both short and long term due to quantitative and qualitative donor T cell defects i.e. reduced perforin, IFN-g and TNF production. Transfer of mixed or matched purified CD4 and CD8 T cells from WT or p75KO donors demonstrated that optimal CTL maturation required p75 signaling in both CD4 and CD8 T cells. Despite defective p75 KO CD4 help for CD8 CTL, p75KO CD4 help for B cells and autoimmunity was intact. These results provide a mechanism by which impaired CD8 CTL could contribute to reduced anti-viral and anti-tumor responses and autoimmunity reported in patients receiving TNF blockers. Our results support the idea that selective p55 blockade may be beneficial by reducing inflammation without compromising CD8 CTL.
Keywords: graft-vs.-host disease, CD8 T cells, cytotoxic T cells, tumor necrosis factor
Introduction
In addition to TcR and CD28 mediated signals, CD8 T cells require a third signal to mature into effector CTL and avoid apoptosis (1, 2). In pathogen models, IL-12 and IFN-a have been shown to act in a signal 3 manner and directly promote CD8 CTL effector function, however the relative importance of either molecule varies with the pathogen (3). CD8 CTL play an important role in elimination of certain pathogens, typically accompanied by activation of the innate immune system and endogenous IL-12 or IFN-a. However, CD8 CTL arising in the non-pathogen setting (i.e. anti-tumor responses) likely occurs in the absence of IL-12 and IFN-a and may therefore use other molecules as a third signal.
A useful model for generating in vivo CD8 CTL in the absence of pathogen infection is the parent-into-F1 (p→F1) model in which homozygous parental strain T cells are transferred into normal non-irradiated F1 mice (reviewed in (4, 5). For example, the transfer of C57Bl/6 (B6) splenocytes into B6D2F1 hosts (B6→BDF1) initially results in donor CD4 T cell recognition of F1 host allogeneic MHC II and the provision of help to: 1) F1 B cells that then undergo polyclonal activation and expansion over the next seven days; and 2) donor (B6) CD8 T cells that recognize allogeneic host MHC I, mature into CTL effectors and reverse host splenic lymphoproliferation by eliminating host splenocytes from days 7–14 after transfer (5).
Using this model, we have previously demonstrated that in vivo TNF blockade during the first week after donor cell transfer completely abrogated donor anti-host CD8 CTL maturation and converted two week phenotype from acute to chronic GVHD (6). TNF blockade after day 7 did not alter acute GVHD phenotype (6), supporting the idea that TNF has a role in donor CD8 CTL maturation, possibly as a signal 3 function, separate from its well known effector role.
TNF signaling in T cells results from TNF binding to one of two receptors: 1) TNF receptor 1 (TNFR1) or p55 expressed on nearly all cells and primarily associated with the inflammatory and apoptotic activity of TNF; and 2) TNFR2 or p75 whose expression is restricted to a subpopulation of immune cells and other cells and is involved in T cell survival and costimulation (7–9). To determine whether the requirement for TNF in CD8 CTL maturation in p→F1 acute GVHD mice requires TNF signaling directly on donor T cells consistent with a signal 3 role, (as opposed to acting indirectly through host APC), we used the B6→BDF1 model of anti-F1 CD8 CTL generation and transferred donor T cells deficient in TNFR p75 and/or p55 into wild type (TNFR intact) BDF1 hosts. Our results indicate a critical role for p75 signaling on donor CD4 and CD8 T cells for optimal CD8 effector CTL maturation.
Materials and Methods
Mice
6–8 week old male wild type C57Bl/6 (B6 WT)(H-2b); TNFR p55 deficient B6.Tnfrsf1atm1Imx/J (p55KO), p75 deficient B6.Tnfrsf1btm1Imx/J (p75KO) or double deficient B6.Tnfrsf1atm1ImxTnfrsf1btm1Imx/J (p55/75KO) (all H-2b), and B6D2F1 (BDF1) (H-2b/d) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All animal procedures were pre-approved by the Institutional Animal Care and Use Committee at the Uniformed Services University of Health Sciences.
Induction of GVHD
Single cell suspensions of B6 WT or TNFR deficient donor splenocytes were prepared as described (10) and transferred into age matched BDF1 hosts by tail vein injection. The percentages of donor CD4 and CD8 T cells populations were analyzed by flow cytometry and donor inoculum adjusted prior to transfer to achieve the desired amount of donor CD4 and CD8 T cells as indicated in the text and respective figure legends. In some experiments, acute GVHD was induced using purified donor T cell subsets achieved through negative selection using Dynal mouse CD4 or CD8 negative isolation kits (Invitrogen, Carlsbad, CA) which deplete B cells, NK cells, monocytes/macrophages, dendritic cells, granulocytes, platelets, erythrocytes and either CD8 or CD4 respectively. Purity was confirmed by flow cytometry and was typically > 95%.
Flow cytometric analysis
Spleen cells were first incubated with anti-murine Fcγ receptor II/III mAb, 2.4G2 for 10 min and then stained with saturating concentrations of Alexa Fluor 488-conjugated, APC-conjugated, biotin-conjugated, PE-conjugated, FITC-conjugated, PerCPCy5.5-conjugated, and Pacific Blue-conjugated mAb against CD4, CD8, B220, H-2Kdd, I-Ad, CD11b and CD11c purchased from either BD Biosciences (San Jose, CA), BioLegend (San Diego, CA), eBioscience (San Diego, CA), or Invitrogen (Carlsbad, CA). Biotinylated primary mAb were detected using PE-Texas Red-streptavidin (BD Biosciences, San Jose, CA). Cells were fixed in 1% paraformaldehyde before reading.
Ex vivo intracellular staining for perforin, IFN-g and TNF was performed using antibodies and reagents purchased from BD PharMingen (San Diego, CA) or Biolegend (San Diego, CA) and staining performed according to the manufacturer’s instructions. Importantly, there was no in vitro re-stimulation or use of Golgi blocking agents. Following completion of the staining protocol, cells were analyzed by flow cytometry immediately.
Multi-color flow cytometric analyses were performed using a BD LSRII flow cytometer (BD Biosciences, San Jose, CA). Gating strategy: lymphocytes were gated by forward and side scatter and fluorescence data were collected for a minimum of 10,000 gated cells. Studies of donor T cells were performed on a minimum of 5,000 cells collected using a lymphocyte gate that was positive for CD4 or CD8 and negative for MHC class I of the uninjected parent (H-2Kd negative). B cells were gated as positive for B220 and either positive (host origin) or negative (donor origin) for MHC Class II of the uninjected parent (I-Ad). Short lived effector CD8 CTL (SLEC) were assessed as KRLG-1 positive, CCR7 negative gated donor CD8 T cells. Host DC and macrophages were identified as I-Ad positive and CD11c or CD11b positive respectively using a broad forward and side scatter gate.
Cytokine Expression by Real Time PCR
Real time PCR was performed as described (11). Briefly, splenocytes (1 × 107) were homogenized in 1 ml of RNA-STAT-60 (Tel-Test, Friendswood, TX). cDNA was synthesized from mRNA using TaqMan® Reverse Transcription Reagents kit (Applied Biosystems, Foster City, CA). Real-time PCR was performed using pre-made primers and probes from TaqMan® Gene Expression Assays and TaqMan® Universal PCR Master Mix (Applied Biosystems) for the following targets: IFN-g, IL-10, IFN-g and IP10 with 18s rRNA as an internal control. The calculation of relative gene expression differences was done by comparative 2−ΔΔCT method. The result was expressed as fold change in the experimental groups compared to uninjected B6D2F1 control. Mice were tested individually and values shown as group mean ± SE.
Kidney Histopathology
Kidney tissue was fixed in 10% buffered formalin and processed for routine paraffin embedding and histological sectioning. Three-micron-thick sections, stained with periodic acid-Schiff stains, were blindly scored by a renal pathologist (MH). The severity of proliferative glomerulonephritis were determined according to a previously described semi quantitative (1+ to 4+) scoring system developed for a murine model of lupus nephritis (12, 13).
Serological studies
Mice were bled at the times indicated and sera tested by ELISA for the presence of IgG antibodies to ssDNA as described (11).
Statistical Analysis
Statistical comparisons (t test and Anova) were performed using Prism 5.0 (Graphpad Software, San Diego, CA).
Results
Acute GVHD phenotype at day 14 is absolutely dependent on donor T cell signaling through the p75 receptor
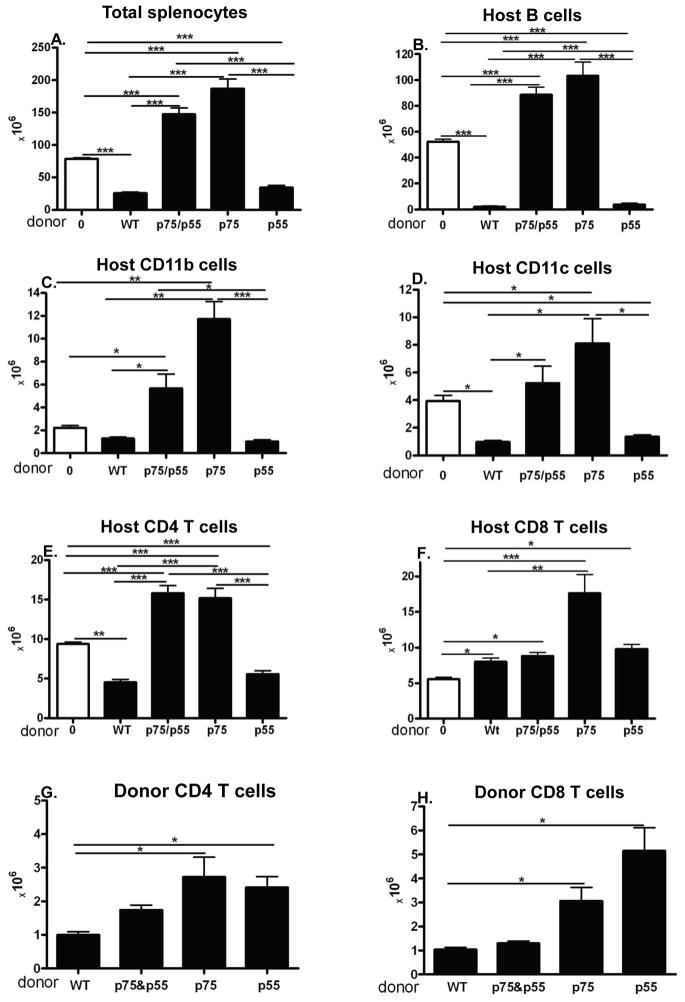
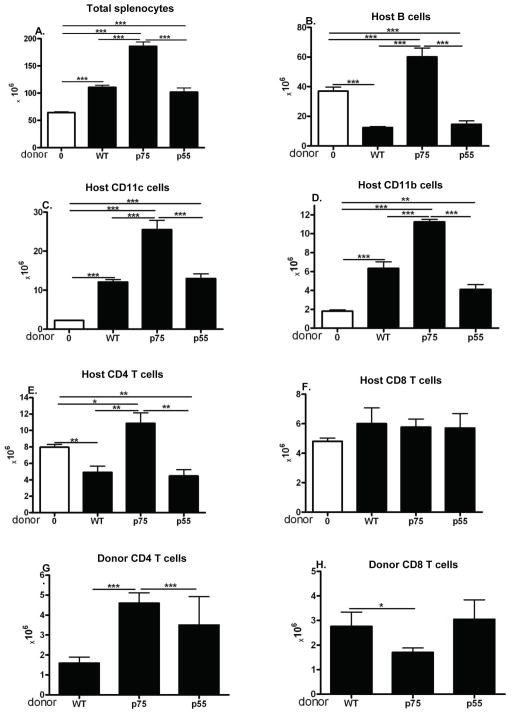
At 14 days after donor cell transfer, B6→F1 acute GVHD mice exhibit a cytotoxic phenotype characterized by significant engraftment of both CD4 and CD8 donor T cells and profound elimination of host splenocytes mediated by donor CD8 CTL specific for host MHC I (reviewed in (5, 14). By contrast, DBA→F1 chronic GVHD mice exhibit a stimulatory phenotype at day 14 characterized by substantial engraftment of CD4 but not CD8 donor T cells and significant expansion of host B cells, T cells and APC vs. control F1 mice. To address whether signaling through the donor T cell TNFR is required for donor CD8 CTL maturation and the development of a cytotoxic phenotype, F1 mice received donor T cells deficient in either p55, p75 or both p55/p75 TNFR and phenotype assessed at day 14. Donor splenocytes were adjusted so that all mice received comparable numbers of CD8 T cells. Compared to uninjected control F1 mice (Fig. 1A–1F, column 1 from left), WT→F1 acute GVHD mice (Fig. 1A–1F, column 2 from left) exhibit the characteristic day 14 cytotoxic phenotype i.e. significant reductions in total splenocytes (Fig. 1A), host B cells (Fig. 1B), CD11b+ APC (Fig. 1C), conventional (CD11c+) dendritic cells (cDC)(Fig. 1D) and CD4 T cells (Fig. 1E). Host CD8 T cells are not significantly reduced vs. uninjected control F1 mice (Fig. 1F) consistent with their greater resistance to elimination by donor CTL relative to other host splenocyte populations (15). As expected, WT donor CD4 and CD8 T cells are present in roughly equivalent numbers (Figs. 1G and 1H, column 1). The pattern seen for WT→F1 mice was not significantly altered in p55KO→F1 mice (Figs. 1A–F, column 5) with the exception of greater donor CD8 T cell engraftment (Fig. 1H) consistent with a vigorous donor anti-host response (16). By contrast, p75KO→F1 mice exhibit a significant increase in all host splenocyte populations not only compared to WT→F1 but also and importantly compared to uninjected control F1 mice (Figs. 1A–F, column 4). The increase in host cells seen for p75KO→F1 mice indicates that initial donor CD4 driven host expansion from days 1–7 (15) is present; however the elimination phase from days 7–14 mediated by donor CD8 CTL is defective consistent with a stimulatory phenotype and suggestive of chronic GVHD long term. The results for p55/p75 KO→F1 mice (Fig. 1, column 3) either mirror those of p75KO→F1 mice (Figs. 1A, 1B, 1D and 1E) or are intermediate between WT→F1 and p75KO→F1 (Figs. 1C, 1F). In the p→F1 model, donor T cell engraftment peaks at ~day 10 followed by contraction, particularly for donor CD8 T cells (15). The significantly greater engraftment of donor CD4 and CD8 T cells in p75KO→F1 mice vs. WT→F1 mice (Figs. 1G, 1H) in the setting of a failure to eliminate host cells is suggestive of impaired CTL effector function and/or delayed maturation.
Fig. 1. Donor T cell signaling through TNFR p75 is critical for induction of acute GVHD phenotype at day 14.
Normal (TNFR intact) BDF1 mice were either uninjected or injected with splenocytes from B6 WT, B6 p55KO, B6 p75KO or B6 p55/p75KO donor cells. At 14 days after donor cell transfer, F1 spleens were assessed for donor and host lymphocyte populations by flow cytometry as described in Methods. Group means ± SE are shown for: A) total splenocytes; B) host B cells; C) host CD11b+ APC; D) host cDC (CD11c+); E) host CD4 T cells; F) host CD8 T cells; G) donor CD4 T cells; and H) donor CD8 T cells (n= 5 mice/group). For all figures, * p<0.0; ** p< 0.01, ***p<0.005. Donor splenocytes were examined by flow cytometry and adjusted so that all groups received ~4 × 106 donor CD8 T cells and 6.7 ± 1.2 × 106 donor CD4 T cells.
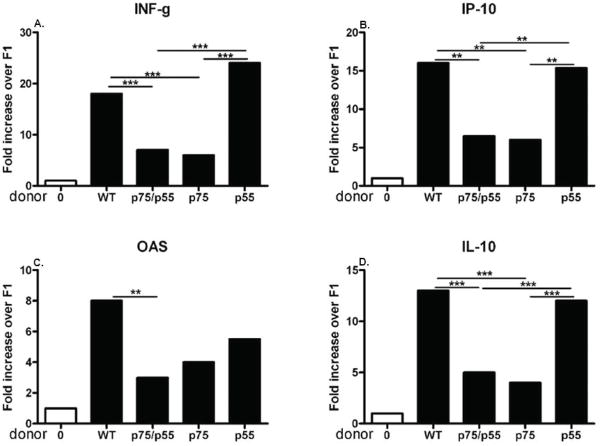
Spleens from the cohort shown in Fig. 1 were also tested for cytokine gene expression by real time PCR (Fig 2). Cytokine genes important in CD8 CTL effector function such as IFN-g, IP-10 and OAS are increased in WT→F1 mice (Fig. 2A–C) as previously demonstrated (17, 18). Increased IL-10 gene expression (Fig. 2D) is also typical of day 14 B6 WT→F1 mice GVHD (18) and contributes to both the exhausted CTL phenotype (19) and memory CD8 generation (20). Cytokine gene expression in p55KO→F1 mice does not differ significantly from WT→F1 mice however p75KO→F1 mice exhibit reduced values for all cytokines and reach significance for all except OAS vs. WT→F1 mice. Taken together, the results of Figs. 1 and 2 indicate that donor T cell p75 but not p55 signaling is critical for the induction of a day 14 acute GVHD cytotoxic phenotype consistent with impaired donor CD8 CTL elimination of host cells.
Fig. 2. Cytokine gene expression typical of day 14 acute GVHD is defective in p75KO→F1 mice.
Spleens harvested from the cohort described in Fig. 1 at day 14 were also tested for cytokine gene expression by real time-PCR as described in Methods. As in Fig. 1, mice were tested individually and values expressed as group mean ± SEM are shown for: A) IFN-g, B) OAS, C) IP-10, and D) IL-10.
Defective donor T cell p75 signaling converts acute GVHD to chronic GVHD long term
To address whether the day 14 stimulatory phenotype seen in Fig. 1 for p75KO→F1 mice is indicative of a long term chronic GVHD phenotype or whether donor CD8 CTL maturation is merely delayed and mice eventually exhibit acute GVHD (i.e. elimination of host splenocytes), F1 mice were injected using a protocol similar to that in Fig. 1 and mice followed long term. Despite using a low donor cell dose to reduce acute GVHD related mortality and permit long term survival, deaths were seen in all GVHD groups. The overall number of deaths/group was greater in WT→F1 (4/8) and in p55KO→F1 (3/7) vs. that of p75KO→F1 (1/7) or p55/075KO→F1 (1/8) however the differences did not reach statistical significance.
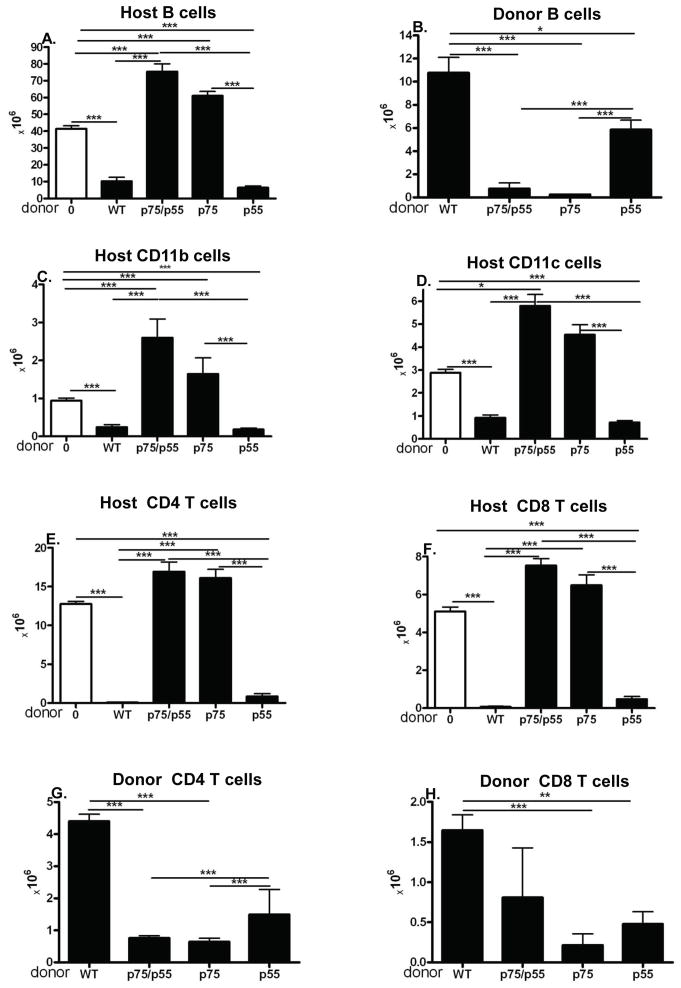
At 20 weeks splenocytes from surviving mice were analyzed by flow cytometry (Fig. 3). Both WT→F1 and p55KO→F1 mice (Fig. 3, columns 2 & 5) exhibit a typical long term acute GVHD phenotype (15) i.e. significant elimination of host B cells (Fig. 3A) with repopulation by donor B cells (Fig. 3B) and elimination of host CD11b APC (Fig. 3C), cDC (Fig. 3D), CD4 (Fig. 3E) and CD8 T cells (Fig. 3F) with demonstrable engraftment of both donor CD4 (Fig. 3G) and CD8 T cells (Fig. 3H). P55KO→F1 mice did not exhibit a phenotype significantly different from WT→F1 with the exception of a mild but significant reduction in donor B cells repopulation. Interestingly engraftment of p55KO donor T cells is also significantly reduced vs. WT→F1 donor cells yet the elimination of all host cell populations is comparable to that of WT→F1 indicating that reduced p55KO donor T cell engraftment is not associated with impaired acute GVHD phenotype long term. By contrast p75KO→F1 mice (Fig. 3, column 4) exhibit host B cell expansion (Fig. 3A) with little detectable repopulation by donor B cells (Fig. 3B), no significant elimination of host CD11b+ APC, DC, CD4 or CD8 T cells vs. control F1 (Figs. 3C–3F). Engraftment of donor p75KO CD4 and CD8 T cells is significantly reduced vs. WT →F1 donor T cells (Figs. 3G & H). Together, these features are consistent with a chronic GVHD phenotype (5, 17, 18). Double KO p55/p75KO→F1 mice for the most part resemble that of p75KO→F1 mice and do not exhibit a clearly distinct phenotype.
Fig. 3. Long term, p75KO→F1 mice exhibit chronic rather than acute GVHD.
Donor and host combinations are as described for Fig. 1. Mice were assessed at 20 weeks by flow cytometry for the following splenic lymphocyte subpopulations: A) host B cells; B) donor B cells; C) host CD11B+ APC; D) host cDC (CD11C+); E) host CD4 T cells; F) host CD8 T cells; G) donor CD4 T cells; and H) donor CD8 T cells. Mice received unfractionated donor splenocytes containing 7.1 ± 0.7 × 106 CD4 T cells and 4.6 ± 0.6 × 106 CD8 T cells. For normal F1, n=5; WT→F1 and p55→F1, n=4; p75KO→F1, n=6 and p55/p75 KO→F1, n=7.
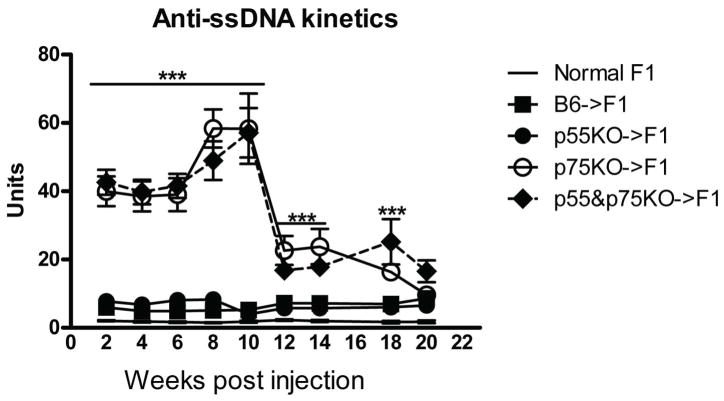
Cardinal features of chronic GVHD in the p→F1 model are lupus-like autoantibodies and immune complex glomerulonephritis (4, 5). For BDF1 hosts, severe lupus-like ICGN is seen following transfer of DBA parental cells (4+ proteinuria, nephrotic syndrome) (13) whereas a mild nephritis follows transfer of B6 CD4 T cell donors (≤ 2+ proteinuria) unless higher donor cell doses (≥15 × 106 CD4 T cells) are used (10, 21). Accordingly, we observed that proteinuria was ≤ 2+ and glomerular scores were ≤ 1+ for all groups with no significant differences between groups (data not shown) and consistent with the relatively low numbers of donor CD4 T cells transferred (<8 × 106). Nevertheless, despite the low intensity of the chronic GVHD reaction, striking and significant differences were seen in serum anti-ssDNA ab, a non-specific marker of host B cell activation characteristic of chronic GVHD (10). Only p75KO→F1 and p55/p75KO→F1 mice exhibited significantly elevated serum anti-ssDNA ab levels vs. control F1 as early as 2 weeks and persisting through week 14 (Fig. 4). The two curves for p75KO→F1 and p55/p75KO→F1 were not significantly different further supporting the idea that p55/p75KO→F1 mice have a phenotype similar to p75kO→F1 mice. No significant elevations over uninjected controls were seen for WT→F1 or p55KO→F1 mice.
Fig. 4. Lupus-like autoantibodies are seen only in F1 mice receiving p75KO donor T cells.
Sera was tested for anti-ssDNA antibodies on the cohort described in Fig. 3 at the indicated times as described in Methods.
Taken together these results demonstrate that both WT→F1 and p55KO→F1 mice exhibit an acute GVHD phenotype long term whereas p75KO→F1 and p55/p75KO→F1 mice exhibit a chronic GVHD phenotype consistent with a defect in donor CD8 effector maturation and function in the setting of donor CD4 driven host B cell expansion and autoimmunity.
CD8 CTL effector phenotype requires donor T cell signaling only through the p75 TNFR
In B6 WT→F1 mice, donor CD8 numbers peak at ~ day 10 and mediate the decline in host lymphocyte expansion (15). By contrast, chronic GVHD in this model results from a failure of donor CD8 CTL maturation in the context of intact donor CD4-driven host splenocyte expansion. Thus, the foregoing results demonstrating that p75KO→F1 mice exhibit a chronic GVHD phenotype short and long term strongly support a defect in p75KO donor CD8 CTL effector function coupled with intact donor CD4 T cell help for host B cells. To determine the integrity of initial maturation of donor CD8 T cells, GVHD was induced using a protocol similar to Fig. 1 and mice assessed at the peak of donor CD8 CTL expansion (day 10) (15). Because p75/p55 KO→F1 mice did not exhibit a unique phenotype but were either intermediate or similar to p75KO→F1 mice, this group is omitted to focus on the dichotomy between p55KO→F1 vs. p75KO→F1 mice. Compared to control F1 mice (Fig. 5A–5F, column 1), WT→F1 mice (Fig. 5A–5F, column 2) exhibit: a) a mild increase in total splenocytes (Fig. 5A); b) significant elimination of host B cells (Fig. 5B); c) expansion of host DC and CD11b+ APC (Figs. 5C–5D); d) reduction of host CD4 T cells (Fig. 5E) but not host CD8 T cells (Fig. 5F); and e) engraftment of both CD4 and CD8 B6 WT donor T cells (Figs. 5G, 5H, column 1). This pattern confirms previous work at day 10 demonstrating that host B cells and CD4 T cells are more sensitive to elimination and are eliminated earlier than host APC or CD8 T cells (15). Values for p55 KO→F1 mice (Fig. 5A–5F, column 4, Figs. 5G, 5H, column 3) do not differ significantly from WT→F1 mice. By contrast, p75KO→F1 mice (Figs. 5A–5F, column 3) exhibit a significant increase in total splenocytes, host B cells, host DC, host CD11b+ APC and host CD4 T cells (Figs. 5A–5E) compared to either WT→F1 or p55KO→F1 mice. The increase in host cells seen in p75KO→F1 mice is an indicator of impaired donor CD8 CTL elimination of intact donor CD4-driven host splenocyte expansion. Interestingly, p75 KO→F1 mice exhibit significantly greater donor CD4 engraftment vs. WT→F1 or p55R KO→F1 mice (Fig. 5G) yet CD8 T cell engraftment is mildly but significantly reduced vs. WT CD8 T cells (Fig. 5H) consistent with a mild but significant quantitative defect in donor CD8 T cell expansion. A second day 10 experiment gave similar results however the reduction in engrafted donor CD8 T cells for p75KO→F1 mice did not reach statistical significance as it did in Fig. 5H (data not shown).
Fig 5. Peak day 10 donor CD8 T cell engraftment is defective in p75KO→F1 mice.
Acute GVHD was induced in BDF1 hosts following the transfer of B6 WT, p55KO or p75KO donor splenocytes containing 7.15 ± 0.35 × 106 CD4 and 4.35 ± 0.45 × 106 CD8 T cells. F1 hosts were assessed by flow cytometry on day 10 for: A) total splenocytes; B) host B cells; C) host cDC; D) host CD11b+ APC; E) host CD4 T cells; F) host CD8 T cells; G) donor CD4 T cells and H) donor CD8 T cells. Results are shown as group mean ± SE (n= 5 mice/group).
Defective p75KO donor CD8 CTL elimination of host cells can be partially compensated by transferring greater numbers
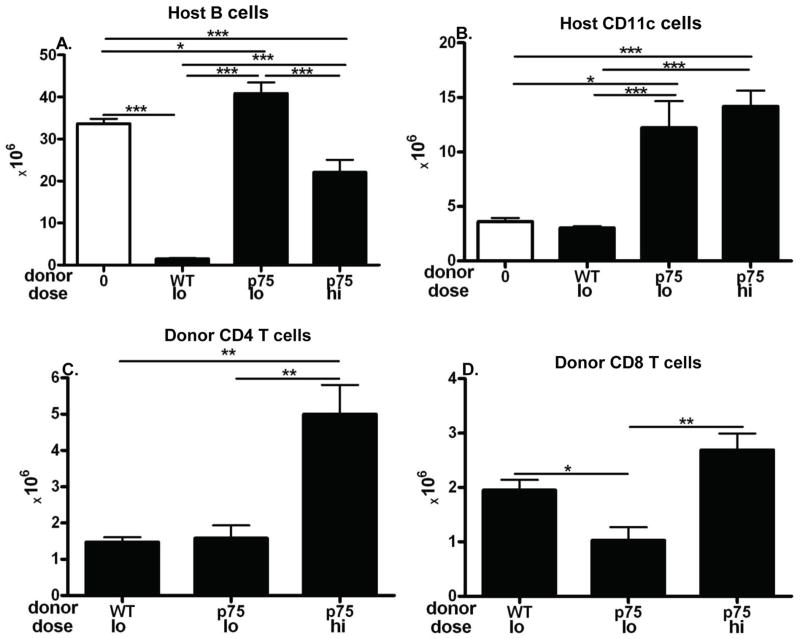
We have previously demonstrated that transferring standard doses donor cells with qualitative CTL defects (i.e. perforin or FasL defective) results in an intermediate phenotype at day 14 due to impaired CTL elimination of host splenocytes (22, 23). Higher doses of qualitatively defective donor cells improved but did not fully correct the impaired acute GVHD phenotype (22, 23). Thus, qualitative defects in CD8 T cell function can be partially but not fully compensated by quantitative measures. To address whether the impaired elimination of host cells in p75KO→F1 mice is due solely to the quantitative defect (shown in Fig. 5H as a significant reduction in peak donor CD8 T cell numbers) or whether a qualitative aspect also contributes, we examined the effect of higher donor cell transfers. F1 mice received either: a) low dose WT or p75KO donor cells (doses comparable to those used in Figs. 1–5) or; b) high dose p75KO donor cells and mice assessed at day 14. Compared to uninjected F1 mice, low dose WT→F1 exhibited the expected cytotoxic phenotype (Fig. 6, columns 1 & 2) consisting of profound elimination of host B cells (Fig. 6A), no increase in host CD11c+ cells (Fig. 6B), and engraftment of both donor CD4 and CD8 T cells subsets (Figs. 6C–6D). Similarly low dose p75KO→F1 (Fig. 6, column 3) reproduced the day 14 phenotype shown in Fig. 1A i.e., a failure to eliminate host B cells or CD11c+ cells (Figs. 6A–6B), normal engraftment of donor CD4 T cells (Fig. 6C) coupled with a mild but significant reduction in donor CD8 T cell engraftment. High dose p75KO→F1 mice (Fig. 6, column 4) exhibited significantly greater elimination of host B cells vs. lo dose p75KO→F1 mice (Fig. 6A) however B cell numbers were still significantly greater than that of WT→F1 mice consistent with impaired donor CD8 CTL killing. High dose p75KO→F1 mice did not exhibit significantly greater killing of host DC (Fig. 6B), host CD11b+APC or host CD4 T cells (data not shown). Importantly, failure to correct host killing in high dose p75KO→F1 mice occurred in conjunction with donor CD4 and CD8 T cells engraftment levels that were comparable to or significantly greater those of WT→F1 mice (Figs. 6C–6D, columns 1 and 3). These results confirm our day 14 data in Fig. 1 in which p75KO→F1 mice exhibit defective elimination of donor CD4 expanded host B cell and APC (FIgs. 1A–1C) despite significantly greater donor CD8 T cell engraftment compared to WT →F1 mice (Fig. 1H). Thus, transferring donor cell numbers sufficient to engraft p75KO donor cells in numbers ≥ those of WT donor cells improves but does not fully correct the defects in host splenocyte elimination. These results support the conclusion that defective donor T cell p75 signaling results in qualitative defects in effector CTL function in addition to quantitative defects.
Fig. 6. Defective donor CD8 CTL killing by p75KO donor T cells is only partially corrected by normalizing engraftment.
Acute GVHD was induced in BDF1 hosts following the transfer of low dose B6 WT or p75KO splenocytes (6.6 – 6.8 × 106 CD4 and 4.8–4.9 × 106 CD8 T cells) or high dose p75KO donor cells (8.8 × 106 CD4 and 6.4 × 106 CD8 T cells). F1 hosts were assessed at day 14 by flow cytometry for lymphocyte subsets as described in Fig. 1 and shown as: A) host B cells; B) host cDC; C) donor CD4 and D) donor CD8 T cells. Results are shown as group mean ± SE (n= 5 mice/group except for high dose p75KO→F1, n=3).
Optimal donor CD8 CTL killing requires p75 signaling by both donor CD4 and CD8 T cells
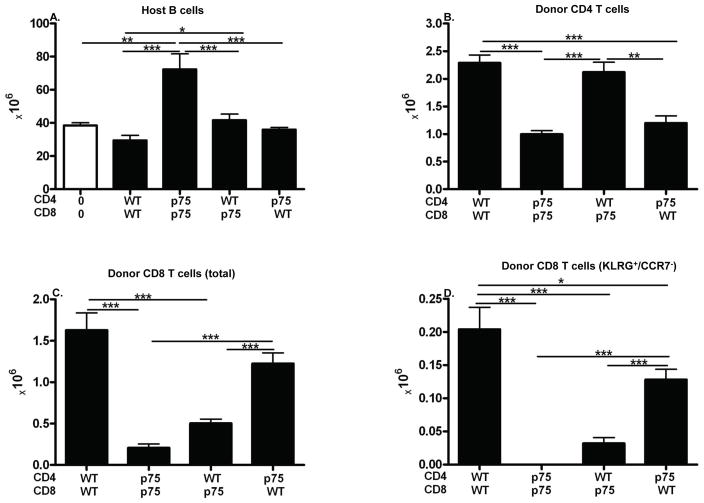
The foregoing results demonstrate that optimal CD8 CTL elimination of host splenocytes requires TNFR signaling through p75 on donor T cells. It is not clear whether this effect is mediated by p75 signaling through CD8 T cells alone, through CD4 T cells alone or through both. To address his question, purified donor CD4 and CD8 T cells from both WT and p75 KO donors were transferred into WT BDF1 mice in all four possible matched or mixed combinations and phenotype assessed during peak donor CD8 CTL expansion and maturation (day 10). As shown above in Fig. 6, defects in p75 KO donor cells can be improved using larger numbers of donor cells. Therefore, to better demonstrate possible mild contributory defects in CD4 T cells, sub-threshold numbers of CD4 donor cells were transferred in conjunction with numbers of CD8 T cells at or above threshold (6.0 ± 0.3 × 106 CD4 and CD8 T cells) resulting in a CD4:CD8 ratio of 1:1 rather than the typical 2:1. Using host B cell elimination as a surrogate marker of donor anti-host CD8 CTL function (15, 24), F1 hosts receiving matched WT donor T cells i.e. WT CD4 + WT CD8 →F1 (matched WT control) exhibit a mild reduction in host B cells vs. control uninjected F1 mice (Fig. 7A, columns 1 & 2, p=n.s.) consistent with the day 10 read out at which point donor CD8 CTL maturation typically has reversed the initial donor CD4 mediated host B cell expansion however elimination of host B cells is not complete until ~ day 14 (15). By contrast, B cells in p75KO CD4 + p75KO CD8→F1 mice (matched p75 control) are significantly elevated vs. uninjected control F1 (Fig. 1A, columns 3 &1) indicative of an intact donor CD4 driven expansion phase but a failure of donor CD8 CTL-mediated elimination phase consistent with a stimulatory chronic GVHD phenotype and confirming our results in Fig. 1A using unfractionated p75KO donor splenocytes. For F1 mice receiving mixed donor T cells, i.e. WT CD4 + p75KO CD8→F1 or p75KO CD4 + WT CD8→F1 (Fig. 7A, columns 4 & 5), both groups exhibit B cell elimination values comparable to matched WT CD4 + WT CD8->F1 control (Fig. 7A, column 2) and are significantly reduced vs. the matched p75KO CD4 + p75KO CD8→F1 control (Fig. 7A, column 3). These results support a role for p75 signaling in both CD4 and CD8 T cells.
Fig. 7. Optimal donor CD8 CTL function requires p75 signaling in both CD4 and CD8 T cells.
CD4 and CD8 T cells were purified from WT and p75 KO donor spleens and combined as described in Methods. BDF1 mice received 6.0–6.7 × 106 CD4 and 5.7–6.2 × 106 CD8 T cells that were either matched or mixed according to donor strain. At day 10, host spleens were analyzed by flow cytometry for numbers of: A) host B cells; B) donor CD4 T cells; C) donor CD8 T cells; and D) KLRG-1 positive donor CD8 T cells. Results are shown as group mean ± SE (n= 5 mice/group for all groups).
Specifically, the defective host B cell killing (increased host B cell numbers vs. control F1) seen for p75KO CD4 +p75KO CD8 →F1 mice (Fig. 7A, column 3) can be significantly improved by substituting either WT CD4 T (Fig. 7A, column 4) or WT CD8 T cells (Fig. 7A, column 5). Conversely, B cell killing for WT CD4 + WT CD8→F1 (Fig. 7A, column 2) is not significantly altered by the substitution of only one p75KO T cell subset (Fig. 7A, columns 4 or 5). The results in Fig. 7A demonstrate that defective p75 signaling in both CD4 or CD8 T cells impairs optimal CD8 CTL maturation as measured by host B cell killing however the defect in each subset can be significantly mitigated by pairing with the appropriate WT subset.
This conclusion is further supported by donor engraftment results. CD4 T cell engraftment levels (Fig. 7B) support a critical role for CD4 p75 signaling in optimal CD4 engraftment. Engraftment of WT CD4 T cells does not differ significantly whether paired with WT or p75KO CD8 T cells (Fig. 7B, columns 1 & 3). Engraftment of p75KO CD4 engraftment is significantly reduced vs. WT CD4 regardless of the source of CD8 T cells. Specifically, both p75KO CD4 + p75KO CD8→F1 and p75RKO CD4 + WT CD8→F1 mice (Fig. 7B, columns 2 & 4) exhibit a significant reduction in donor CD4 T cell engraftment vs. F1 mice receiving WT CD4 T cells in either combination (WT CD4 + WT CD8→F1 or WT CD4 + p75KO CD8→F1, Fig. 7B, columns 1 & 3). Thus, p75 signaling is critical for optimal donor CD4 T cell engraftment and is not altered by mixed pairing of CD8 T cells.
Similarly, donor CD8 T cell engraftment levels (Fig. 7C) support a role for intrinsic CD8 p75 signaling in optimal engraftment. Compared to the matched WT control (Fig. 7C, column 1), matched p75KO control mice (Fig. 7C, column 2) exhibit significantly reduced engraftment of donor CD8 T cells. These results are consistent with the day 10 peak CD8 engraftment results demonstrating a mild but significant reduction in CD8 engraftment for p75KO→F1 mice (Fig. 5H). They differ from our day 14 results in Fig. 1 using unfractionated cells possibly as a consequence of the greater numbers of donor cells used in that experiment. Defective CD8 T cell engraftment seen in the matched p75 KO control mice (Fig. 7C, column 2) is mildly but not significantly boosted when paired with WT CD4 T cells (Fig. 7C, column 3) indicative of an intrinsic p75 mediated CD8 defect. Of note, engraftment of WT CD8 T cells is mildly but not significantly reduced when paired with p75KO CD4 T cells vs. WT CD4 T cells (Fig. 7C, columns 1 vs. 4) consistent with a mild helper defect for p75 KO CD4 T cells.
Lastly, KLRG-1 expression is a marker of CD8 CTL effector maturation and KLRG-1pos/CCR7neg CD8 T cells represent short lived effector cells (SLEC) (19). As shown in Fig. 7D, SLEC numbers are profoundly defective in p75KO matched control mice (Fig. 7D, column 2) vs. that of the matched WT control (Fig. 7D, column 1) with values below the limits of detection. Reduced p75KO CD8 SLEC numbers seen in p75KO matched mice (Fig. 7D, column 2) are mildly improved but not corrected by pairing with normal CD4 help (WT CD4 + p75KO CD8→F1, column 3) consistent with a significant intrinsic defect in p75RKO CD8 T cells. Conversely, WT SLEC CD8 numbers seen in WT control mice is moderately but significantly reduced when paired with p75KO CD4 T cells vs. WT CD4 + T cells (Fig. 7D, columns 1 & 4) further supporting a mild but contributory role for CD4 p75 signaling in providing help for normal CD8 CTL effector maturation. Of note, mice receiving mixed WT CD4 and p75KO CD8 donor T cells exhibit significantly reduced CD8 engraftment and KLRG-1 upregulation vs. WT matched control (Figs. 7C–7D, columns 1 and 3) however this was not associated with a significant impairment of host B cell killing (Fig. 7A, column 4) indicating the mild nature of the p75 KO defect when only one subset if affected.
Taken together, the results in Fig. 7 demonstrate that p75 signaling is critical for optimal donor CD4 and CD8 engraftment, for optimal CD4 help for CD8 CTL elimination of B cells and for optimal effector marker upregulation (KLRG-1).
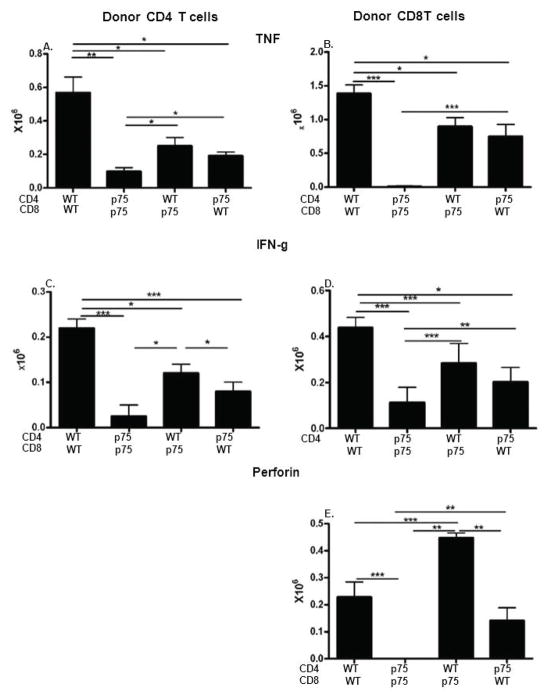
p75KO donor T cell signaling is required for optimal CD4 and CD8 intracellular cytokine expression
Splenic donor T cells from the cohort shown in Fig. 7 were analyzed by flow cytometry for intracellular markers characteristic of effector maturation i.e., TNF, IFN-g, and perforin (Fig. 8). Representative staining plots are shown in Supplemental Fig 1. These markers typically peak between days 7–10 and accordingly WT matched controls exhibit detectable numbers of cytokine expressing cells for all cytokines except perforin expressing CD4 T cells (Fig. 8A–8E, column 1). Compared to the WT matched control, p75 matched control mice (Figs. 8A–8E, column 2 vs. 1) exhibit significant reductions in the numbers of: 1) CD4 T cells expressing TNF and IFN-g (Figs. 8A, 8C); and 2) undetectable numbers of CD8 T cells expressing TNF, IFN-g and perforin (Figs. 8B, 8D, 8E). Numbers of perforin expressing CD4 T cells were too small for all groups to allow meaningful conclusions (data not shown). The results for the two matched control groups demonstrate that when both donor T cell subsets are p75 defective, significant qualitative defects in both donor CD4 and CD8 T cell effector maturation can be seen.
Fig. 8. Intact p75 signaling is required for optimal donor CD4 and CD8 intracellular expression of effector CTL markers.
Spleens from the cohort in Fig. 7 were examined on day 10 by flow cytometry for intracellular expression of: TNF (A,B); IFN-g (C,D), and perforin (E) on donor CD4 T (A, C) or CD8 (B, D, E) T cells.
Mixing of donor T cell subsets improves but does not correct the defects seen for matched p75 KO donor cells further supporting the idea that both CD4 and CD8 p75 signaling is required for optimal CD8 effector maturation. In general, mixed subsets (Figs. 8A–8E, columns 3 & 4) exhibit values that are intermediate between the matched WT or p75 KO controls (Figs. 8A–8E, columns 1 and 2). In particular, pairing of WT CD4 T cells with p75KO CD8 T cells (Figs. 8A–8E, column 3) results in a significant boost in all CD4 and CD8 parameters vs. matched p75KO control (Figs. 8A–8E, column 2) however these values do not reach those of the matched WT control (Figs. 8A–8D, column 1). Thus, WT CD4 T cells can boost but not normalize defective p75 KO CD8 T cell IFN-g, TNF and perforin expression (Fig. 8B, 8D, 8E, columns 3 vs. 2 indicating that even with a normal source of CD4 help, p75KO CD8 T cells remain intrinsically abnormal.
Similarly, p75 KO CD4 T cells exhibit a mild to moderate defect in their help for CD8 T cells as shown by pairing of p75KO CD4 with WT CD8 T cells and compared to values of the WT control (Fig. 8, columns 1 & 4). This helper defect is best seen as a significant reduction in WT CD8 expression of IFN-g and a non-significant reduction in perforin expression when paired with p75 CD4 T cells vs. WT CD4 T cells (Figs 8D & 8E, columns 1 & 4). There was also a significant reduction in p75KO CD4+ WT CD8 TNF expression in CD8 T cells however the mean value was increased due to mouse to mouse variability. The results in Fig. 8 further support the conclusion from Fig. 7 that optimal quantitative and qualitative CD8 CTL effector maturation requires p75 signaling by both CD4 and CD8 T cells.
Discussion
CD8 CTL generation in the p→F1 model has been previously shown to be critically dependent on TNF (6). In B6→BDF1 mice, anti-TNF mAb treatment during days 0–5 prevented donor CD8 CTL maturation and converted phenotype from acute to chronic GVHD whereas TNF blockade on day 7 had little effect, supporting the idea that TNF is critical in CD8 CTL initiation rather than acting later at the effector phase. It was not clear from that study however whether TNF acted directly on donor T cells in a signal 3/costimulatory role or acted indirectly, perhaps through host APC. Our results confirm the former possibility by demonstrating that TNF signaling through donor T cell TNFR p75 but not p55 is absolutely required for CD8 CTL effector maturation. CD4 T cell signaling through p75 is required for optimal CD4 help for CD8 maturation and CD8 T cell signaling through p75 is required for optimal CTL effector function. Interestingly, direct measurement of p75 KO donor cell quantitative and qualitative parameters, e.g., donor T cell engraftment and intracellular effector markers revealed only mild defects for mice receiving unfractionated donor cells. By contrast, indirect parameters of donor CD8 CTL function, e.g., elimination of host splenocytes, revealed profound and long lasting defects such that at two weeks, p75 KO→F1 mice exhibited a stimulatory phenotype rather than the expected cytotoxic phenotype which persisted long term with p75 KO→F1 mice exhibiting chronic rather than acute GVHD. These results are consistent with previous work demonstrating that host B cell depletion is a more sensitive indicator of in vivo donor CD8 CTL activity than are CTL assays performed in vitro, ex vivo or even in vitro (15, 24).
Because chronic GVHD in this model is mediated solely by donor CD4 T cells whereas acute GVHD requires both CD4 and CD8 donor T cells (5), the conversion from acute to chronic GVHD in p75KO→F1 mice indicates a significant defect in donor p75KO CD8 T cell maturation. In support of this idea, dose response studies demonstrated that transferring sufficient donor cells to achieve p75 KO T cell engraftment greater than that of WT donor cells improved but did not correct the profound defects in host B cell elimination in p75KO→F1 mice. These results provide indirect evidence for a significant qualitative defect in p75KO CD8 effector function. Direct evidence for a p75KO donor cell qualitative defect was best seen at donor cell doses just above the threshold for GVHD induction and consisted of defective intracellular expression of IFN-g and TNF for both CD4 and CD8 T cells and defective perforin expression for donor CD8 T cells. Together these results support the conclusion that p75 signaling in both ag-specific CD4 and CD8 T cell subsets is critical for ag-specific CD8 CTL maturation and effector function. By contrast, no defects were seen in acute GVHD phenotype short or long term when p55 KO donor cells were transferred. These results appear to be at variance with results in a murine model of acute GVHD using irradiated bone marrow transplant recipients and demonstrating that p55 but not p75 signaling is critical for acute GVHD (25). This discrepancy likely reflects the strong inflammatory cytokine storm and inflammation elicited by the conditioning regimen in that model and the well known role of p55 signaling in TNF-mediated inflammation (26, 27). By contrast, in the p→F1 model, there is no conditioning regimen and no similar cytokine storm thus mitigating the role of p55 mediated inflammation.
It has been previously demonstrated that the CD8 CTL anti-pathogen response requires a third signal in addition to TcR stimulation and CD28 mediated costimulation (1, 3). Two well recognized signal 3 molecules are IL-12 and IFN (1, 3). TNFR superfamily members also influence T cell activation and maturation by providing costimulation signals separate from CD28 signaling. OX-40, 4-B11, CD27 HVEM, CD30, and GITR can all provide signals important in the initiation of immune responses and in long lived memory (28). In addition, TNFR p75 signaling can provide a costimulatory signal for T cells (28) that ensures optimal IL-2 production and T cell survival during clonal expansion and enhances memory pool formation by conferring protection from apoptosis (29–31). As a result, p75 KO T cells have been reported to exhibit a reduced accumulation of effector cells as shown by reduced numbers of ag-specific intracellular IFN-g expressing cells and reduced clearance of Listeria monocytogenes (29). Our results, while consistent with these quantitative defects, differ in that we also demonstrate a qualitative defect in p75KO CD8 CTL killing and further, that this defect is not corrected by normalization of p75KO T cell numbers.
A second novel finding of our study regards the qualitative defect in p75KO CD4 T cell function. As discussed above, p75KO→F1 mice exhibit chronic rather than acute GVHD due to defective CD8 CTL maturation. Chronic GVHD in the p→F1 model is mediated solely by donor CD4 T cell recognition allogeneic host MHC II and the provision of cognate CD4 T cell help to host B cells resulting in B cell hyperactivity and autoantibody production (32). Although p75KO CD4 T cells are defective in their help for CD8 CTL maturation, they are nevertheless able to provide help to host B cells and induce lupus-like autoantibodies and chronic GVHD. Thus, p75 signaling is much more important for CD4 T cell help to CD8 CTL than it is for CD4 help to B cells. This functional disparity in CD4 T cell help is reminiscent of our findings with another TNFR super family member, Fas, in which Fas defective donor CD4 T cells exhibit significantly impaired help for CD8 CTL but no detectable defect in their ability to provide help to B cells and drive chronic, lupus-like GVHD (10).
Lastly, our results may have relevance to patients receiving therapeutic TNF blockers. Our demonstration of a critical role for p75 signaling in optimal in vivo CD8 CTL function raises the concern TNF blockers may impair the CD8 CTL contribution to anti-viral and anti-tumor responses. For example, CD8 CTL are strongly linked to recovery from EBV infection (33) and TNF is critical for the development of HBV-specific CTL (34). Incidents of reactivation of EBV, HBV, varicella-zoster and less commonly hepatitis C have been reported in patients on TNF blockers (35–37). Although the numbers are small, in the absence of long term prospective studies, a definitive statement regarding the safety of TNF blockers in viral conditions cannot be made and, in the case of HBV, prophylactic anti-viral therapy and close clinical monitoring has been suggested (38, 39).
Regarding tumors, estimating the cancer risk of TNF blockers separate from that of the underlying condition and the concomitant use of immunosuppressives is complicated by the long latency of tumors, their relative low incidence and methodological concerns e.g., discrepancies between observational studies and meta analyses (40, 41). Accordingly, reports of an increase in malignancies (42), particularly hematological malignancies (43), in patients receiving TNF blockers have not been uniformly confirmed (40, 44). Nevertheless, evidence supporting an increased risk of cancer, particularly lymphomas, in children and adolescents receiving TNF blockers (45), prompted the FDA to issue a warning (46). TNF signaling particularly through the p75 has been shown to be critical to anti-tumor T cell responses (47). Moreover, Fas and perforin pathways are important in normal lymphocyte homeostasis (48–50). Thus, impairment of p75 T cell signaling by TNF blockers could impair T cell mediated tumor surveillance.
Lastly, our results may be relevant to the lupus-like autoimmunity reported in patients receiving TNF blockers (47, 51, 52) and suggest a possible mechanism by which this could occur. In both humans and mice, CD4 T cells are necessary and sufficient for lupus development (53–57) and CD8 T cells, to include CTL, (5, 58–63) act to down regulate lupus. Based on our results demonstrating that p75 signaling is important for CD8 effector maturation but not for CD4 T cell help to B cells, it is possible that patients who experience a lupus-like tolerance break (i.e. CD4 driven- B cell hyperactivity) while on TNF blockers may exhibit an impairment in the ability of down regulatory CD8 T cells to restore tolerance without a concomitant impairment of CD4 T cells to provide help to autoreactive B cells and autoantibody production. For this scenario to occur, patients would first need to encounter a lupus trigger which may account for the relative low frequency of autoimmunity in the setting of TNF blockers. Once tolerance is lost, the balance between lupus and CD8 mediated down regulation may be shifted such that autoimmunity is favored. The possible benefit of therapeutic selective p55 or p75 blockade was put forth roughly a decade ago (64) and remains under active study (9, 65). Moreover, soluble TNF efficiently activates only p55 whereas membrane TNF is largely responsible for p75 activation (9, 66). By extension, our results support the conclusion that it is membrane bound rather than soluble TNF binding to donor T cell p75 that is critical for CD8 CTL maturation in the absence of pathogens. In light of the beneficial role of CD8 T cells in infections, tumors and autoimmunity, our results demonstrating a critical role for p75 signaling in CTL maturation provide support for further investigation of TNF blockers that selectively inhibit p55 and spare p75, to include differential targeting of membrane vs. soluble TNF.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health AI047466 (CSV).
Abbreviations used
- BDF1
B6D2F1
- B6
C57Bl/6
- DBA
DBA/2J
- HVG
host-vs.-graft
- KO
knock out
- p→F1
parent-into-F1
- p55
TNFR1 p55
- p75
TNFR2 p75
- p55KO
B6.Tnfrsf1atm1Imx/J
- p75KO
B6.Tnfrsf1btm1Imx/J
- p55/p75KO
B6.Tnfrsf1atm1ImxTnfrsf1btm1Imx/J
- SLEC
short lived effector cells; tumor necrosis factor receptor
- WT
wild type
References
- 1.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 2.Curtsinger JM, Gerner MY, Lins DC, Mescher MF. Signal 3 availability limits the CD8 T cell response to a solid tumor. J Immunol. 2007;178:6752–6760. doi: 10.4049/jimmunol.178.11.6752. [DOI] [PubMed] [Google Scholar]
- 3.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenberg RA, Via CS. T cells, murine chronic graft-versus-host disease and autoimmunity. J Autoimmun. 2012;39:240–247. doi: 10.1016/j.jaut.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Via CS. Advances in lupus stemming from the parent-into-F1 model. Trends Immunol. 2010;31:236–245. doi: 10.1016/j.it.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Via CS, Shustov A, Rus V, Lang T, Nguyen P, Finkelman FD. In vivo neutralization of TNF-alpha promotes humoral autoimmunity by preventing the induction of CTL. J Immunol. 2001;167:6821–6826. doi: 10.4049/jimmunol.167.12.6821. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal BB, Eessalu TE, Hass PE. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature. 1985;318:665–667. doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- 8.Leist M, Gantner F, Jilg S, Wendel A. Activation of the 55 kDa TNF receptor is necessary and sufficient for TNF-induced liver failure, hepatocyte apoptosis, and nitrite release. J Immunol. 1995;154:1307–1316. [PubMed] [Google Scholar]
- 9.Faustman D, Davis M. TNF receptor 2 pathway: drug target for autoimmune diseases. Nat Rev Drug Discov. 2010;9:482–493. doi: 10.1038/nrd3030. [DOI] [PubMed] [Google Scholar]
- 10.Puliaeva I, Puliaev R, Shustov A, Haas M, Via CS. Fas expression on antigen-specific T cells has costimulatory, helper, and down-regulatory functions in vivo for cytotoxic T cell responses but not for T cell-dependent B cell responses. J Immunol. 2008;181:5912–5929. doi: 10.4049/jimmunol.181.9.5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soloviova K, Puliaiev M, Foster A, Via CS. The parent-into-F1 murine model in the study of lupus-like autoimmunity and CD8 cytotoxic T lymphocyte function. Methods Mol Biol. 2012;900:253–270. doi: 10.1007/978-1-60761-720-4_12. [DOI] [PubMed] [Google Scholar]
- 12.Passwell J, Schreiner GF, Nonaka M, Beuscher HU, Colten HR. Local extrahepatic expression of complement genes C3, factor B, C2, and C4 is increased in murine lupus nephritis. J Clin Invest. 1988;82:1676–1684. doi: 10.1172/JCI113780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster AD, Haas M, Puliaeva I, Soloviova K, Puliaev R, Via CS. Donor CD8 T cell activation is critical for greater renal disease severity in female chronic graft-vs.-host mice and is associated with increased splenic ICOS(hi) host CD4 T cells and IL-21 expression. Clin Immunol. 2010;136:61–73. doi: 10.1016/j.clim.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puliaeva I, Soloviova K, Puliaiev M, Lang T, Puliaev R, Via CS. Enhancement of suboptimal CD8 cytotoxic T cell effector function in vivo using antigen-specific CD80 defective T cells. J Immunol. 2011;186:291–304. doi: 10.4049/jimmunol.0902370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puliaev R, Puliaeva I, Welniak LA, Ryan AE, Haas M, Murphy WJ, Via CS. CTL-promoting effects of CD40 stimulation outweigh B cell-stimulatory effects resulting in B cell elimination and disease improvement in a murine model of lupus. J Immunol. 2008;181:47–61. doi: 10.4049/jimmunol.181.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster AD, Soloviova K, Puliaeva I, Puliaiev M, Puliaev R, Finkelman F, Via CS. Donor CD8 T cells and IFN-gamma are critical for sex-based differences in donor CD4 T cell engraftment and lupus-like phenotype in short-term chronic graft-versus-host disease mice. J Immunol. 2011;186:6238–6254. doi: 10.4049/jimmunol.1001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puliaeva I, Puliaev R, Via CS. Therapeutic potential of CD8+ cytotoxic T lymphocytes in SLE. Autoimmunity Rev. 2009;8:219–223. doi: 10.1016/j.autrev.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rus V, Svetic A, Nguyen P, Gause WC, Via CS. Kinetics of Th1 and Th2 cytokine production during the early course of acute and chronic murine graft-versus-host disease. Regulatory role of donor CD8+ T cells. J Immunol. 1995;155:2396–2406. [PubMed] [Google Scholar]
- 19.Parish IA, Kaech SM. Diversity in CD8(+) T cell differentiation. Curr Opin Immunol. 2009;21:291–297. doi: 10.1016/j.coi.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foulds KE, Rotte MJ, Seder RA. IL-10 is required for optimal CD8 T cell memory following Listeria monocytogenes infection. J Immunol. 2006;177:2565–2574. doi: 10.4049/jimmunol.177.4.2565. [DOI] [PubMed] [Google Scholar]
- 21.Rus V, Nguyen V, Puliaev R, Puliaeva I, Zernetkina V, Luzina I, Papadimitriou JC, Via CS. T cell TRAIL promotes murine lupus by sustaining effector CD4 Th cell numbers and by inhibiting CD8 CTL activity. J Immunol. 2007;178:3962–3972. doi: 10.4049/jimmunol.178.6.3962. [DOI] [PubMed] [Google Scholar]
- 22.Shustov A, Luzina I, Nguyen P, Papadimitriou JC, Handwerger B, Elkon KB, Via CS. Role of perforin in controlling B-cell hyperactivity and humoral autoimmunity. J Clin Invest. 2000;106:R39–47. doi: 10.1172/JCI8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Via CS, Nguyen P, Shustov A, Drappa J, Elkon KB. A major role for the Fas pathway in acute graft-versus-host disease. J Immunol. 1996;157:5387–5393. [PubMed] [Google Scholar]
- 24.Puliaev R, Nguyen P, Finkelman FD, Via CS. Differential requirement for IFN-gamma in CTL maturation in acute murine graft-versus-host disease. J Immunol. 2004;173:910–919. doi: 10.4049/jimmunol.173.2.910. [DOI] [PubMed] [Google Scholar]
- 25.Hill GR, Teshima T, Rebel VI, Krijanovski OI, Cooke KR, Brinson YS, Ferrara JL. The p55 TNF-alpha receptor plays a critical role in T cell alloreactivity. J Immunol. 2000;164:656–663. doi: 10.4049/jimmunol.164.2.656. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Tawara I, Toubai T, Reddy P. Pathophysiology of acute graft-versus-host disease: recent advances. Transl Res. 2007;150:197–214. doi: 10.1016/j.trsl.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 29.Kim EY, Priatel JJ, Teh SJ, Teh HS. TNF receptor type 2 (p75) functions as a costimulator for antigen-driven T cell responses in vivo. J Immunol. 2006;176:1026–1035. doi: 10.4049/jimmunol.176.2.1026. [DOI] [PubMed] [Google Scholar]
- 30.Kim EY, Teh HS. TNF type 2 receptor (p75) lowers the threshold of T cell activation. J Immunol. 2001;167:6812–6820. doi: 10.4049/jimmunol.167.12.6812. [DOI] [PubMed] [Google Scholar]
- 31.Kim EY, Teh HS. Critical role of TNF receptor type-2 (p75) as a costimulator for IL-2 induction and T cell survival: a functional link to CD28. J Immunol. 2004;173:4500–4509. doi: 10.4049/jimmunol.173.7.4500. [DOI] [PubMed] [Google Scholar]
- 32.Morris SC, Cheek RL, Cohen PL, Eisenberg RA. Autoantibodies in chronic graft versus host result from cognate T-B interactions. J Exp Med. 1990;171:503–517. doi: 10.1084/jem.171.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rickinson AB, Moss DJ. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu Rev Immunol. 1997;15:405–431. doi: 10.1146/annurev.immunol.15.1.405. [DOI] [PubMed] [Google Scholar]
- 34.Kasahara S, Ando K, Saito K, Sekikawa K, Ito H, Ishikawa T, Ohnishi H, Seishima M, Kakumu S, Moriwaki H. Lack of tumor necrosis factor alpha induces impaired proliferation of hepatitis B virus-specific cytotoxic T lymphocytes. J Virol. 2003;77:2469–2476. doi: 10.1128/JVI.77.4.2469-2476.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SY, Solomon DH. Tumor necrosis factor blockade and the risk of viral infection. Nat Rev Rheumatol. 2010;6:165–174. doi: 10.1038/nrrheum.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shale MJ. The implications of anti-tumour necrosis factor therapy for viral infection in patients with inflammatory bowel disease. Br Med Bull. 2009;92:61–77. doi: 10.1093/bmb/ldp036. [DOI] [PubMed] [Google Scholar]
- 37.Brunasso AM, Puntoni M, Gulia A, Massone C. Safety of anti-tumour necrosis factor agents in patients with chronic hepatitis C infection: a systematic review. Rheumatology (Oxford) 2011;50:1700–1711. doi: 10.1093/rheumatology/ker190. [DOI] [PubMed] [Google Scholar]
- 38.Carroll MB, Forgione MA. Use of tumor necrosis factor alpha inhibitors in hepatitis B surface antigen-positive patients: a literature review and potential mechanisms of action. Clin Rheumatol. 2010;29:1021–1029. doi: 10.1007/s10067-010-1523-2. [DOI] [PubMed] [Google Scholar]
- 39.Carroll MB, Bond MI. Use of tumor necrosis factor-alpha inhibitors in patients with chronic hepatitis B infection. Semin Arthritis Rheum. 2008;38:208–217. doi: 10.1016/j.semarthrit.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Solomon DH, Mercer E, Kavanaugh A. Observational studies on the risk of cancer associated with tumor necrosis factor inhibitors in rheumatoid arthritis: a review of their methodologies and results. Arthritis Rheum. 2012;64:21–32. doi: 10.1002/art.30653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Askling J, Fahrbach K, Nordstrom B, Ross S, Schmid CH, Symmons D. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119–130. doi: 10.1002/pds.2046. [DOI] [PubMed] [Google Scholar]
- 42.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 43.Nair B, Raval G, Mehta P. TNF-alpha inhibitor etanercept and hematologic malignancies: report of a case and review of the literature. Am J Hematol. 2007;82:1022–1024. doi: 10.1002/ajh.20926. [DOI] [PubMed] [Google Scholar]
- 44.Leombruno JP, Einarson TR, Keystone EC. The safety of anti-tumour necrosis factor treatments in rheumatoid arthritis: meta and exposure-adjusted pooled analyses of serious adverse events. Ann Rheum Dis. 2009;68:1136–1145. doi: 10.1136/ard.2008.091025. [DOI] [PubMed] [Google Scholar]
- 45.Diak P, Siegel J, La Grenade L, Choi L, Lemery S, McMahon A. Tumor necrosis factor alpha blockers and malignancy in children: forty-eight cases reported to the Food and Drug Administration. Arthritis Rheum. 2010;62:2517–2524. doi: 10.1002/art.27511. [DOI] [PubMed] [Google Scholar]
- 46.FDA. Cancer Warnings Required for TNF Blockers. 2009 http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm175803.htm.
- 47.Alessandri C, Scrivo R, Spinelli FR, Ceccarelli F, Magrini L, Priori R, Valesini G. Autoantibody production in anti-TNF-alpha-treated patients. Ann N Y Acad Sci. 2007;1110:319–329. doi: 10.1196/annals.1423.034. [DOI] [PubMed] [Google Scholar]
- 48.de Saint Basile G, Fischer A. The role of cytotoxicity in lymphocyte homeostasis. Curr Opin Immunol. 2001;13:549–554. doi: 10.1016/s0952-7915(00)00257-0. [DOI] [PubMed] [Google Scholar]
- 49.Kagi D, Odermatt B, Mak TW. Homeostatic regulation of CD8+ T cells by perforin. Eur J Immunol. 1999;29:3262–3272. doi: 10.1002/(SICI)1521-4141(199910)29:10<3262::AID-IMMU3262>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 50.Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2000;290:1354–1358. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- 51.Soforo E, Baumgartner M, Francis L, Allam F, Phillips PE, Perl A. Induction of systemic lupus erythematosus with tumor necrosis factor blockers. J Rheumatol. 2010;37:204–205. doi: 10.3899/jrheum.081312. [DOI] [PubMed] [Google Scholar]
- 52.Williams EL, Gadola S, Edwards CJ. Anti-TNF-induced lupus. Rheumatology (Oxford) 2009;48:716–720. doi: 10.1093/rheumatology/kep080. [DOI] [PubMed] [Google Scholar]
- 53.Peng SL, Fatenejad S, Craft J. Induction of nonpathologic, humoral autoimmunity in lupus-prone mice by a class II-restricted, transgenic alpha beta T cell. Separation of autoantigen-specific and -nonspecific help. J Immunol. 1996;157:5225–5230. [PubMed] [Google Scholar]
- 54.Mohan C, Adams S, Stanik V, Datta SK. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J Exp Med. 1993;177:1367–1381. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koshy M, Berger D, Crow MK. Increased expression of CD40 ligand on systemic lupus erythematosus lymphocytes. J Clin Invest. 1996;98:826–837. doi: 10.1172/JCI118855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koh DR, Ho A, Rahemtulla A, Fung-Leung WP, Griesser H, Mak TW. Murine lupus in MRL/lpr mice lacking CD4 or CD8 T cells. Eur J Immunol. 1995;25:2558–2562. doi: 10.1002/eji.1830250923. [DOI] [PubMed] [Google Scholar]
- 57.Santoro TJ, Portanova JP, Kotzin BL. The contribution of L3T4+ T cells to lymphoproliferation and autoantibody production in MRL-lpr/lpr mice. J Exp Med. 1988;167:1713–1718. doi: 10.1084/jem.167.5.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fan GC, Singh RR. Vaccination with minigenes encoding V(H)-derived major histocompatibility complex class I-binding epitopes activates cytotoxic T cells that ablate autoantibody-producing B cells and inhibit lupus. J Exp Med. 2002;196:731–741. doi: 10.1084/jem.20020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karpouzas GA, La Cava A, Ebling FM, Singh RR, Hahn BH. Differences between CD8+ T cells in lupus-prone (NZB × NZW) F1 mice and healthy (BALB/c × NZW) F1 mice may influence autoimmunity in the lupus model. Eur J Immunol. 2004;34:2489–2499. doi: 10.1002/eji.200424978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hahn BH, Ebling F, Singh RR, Singh RP, Karpouzas G, La Cava A. Cellular and molecular mechanisms of regulation of autoantibody production in lupus. Ann N Y Acad Sci. 2005;1051:433–441. doi: 10.1196/annals.1361.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature. 2010;467:328–332. doi: 10.1038/nature09370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim HJ, Wang X, Radfar S, Sproule TJ, Roopenian DC, Cantor H. CD8+ T regulatory cells express the Ly49 Class I MHC receptor and are defective in autoimmune prone B6-Yaa mice. Proc Natl Acad Sci U S A. 2011;108:2010–2015. doi: 10.1073/pnas.1018974108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang L, Bertucci AM, Ramsey-Goldman R, Burt RK, Datta SK. Regulatory T cell (Treg) subsets return in patients with refractory lupus following stem cell transplantation, and TGF-beta-producing CD8+ Treg cells are associated with immunological remission of lupus. J Immunol. 2009;183:6346–6358. doi: 10.4049/jimmunol.0901773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kollias G, Kontoyiannis D. Role of TNF/TNFR in autoimmunity: specific TNF receptor blockade may be advantageous to anti-TNF treatments. Cytokine Growth Factor Rev. 2002;13:315–321. doi: 10.1016/s1359-6101(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 65.Shibata H, Yoshioka Y, Ohkawa A, Minowa K, Mukai Y, Abe Y, Taniai M, Nomura T, Kayamuro H, Nabeshi H, Sugita T, Imai S, Nagano K, Yoshikawa T, Fujita T, Nakagawa S, Yamamoto A, Ohta T, Hayakawa T, Mayumi T, Vandenabeele P, Aggarwal BB, Nakamura T, Yamagata Y, Tsunoda S, Kamada H, Tsutsumi Y. Creation and X-ray structure analysis of the tumor necrosis factor receptor-1-selective mutant of a tumor necrosis factor-alpha antagonist. J Biol Chem. 2008;283:998–1007. doi: 10.1074/jbc.M707933200. [DOI] [PubMed] [Google Scholar]
- 66.Cabal-Hierro L, Lazo PS. Signal transduction by tumor necrosis factor receptors. Cell Signal. 2012;24:1297–1305. doi: 10.1016/j.cellsig.2012.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.