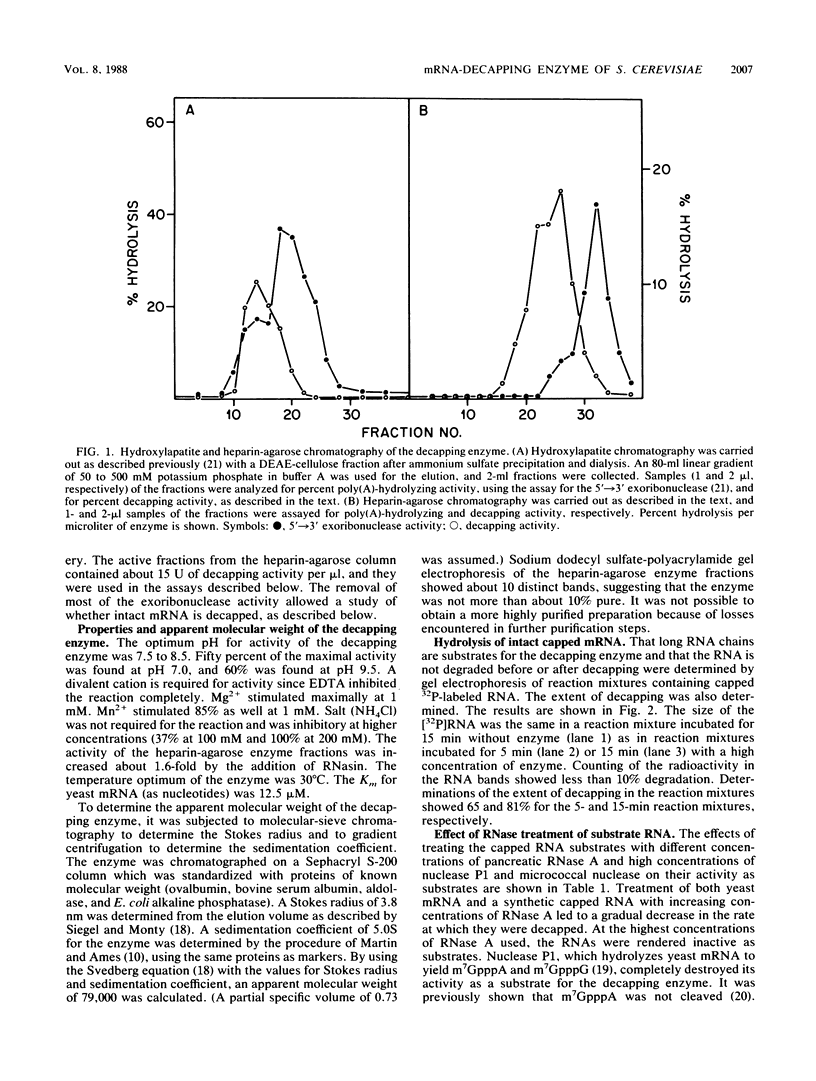
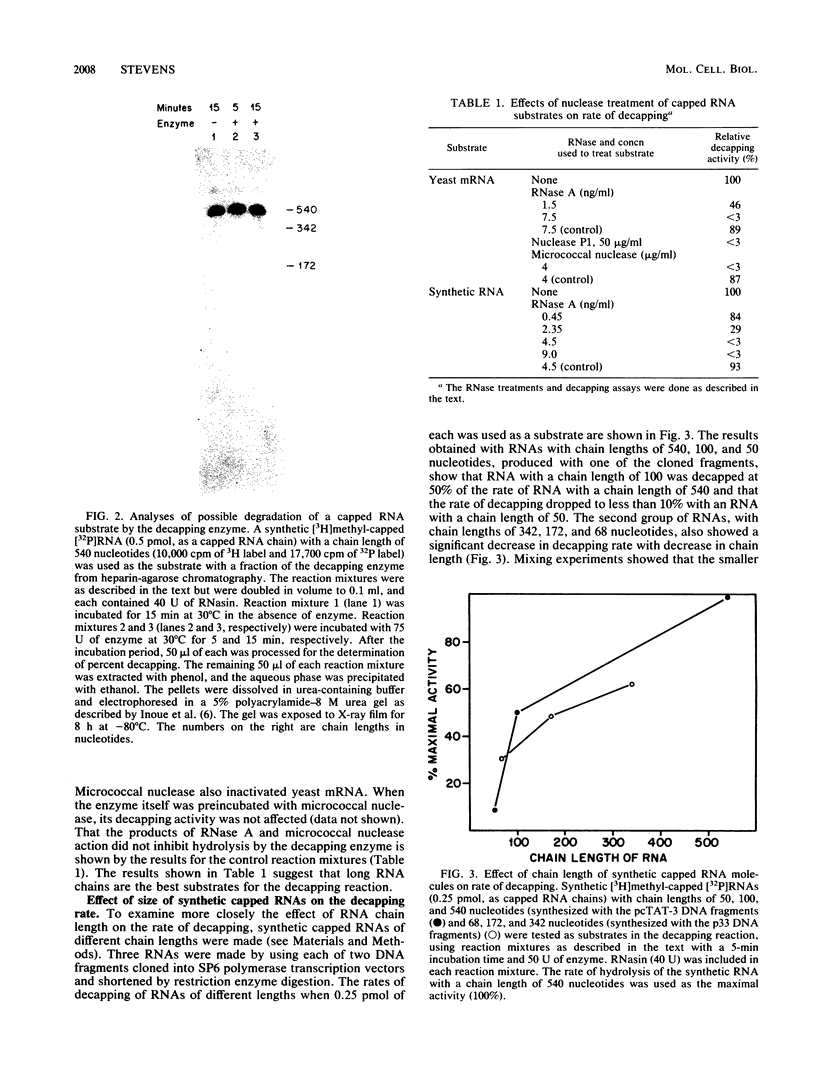
Abstract
An enzyme that hydrolyzes one PPi bond of the cap structure of mRNA, yielding m7GDP and 5'-p RNA was purified from Saccharomyces cerevisiae to a stage suitable for characterization. The specificity of the enzyme was studied, using both yeast mRNA and synthetic RNAs labeled in the cap structure. A synthetic capped RNA (540 nucleotides) was not reduced in size, while as much as 80% was decapped. Yeast mRNA treated with high concentrations of RNase A, nuclease P1, or micrococcal nuclease was inactive as a substrate. The use of synthetic capped RNAs of different sizes (50 to 540 nucleotides) as substrates showed that the larger RNA can be a better substrate by as much as 10-fold. GpppG-RNA was hydrolyzed at a rate similar to that at which 5'-triphosphate end group were not hydrolyzed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Janda M. cDNA cloning and in vitro transcription of the complete brome mosaic virus genome. Mol Cell Biol. 1984 Dec;4(12):2876–2882. doi: 10.1128/mcb.4.12.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkiewicz M., Sierakowska H., Shugar D. Nucleotide pyrophosphatase from potato tubers. Purification and properties. Eur J Biochem. 1984 Sep 3;143(2):419–426. doi: 10.1111/j.1432-1033.1984.tb08389.x. [DOI] [PubMed] [Google Scholar]
- Contreras R., Cheroutre H., Degrave W., Fiers W. Simple, efficient in vitro synthesis of capped RNA useful for direct expression of cloned eukaryotic genes. Nucleic Acids Res. 1982 Oct 25;10(20):6353–6362. doi: 10.1093/nar/10.20.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., LaFiandra A., Shatkin A. J. 5'-Terminal structure and mRNA stability. Nature. 1977 Mar 17;266(5599):235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- Green M. R., Maniatis T., Melton D. A. Human beta-globin pre-mRNA synthesized in vitro is accurately spliced in Xenopus oocyte nuclei. Cell. 1983 Mar;32(3):681–694. doi: 10.1016/0092-8674(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Inoue T., Sullivan F. X., Cech T. R. New reactions of the ribosomal RNA precursor of Tetrahymena and the mechanism of self-splicing. J Mol Biol. 1986 May 5;189(1):143–165. doi: 10.1016/0022-2836(86)90387-6. [DOI] [PubMed] [Google Scholar]
- Kole R., Sierakowska H., Shugar D. Novel activity of potato nucleotide pyrophosphatase. Biochim Biophys Acta. 1976 Jul 8;438(2):540–550. doi: 10.1016/0005-2744(76)90270-9. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Padgett R. A., Sharp P. A. Recognition of cap structure in splicing in vitro of mRNA precursors. Cell. 1984 Oct;38(3):731–736. doi: 10.1016/0092-8674(84)90268-x. [DOI] [PubMed] [Google Scholar]
- Lee K. L., Isham K. R., Stringfellow L., Rothrock R., Kenney F. T. Molecular cloning of cDNAs cognate to genes sensitive to hormonal control in rat liver. J Biol Chem. 1985 Dec 25;260(30):16433–16438. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Monroy G., Spencer E., Hurwitz J. Characteristics of reactions catalyzed by purified guanylyltransferase from vaccinia virus. J Biol Chem. 1978 Jun 25;253(12):4490–4498. [PubMed] [Google Scholar]
- Nuss D. L., Furuichi Y. Characterization of the m7G(5')pppN-pyrophosphatase activity from HeLa cells. J Biol Chem. 1977 May 10;252(9):2815–2821. [PubMed] [Google Scholar]
- Nuss D. L., Furuichi Y., Koch G., Shatkin A. J. Detection in HeLa cell extracts of a 7-methyl guanosine specific enzyme activity that cleaves m7GpppNm. Cell. 1975 Sep;6(1):21–27. doi: 10.1016/0092-8674(75)90069-0. [DOI] [PubMed] [Google Scholar]
- Rothrock R., Lee K. L., Isham K. R., Johnson A. C., Kenney F. T. Different mechanisms control developmental activation of transcription of genes subject to identical hormonal regulation in adult liver. Biochem Biophys Res Commun. 1987 May 14;144(3):1182–1187. doi: 10.1016/0006-291x(87)91436-7. [DOI] [PubMed] [Google Scholar]
- Scherer G., Schmid W., Strange C. M., Röwekamp W., Schütz G. Isolation of cDNA clones coding for rat tyrosine aminotransferase. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7205–7208. doi: 10.1073/pnas.79.23.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J. mRNA cap binding proteins: essential factors for initiating translation. Cell. 1985 Feb;40(2):223–224. doi: 10.1016/0092-8674(85)90132-1. [DOI] [PubMed] [Google Scholar]
- Shinshi H., Miwa M., Sugimura T. Enzyme cleaving the 5'-terminal methylated blocked structure of messenger RNA. FEBS Lett. 1976 Jun 1;65(2):254–257. doi: 10.1016/0014-5793(76)80492-9. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Sripati C. E., Groner Y., Warner J. R. Methylated, blocked 5' termini of yeast mRNA. J Biol Chem. 1976 May 25;251(10):2898–2904. [PubMed] [Google Scholar]
- Stevens A. An mRNA decapping enzyme from ribosomes of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1150–1155. doi: 10.1016/0006-291x(80)90072-8. [DOI] [PubMed] [Google Scholar]
- Stevens A., Maupin M. K. A 5'----3' exoribonuclease of Saccharomyces cerevisiae: size and novel substrate specificity. Arch Biochem Biophys. 1987 Feb 1;252(2):339–347. doi: 10.1016/0003-9861(87)90040-3. [DOI] [PubMed] [Google Scholar]
- Stevens A. Purification and characterization of a Saccharomyces cerevisiae exoribonuclease which yields 5'-mononucleotides by a 5' leads to 3' mode of hydrolysis. J Biol Chem. 1980 Apr 10;255(7):3080–3085. [PubMed] [Google Scholar]
- Stevens A. Pyrimidine-specific cleavage by an endoribonuclease of Saccharomyces cerevisiae. J Bacteriol. 1985 Oct;164(1):57–62. doi: 10.1128/jb.164.1.57-62.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]




