Abstract
AIM
To evaluate the effects of intensive control of blood glucose and blood pressure on microvascular complications in patients with type II diabetes by comparing the therapeutic effects of intensive and standard treatment in patients with type II diabetes.
METHODS
A total of 107 patients with type II diabetes were randomly assigned into intensive and standard treatment groups. Patients in the intensive treatment group received preterax (perindopril/ indapamide) to control blood pressure, and gliclazide (diamicron) MR to control blood glucose. Patients in the standard treatment group received routine medications or placebo. Urinary microalbumin (UMA), urinary creatinine (UCR), the UMA/UCR ratio, and visual acuity were monitored according to the study design of the ADVANCE trial. Direct ophthalmoscopy and seven-field stereoscopic retinal photography were used to examine the fundi at baseline, and repeated after 5 years of treatment.
RESULTS
The characteristics of patients in both groups were well balanced at baseline. After 5 years of treatment, visual acuity was found to be decreased in the standard group (P=0.04), but remained stable in the intensive group. The severity of diabetic retinopathy had not progressed in patients in the intensive group, but had deteriorated in the standard group (P=0.0006). The UMA/UCR ratio was not obviously changed in patients in the intensive group, whereas it was significantly increased in the standard group (P=0.00).
CONCLUSION
Intensive control of blood glucose and blood pressure can decrease the incidence or slow the progression of microvascular complications in patients with type II diabetes, and maintain stable vision.
Keywords: diabetes mellitus, intensive therapy, microvascular complications, diabetic retinopathy
Introduction
Diabetic retinopathy (DR) is one of the microvascular complications caused by hyperglycemia. Furthermore, hypertension can promote the progression of DR and induce further damage of retinal vessels. The results of the United Kingdom Prospective Diabetes Study (UKPDS) [1] and Diabetes Control and Complications Trial (DCCT) [2] demonstrated that intensive control of blood glucose can reduce the incidence and progression of DR. The results of the UKPDS [1] also showed that lowering blood pressure can reduce the occurrence and the progression of retinopathy.
The Action in Diabetes and Vascular Disease: Preterax and Diamicron-MR Controlled Evaluation (ADVANCE) study is a 5-year double-blind clinical project conducted in 11 140 patients in 215 centers from 20 countries, of whom 3 293 patients were from 49 centers in China. Compared with the UKPDS, the blood-glucose control was more intensive (HbA1c<6.5%) in ADVANCE, thus ADVANCE is useful for the study of the linear relationship between blood-glucose control and retinopathy [3]. At present, DR is mostly diagnosed based on direct ophthalmoscopic results or medical history. Because these methods for diagnosis and staging for retinopathy are not very accurate, DR was diagnosed by fundus photography in ADVANCE, as this method may be more accurate and more easily followed up.
The current randomized study was a part of ADVANCE, which particularly evaluated the effects of intensive or standard control of blood glucose and blood pressure on the occurrence and development of type II diabetes-related microvascular complications.
SUBJECTS and Methods
Subjects
A total of 107 patients with type II diabetes (with or without hypertension) were enrolled in this study during the period from May 2002 to January 2003. All patients were diagnosed in the Department of Endocrinology and Department of Hypertension of Ruijin Hospital, affiliated to Shanghai Jiaotong University School of Medicine. Both departments were the local centers for the ADVANCE trial.
After a 3-month wash-out period for previous medications, the 107 patients (54 males, 53 females; mean age 66 years, range 55-85) were randomly assigned into two groups. The intensive group (n=53) was treated with gliclazide (diamicron) MR for intensive control of blood glucose, combined with preterax (perindopril plus indapamide) to control blood pressure. The standard group (n=54) was treated with anti-hyperglycemia medications other than gliclazide MR for blood-glucose control and with a placebo for blood-pressure control.
The patients had a history of type II diabetes ranging from 2 months to 34 years, with an average of 7.36 years. In addition to diabetes, 86% of the patients also had concomitant hypertension, and had been treated with medication for an average of 12.8 years (range 0-43 years). Of the 107 patients, 99 had completed 5 years of treatment according to the ADVANCE study design. During the current study, five patients (three in the standard group and two in the intensive group) died from cardiocerebral vascular complications, and one patient in the intensive group died from unrelated causes. Two patients (one in the standard group and the other in the intensive group) were lost to follow-up. Of the 99 patients, one patient in the standard group missed their fundus examination.
Methods
Patients were routinely examined, treated, and followed up by physicians from both clinical departments. Tests for fasting blood glucose, glycated hemoglobin, serum lipid, urinary microalbumin (UMA) and urinary creatinine (UCR) were repeated at the clinic visits every 3 months. The criteria for control of blood sugar and blood pressure in the intensive group were to maintain the glycated hemoglobin at less than 6.5% and blood pressure at less than 140/90 mmHg. Modification of medication in the intensive group was allowed in order to reach these two criteria. No strict criteria for blood sugar or blood pressure were set for the standard group.
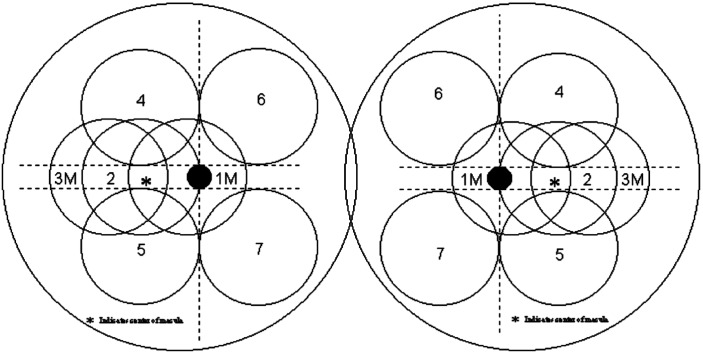
Visual acuity was measured and fundi examined at the Department of Ophthalmology. Patients with severe cataract, shallow anterior chamber, glaucoma, and previous history of eye treatments such as retinal laser photocoagulation, vitrectomy, or cataract surgery, were excluded. The photographs were taken from the enrolled patients at baseline, and repeated after 2 and 5 years of treatment. After the pupils were completely dilated to 6mm with tropicamide phenylephrine eye drops (Santen Pharmaceutical, Tokyo, Japan), retinal photography was conducted according to the refined Early Treatment Diabetic Retinopathy Study (ETDRS) method with seven fields (Figure 1). The images were sent to the Julius Center, University Medical Center, Utrecht, Netherlands, where they were examined within 1 month of receipt.
Figure 1. Fundus model of the refined seven-field ETDRS method.
Statistical Analysis
Data were analyzed using the Wilcoxon test with SPSS software (version 11.5; SPSS Inc., Chicago, IL, USA). P value <0.05 was considered to be statistically significant.
Results
Visual Acuity
Visual acuities of 198 eyes in 99 patients (49 in the intensive group and 50 in the standard group) were examined. The visual acuity was not statistically different between the two groups at baseline (Z=-0.55, P=0.59). However, compared with those in the intensive group, the visual acuity of patients in the standard group had decreased significantly after 5 years (P=0.04, Table 1).
Table 1. Visual acuity of eyes of patients in the intensive and standard groups.
| Group | Time point | Visual acuity |
Z | P | |||
| <0.1 | 0.1–0.3 | 0.3–0.6 | >0.6 | ||||
| Intensive | Baseline | 8 | 7 | 12 | 71 | 0.48 | 0.65 |
| 5 years | 9 | 12 | 8 | 69 | |||
| Standard | Baseline | 6 | 6 | 25 | 63 | 2.04 | 0.04 |
| 5 years | 8 | 8 | 34 | 50 | |||
Fundus
Fundi of 196 eyes in 98 patients were examined (Table 2). No difference was found in fundus images between the intensive and standard groups at baseline (Z=1.16, P=0.25). However, significant progression of retinopathy was found in the standard group after 5 years (Z=3.44, P=0.0006, Figure 2), but not in the intensive treatment group (Z=0.77, P=0.44). Five patients accepted treatment with retinal laser photocoagulation. One patient in the standard group developed retinal branch vein occlusion (BRVO).
Table 2. Fundus of eyes of patients in the intensive and standard groups.
| Group | Time point | Diabetic retinopathy |
BRVO | Severe cataract | Atrophy or blindness | Total | |||
| Stage 0 | Stage I | Stage II | Stage III | ||||||
| Intensive | Baseline | 76 | 7 | 7 | 0 | 1 | 7 | 0 | 98 |
| 5 years | 70 | 8 | 5 | 4 | 1 | 10 | 0 | 98 | |
| Standard | Baseline | 81 | 6 | 3 | 0 | 0 | 6 | 2 | 98 |
| 5 years | 58 | 11 | 9 | 5 | 1 | 12 | 2 | 98 | |
Figure 2. Fundus changes in the right eye of a patient in the standard group (male, 70 years old, 8-year history of diabetes and 10-year history of hypertension).
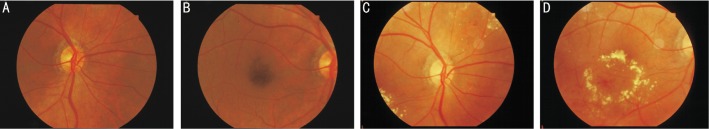
A, B: the fundus at baseline; C, D: the fundus at 5 years after standard treatment, showing microaneurysm, bleeding and exudation on the retina.
UMA/UCR
The UMA/UCR ratios at baseline and 5 years after treatment were not significantly different in the intensive group, but had significantly increased at 5 years in the standard group (P=0.00, Table 3).
Table 3. UMA/UCR of patients in the intensive and standard groups.
| Group | Time point | Cases | UMA/UCR (mg/mmoL) |
Z | P | ||
| Average | Minimum | Maximum | |||||
| Intensive | Baseline | 49 | 17.8 | 0.37 | 283.2 | -1.25 | 0.21 |
| 5 years | 49 | 7.5 | 0.42 | 70.44 | |||
| Standard | Baseline | 50 | 3.41 | 0.25 | 27.00 | -3.78 | 0.00 |
| 5 years | 50 | 18.96 | 0.01 | 354.8 | |||
Discussion
The results of this study show that intensive control of blood glucose was able to reduce the progression of DR and DN, which are the two most common microvascular complications of type II DM. The results from a clinical trial indicated that intensive control could reduce the risk of new or worsening nephropathy by 21%, compared with standard control [3]. Meanwhile, another result of the trial was that the incidence of microvascular complications decreased by 9% and UMA decreased by 20% in the intensive group compared with the standard group [4]. UMA is an index of early pathological change in renal microvascular complications. Lunetta et al [5] reported that abnormalities in the UMA/UCR ratio were related to the incidence and severity of DR. Patients often had high UMA concomitant with severe DR. Patients with persisting low UMA may have decreased light sensitivity in the parafovea before the occurrence of DR, and low UMA is considered to be the earliest pathological change of DR. Although the mechanism of UMA elevation is unknown, this abnormality is known to be due to functional aberration in microvascular endothelial cells, and will induce microvascular complications. Zoungas et al [6] found that, compared with no intervention, combination treatment could reduce the risk of new or worsening nephropathy by 33%, the risk of new-onset macroalbuminuria by 54%, and the risk of new-onset microalbuminuria by 26%. Moreover, the combination treatment was associated with an 18% reduction in the risk of death from various causes. In our study, the UMA/UCR ratio was not significantly different in the intensive group between baseline and 5 years after treatment, but was significantly elevated in the standard group, indicating that the function of the glomeruli was damaged in those patients with long-term, poorly controlled diabetes and hypertension. The deteriorations in renal glomerular function were related to the worsening of diabetic microangiopathy. Intensive control of blood pressure and glucose, combined with close HbA1c monitoring and guidance, were helpful to slow down the progression of renal lesions, and to protect glomerular functions.
HbA1c is formed from the principal part of hemoglobin and glucose via the action specific enzymes. The percentage of HbA1c in hemoglobin reflects the average glucose level within 3 months, which may reflect the long-term status of blood glucose, and help to guide the therapeutic strategy for these patients. In the intensive group of the ADVANCE study, blood glucose was controlled intensively (HbA1c<6.5%), resulting in better control of DR. The results of the UKPDS study indicated that every decrease of 0.9% in HbA1c could lead to a decrease of 21% in the risk of retinopathy [1].
The control of blood pressure or blood glucose may have different influences on the incidence of diabetes-related vascular complications. The results of the UKPDS [1] study showed that satisfactory blood-pressure control has a greater contribution to reducing the risk of vessel complications than does blood-glucose control. Intensive control of blood pressure could decrease the incidence of diabetes-related vascular diseases and other complications. In addition, patients with diabetes who have low blood pressure have fewer complications than patients without diabetes who have hypertension [1]. However, there have been some conflicting results from other authors. Beulens [7] reported that fewer patients on blood pressure-lowering treatment experienced incidence or progression of retinopathy compared with patients on placebo, but the difference was not significant (OR 0.78; 95% CI 0.57–1.06; P=0.12). Blood pressure-lowering treatment reduced the occurrence of macular oedema (P=0.016) and arteriovenous nicking compared with placebo (P=0.025). Compared with standard glucose control, intensive glucose control did not reduce the incidence or progression of retinopathy (P=0.27). These findings are consistent with the results of many other studies [8], [9].
In the current study, seven-field stereoscopic photography of both eyes were conducted at baseline assessment and 5 years after treatment. The staging system for DR reported at the 2002 International Ophthalmology Conference in Sydney [10] was used in this study. Stage 0 means no change in the fundus, while stages 1-3 refer to increasing severities of non-proliferative retinopathy. The results showed that new retinopathy occurred in 19 of 196 eyes, and one patient in the standard group developed BRVO, which is somewhat lower than reported in the literature. Cataract might be one of the reasons for this lower percentage of retinopathy. No difference in the severity of DR was found between the two groups at baseline. However, after 5 years of treatment, the severity of DR had also remained stable in the intensive group, but had worsened in the standard group. No proliferative retinopathy was found in our study. In addition, there was no difference in visual acuity between the two groups at baseline. However, after 5 years of treatment, visual acuity had remained stable in the intensive group, but had decreased in the standard group. These results suggested that intensive control of blood glucose and blood pressure could protect retinal vessels and vision from damage and prevent retinopathy.
Retinal laser photocoagulation treatment can be used as an indicator of microvascular changes in patients with diabetes. Although this indicator is specific, it is not particularly, sensitive and is affected by medical practice and individual health-care systems. The HOPE report [11] showed that ramipril could not decrease the incidence of retinal laser photocoagulation treatment. However, the UKPDS study suggested that medication could decrease microvascular change by one-third, and thus in turn decrease the requirement for retinal laser photocoagulation [1]. The incidence of retinal laser photocoagulation treatment was different in some reports (UKPDS study 1.7%, HOPE study 2.2%). Only five patients in our study accepted retinal laser photocoagulation.
Overall, DR cannot be cured, but blindness can be prevented. Such prevention needs cooperation between both patients and physicians from internal medicine, endocrinology, and ophthalmology to closely and coordinately control blood pressure, glucose, serum lipid and other risk factors. Routine fundus examination, regular follow-up, and proper treatment could prevent diabetes-related blindness.
Footnotes
Foundation items: French Pharmaceutical Company Servier, Australian National Health and Medical Research Council (No. NCT00145925); National Natural Science Foundation of China (No. 60978030); Shanghai Leading Academic Discipline Project (No. S30205)
References
- 1.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 2.Albers JW, Herman WH, Pop-Busui R, Feldman EL, Martin CL, Cleary PA, Waberski BH, Lachin JM. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care. 2010;33(5):1090–1096. doi: 10.2337/dc09-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 4.ADVANCE Collaborative Group. MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M, Glasziou P, Grobbee DE, Hamet P, Heller S, Liu LS, Mancia G, Mogensen CE, Pan CY, Rodgers A, Williams B. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 5.Lunetta M, Infantone L, Calogero AE, Infantone E. Increased urinary albumin excretion is a marker of risk for retinopathy and coronary heart disease in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 1998;40(1):45–51. doi: 10.1016/s0168-8227(98)00024-2. [DOI] [PubMed] [Google Scholar]
- 6.Zoungas S, de Galan BE, Ninomiya T, Grobbee D, Hamet P, Heller S, MacMahon S, Marre M, Neal B, Patel A, Woodward M, Chalmers J, ADVANCE Collaborative Group. Cass A, Glasziou P, Harrap S, Lisheng L, Mancia G, Pillai A, Poulter N, Perkovic V, Travert F. Combined effects of routine blood pressure lowering and intensive glucose control on macrovascular and microvascular outcomes in patients with type 2 diabetes: New results from the ADVANCE trial. Diabetes Care. 2009;32(11):2068–2074. doi: 10.2337/dc09-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beulens JW, Patel A, Vingerling JR, Cruickshank JK, Hughes AD, Stanton A, Lu J, McG Thom SA, Grobbee DE, Stolk RP, AdRem Project Team and ADVANCE Management Committee ADVANCE management committee. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trial. Diabetologia. 2009;52(10):2027–2036. doi: 10.1007/s00125-009-1457-x. [DOI] [PubMed] [Google Scholar]
- 8.Brito JP, Montori VM. Intensive BP control and/or glucose control did not reduce microvascular events in hypertensive type 2 diabetes. Ann Intern Med. 2012;157(8):JC4–7. doi: 10.7326/0003-4819-157-8-201210160-02007. [DOI] [PubMed] [Google Scholar]
- 9.ACCORD Study Group; ACCORD Eye Study Group. Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, Hubbard L, Esser BA, Lovato JF, Perdue LH, Goff DC, Jr, Cushman WC, Ginsberg HN, Elam MB, Genuth S, Gerstein HC, Schubart U, Fine LJ. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363(3):233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT, Global Diabetic Retinopathy Project Group Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 11.Heart Outcomes Prevention Evaluation (HOPE) Study Investigators Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355(9200):253–259. [PubMed] [Google Scholar]