Abstract
AIM
To determine the effect of multiple injections of ranibizumab or bevacizumab on retinal nerve fiber layer (RNFL) and intraocular pressure (IOP) in patients with age-related macular degeneration (AMD).
METHODS
This retrospective study includes 35 eyes of 35 patients treated with intravitreal bevacizumab (IVB, 1.25mg/0.05mL) and 30 eyes of 30 patients with intravitreal ranibizumab (IVR, 0.5mg/0.05mL) who had Fast RNFL analysis (Stratus™); IOP measurements were taken 30 minutes and 24 hours after each injection.
RESULTS
The mean ages were 68.0±7.5 and 69.1±7.7 years in the IVR and IVB groups, respectively (P=0.55). They underwent (6.3±1.9) and (5.1±1.3) injections (P=0.07) over (13.6±2.1) and (14.05±2.6) months (P=0.45) in the IVR and IVB groups, respectively. Changes in overall and temporal RNFL thickness in IVR-treated eyes (105.3±6.9µm and 74.4±11.2µm) were not different from those in untreated eyes in the IVR group (104.6± 8.4µm and 75.1±12.6µm) (P=0.57 and P=0.41, respectively). Similarly, overall and temporal RNFL thickness in IVB-treated eyes (105.8±8.1µm and 74.5±11.8µm) were not different from those in untreated eyes in the IVB group (104.6±8µm and 74.8±12.9µm) (P=0.42 and P=0.80, respectively). The frequencies of IOP rise (P=0.60) and changes in RNFL thickness from baseline (P=0.16) were comparable between groups.
CONCLUSION
Repeated intravitreal injection of ranibizumab or bevacizumab does not seem have adverse effects on RNFL thickness or IOP in wet AMD patients.
Keywords: anti-VEGF, bevacizumab, ranibizumab, retinal nerve fiber layer, intraocular pressure, adverse effect
INTRODUCTION
In recent years, intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) agents, namely bevacizumab and ranibizumab, have been the primary treatment for age-related macular degeneration (AMD) and macular edema of vascular origin. However, non-arteritic anterior ischemic optic neuropathy and anterior ischemic optic neuropathy with central retinal artery and vein occlusion have been reported as complications of intravitreal bevacizumab and ranibizumab injections, respectively[1],[2]. While temporary intraocular pressure (IOP) spikes are common following a single intraocular injection, a growing number of recent reports describe permanent IOP elevations after multiple intravitreal injections[3]-[8]. VEGF is a critical neuroprotective factor involved in the adaptive response to retinal ischemia; therefore, VEGF inhibition may adversely affect retinal structures and functions, especially during long-term treatment with intravitreal injections of anti-VEGF agents[9].
Here, we evaluated the effect of multiple intravitreal injections of bevacizumab or ranibizumab on retinal nerve fiber layer (RNFL) thickness and IOP in patients with wet AMD.
SUBJECTS AND METHODS
This retrospective, observational, consecutive case series included 35 eyes of 35 patients with wet AMD treated with intravitreal bevacizumab (IVB, 1.25mg/0.05mL; Altuzan®, Genentech, San Francisco, CA, USA), and 30 eyes of 30 patients with wet AMD treated with intravitreal ranibizumab (IVR, 0.5mg/0.05mL; Lucentis®, Genentech, USA). This study included eligible patients who had been treated at the same clinic (Ophthalmology Department at GATA Military Hospital in Ankara) between May 2009 and January 2012. All subjects had refraction ±3 diopters. We excluded patients who had fewer than three intravitreal injections during a minimum of 1-year follow-up; those with ocular diseases and disorders affecting RNFL thickness, including anomalous optic disc with cup/disc ratios of more than 0.4; and patients with narrow angles (Shaffer grade≤2); pigment dispersion; or pseudoexfoliation in the anterior chamber. Patients with systemic pathologies other than controlled hypertension were also considered ineligible. This study adhered to the tenets of the Declaration of Helsinki; informed consent was obtained from the subjects prior to treatment, and our Institutional Review Board approved the protocol. All patients included in the study submitted to a detailed clinical examinations, including visual acuity assessment with an ETDRS' chart, IOP measurement with the same applanation tonometer, and RNFL thickness with the same optical coherence tomography (OCT) instrument, both at baseline and during follow-up (monthly for the IVR group or every 6 weeks for the IVB group). After three loading doses in both groups, we implanted an “as needed” protocol as described in the PrONTO Study [10]. None of the patients had glaucoma or ocular medication history prior to participation. Intraocular injections were performed according to the guidelines described by Aiello et al[11],[12]. IOP measurements and slit-lamp examinations were performed twice: 30 minutes and 24 hours after each injection. OCT measurements were performed 1 to 7 days before injection. No prophylactic antiglaucomatous agents were used before or after injection, except for patients with IOP values exceeding 30mmHg at 30 minutes or 25mmHg at 24 hours.
We performed sequential RNFL thickness analysis with OCT (Stratus™, Carl Zeiss Meditec AG, Jena, Germany) using an automated computer algorithm (Fast RNFL). Only those optic nerve head (ONH) scans meeting specific qualification criteria were included for data analysis. The criteria were: 1) baseline and follow-up images centered on the optic disc without scan circle displacement; 2) signal strength of at least 7; 3) measurement by the same technician at the same time interval between 8:30 and 10:00 a.m. Qualified scans were analyzed for differences regarding RNFL thickness in quadrants within and between groups.
Statistical Analysis
Descriptive statistics were performed with SPSS, version 15.0 (SPSS, Chicago, IL). We performed t tests to compare parametric data (age, IOP, RNFL). For comparisons of nominal data (sex, IOP rise), Chi-squared tests were used. A P value of 0.05 or less was considered to be significant.
RESULTS
Patient demographics and the number of injections and follow-ups are shown in Table 1.
Table 1. Patient demographics and number of injections and follow-ups by group.
| IVR(n=30) | IVB(n=35) | P | |
| Mean age (a) | 68.0±7.5 | 69.1±7.7 | 0.55 |
| Female/Male | 19/11 | 17/18 | 0.23 |
| Follow-up (months) | 13.6±2.1 | 14.05±2.6 | 0.45 |
| Injections | 6.3±1.9 | 5.1±1.3 | 0.07 |
IVR: Intravitreal ranibizumab; IVB: Intravitreal bevacizumab.
x±s
A review of patient medical records also showed that no paracentesis was needed to reduce IOP immediately after intraocular injections. No remarkable IOP spikes or uveitogenic inflammation requiring intervention were noted on the first-day slit-lamp examination or at subsequent follow-up visits.
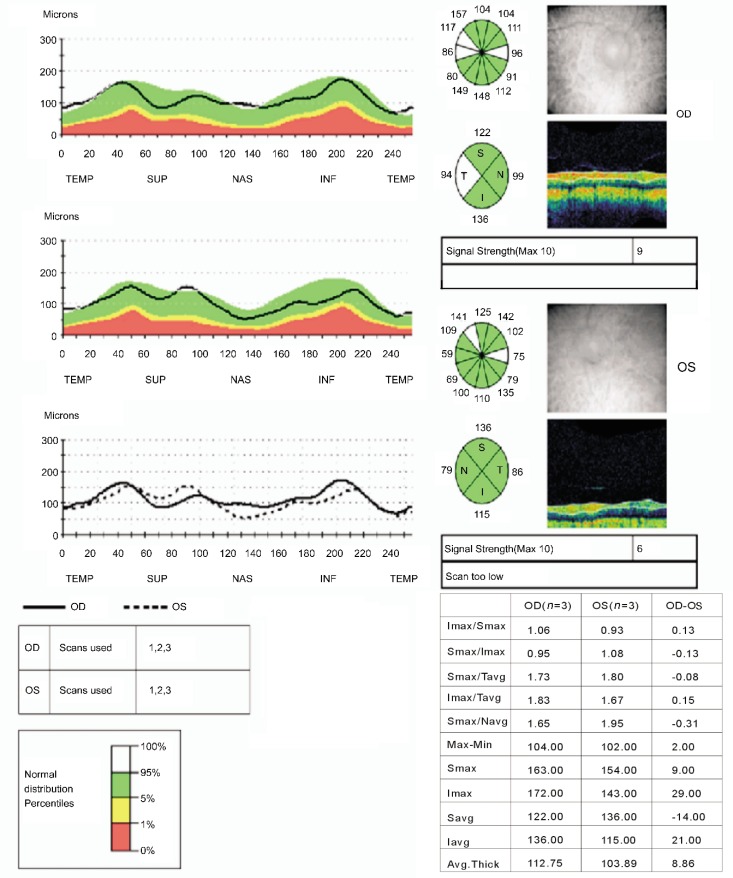
Initial and final quadratic (inferior, superior, nasal, and temporal) and overall RNFL thickness values were not statistically different between the treated and untreated eyes or between groups (all P>0.05) (Table 2 and Figure 1).
Table 2. Effects of ranibizumab or bevacizumab on IOP and RNFL thickness.
| 1Variable | IVR |
IVB |
||||
| Pre-injection | Post-injection | P | Pre-injection | Post-injection | P | |
| 2IOP (mmHg) | 14.7±3.9 | 16.1±2.3 | 0.22 | 15.5±2.5 | 15.2±3.3 | 0.30 |
| 3IOP rise, n(%) | 25 (13.1%) | 21(11.9%) | 0.72 | |||
| RNFL, temporal (µm) | 74.4±11.2 | 75.1±12.6 | 0.41 | 74.5±11.8 | 74.8±12.9 | 0.80 |
| RNFL, overall (µm) | 105.3±6.9 | 104.6±8.4 | 0.57 | 105.8±8.1 | 104.6±8.1 | 0.42 |
1Variables recorded at each follow-up examination were considered; 2 Measured 30 minutes after injection; 3Increase of ≥20% of baseline IOP or ≥24mmHg.
x±s
Figure 1. RNFL analysis in the temporal quadrant (in ocular dextra: OD) and overall, including temporal (in ocular sinistra: OS).
DISCUSSION
In this study, multiple injections of bevacizumab or ranibizumab had discernible effects on RNFL. There was also no difference between the groups regarding IOP fluctuation or RNFL changes from baseline (Table 2).
Demographic features were comparable between the groups. Fewer injections were needed in the IVB group during similar follow-up times (Table 1).
Among currently available anti-VEGF therapies, ranibizumab and bevacizumab are the most commonly used to treat wet AMD patients. In this study, we employed a bevacizumab dose of 1.5mg/0.05ml, which is common in our clinic and is the same volume used in ranibizumab injections. A review of the current literature suggests that intravitreal anti-VEGF agents are generally safe and effective for wet AMD treatment for up to 2-3 years[12]-[15].
In the context of in vitro and in vivo molecular characteristics and pharmacological properties, ranibizumab and bevacizumab are considered to have overlapping but distinct therapeutic potentials[16]. VEGF is a well-known neurotrophic factor, and anti-VEGF drugs interact with circulating VEGF molecules to prevent them from binding to VEGF receptor-2 (VEGFR2). Expression of VEGFR2 was detected in several neuronal cell layers of the retina, where it was shown to be involved in retinal neuroprotection and the adaptive response to retinal ischemia[9]. Insufficient activation of this neurotrophic cytokine in chronically anti-VEGF-treated eye may result in deleterious downstream effects on RNFL. In addition, Avci et al[17] showed in a transmission electron microscopy (TEM) study with rabbits that bevacizumab and pegaptanib caused dose-dependent apoptotic changes in photoreceptors. Nevertheless, many experimental studies with repeated intravitreal injections of ranibizumab or bevacizumab showed no toxic effect on the retina[18],[19]. Maturi et al[20] showed no significant measurable photoreceptor toxicity following intravitreal bevacizumab treatment in AMD during an electrodiagnostic study. The CATT (Comparison of AMD Treatments Trials) study suggested that differences in rates of serious adverse events, which seem to be in favor of ranibizumab, should be further evaluated[15].
Intravitreal injection of anti-VEGF agents is reported to cause both transient and sustained elevations of IOP with volume increase[5], [21]-[23]. Compared to other intravitreal agents, IOP rise was relatively rare; however, IOP has been shown to rise up to 36mmHg after bevacizumab injection[21],[24] but usually returns to baseline within 30-60 minutes[21]. IOP spikes up to 34mmHg (one case with IVB) were observed at 30-minute examination in our study, but rates of IOP rise (13.1% vs. 11.9%) were comparable between groups. None of the patients required chronic medication at 24 hours after injection. Trabecular meshwork obstruction by impurities or the drug itself or intraocular inflammation have been assumed to be responsible for sustained IOP elevations in the weeks and months after injection[4]-[8]. IOP fluctuations following repeat intraocular anti-VEGF injections and chronic suppression of neuroprotective VEGF could theoretically result in RNFL damage, but none of the patients in our study exhibited sustained IOP rise or inflammatory reactions that required intervention. However, patients vulnerable to glaucomatous damage were excluded from the study.
We evaluated OCT data that were obtained under strict criteria. We took into consideration the refractive errors, as well as optic disc size, to minimize measurement errors[25]. There is still ongoing controversy regarding the association of signal strength to RNFL thickness. This was especially evident in cases with glaucoma-induced optic atrophy[26]. In this study, we excluded those cases with a propensity for RNFL damage and used good scan quality (≥7) to avoid low signal strength artifacts in order to overcome potential biases of OCT data evaluation. Cheng et al[27] performed in vitro and in vivo studies and showed that repeat injections of bevacizumab were not toxic to retinal ganglion cells. In a retrospective study of AMD patients treated with multiple injections (16.0±5.5) with longer follow-up times (27.0±9.7 months), Horsley et al[28] demonstrated that neither IVB nor IVR were toxic to the optic nerve. Our findings are consistent with those of Horsley et al[28] and we additionally provide quadratic analysis results of RNFL thickness.
Admittedly, our retrospective study design, limited sample size, and relatively short follow-up period precludes drawing firm conclusions regarding the long-term safety and/or efficacy of anti-VEGF treatments on RNFL. In addition, the results would likely have been different if bevacizumab injections were 2.5mg/0.1mL instead of 1.25mg/0.05mL. Effects of these drugs on AMD progression were not one of the outcomes of this study; however, 25 of 30 (83%) IVR patients and 28 of 35 (80%) of IVB patients had no need for maintenance therapy after 1 year. While the IVR group received injections once a month, the IVB group was treated every 6 weeks, similar to most studies using IVB. This may explain the difference in the number of injections between the groups. There were, however, no discernible differences with regard to IOP and RNFL values during the mean 14 months of follow-up between the two groups. We believe that further prospective studies with longer follow-up times in larger study populations are needed to support our findings.
In conclusion, multiple intravitreal injections of the anti-VEGF agents bevacizumab and ranibizumab seem to have no adverse effect on RNFL thickness or IOP.
References
- 1.Ganssauge M, Wilhelm H, Bartz-Schmidt KU, Aisenbrey S. Non-arteritic anterior ischemic optic neuropathy (NA-AION) after intravitreal injection of bevacizumab (Avastin) for treatment of angoid streaks in pseudoxanthoma elasticum. Graefes Arch Clin Exp Ophthalmol. 2009;247(12):1707–1710. doi: 10.1007/s00417-009-1184-5. [DOI] [PubMed] [Google Scholar]
- 2.Battaglia Parodi M, Iacono P, Cascavilla ML, Zucchiatti I, Kontadakis DS, Vergallo S, Bandello F. Sequential anterior ischemic optic neuropathy and central retinal artery and vein occlusion after ranibizumab for diabetic macular edema. Eur J Ophthalmol. 2010;20(6):1076–1078. doi: 10.1177/112067211002000609. [DOI] [PubMed] [Google Scholar]
- 3.Tseng JJ, Vance SK, Della Torre KE, Mendonca LS, Cooney MJ, Klancnik JM, Sorenson JA, Freund KB. Sustained increased intraocular pressure related to intravitreal antivascular endothelial growth factor therapy for neovascular age-related macular degeneration. J Glaucoma. 2012;21(4):241–247. doi: 10.1097/IJG.0b013e31820d7d19. [DOI] [PubMed] [Google Scholar]
- 4.Sniegowski M, Mandava N, Kahook MY. Sustained intraocular pressure elevation after intravitreal injection of bevacizumab and ranibizumab associated with trabeculitis. Open Ophthalmol J. 2010;4:28–29. doi: 10.2174/1874364101004010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahook MY, Kimura AE, Wong LJ, Ammar DA, Maycotte MA, Mandava N. Sustained elevation in intraocular pressure associated with intravitreal bevacizumab injections. Ophthalmic Surg Lasers Imaging. 2009;40(3):293–295. doi: 10.3928/15428877-20090430-12. [DOI] [PubMed] [Google Scholar]
- 6.Good TJ, Kimura AE, Mandava N, Kahook MY. Sustained elevation of intraocular pressure after intravitreal injections of anti-VEGF agents. Br J Ophthalmol. 2011;95(8):1111–1114. doi: 10.1136/bjo.2010.180729. [DOI] [PubMed] [Google Scholar]
- 7.Adelman RA, Zheng Q, Mayer HR. Persistent ocular hypertension following intravitreal bevacizumab and ranibizumab injections. J Ocul Pharmacol Ther. 2010;26(1):105–110. doi: 10.1089/jop.2009.0076. [DOI] [PubMed] [Google Scholar]
- 8.Bakri SJ, McCannel CA, Edwards AO, Moshfeghi DM. Persistent ocular hypertension following intravitreal ranibizumab. Graefes Arch Clin Exp Ophthalmol. 2008;246(7):955–958. doi: 10.1007/s00417-008-0819-2. [DOI] [PubMed] [Google Scholar]
- 9.Nishijima K, Ng YS, Zhong L, Bradley J, Schubert W, Jo N, Akita J, Samuelsson SJ, Robinson GS, Adamis AP, Shima DT. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171(1):53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, Davis JL, Flynn HW, Jr, Esquiabro M. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148(1):43–58. doi: 10.1016/j.ajo.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Aiello LP, Brucker AJ, Chang S, Cunningham ET, Jr, D'Amico DJ, Flynn HW, Jr, Grillone LR, Hutcherson S, Liebmann JM, O'Brien TP, Scott IU, Spaide RF, Ta C, Trese MT. Evolving guidelines for intravitreous injections. Retina. 2004;24(5 Suppl):S3–19. doi: 10.1097/00006982-200410001-00002. [DOI] [PubMed] [Google Scholar]
- 12.Jeganathan VS, Verma N. Safety and efficacy of intravitreal anti-VEGF injections for age-related macular degeneration. Curr Opin Ophthalmol. 2009;20(3):223–225. doi: 10.1097/ICU.0b013e328329b656. [DOI] [PubMed] [Google Scholar]
- 13.Ladas ID, Karagiannis DA, Rouvas AA, Kotsolis AI, Liotsou A, Vergados I. Safety of repeat intravitreal injections of bevacizumab versus ranibizumab: our experience after 2,000 injections. Retina. 2009;29(3):313–318. doi: 10.1097/IAE.0b013e31819a5f98. [DOI] [PubMed] [Google Scholar]
- 14.Schmucker C, Ehlken C, Hansen LL, Antes G, Agostini HT, Lelgemann M. Intravitreal bevacizumab (Avastin) vs. ranibizumab (Lucentis) for the treatment of age-related macular degeneration: a systematic review. Curr Opin Ophthalmol. 2010;21(3):218–226. doi: 10.1097/ICU.0b013e3283386783. [DOI] [PubMed] [Google Scholar]
- 15.CATT Research Group. Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer CH, Holz FG. Preclinical aspects of anti-VEGF agents for the treatment of wet AMD: ranibizumab and bevacizumab. Eye (Lond) 2011;25(6):661–672. doi: 10.1038/eye.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avci B, Avci R, Inan UU, Kaderli B. Comparative evaluation of apoptotic activity in photoreceptor cells after intravitreal injection of bevacizumab and pegaptanib sodium in rabbits. Invest Ophthalmol Vis Sci. 2009;50(7):3438–3446. doi: 10.1167/iovs.08-2871. [DOI] [PubMed] [Google Scholar]
- 18.Zayit-Soudry S, Zemel E, Loewenstein A, Perlman I. Safety evaluation of repeated intravitreal injections of bevacizumab and ranibizumab in rabbit eyes. Retina. 2010;30(4):671–681. doi: 10.1097/IAE.0b013e3181c0858c. [DOI] [PubMed] [Google Scholar]
- 19.Sari A, Adiguzel U, Canacankatan N, Yilmaz N, Dinc E, Oz O. Effects of intravitreal bevacizumab in repeated doses: an experimental study. Retina. 2009;29(9):1346–1355. doi: 10.1097/IAE.0b013e3181b26343. [DOI] [PubMed] [Google Scholar]
- 20.Maturi RK, Bleau LA, Wilson DL. Electrophysiologic findings after intravitreal bevacizumab (Avastin) treatment. Retina. 2006;26(3):270–274. doi: 10.1097/00006982-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Falkenstein IA, Cheng L, Freeman WR. Changes of intraocular pressure after intravitreal injection of bevacizumab (Avastin) Retina. 2007;27(8):1044–1047. doi: 10.1097/IAE.0b013e3180592ba6. [DOI] [PubMed] [Google Scholar]
- 22.Kim JE, Mantravadi AV, Hur EY, Covert DJ. Short-term intraocular pressure changes immediately after intravitreal injections of anti-vascular endothelial growth factor agents. Am J Ophthalmol. 2008;146(6):930–934. doi: 10.1016/j.ajo.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Hollands H, Wong J, Bruen R, Campbell RJ, Sharma S, Gale J. Short-term intraocular pressure changes after intravitreal injection of bevacizumab. Can J Ophthalmol. 2007;42(6):807–811. doi: 10.3129/i07-172. [DOI] [PubMed] [Google Scholar]
- 24.Bakri SJ, Pulido JS, McCannel CA, Hodge DO, Diehl N, Hillemeier J. Immediate intraocular pressure changes following intravitreal injections of triamcinolone, pegaptanib, and bevacizumab. Eye (Lond) 2009;23(1):181–185. doi: 10.1038/sj.eye.6702938. [DOI] [PubMed] [Google Scholar]
- 25.Bayraktar S, Bayraktar Z, Yilmaz OF. Influence of scan radius correction for ocular magnification and relationship between scan radius with retinal nerve fiber layer thickness measured by optical coherence tomography. J Glaucoma. 2001;10(3):163–169. doi: 10.1097/00061198-200106000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Wu Z, Huang J, Dustin L, Sadda SR. Signal strength is an important determinant of accuracy of nerve fiber layer thickness measurement by optical coherence tomography. J Glaucoma. 2009;18(3):213–216. doi: 10.1097/IJG.0b013e31817eee20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng CK, Peng PH, Tien LT, Cai YJ, Chen CF, Lee YJ. Bevacizumab is not toxic to retinal ganglion cells after repeated intravitreal injection. Retina. 2009;29(3):306–312. doi: 10.1097/IAE.0b013e3181909404. [DOI] [PubMed] [Google Scholar]
- 28.Horsley MB, Mandava N, Maycotte MA, Kahook MY. Retinal nerve fiber layer thickness in patients receiving chronic anti-vascular endothelial growth factor therapy. Am J Ophthalmol. 2010;150(4):558–561. doi: 10.1016/j.ajo.2010.04.029. [DOI] [PubMed] [Google Scholar]