Abstract
AIM
To investigate the effectiveness and feasibility of inducing myopia in guinea pigs by flickering light (FL) stimulation with different frequencies.
METHODS
Seventy 2-week-old guinea pigs were randomly assigned to six groups: five FL groups and a control group (n=12 for each). Animals in the five FL groups were raised under 500lx illumination with a duty diurnal cycle of 50% at a flash rate of 5, 1, 0.5, 0.25 and 0.1Hz respectively. Those in the control group were reared under steady 250lx illumination. Refraction, axial length, and radius of curvature were measured before and at 2, 4, 6, 8, 10 and 12 weeks after treatment. At week 12, the eyeballs were taken out and three ocular dimensions and dry weight of sclera were measured.
RESULTS
A myopic shift and axial eye length increase developed in the five FL groups. Stimulation at 0.5Hz caused greater changes in myopic shift, axial elongation, eyeball dimension, and dry weight of sclera than stimulation at other frequencies. Compared with controls, eyes in 0.5Hz group were approximately -5.5±1.5D more myopic with increase in horizontal, vertical, axial dimensions by 0.89±0.3mm, 0.69±0.2mm, 1.12±0.2mm respectively and with increase in dry weight of sclera by 0.44mg.
CONCLUSION
Chronic exposure to periodic illumination at temporal frequency is attended by development of excessive ocular enlargement and myopic refractive error. Emmetropization could be disrupted differently by frequency alteration.
Keywords: myopia, flickering illumination, stimulus, guinea pig, animal model, eye development
INTRODUCTION
Myopia is a complex trait influenced by unidentified genetic and environmental factors as yet[1]. Early visual experience is well known to play a critical role in controlling eye growth during development[2]. It has been suggested that the early stages of human visual processing involve analysis by a parallel set of channels that may be defined in terms of their spatial and temporal tuning characteristics [3]. Until now, temporal frequency selectivity has received less attention [4], so the research of flickering light (FL) with different frequency might supply a better understanding of how visual experience regulates ocular growth.
In this respect, it remains controversial whether prolonged exposure to flickering light is associated with refractive development[5]. Schwahn and Schaeffel[6] reported that defocus-induced and deprivation-induced myopia in chickens could both be suppressed by flickering light (12 and 6Hz produced by rotating chopper disks). Contrarily, Cynader et al[7] and Cremieux et al[8] found strobe-reared cats were more myopic than normal cats (a 9-musec strobe flash every 2 seconds). A study by Yu et al[9] reported myopia could be induced in C57BL/6 mice under illumination with a duty cycle of 50% at a flash rate of 2Hz. The myopic shift induced by flickering light was smaller than that induced by deprivation. Similar results were found in guinea pig models [10].
The great variability in the selected flicker parameters in different species may account for the discrepancy in previous works. These results could be better understood if additional temporal frequencies were tested. Given that FL at 2Hz affects emmetropization, other frequencies may also change refractive development. The question under study here is how myopia development is related to the flicker frequencies and one possible hypothesis is that myopia increases with flicker frequency. As an artificial light, FL has a variety of frequencies. Is myopia more susceptible to a certain frequency, or the higher the frequency, the more myopic in refraction? The guinea pigs have a well-developed visual system[11] and they reliably develop refractive errors as expected from imposed defocus[11]. However, the effects of disruption of the diurnal light/dark cycles have not yet been studied in the guinea pig in as much detail as in the chicken. Therefore, the current study was performed to find out whether exposure to flickering light at certain frequencies might be an easy way to induce myopia in the guinea pig model. In doing so, we hoped to examine changes in eye shape after flickering challenge, and then, to establish an animal myopic model based on abnormal temporal tuning characteristics.
MATERIALS AND METHODS
Animals and experimental design
Seventy guinea pigs (English short hair stock, tricolor strain) were obtained at an average age of two weeks. The guinea pigs were examined carefully by slit lamp; animals with cortical or nuclear opacities were excluded. All treatments were in agreement with the Association for Research in Vision and Ophthalmology Statement for Use of Animals in Ophthalmic and Vision Research. The animal research was approved by the Animal Care and Ethics Committee in Fudan University, Shanghai, China.
Illuminative conditions and procedures
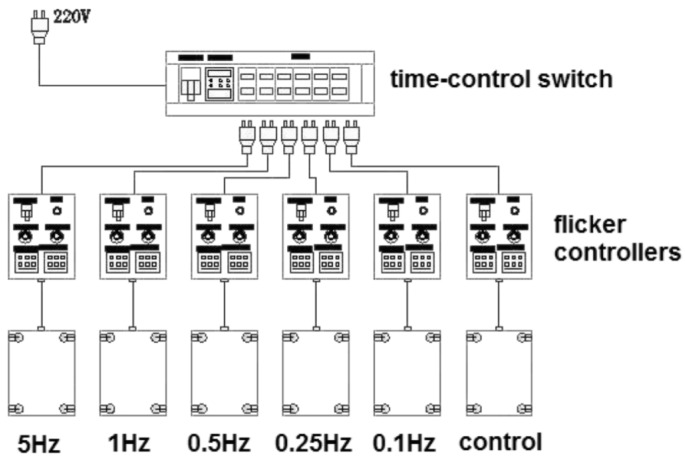
Seventy guinea pigs were raised in a darkroom with no light access from outside (temperature, 20-22°C, humidity, 55%-65%) [6]. Four guinea pigs were placed in one cage (dimension: 50.0cm×40.0cm×60.0cm, mesh size 1.5cm×5.0cm). Each cage was covered to ensure complete darkness. All the cages were well ventilated to maintain constant temperature inside. Four light emitting diodes (narrow spectra around 505nm; color temperature: 2850K) were installed in the four upper corners of each cage at a height of 50cm above the cage floor, providing a maximal luminance of 500lx. These lamps were synchronized in terms of their timing and intensity of illumination by flicker controllers (Yinuo Automation Co., LTD, Changsha, China. linear output, analogue signal, alternating-current pulse 220V). When the lights were on, a high precise silicon photodiode (AR813, China) was utilized to make sure the luminance was 500lx. All the controllers were manipulated by a time-control switch. The light was turned on at 6:00 AM and turned off at 6:00 PM. Experimental protocols were schematically represented in Figure 1.
Figure 1. The experimental animals were assigned to six groups: five FL groups and one control group.
The FL groups were raised with a duty diurnal cycle of 50% at a flash rate of 5, 1, 0.5, 0.25 and 0.1Hz respectively. Animals in control group were reared under steady illumination. All the controllers were manipulated by a time-control switch.
Seventy animals were randomized into six groups: five FL groups and one control group (n=12 for each). Animals in FL groups were exposed to 500lx illumination with a duty diurnal cycle of 50% at a flash rate of 5Hz (duty cycle=50%, 0.1 second and then in the dark for 0.1 second, with this analogize), 1, 0.5, 0.25 and 0.1Hz respectively. Those in the control group were raised under steady illumination of 250lx 12 hours per day, because the periodic stimulation with trains of on-off light pulses reduced the delivered light per time unit by 50% compared with the two 0-500lx FL groups.
Optical and biometric measurements
All measurements were performed prior to treatment and after 2, 4, 6, 8, 10 and 12 weeks of treatment. All measurements were performed by researchers who were masked to the identity of the experimental groups. The refraction of each eye was examined by streak retinoscopy in the dark. Animals were not anaesthetized, and 1% cyclopentolate hydrochloride (ALCON, Belgium) was topically administered to dilate the pupils prior to the examination. Measurements were taken in the horizontal meridian, 90° to the sagittal plane of the head and 30° temporal to the optic axis. Refractive errors were recorded as the mean refractions of horizontal and vertical meridians, as previously described[12].
The ocular component dimensions in vivo were measured using A-scan ultrasound (11MHz; SW1000, Suowei Co., LTD, China) in anaesthetized guinea pigs with administration of 0.5% proparacaine hydrochloride (Alcon, Belgium). Measurements were accepted when clear traces of various components of eyes with consistent waves and amplitudes were detected[12]. Ten repeated measurements were taken and the mean was recorded for each of the axial components.
Radius of corneal curvature (CRC) was measured in alert guinea pigs with a keratometer (OM-4; Topcon, Tokyo, Japan) combined with a plus 8.0D aspherical lens. A set of stainless steel ball-bearings was used for calibration[12].
Ocular dimensions and weights
At the 12-week follow-up observation, animals were sacrificed with an overdose of sodium pentobarbitone. The eyeball was enucleated and three ocular dimensions were measured with a digital vernier caliper in all groups: horizontal length (nasal-temporal), vertical length (superior-inferior), and axial length (anterior-posterior). A punch of the posterior sclera was removed 1-2mm nasal to the optic nerve with a 6mm surgical trephine. The retina and choroid were carefully removed from the scleral tissue, which was dried in an oven for 24 hours and then, immediately weighed with an electronic balance to obtain the dry weight of the sample[13].
Changes in body weight were used to assess the effect of physical development. Each guinea pig was weighed using an electronic balance (Kelang, JA1203N, China) after being placed into a small container, followed by other measurements which were detailed in previous sections.
Statistical Analysis
The raw data were processed using GRAPHPADPRISM (GRAPHPAD Software, Inc., San Diego, CA). Only data of the left eyes were registered for analysis in this study. After confirmation of normal distribution of the data, statistical comparison was performed using one-way analysis of variance (ANOVA) followed by Multiple comparisons. All results were expressed as mean±SD and differences were considered significant at P <0.05.
RESULTS
Optical and biometric measurements
There were no significant differences in refraction, axial length, or corneal radius of curvature at any time-point between the right and left eyes of all animals in each group (P>0.05). There was no significant difference in biometric data among the six groups before exposure to flickering light of different frequencies (P<0.05).
Table 1 summarizes the mean biometric results from each group over the 12 weeks and the statistical results of the comparison between FL groups and the control. The CRC in all six groups increased gradually with treatment time as compared to the time-point at the beginning (P<0.05), but no inter-group differences were found at each time-point (P>0.05). The mean refraction of the controls became less hyperopic throughout the experimental period, with a reduction in hyperopia of nearly 1.5D over the 12 weeks. The mean refraction in all the groups was around 4.5D at the beginning of the experiments, but a greater decrease in mean refractive power for the FL groups was apparent. Significant differences between the groups were observed at the end.
Table 1. Mean biometric results from guinea pigs reared under the different lighting conditions over twelve weeks and the statistical results of the comparison between groups at each time-point.
| Groups | Time-point (week) eyes (n=12) |
|||||||
| 0 week | 2 weeks | 4 weeks | 6 weeks | 8 weeks | 10 weeks | 12 weeks | ||
| Refraction (D) | 5Hz | 4.02±0.19 | 2.33±0.87 | 1.35±0.68 | 0.81±1.12 | 0.67±1.24 | 0.55±1.17 | 0.53±1.15 |
| 1Hz | 4.17±0.25 | 1.98±0.58 | 0.58±0.58 | -0.11±0.73 | -0.46±0.73 | -0.77±0.91 | -0.63±1.23 | |
| 0.5Hz | 4.23±0.23 | 0.52±0.76 | -0.92±1.07 | -1.69±1.01 | -2.02±1.21 | -2.34±1.34 | -2.62±1.23 | |
| 0.25Hz | 4.13±0.25 | 1.42±0.68 | 0.46±1.01 | 0.17±1.03 | 0.08±1.49 | 0.15±1.51 | 0.17±1.66 | |
| 0.1Hz | 4.23±0.19 | 2.08±0.53 | 1.29±1.02 | 1.17±0.89 | 0.92±0.97 | 0.81±0.91 | 0.81±1.14 | |
| Control | 4.23±0.13 | 3.81±0.40 | 3.25±0.56 | 3.08±0.51 | 2.92±0.56 | 2.83±0.61 | 2.70±0.67 | |
| F | 1.86 | 33.2 | 31.4 | 36.3 | 27.4 | 29.2 | 26.2 | |
| P | 0.114 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Axial length(mm) | 5Hz | 6.95±0.22 | 7.39±0.15 | 7.69±0.25 | 7.82±0.16 | 7.96±0.98 | 8.05±0.09 | 8.17±0.13 |
| 1Hz | 6.91±0.09 | 7.72±0.11 | 7.96±0.12 | 8.00±0.12 | 8.05±0.15 | 8.19±0.04 | 8.29±0.08 | |
| 0.5Hz | 6.86±0.14 | 7.79±0.16 | 8.28±0.42 | 8.35±0.27 | 8.41±0.19 | 8.46±0.16 | 8.47±0.16 | |
| 0.25Hz | 6.89±0.09 | 7.34±0.14 | 7.68±0.07 | 7.81±0.03 | 7.95±0.11 | 8.04±0.19 | 8.25±0.29 | |
| 0.1Hz | 6.89±0.10 | 7.46±0.10 | 7.77±0.07 | 7.82±0.06 | 7.94±0.64 | 7.98±0.04 | 8.05±0.29 | |
| Control | 6.89±0.09 | 7.33±0.07 | 7.48±0.08 | 7.57±0.09 | 7.62±0.06 | 7.76±0.25 | 7.79±0.21 | |
| F | 0.56 | 30.3 | 20.5 | 37.9 | 51.0 | 27.6 | 11.4 | |
| P | 0.73 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Radius of corneal curvature (mm) | 5Hz | 3.15±0.06 | 3.39±0.13 | 3.49±0.27 | 3.51±0.16 | 3.53±0.09 | 3.58±0.17 | 3.64±0.32 |
| 1Hz | 3.15±0.06 | 3.36±0.12 | 3.46±0.26 | 3.49±0.15 | 3.52±0.09 | 3.58±0.13 | 3.64±0.24 | |
| 0.5Hz | 3.17±0.13 | 3.32±0.15 | 3.43±0.11 | 3.48±0.08 | 3.52±0.08 | 3.56±0.05 | 3.59±0.03 | |
| 0.25Hz | 3.26±0.29 | 3.31±0.22 | 3.43±0.14 | 3.46±0.12 | 3.48±0.13 | 3.55±0.08 | 3.62±0.09 | |
| 0.1Hz | 3.29±0.29 | 3.33±0.20 | 3.44±0.22 | 3.47±0.11 | 3.49±0.11 | 3.56±0.09 | 3.63±0.11 | |
| Control | 3.14±0.11 | 3.33±0.17 | 3.46±0.16 | 3.47±0.08 | 3.49±0.04 | 3.55±0.04 | 3.61±0.09 | |
| F | 1.43 | 0.34 | 0.15 | 0.29 | 0.42 | 0.15 | 0.07 | |
| P | 0.23 | 0.89 | 0.98 | 0.92 | 0.83 | 0.98 | 0.99 | |
“P” represents the significance of the inter-group differences using ANOVA.
x±s
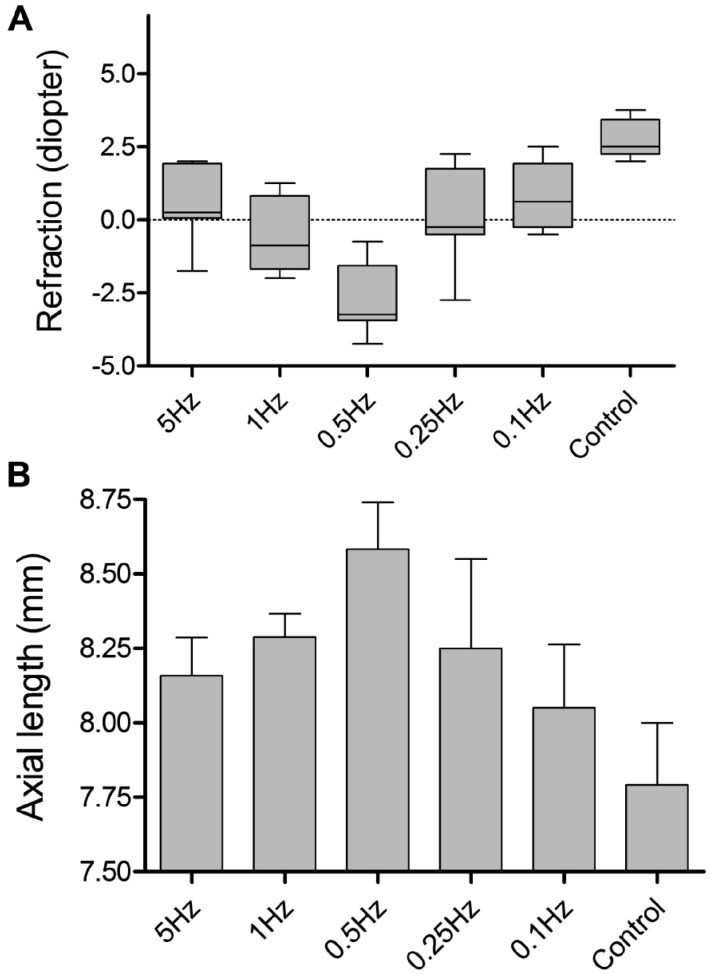
By the end of experiment, stimulation at 0.5Hz had caused the development of a larger myopic shift in refraction and more axial elongation than stimulation at other frequencies (Figure 2 A, B). Similar trend was observed in the proportion of axial length.
Figure 2. Changes of refraction (A) and axial length (B) in the 12th week.
A: ANOVA with Bonferroni: except for 5Hz vs 1Hz, 5Hz vs 0.25Hz, 5Hz vs 0.1Hz, 1Hz vs 0.25Hz, 1Hz vs 0.1Hz, 0.25Hz vs 0.1Hz, the differences of refraction for all remaining comparisons were statistically significant, P<0.05. B: Except for 5Hz vs 1Hz, 5Hz vs 0.25Hz, 5Hz vs 0.1Hz, 1Hz vs 0.25Hz, 1Hz vs 0.1Hz, 0.25Hz vs 0.1Hz, the differences of axial length for all remaining comparisons were statistically significant, P<0.05.
Ocular dimensions and weights
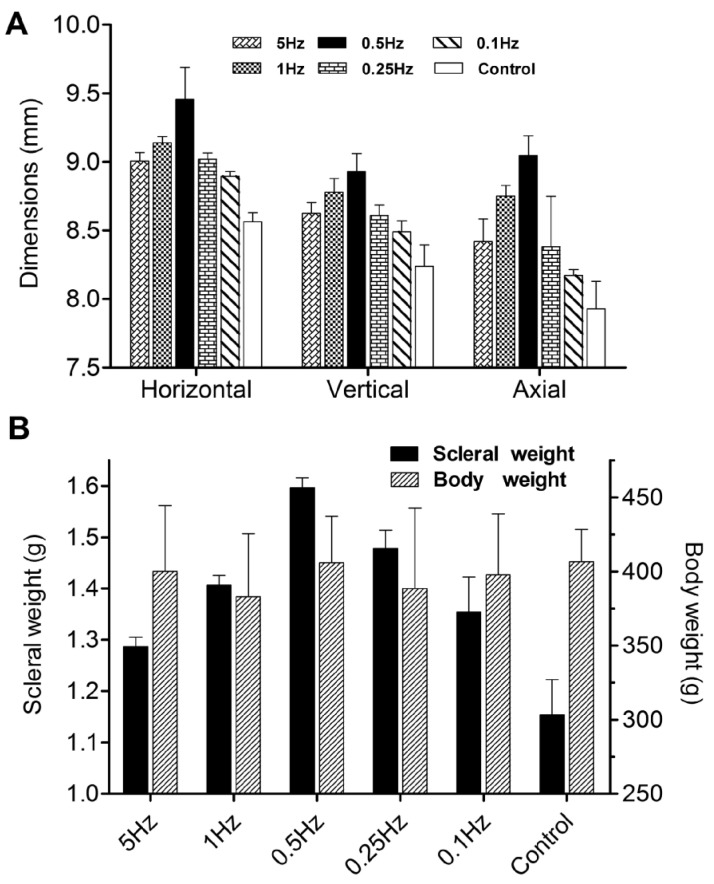
At the 12-week follow-up observation, eyeballs in all six groups were taken out. Their three dimensions were shown in Figure 3A, and body weight and dry weight of the scleral samples were shown in Figure 3B. Table 2 summarized the results from each group and the statistical results of the comparison. There were no statistically significant differences in body weight among the six groups. Compared with the guinea pigs raised at 0.5Hz flickering light, those raised at higher and lower frequencies became less myopic and had shorter eyes.
Figure 3. Changes of three dimensions (A) and weights (B) in the 12th week.
A: ANOVA with Bonferroni: except for 5Hz vs 1Hz, 5Hz vs 0.25Hz, 5Hz vs 0.1Hz, 1Hz vs 0.25Hz, 0.25Hz vs 0.1Hz, the differences of horizontal dimension for all remaining comparisons were statistically significant, P<0.05; except for 5Hz vs 0.25Hz, 0.25Hz vs 0.1Hz, the differences of vertical dimension for all remaining comparisons were statistically significant, P<0.05; except for 5Hz vs 0.25Hz, 0.25Hz vs 0.1Hz, the differences of axial dimension for all remaining comparisons were statistically significant, P<0.05. B: Except for 1Hz vs 0.1Hz, the differences of scleral weights for all remaining comparisons were statistically significant, P<0.05. There were no statistically significant differences in body weight among 6 groups P>0.05.
Table 2. Ocular dimensions and weights in the 12th week in six groups.
| Groups | Three dimension (mm) |
Weight (g) |
|||
| Horizontal | Vertical | Axial | Sclera | Body | |
| 5Hz | 9.01±0.06 | 8.62±0.08 | 8.42±0.16 | 1.29±0.02 | 400.0±44.5 |
| 1Hz | 9.14±0.05 | 8.78±0.09 | 8.75±0.08 | 1.41±0.02 | 382.9±42.6 |
| 0.5Hz | 9.46±0.23 | 8.93±0.13 | 9.05±0.14 | 1.59±0.02 | 405.8±31.4 |
| 0.25Hz | 9.02±0.05 | 8.60±0.07 | 8.38±0.37 | 1.47±0.04 | 388.3±54.6 |
| 0.1Hz | 8.89±0.03 | 8.49±0.08 | 8.17±0.04 | 1.35±0.07 | 397.5±41.4 |
| Control | 8.56±0.07 | 8.23±0.15 | 7.93±0.20 | 1.15±0.07 | 406.5±21.9 |
| F | 89.3 | 59.3 | 50.2 | 146.2 | 0.65 |
| P | <0.05 | <0.05 | <0.05 | <0.05 | >0.05 |
By week 12 after experiment, eyes of 0.5Hz group had been -5.5±1.5D more myopic in refraction as compared with controls; however, when the offset of small eye artifact was taken into account, as the previous studies[14], [15] described, the amended refractions of six groups were 5Hz: -4.80D, 1Hz: -7.13D, 0.5Hz: -10.81D, 0.25Hz: -5.65D, 0.1Hz: -4.33D, and controls: -1.06D. Meanwhile, eyes of 0.5Hz group were approximately 0.44mg heavier in scleral weight, with increase in horizontal, vertical, axial dimensions by 0.89±0.3mm, 0.69±0.2mm, 1.12±0.2mm respectively.
DISCUSSION
The key observation was that periodic illumination at defined temporal frequency led to an accelerated shift towards myopia, which was accompanied by development of longer eyes. Meanwhile, steady illumination ensured normal axial eye growth. Induction of refractive changes from flickering light has been described in some different species [8], [9]; however, little work has been reported in guinea pigs. Our findings agree in general with the results of Yu et al [9], but the underlying mechanisms have remained unclear. The present study suggests a novel method for inducing myopia in guinea pigs for research purposes.
At week 12, there was no difference in body weights among 6 groups. This suggests that the difference in ocular weight is not related to that in body weight. Further, CRC increased steadily throughout the experimental period, and there was no significant difference between eyes in each group at each time point. This implies that the effects of flickering light on refractive development are mainly due to changes in axial elongation. Previous studies[15]-[17] have also demonstrated that there are independent control mechanisms for eye growth in the anterior and posterior segment.
As an artificial light, FL owns a variety of frequencies. Our study focused on a limited range of low frequencies and not all adjacent frequencies between experimental groups were statistically different. But on the whole, flickering light at temporal frequencies above and below 0.5Hz caused fewer enlargement and less myopic shift in refraction than that at 0.5Hz. As for this point, Umino et al [18] claimed visual sensitivity was generally the greatest for intermediate spatial and temporal frequencies and attenuated at higher and lower frequencies. The mechanism of this myopia remains unclear but one alternative explanation is that periodic stimulation, with trains of on-off light pulses, reduces the amount of light delivered per unit time by 50%, and interrupts any spatiotemporal processing in the retina. At the most “effective” frequency, 0.5Hz, the retina is stimulated by intense light for 1 second and then in the dark for 1 second. This may not provide sufficient information for successful emmetropization. Thus, the retinal circuits may be activated to induce myopia. At the lower temporal frequencies tested, 0.25Hz and 0.1Hz, light-pulses were respectively 2 seconds and 5 seconds long. This condition therefore may approach the condition of continuous illumination, allowing sufficient integration of activation by spatiotemporally patterned retinal images, to activate mechanisms that oppose the development of myopia. At the higher temporal frequencies tested, 1Hz and 5Hz, light-pulses were respectively 500 msec and 100 msec long, separated by dark intervals of equal duration. Such brief exposures may not permit retinal circuits to analyze spatio-temproal patterns, which is necessary to guide emmetropization. However, the image of a grating moving across the retina (as a result of either object-motion or eye-movement) may also be seen as one type of flickering light at any point in the retina[18]. The shorter intervals between one pulse and the next may simulate the actions of real images in the retina, and then, the retinal circuits are activated to prevent myopia.
Our work represents an attempt to expand our knowledge of how the temporal properties of periodic visual stimuli influence ocular growth and refraction. Studies of this sort in animal models might reveal important features of the retinal circuitry by which visual stimuli regulate eye growth. This would make the guinea pig a promising animal model for future studies.
Acknowledgments
The authors thank Zi-Mei Zhou, Georg. Dorninger, and Haleena Ramsahye who reviewed and edited this paper.
Footnotes
Foundation items: Foundation for Shanghai Municipal Health Bureau (No. 2010147), National Natural Science Foundation of China (No. 81100689), Foundation for Shanghai Jinshan Health Bureau (No. JWKJ-KTYQ-201203)
REFERENCES
- 1.Zhou X, Huang Q, An J, Lu RX, Qin XY, Jiang LQ, Li Y, Wang JH, Chen JF, Qu J. Genetic deletion of the adenosine A2A receptor confers postnatal development of relative myopia in mice. Invest Ophthalmol Vis Sci. 2010;51(9):4362–4370. doi: 10.1167/iovs.09-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ip JM, Rose KA, Morgan IG, Burlutsky G, Mitchell P. Myopia and the urban environment: findings in a sample of 12-year-old Australian school children. Invest Ophthalmol Vis Sci. 2008;49(9):3858–3863. doi: 10.1167/iovs.07-1451. [DOI] [PubMed] [Google Scholar]
- 3.Bex PJ, Verstraten FA, Mareschal I. Temporal and spatial frequency tuning of the flicker motion aftereffect. Vision Res. 1996;36(17):2721–2727. doi: 10.1016/0042-6989(96)00004-1. [DOI] [PubMed] [Google Scholar]
- 4.Hulvershorn J, Bloy L, Gualtieri EE, Leigh JS, Elliott MA. Spatial sensitivity and temporal response of spin echo and gradient echo bold contrast at 3 T using peak hemodynamic activation time. Neuroimage. 2005;24(1):216–223. doi: 10.1016/j.neuroimage.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 5.Blair NP, Wanek JM, Mori M, Shahidi M. Abnormal retinal vascular oxygen tension response to light flicker in diabetic rats. Invest Ophthalmol Vis Sci. 2009;50(11):5444–5448. doi: 10.1167/iovs.09-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwahn HN, Schaeffel F. Flicker parameters are different for suppression of myopia and hyperopia. Vision Res. 1997;37(19):2661–2673. doi: 10.1016/s0042-6989(97)00114-4. [DOI] [PubMed] [Google Scholar]
- 7.Cynader M, Berman N, Hein A. Cats reared in stroboscopic illumination: effects on receptive fields in visual cortex. Proc Natl Acad Sci U S A. 1973;70(5):1353–1354. doi: 10.1073/pnas.70.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cremieux J, Orban GA, Duysens J, Amblard B, Kennedy H. Experimental myopia in cats reared in stroboscopic illumination. Vision Res. 1989;29(8):1033–1036. doi: 10.1016/0042-6989(89)90117-x. [DOI] [PubMed] [Google Scholar]
- 9.Yu Y, Chen H, Tuo J, Zhu Y. Effects of flickering light on refraction and changes in eye axial length of C57BL/6 mice. Ophthalmic Res. 2011;46(2):80–87. doi: 10.1159/000323179. [DOI] [PubMed] [Google Scholar]
- 10.Cheng ZY, Li JH, Li R, Xie YB. Effects of flashing light on ocular growth and development of myopia in pigmented guinea pigs. Zhonghua Yan Ke Za Zhi. 2004;40(9):601–604. [PubMed] [Google Scholar]
- 11.Armitage JA, Bui BV, Gibson R, Vingrys AJ. Postnatal development of flicker sensitivity in guinea pigs. Clin Exp Optom. 2001;84(8):270–275. doi: 10.1111/j.1444-0938.2001.tb05037.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhou X, Lu F, Xie R, Jiang L, Wen J, Li Y, Shi J, He T, Qu J. Recovery from axial myopia induced by a monocularly deprived facemask in adolescent (7-week-old) guinea pigs. Vision Res. 2007;47(8):1103–1111. doi: 10.1016/j.visres.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Liu R, Qian YF, He JC, Hu M, Zhou XT, Dai JH, Qu XM, Chu RY. Effects of different monochromatic lights on refractive development and eye growth in guinea pigs. Exp Eye Res. 2011;92(6):447–453. doi: 10.1016/j.exer.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Glickstein M, Millodot M. Retinoscopy and eye size. Science. 1970;168(3931):605–606. doi: 10.1126/science.168.3931.605. [DOI] [PubMed] [Google Scholar]
- 15.Jiang L, Schaeffel F, Zhou X, Jin X, Pan M, Ye L, Wu X, Huang Q, Lu F, Qu J. Spontaneous axial myopia and emmetropization in a strain of wild-type guinea pig (Cavia porcellus) Invest Ophthalmol Vis Sci. 2009;50(30):1013–1019. doi: 10.1167/iovs.08-2463. [DOI] [PubMed] [Google Scholar]
- 16.Lu F, Zhou X, Jiang L, Fu Y, Lai X, Xie R, Qu J. Axial myopia induced by hyperopic defocus in guinea pigs: A detailed assessment on susceptibility and recovery. Exp Eye Res. 2009;89(1):101–108. doi: 10.1016/j.exer.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 17.Backhouse S, Phillips JR. Effect of induced myopia on scleral myofibroblasts and in vivo ocular biomechanical compliance in the guinea pig. Invest Ophthalmol Vis Sci. 2010;51(12):6162–6171. doi: 10.1167/iovs.10-5387. [DOI] [PubMed] [Google Scholar]
- 18.Umino Y, Solessio E, Barlow RB. Speed, spatial, and temporal tuning of rod and cone vision in mouse. J Neurosci. 2008;28(1):189–198. doi: 10.1523/JNEUROSCI.3551-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]