Abstract
AIM
To compare the clinical efficacy of intravitreal injections of bevacizumab and ranibizumab for treating Chinese patients with neovascular age-related macular degeneration (AMD).
METHODS
Among 60 Chinese patients with exudative AMD (60 eyes), 28 received intravitreal bevacizumab injections (1.25mg) and 32 received intravitreal ranibizumab injections (0.5mg), once a month for 3 months and were followed for a total of 6 months. Monthly optical coherence tomography (OCT) was used to determine whether the patients received additional treatments during the follow-up. We compared the baseline and 6-month follow-up values of mean best-corrected visual acuity (BCVA) and central retinal thickness (CRT) in both groups of patients. We also compared the occurrence of adverse events.
RESULTS
At the 6-month follow-up, the mean BCVA (logMAR) of the bevacizumab and ranibizumab treatment groups improved from the baseline measurements of 0.72±0.23 and 0.73±0.22 to 0.47±0.14 and 0.45±0.20, respectively (P<0.05 for both groups). However, the change was not significantly different between the two groups. As evaluated by OCT, CRT decreased from 366.71±34.72µm and 352±36.9µm at baseline to 250.86±41.51µm and 243.22±41.38µm in the bevacizumab and ranibizumab groups, respectively (P<0.05 for both groups). However, the change was not significantly different between the two groups. There were no severe local adverse reactions or systemic adverse events.
CONCLUSION
Intravitreal bevacizumab and ranibizumab have equivalent effects on BCVA and CRT and appeare safe over the short-term.
Keywords: age-related macular degeneration, choroidal neovascularization, bevacizumab (avastin), ranibizumab (lucentis)
Introduction
Age-related macular degeneration (AMD), also referred to as “senile macular degeneration” occurs primarily in individuals over 50 years and always involves both eyes. AMD causes vision deterioration, with or without deformation, discoloration, or a central dark point, is one of the primary reasons for blindness in the elderly in developed countries, and seriously reduces the quality of life. With China's aging population and improved living standards, the incidence of AMD is gradually rising and affecting younger people[1],[2].
Clinically, AMD is classified into two types: dry (atrophic) and wet (exudative). Dry AMD is characterized by macular drusen, hyperplasia, and retinal pigment epithelium atrophy, along with slow vision loss. Wet AMD is a major cause of blindness, and patients exhibit choroidal neovascularization (CNV) with subretinal hemorrhage, exudation, subretinal fibrosis, or vitreous hemorrhage[3], leading to serious and, if untreated, irreversible decline in visual acuity in the short term. Therefore, wet AMD therapy focuses on treating CNV.
Vascular endothelial growth factor (VEGF) is a cytokine that promotes angiogenesis and vessel wall permeability, thereby playing an important role in CNV formation[4]. Anti-VEGF drugs safely and effectively inhibit CNV development, stabilize and improve visual acuity, and reduce macular edema[5],[6]. Bevacizumab and ranibizumab are anti-VEGF drugs that have been widely used to treat ophthalmological choroidal neovascular disease, including wet AMD. Although recent studies performed in western countries have compared these two drugs and found their clinical efficacy to be comparable[7]-[9], little is known about their comparative clinical efficacy in a native Asian population. This study assessed the clinical efficacy of these two drugs in Chinese patients with wet AMD.
SUBJECTS AND METHODS
Subjects
After slit-lamp indirect ophthalmoscopy, fundus fluorescein angiography (FFA), indocyanine green angiography (ICGA), and optical coherence tomography (OCT) examinations, 60 patients over the age of 50 years were diagnosed with wet AMD. Patients with pathologic myopia, uveitis, ocular trauma or diabetic retinopathy lesions, polypoidal choroidal vasculopathy, end-stage scarring CNV, one eye, history of cardio-cerebral vascular disease, or history of previous treatment with photodynamic therapy (PDT), transpupillary thermotherapy (TTT), or retinal laser photocoagulation were excluded from this study. We selected 28 patients (28 eyes) diagnosed with wet AMD who accepted intravitreal injections with bevacizumab (Avastin; Roche, Basel, Switzerland) at our hospital from October 2008 to October 2009, and 32 patients (32 eyes) diagnosed with wet AMD who accepted intravitreal injections with ranibizumab (Lucentis; Novartis, Basel, Switzerland) at our hospital from August 2010 to June 2012 (baseline data shown in Table 1). Although these two arms of the study were run consecutively, they both involved the same ophthalmic surgeons. The bevacizumab group included 13 males and 15 females, with a mean age of 66.8±8.6 years, and the ranibizumab group comprised 17 males and 15 females, with a mean age of 67.9±10.3 years. The baseline best-corrected visual acuity (BCVA, logMAR) was 0.72±0.23 in the bevacizumab group and 0.73±0.22 in the ranibizumab group. The baseline central retinal thickness (CRT) was 366.71±34.72µm in the bevacizumab group and 352±36.9µm in the ranibizumab group (Table 1). These mean ages, visual acuities, and central retinal thicknesses were not significantly different between the two groups of patients. All patients signed informed consent forms. Bevacizumab intravitreal injections to treat wet AMD were part of the AMD Clinical Characteristics and Interventions Research Project in China, and were approved by the hospital's Ethics Committee. Ranibizumab was provided by Novartis as part of the ranibizumab drug donation program, and was also approved by the Ethics Committee.
Table 1. Baseline data before treatment.
| Bevacizumab | Ranibizumab | P | |
| Gender, n (%) | 0.779 | ||
| Female | 15 (53.57) | 15 (46.88) | |
| Male | 13 (46.43) | 17 (53.12) | |
| Age (a) | 0.694 | ||
| 50-59 | 4 (14.29) | 9 (28.12) | |
| 60-69 | 9 (32.14) | 9 (28.12) | |
| 70-79 | 12 (42.86) | 10 (31.25) | |
| 80-89 | 3 (10.71) | 4 (12.5) | |
| BCVA (logMAR) | 0.72±0.23 | 0.73±0.22 | 0.812 |
| CRT (µm) | 366.71±34.72 | 352±36.9 | 0.12 |
| IOP (mmHg, MEDIAN) | 14 | 13.5 | 0.765 |
| Blood pressure (mmHg, MEDIAN) | |||
| Systolic blood pressure | 121.89±14.24 | 122.19±10.05 | 0.93 |
| Diastolic blood pressure | 80.71±10.88 | 82.28±7.33 | 0.51 |
x±s
Methods
Sixty patients underwent monthly injections for 3 months and attended monthly follow-ups for 3 additional months after treatment. The intravitreal injections of bevacizumab and ranibizumab were 1.25mg (0.05mL) and 0.5mg (0.05mL), respectively, and were administered in an operating room. Three days prior to the procedures and immediately after topical anesthesia, antibiotic drops of povidone-iodine were used to clean the eyelid and conjunctival sac. The treatment drugs were injected into the vitreous cavity through the pars plana. The patients were assessed on the first postoperative day, and then followed monthly for 6 months. Each follow-up visit included visual acuity and intraocular pressure assessments, a mydriasis fissures lamp examination, and OCT measurement. The review results determined whether additional therapy was required. The standards for additional treatment were: OCT showing active neovascularization with or without decreased vision; new-onset or persistent subretinal hemorrhage, effusion, or new-onset or persistent macular edema; macula thickness >250µm; or decreased visual acuity no less than two-line (excluding other factors leading to vision loss). Treatment clinical efficacy and safety were compared after 6 months (The ophthalmic surgeons involved in both arms were the same).
Statistical Analysis
Statistical Package for the Social Sciences software (SPSS v17.0, SPSS Inc., Chicago, IL, USA) was used for data processing. Snellen chart BCVA measurements were converted to logMAR [10]. Each dataset showed normal distribution by the Kolmogorov-Smirnov test. BCVA, CRT, intraocular pressure, and blood pressure were analyzed with one-sample t-tests within groups and independent sample t-tests between groups. Classification count data were analyzed using the Chi-square test, and if the sample size was <40, then Fisher's exact method was used. P<0.05 was considered statistically significant.
Results
Vision
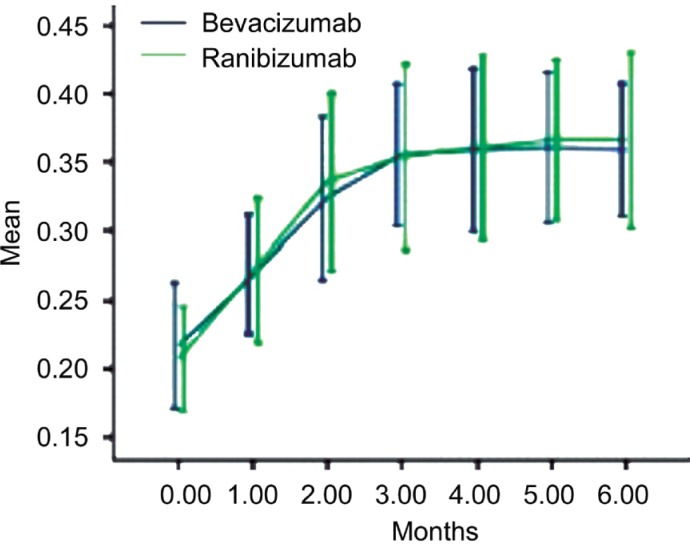
Before treatment, BCVA (logMAR) values of the bevacizumab and ranibizumab groups were 0.72±0.23 and 0.73±0.22, respectively. After three consecutive injections, the two groups of patients had mean BCVA values of 0.49±0.15 and 0.48±0.23, respectively. At the 6-month follow-up, the mean BCVA (logMAR) of the two groups was (0.47±0.14) and (0.45±0.20), respectively (P<0.05). The two sets of visual acuity increases were significant compared to baseline values (t-test, P<0.05), and there was no significant difference in visual acuity improvement between the two groups (P=0.567; Figure 1, Table 2).
Figure 1. BCVA changes over 6 months.
Error bars represent 95% confidence interval (CI).
Table 2. Changes in BCVA over time.
| Baseline | 1 month | 2 months | 3 months | 6 months | |
| Bevacizumab | 0.72±0.23 | 0.6±0.18 | 0.52±0.18 | 0.49±0.15 | 0.47±0.14 |
| Ranibizumab | 0.73±0.22 | 0.61±0.25 | 0.49±0.24 | 0.48±0.23 | 0.45±0.20 |
| P | 0.812 | 0.87 | 0.46 | 0.91 | 0.75 |
x±s
CRT
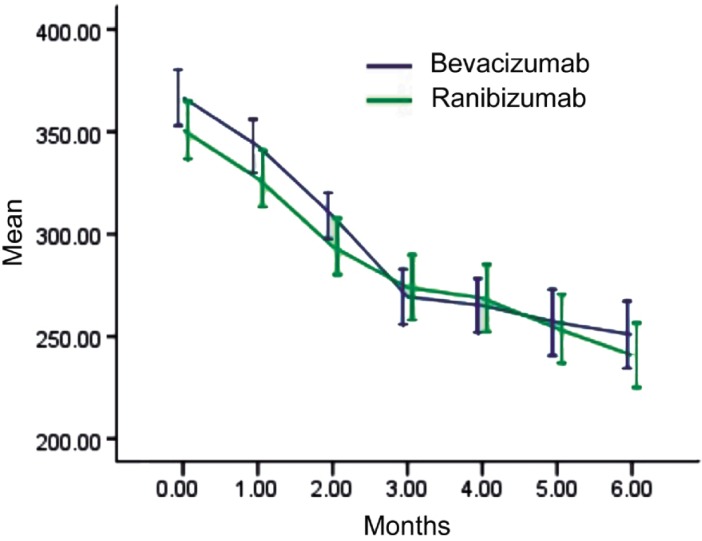
CRT showed declining trends in both groups after injection. In the bevacizumab group (28 patients, 28 eyes), preoperative CRT as assessed by OCT was 366.71±34.72µm. At the 6-month review, CRT was significantly reduced to 250.86±41.51µm (P<0.05). In the ranibizumab group (32 cases, 32 eyes), preoperative CRT was 352±36.9µm and was significantly reduced to 243.22±41.38µm at 6 months (t-test, P<0.05). The 6-month CRT values were not significantly different between the two groups (P=0.483, Figure 2).
Figure 2. CRT changes over 6 months.
Error bars represent 95% confidence intervals (CI).
Complications, intraocular pressure, and blood pressure changes
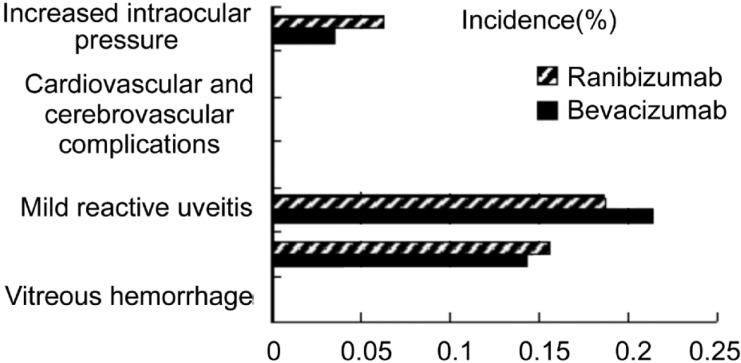
In the bevacizumab and ranibizumab groups, 6 (21.43%) and 6 (18.75%) eyes had mild reactive uveitis (self-healed within 2 weeks), 4 (14.3%) and 5 (15.63%) eyes had subconjunctival hemorrhage (absorption after 2 weeks), and 1 (3.57%) and 2 (6.25%) eyes had postoperative increased intraocular pressure (<25mmHg), respectively. No serious ocular complications, such as retinal detachment, vitreous hemorrhage, or infectious endophthalmitis, or systemic adverse events, such as cerebrovascular disease, were observed in either group (Figure 3).
Figure 3. Incidence of adverse events.
Intraocular pressure was not significantly changed in either group (bevacizumab group, P=0.423; ranibizumab group, P=0.847), and an independent sample t-test showed no significant difference between them. We did not observe any significant differences in systolic or diastolic blood pressures over time or between groups (all P>0.05).
In the bevacizumab group, three patients (3 eyes, 10.71%) underwent one additional treatment, and one eye (3.57%) had two additional treatments. In the ranibizumab group, 6 patients (6 eyes, 18.75%) exhibited CNV recurrence on review OCT (five patients with vision loss of two lines or more, 1 person without declining eyesight). Among these, 5 cases (5 eyes, 15.63%) had one additional treatment and one patient (1 eye, 3.13%) underwent two additional treatments. Vision stabilized after the secondary treatments, and review OCT showed that the lesion areas were significantly reduced. At 6 months, the bevacizumab and ranibizumab groups had received 3.18 and 3.22 injections per patient, respectively.
Discussion
VEGF is considered a highly specific vascular endothelial cellular regulatory factor that is closely related to angiogenesis and vascular permeability and is the most important physiological factor that regulates vascular growth. Using immunohistochemical analysis, another study showed that surgical resections of CNV films in AMD patients exhibited a high correlation between VEGF expression and AMD[11], thereby providing a theoretical basis for anti-VEGF treatment for wet AMD.
Bevacizumab was the first anti-VEGF drug approved, and is a first-line drug for metastatic colon cancer. It is a restructuring of the full-length humanized monoclonal IgG1 antibody (relative molecular mass of approximately 148 000 kDa). Its half-life in the vitreous cavity is about 9.8 days. Its mechanism of action involves competitive inhibition of VEGF and its specific receptors, thereby inhibiting vascular endothelial cell proliferation and reducing angiogenesis[12]. Ranibizumab is the second generation of humanized monoclonal antibody fragments and is a restructuring of the anti-VEGF Fab portion (relative molecular mass of approximately 48 000 kDa). Its half-life in the vitreous cavity is also about 9 days[13]. It can reduce immunogenicity of the non-associative humanized fragment and two portions of the rat high-affinity antigenic determinant. It has specific affinity for all human VEGF subtypes, inhibits vascular leakage and neovascularization, and is therapeutic for CNV.
A number of clinical studies have demonstrated the effectiveness of ranibizumab and bevacizumab for the treatment of wet AMD [14]-[17]. We found that both drugs exerted similar clinical efficacies and safety profiles for the treatment of wet AMD. The mean BCVA of both groups improved significantly after treatment, and their final values were not significantly different. Accordingly, mean central retinal thicknesses declined significantly as a result of treatment and their final values did not differ significantly by group. However, it is possible that differences between the two drugs were masked by the small sample size and short follow-up time of this study. It has been suggested that because bevacizumab has a molecular mass approximately three times greater than that of ranibizumab, its retinal penetration may not be as effective. However, connections between retinal cells are loose in AMD patients with retinal edema, and bevacizumab macromolecules can easily penetrate the retina; therefore, clinical effects due to molecular weight differences are not obvious in this population [18]. Follow-up OCT assessments suggested that the two groups of patients had comparable CNV recurrence, macular edema, and subretinal effusion. The bevacizumab group had an average of 3.18 treatments per patient, whereas the ranibizumab group had 3.22 treatments per patient. Anterior chamber inflammation and subconjunctival hemorrhage are common during intravitreal injections and may be improved by symptomatic treatment. We did not observe serious complications such as retinal hemorrhage, complicated cataract, retinal detachment, vitreous hemorrhage, and infectious endophthalmitis or systemic adverse events such as cerebrovascular accidents. With its greater molecular weight, slightly longer half-life, and relatively slow drug metabolism in the vitreous cavity, bevacizumab could more readily cause strokes, hypertension, and systemic adverse events. However, bevacizumab injection frequency was slightly lower, which effectively reduced the risk of local adverse events due to injection. Overall, there were no obvious differences in the clinical safety of the two drugs. The higher price of ranibizumab ($2 000 per injection[19]) limits its use in developing countries and undeveloped regions where patient income levels are low. In contrast, bevacizumab is about 40-fold less expensive[20]. Given the lack of differences between the drugs in clinical curative effects and safety over the short term, bevacizumab is presently the preferred treatment choice for the majority of Chinese patients.
The main limitation of our study is its non-randomized design, that it is mostly on retrospective observation, and that the investigators were not masked to treatment modality. Moreover, it assessed a small sample size over a relatively short follow-up period (6 months). However, our results show that in the short term, bevacizumab and ranibizumab can effectively stabilize vision or improve eyesight, reduce macular area retinal thickness, and improve fundus CNV leakage and retinal effusion. Both drugs were safe, with a low incidence of adverse events. Our conclusion in comparing the safety and efficacy of intravitreal bevacizumab and ranibizumab in patients with exudative AMD in China is similar to those of larger randomized clinical trials that focused on foreign AMD patients[7]-[9]. Further clarification of their efficacy and safety for Chinese patients will require larger sample sizes and longer follow-up, preferably as part of a prospective multicenter clinical research study.
References
- 1.Zou HD, Zhang X, Xu X, Wang FH, Zhang SJ. Age-related macular degeneration prevalence survey of Caojiadu street Jing'an district in Shanghai. ZhonghuaYanke Zhazhi. 2005;41(1):15–19. [PubMed] [Google Scholar]
- 2.Lou ZL, Chen G, Tang R. Age-related macular degeneration prevalence survey of agency personnel in Changsha city. Ophthalmol Res. 2008;26(11):822–823. [Google Scholar]
- 3.Ni Z. 1st edition. Shanghai: Shanghai Scientific Popularization Publishing House; 2002. Pathological anatomy and clinical of ophthalmology; pp. 281–282. [Google Scholar]
- 4.Sheng YJ. Advances in the treatment of age-related macular degeneration. J Clin Ophthalmol. 2004;12(5):471. [Google Scholar]
- 5.Bressler NM. Antiangiogenic approaches to aged-related macular degeneration today. Ophthalmology. 2009;116(10 Suppl):S15–23. doi: 10.1016/j.ophtha.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 6.Schouten JS, La Heij EC, Webers CA, Lundqvist IJ, Hendrikse F. A systematic review on the effect of bevacizumab in exudative age-related macular degeneration. Greafes Arch Clin Exp Ophthalmol. 2009;247(1):1–11. doi: 10.1007/s00417-008-0952-y. [DOI] [PubMed] [Google Scholar]
- 7.Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, Reeves BC. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119(7):1399–1411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL., 3rd Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. [Google Scholar]
- 9.Krebs I, Schmetterer L, Boltz A, Told R, Vécsei-Marlovits V, Egger S, Schönherr U, Haas A, Ansari-Shahrezaei S, Binder S, MANTA Research Group A randomised double-masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age-related macular degeneration. Br J Ophthalmol. 2013;97(3):266–271. doi: 10.1136/bjophthalmol-2012-302391. [DOI] [PubMed] [Google Scholar]
- 10.Hera R, Keramidas M, Peoc'h M, Mouillon M, Romanet JP, Feige JJ. Expression of VEGF and angiopoietins in subfoveal membranes from patients with age-related macular degeneration. Am J Ophthalmol. 2005;139(4):589–596. doi: 10.1016/j.ajo.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 11.Hornig C, Weich HA. Soluble VEGF receptors. Angiogenesis. 1999;3(1):33–39. doi: 10.1023/a:1009033017809. [DOI] [PubMed] [Google Scholar]
- 12.Tatar O, Adam A, Shinoda K, Stalmans P, Eckardt C, Lüke M, Bartz-Schmidt KU, Grisanti S. Expression of VEGF and PEDF in choroidal neovascular membranes following verteporfin photodynamic therapy. Am J Ophthalmol. 2006;142(1):95–104. doi: 10.1016/j.ajo.2006.01.085. [DOI] [PubMed] [Google Scholar]
- 13.O'Shea JG. Response to replacing ranibizumab with bevacizumab on the Pharmaceutical Benefits Scheme: where does the current evidence leave us. Clin Exp Optom. 2012;95(5):541–543. doi: 10.1111/j.1444-0938.2012.00736.x. [DOI] [PubMed] [Google Scholar]
- 14.Senger DR, Van de Water L, Brown LF, Nagy JA, Yeo KT, Yeo TK, Berse B, Jackman RW, Dvorak AM, Dvorak HF. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 1993;12(3–4):303–324. doi: 10.1007/BF00665960. [DOI] [PubMed] [Google Scholar]
- 15.Kaiser PK, Brown DM, Zhang K, Hudson HL, Holz FG, Shapiro H, Schneider S, Acharya NR. Angiographic and optical coherence tomographic results of the MARINA study of ranibizumb in neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007;144(6):850–857. doi: 10.1016/j.ajo.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Holladay JT. Visual acuity measurements. J Cataract Refract Surg. 2004;30(2):287–290. doi: 10.1016/j.jcrs.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Joeres S, Heussen FM, Treziak T, Bopp S, Joussen AM. Bevacizumab (Avastin) treatment in patients with retinal angiomatous proliferation. Graefes Arch Clin Exp Ophthalmol. 2007;245(11):1597–1602. doi: 10.1007/s00417-007-0580-y. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Liu ZL, Sun P, Gu F. Intravitreal bevacizumab for treatment of subfoveal idiopathic choroidal neovascularization: results of a 1-year prospective trial. Am J Ophthalmol. 1712;153(2):300–306. doi: 10.1016/j.ajo.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Raftery J, Clegg A, Jones J, Tan SC, Lotery A. Ranibizumab (Lucentis) versus bevacizumab (Avastin): modelling cost effectiveness. Br J Ophthalmol. 2007;91(9):1244–1246. doi: 10.1136/bjo.2007.116616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abeysiri P, Johnston NR, Molteno AC. Price of bevacizumab for intravitreal injection in New Zealand. Clin Experiment Ophthalmol. 2010;38(1):38–84. doi: 10.1111/j.1442-9071.2010.02196.x. [DOI] [PubMed] [Google Scholar]