Abstract
Background:
Pneumocystis jirovecii causes Pneumocystis pneumonia (PCP) in immunocompromised patients with a high rate of morbidity and mortality. Colonization with this fungus may stimulate pulmonary inflammation or lead to PCP in susceptible patients. The epidemiology of this infection and routs of its transmission has poorly studied in Iran. We examined Pneumosystis colonization in patients with various lung underlying diseases.
Methods:
Bronchoalveolar lavage (BAL) fluids of 458 patients with different underlying diseases or pulmonary signs were collected between August 2010 and January 2012. Patients were divided into four groups: transplant recipients, malignant patients, immunosuppressive drug recipients and patients with other different lung diseases. A sensitive nested-PCR method targeted 18S ribosomal RNA gene was used for investigating P. jirovecii in the specimens.
Results:
P. jirovecii DNA was detected in 57 out of 458 (12.5%) BAL samples by nested-PCR. Colonization rate in malignant patients, transplant recipients, immunosuppressive therapy recipients and patients with other various lung diseases was 21.7%, 20.3%, 12.7% and 7.3%, respectively. The enzyme BanI cuts all PCR products producing fragments with the size of 228 and 104 base pair. This finding as well as sequencing of four random positive samples validated and reconfirmed the PCR results. P. jirovecii cysts were found in 5 out of 57 PCR positive samples.
Conclusion:
A significant number of patients with pulmonary diseases were colonized by P. jirovecii that can develop to PCP in these patients or they may transmit the fungus to other susceptible patients.
Keywords: Pneumocystis jirovecii, Pneumocystis pneumonia, Colonization, Nested-PCR
Introduction
Pneumocystis jirovecii (previously called Pneumocystis carinii) is an unusual opportunistic fungus that was formerly considered as a protozoon. However, considering sequence homologies in 18S ribosomal RNA gene, this organism was reclassified to the class Archiascomycetes, phylum Ascomycota of the fungal kingdom (1, 2). P. jirovecii causes pneumocystis pneumonia (PCP) in immunodeficient patients especially in HIV infected persons and other larger immunosuppressed groups such as transplant recipients, patients with autoimmune disorders (who treated with steroid or monoclonal antibodies directed against cell mediated immune system mediators), malignant patients and malnourished children (1, 3–5). In the lack of a culture system for isolating P. jirovecii from clinical samples, the laboratory diagnosis of PCP has depended on microscopic detection of organism with conventional staining methods like toluidine blue O, Grocott-Gomori methenamin silver, Giemsa, Calcofluor White or direct and indirect fluorescence immunocytochemical staining (6–8).
Recently PCR technology has improved sensitivity of the diagnosis of PCP and reduced need to invasively obtained clinical specimens such as lung biopsy. Nested-PCR approach has made detection of P. jirovecii from non-invasive clinical samples such as induced sputa or oropharyngeal washings more sensitive and specific than single-step standard PCR (7, 9). By application of these techniques, colonization with P. jirovecii have been demonstrated in some groups of patients with mild immunosuppression (10). These techniques have also shown that P. jirovecii can be carried in normal healthy individuals or asymptomatic patients with only mild immunosuppression induced by HIV infection or in patients requiring long term glucocorticoid therapy for underlying malignancy, and in immunocompetent individuals with chronic pulmonary diseases (7, 8). The colonization may surrogate to PCP on the conditions that underlying diseases go to a severe stage or in the absence of correct treatments. For this reason detection of carriage or colonization could be important for understanding the nature of epidemiological or clinical aspects of PCP.
Although the incidence of PCP in developed countries has reduced as a result of prophylaxis, but in the developing countries, it is a significant concern because of limited care resources and absence of enough data about epidemiology of the infection. PCP can causes death in over than 40% of cases especially in sever immunocompromised patients (5, 9, 11).
In the present study we have ascertained P. jirovici colonization in a relatively large group of non-HIV-infected patients with different pulmonary diseases undergoing diagnostic bronchoscopy. A specific nested-PCR method was used to detect DNA of P. jirovecii in the collected bronchoalveolar lavage (BAL) samples. The risk factors correlated with colonization were also investigated in different groups of patients. Combination of our results and other recent data may improve our understanding about Pneumocystis carriage and routs of its transmission among susceptible patients.
Materials and Methods
A total of 458 BAL fluids taken from non-HIV-infected patients with different pulmonary signs and symptoms and without evidenced PCP symptoms were included in the study. The BAL samples were obtained between August 2010 and January 2012, mainly from patients referred for bronchoscopy to pulmonology ward of Shariati Hospital in Tehran, Iran; or who their clinical specimens were submitted to Medical Mycology laboratory at Tehran University of Medical Sciences. Clinical information of each sample was gathered by medical record review.
Colonization or subclinical infection with P. jirovecii was defined when a patient did not have specific symptoms or history of PCP, and showed a positive nested-PCR result indicating presence of P. jirovecii DNA in his or her respiratory secretions (9). BAL fluids were collected gradually and stored in −20°C. The samples were centrifuged at 5000 rpm for 5 min, and a 300 μl aliquot of sediment was digested with 100 μg/ml of proteinase K (Gibco BRL- Life Technologies) at 56°C for 2 hour.
DNA of the digested sediments were extracted with equal volume of phenol-chloroform and once again with chloroform. DNA was precipitated with equal volume of 2-propanol and 1/10 volume 3 M sodium acetate at −20°C for 20 min and pelleted by centrifugation at 10000 rpm for 15 min. After a wash with 0.3 ml 70% ethanol, the pellet was air-dried and resuspended in 50 μl distilled water.
Nested-PCR protocol for amplification of partial 18SrRNA gene of P. jirovecii was performed based on the method described by Umera et al. (2). External primers namely Pjf9 (5′-TTCGGGGCTTACTTTGGTC-3′) and Pjr4 (5′-GTAGTTAGTCTTCAATAAATCT-3′) were used in first round, which produced a 710 bp amplicon, and the internal primers Pjf8 (5′-AGGCCTACCATGGTTTCG-3′) and Pjr8 (5′-CTTCGGAGGACCGGGCCGT-3′) were used in the second round to amplify a 332 bp fragment (2).
Each PCR reaction contained 2.5 μl of 10×reaction buffer, 2 mM MgCl2, 500 μM dNTPs mixture, 1.25 U Taq polymerase (Prime Taq, Genet Bio, South Korea), 25 pmol of each primer, 2 μl of DNA template solution and enough distilled water to a final volume of 25 μl. First round was fulfilled under the following condition: 95°C for 4 min, 30 cycles of 94°C for 45 s, 60°C for 1 min and 72°C for 1 min; and a final extension for 10 min in 72°C. Two microliter of the first PCR product was used as DNA template in the second PCR reaction having the following conditions: 95°C for 4 min, 30 cycles of 94°C for 30 s, 60°C for 45 s and 72°C for 45 s; and a final extension of 72°C for 10 min. PCR products were analyzed by electrophoresis on 1.5% agarose gels containing 0.5 μg/ml ethidium bromide and the bands were observed under UV light of transilluminator. To rule out any false positive results, aerosol barrier pipette tips were used and different steps of the preparations including DNA extraction, master mix preparation, PCR reactions and product detection were done in different rooms. Ultra pure water (instead of DNA) as the negative controls, and a plasmid containing the nucleotide sequence of partial 18S rRNA gene of P. jirovecii as the positive control, were included in each PCR run.
To validate the nested-PCR products, the sequences of the 18SrRNA gene of P. jirovecii (GenBank accession number AB266392) was investigated by DNASIS Max software (version 3.0, Hitachi, USA) to find a specific restriction digestion, and the enzyme BanI was chosen to cut the nested PCR products. Positive nested-PCR products of clinical specimens were digested with BanI (New England Bio Labs, UK) for 2 h at 37°C in a total volume of 15 μl containing 7 μl of PCR product and 5 U of the enzyme and then were visualized by electrophoresis onto 2% agarose gels. Furthermore, a total number of 4 randomly selected nested PCR products were purified by using AccuPrep PCR purification kit (Bioneer, South Korea) and subjected to sequencing by the forward primer Pjf8.
Those centrifuged sediments of BAL samples having positive nested-PCR, was subjected to staining with Giemsa and observed by light microscopy at a final magnification of 2000×.
Results
Four hundred and fifty eight subjects were included in this study, including 271 males and 187 females with median age of 50, ranged 7–95 years. Colonization did not seem to be dependent on sex or age of patients. There were 79 transplant recipients (49 lung, 7 kidney,14 bone marrow, 6 liver and 3 heart transplant patients); 63 inherited severe immunodeficiency or receiving immunosuppressant treatment patients, 69 patients with malignancies (33 solid tumors, 29 leukemia and 7 lymphoma patients) and 247 patients with other different lung diseases. Patients in the last group did not have conditions such as primary or acquired immunodeficiency, receiving cytotoxic or immunosuppressive medications, HIV seropositivity, cancers and systemic underlying diseases.
The nested primers Pjf8 and Pjr8 successfully amplified a 332 bp fragment of 18SrRNA gene of P. jirovecii in positive control and in 57 out of 458 BAL samples obtained from patients with pulmonary diseases. No amplification was seen in negative controls (Fig. 1). The nested PCR results of each group of patients were summarized in table 1.
Fig. 1:
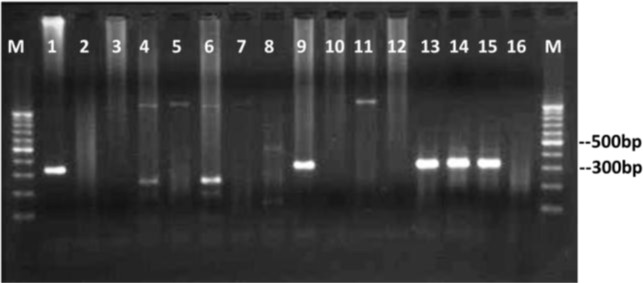
Agarose gel electrophoresis of nested-PCR products of P. jirovecii partial 18SrRNA gene. Lanes 1: positive control, lanes 9 and 13–15: positive clinical samples having specific 330 bp bands representative of P. jirovecii, lanes 2–8 and 10–12: no representative band indicating negative clinical samples, lane 16: negative control. Lanes 4 and 6 have unspecific bands with unexpected size seen in some samples. Lanes M: 100 bp DNA size marker.
Table 1:
Frequency of patients in different groups and results of nested PCR for each group
| Patients | Subjects No. | Positive Nested PCR |
|---|---|---|
| Transplant recipients | 79 | 16 (20.3%) |
| Lung transplantation | 49 | 6 |
| Bone marrow or stem cell transplantation | 14 | 4 |
| Liver transplantation | 6 | 2 |
| Kidney transplantation | 7 | 3 |
| Heart transplantation | 3 | 1 |
| Malignant patients | 69 | 15 (21.7%) |
| Acute myeloid leukemia | 14 | 3 |
| Acute lymphocytic leukemia | 9 | 2 |
| Chronic myeloid leukemia | 2 | 1 |
| Chronic lymphocytic leukemia | 4 | 3 |
| Hodgkin lymphoma | 2 | 0 |
| Non-Hodgkin lymphoma | 5 | 1 |
| Multiple myeloma | 1 | 0 |
| Lung cancer | 20 | 3 |
| Other solid tumors (breast, prostate, brain, thyroid, gastrointestinal) | 13 | 2 |
| Immunosuppressed patients | 63 | 8 (12.7%) |
| Immunosuppressant medication | 54 | 6 |
| Immunodeficiency( Non HIV positive) | 9 | 2 |
| Immunocompetent patients (Any pulmonary diseases) | 247 | 18 (7.3%) |
| Total | 458 | 57 (12.5%) |
Malignant patients and transplant recipients patients showed higher rates of colonization (21.7% and 20.3%, respectively) while immunosuppressive therapy recipient patients showed only 12.7% colonization compared to other patients with various lung diseases with 7.3% colonization. Among the malignant patients, patients with acute myeloid leukemia, chronic lymphocytic leukemia and lung cancer had a higher rate of colonization in contrast to other malignant patients. Lung transplantation recipients showed more colonization in comparison with the other organ transplant recipients. Among immunosuppressive therapy recipients, those patients who had received corticosteroids as immunosuppressant medications, showed increased colonization with P. jirovecii.
According to our sequence analysis, the enzyme BanI cuts the amplified fragments of second round PCR at nucleotide position 228 and produces two fragments of 228 and 104 bp in size (Fig. 2).
Fig. 2:
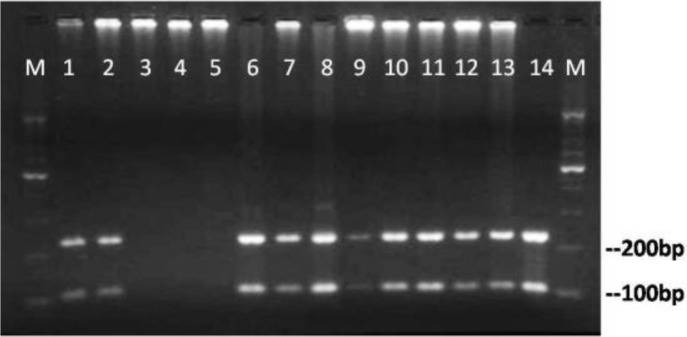
Agarose gel electrophoresis of nested PCR products after digestion with the restriction enzyme BanI presenting two fragments of 228 and 104 base pair. Lanes 1–13: digested products of clinical samples, lane14 digested product of positive control, lanes 3 – 5: no specific PCR product. Lanes M: 100 bp size marker
As it is seen in Fig. 2, all positive nested-PCR products have resulted in expected RFLP products, indicating that the positive PCRs are resulted from amplification of P. jirovecii specific rRNA gene.
Analysis of sequencing results of four PCR products by using online BLAST software showed identity in all nucleotides except a G insertion in one sequence and a T deletion in another sequence (Data not shown). The sequences were deposited in GenBank as Iranian samples with accession numbers JX856143- JX856146. These finding validates and reconfirms our PCR results.
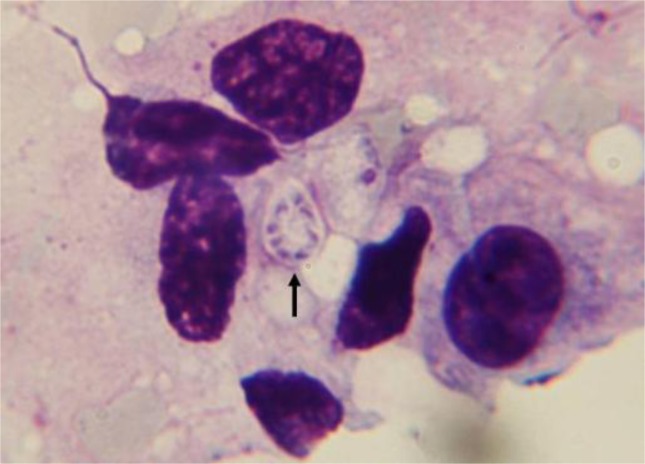
Cytological investigation of those BAL specimens with positive nested-PCR revealed characteristic microscopic appearance of P. jirovecii cysts in 5 out of 57 samples. An example of the microscopically positive samples for P. jirovecii from an immunosuppressive therapy recipient has been shown in Fig. 3. Characteristic 8-nuclei cyst is seen clearly.
Fig. 3:
Giemsa stained preparation of a BAL fluid taken from an immunosuppressed patient representative of Pneumocystis jirovecii cyst containing intracystic sporozoites (2000× magnification)
Discussion
The epidemiology of Pneumocystis infections is still remained undetermined because the detection of the fungus in clinical specimens by microbiological methods such as direct microscopy does not have enough sensitivity. Furthermore, the organism cannot grow on microbiological media. However, molecular approaches mainly PCR-based methods and experimental Pneumocystis infection in animal models have revealed some features of Pneumocystis infections (6). Although retrieval of Pneumocystis DNA may not indicate the presence of vital Pneumocystis in respiratory samples, it can be a significant marker for presenting the fungus in the lung (9).
History of study on Pneumocystis infections in Iran returned to early years after World War II. The first clinical cases of plasma cell pneumonia in Iranian patients were described in malnourished children in a Shiraz orphanage by Post et al. (12) . They showed an epidemic PCP with a high mortality rate during 1962 to 1968 in south Iran by staining of lung necropsies. These studies were continued by comparing the immunoglobulin levels in PCP and non-PCP patients (13). In recent years some studies using staining methods or PCR techniques have done in animal models (14–18). Nevertheless studies on PCP cases have been limited to HIV infected patients based on clinical symptoms rather than laboratory findings (19–23). The most important studies on Pneumocystis in Iran were summarized in table 2. In this study, we thought that nested-PCR technique could be used as a sensitive and specific method for evaluating carriage and colonization with P. jirovecii. We determined the colonization with P. jirovecii in BAL fluids of a significant number of patients with pulmonary diseases. To our knowledge, this is the first molecular epidemiological study to describe Pneumocystis colonization in non-HIV-infected Iranian patients with pulmonary diseases. The study demonstrated that colonization by P. jirovecii often occurs in patients with mild immunosuppression and underlying pulmonary diseases. Patients who carry P. jirovecii in their lung are at risk for PCP in term of level of disruption in their immunity or they could be a possible reservoir for transmitting the fungus in the hospitalized patients. On our findings, colonization with P. jirovecii was detected in 12.5% of all our patients. In patients with predisposing factors such as malignancy and the recipients of organ transplant, P. jirovecii colonization has occurred in higher rates of 21.7% and 20.3% of cases, respectively, while it was detected only in 12.7% of patients with the history of receiving immunosuppressant medications and in 7.3% of patients with other various lung diseases. The higher rates of colonization seen in malignant and transplant patients might be due to severity of their predisposing factors, especially effects of cytotoxicity of drugs used in these groups of patients and consequent impaired cellular immunity. The rate of detection of P. jirovecii DNA in each subgroup especially in hematological malignant patients were increased in whom were in sever phase of their underlying diseases. The rates of colonization with P. jirovecii in immunosuppressant therapy receivers were meaningfully higher than those had not received immunosuppressant (12.7% versus 7.3%).
Table 2:
Summary of some studies carried out on Pneumocystis in Iran
| Study Population | Subjects No. | Diagnostic Specimen | Diagnostic methods | Colonization or Infection with Pneumocystis | Date of Study Publication (reference) |
|---|---|---|---|---|---|
| Orphan children | 40 | Autopsy lung | H&E stain | 12.5% | 1964 (12) |
| Orphan children | 50 | Serum, Respiratory secretions | Immuoeletrophresis, Giemsa stain | NA | 1972 (13) |
| Immunocopetent rats | 158 | Autopsy lung | H&E, GMS, Giemsa stains | 0% | 2006 (14) |
| Immunosuppressed rats | 15 | Respiratory secretions | 100% | ||
| HIV infected patients | 52 | ND | ND | 0% | 2006 (19) |
| Immunosuppressed rats | 20 | BAL, LH, OS | GMS, Giemsa stains PCR |
NA | 2007 (15) |
| HIV infected patients | 12 | BAL | IF stain | 100% | 2007 (20) |
| Immunocopetent rats | 4 | BAL, LH,OS | GMS stain | NA | 2008 (16) |
| Immunosuppressed rats | 13 | PCR | |||
| Immunosuppressed rats | 22 | BAL, LH,OS | GMS, Giemsa stains | NA | 2008 (17) |
| Immunosuppressed patients (Receiving prednisolone) | 2 | BAL | IF stain | 100% | 2008 (21) |
| Immunosuppressed rats | 6 | LH | Giemsa stain | NA | 2008 (18) |
| HIV infected patients | 120 | ND | ND | 22.5% | 2010 (22) |
| HIV infected patients | 177 | ND | ND | 4.5% | 2011 (23) |
BAL: Bronchoalveolar lavage, GMS: Gomori's Methenamine Silver, H & E: Hematoxylin & Eosin, IF: Immunofluorescence, LH: lung homogenate, NA: Not applicable, ND: Not determined, OS: Oral swab, PCR: Polymerase chain reaction
In other part of the world, similar studies have been reported but there are some differences in their results especially in the rate of colonization in different patients groups or in general population (24). Studies of Nevez et al. (25) and Medrano et al. (9) using nested-PCR method have revealed a colonization rate about 20% in healthy immunocompetent adults. Also Nevez et al. (26) and Vergas et al. (27) have reported Pneumocystis colonization in 16% of non-HIV-infected patients with a variety of medical problems, whereas Maskell et al. (10) have reported a higher rate of 44% for this group of patients. Probst et al. (28) have showed that over than 21% of patients with primary lung diseases were colonized with Pneumocystis, however, more recent studies have revealed a wide range of colonization in these population with colonization rates varying 16% to 55% (29–32). In our study, Pneumocystis colonization was found in 12.5% of all samples obtained from patients. Other studies that have assessed similar groups of patients with nested-PCR have showed Pneumocystis colonization prevalence that fluctuated from 2.6% to 55% (33). The differences in these studies could be interpreted by differences in their detection methods or varying predisposing factors of the patients. However the association between these high rates of P. jirovecii colonization and progression of PCP or other lung diseases like COPD is debatable and more studies are needed to establish this association.
Detection of P. jirovecii specific DNA in BAL or other respiratory secretions with nested-PCR can help us to diagnose PCP. Because of some limitations in the study plan we did not follow up patients for later consequences such as developing to PCP, thus we did not have golden standard of positive samples. However, as P. jirovecii colonization in respiratory tract of persons may lead to misdiagnose PCP, a delicate differentiation between infection and colonization is crucial to establish a diagnosis. To meet this goal, performing studies using more specific methods preferably quantitative approach such as real-time PCR looks to be useful. Therefore including samples from patients with proven PCP as well as some normal individual for determining a quantitative cutoff point to discriminate the infection from colonization is necessary. The main goal of this study was only estimation of P. jirovecii colonization and presumptive diagnosing PCP in predisposed patients. Developing a quantitative TaqMan probe real-time PCR in order to improve the situation is ongoing in our laboratory.
Conclusion
Our data showed a high presence of P. jirovecii DNA sources in BAL fluids obtained from patients with different pulmonary diseases. Special attention to these patients is essential for preventing nosocomial infections or emerging drug resistance isolates. More investigations are needed to show the real role of colonized persons as a reservoir of infection for transmission of P. jirovecii. The high presence of P. jirovecii DNA in respiratory samples of patients with chronic pulmonary diseases in this study indicates that the prevalence of colonization with P. jirovecii in these patients and possibly in general Iranian population could be higher than expected estimations.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors.
Acknowledgments
We are delighted for all helps from Tehran University of Medical Sciences and Teikyo University Institute of Medical Mycology, Tokyo, Japan. The authors declare that there is no Conflict of Interests.
References
- 1.Hof H. Pneumocystis jirovecii: A peculiar fungus posing particular problems for therapy and prophylaxis. Mycosis. 2012;55:1–7. [Google Scholar]
- 2.Uemura N, Makimura K, Onozaki M, Otsuka Y, Shibuya Y, Yazaki H, et al. Development of a loop-mediated isothermal amplification method for diagnosing Pneumocystis Pneumonia. J Med Microbiol. 2008;57:50. doi: 10.1099/jmm.0.47216-0. [DOI] [PubMed] [Google Scholar]
- 3.Montes-Cano MA, de la Horra C, Martin-Juan J, Varela JM, Torronteras R, Respaldiza N, et al. Pneumocystis jiroveci Genotypes in the Spanish Population. Clin Infec Dis. 2004;39:123–8. doi: 10.1086/421778. [DOI] [PubMed] [Google Scholar]
- 4.Rodino J, Rincon N, Aguilar YA, Rueda ZV, Herrera M, Velez LA. Microscopic diagnosis of Pneumocystis jirovecii pneumonia in bronchoalveolar lavage and oropharyngeal wash samples of immunocompromised patients with pneumonia. Biomedica. 2011;31(2):222. doi: 10.1590/S0120-41572011000200010. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Martineza MJ, Miro JM, Vallsa ME, et al. Sensitivity and specificity of nested and real-time PCR for the detection of Pneumocystis jiroveci in clinical specimens. Diagn Microbiol Infect Dis. 2006;56:153–60. doi: 10.1016/j.diagmicrobio.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Helweg-Larsen J. Pneumocystis jiroveci: Applied molecular microbiology, epidemiology and diagnosis. Dan Med Bull. 2004;51:251–73. [PubMed] [Google Scholar]
- 7.Sing A, Trebesius K, Roggenkamp A, Russmann H, Tybus K, Pfaff F, et al. Evaluation of Diagnostic Value and Epidemiological Implications of PCR for Pneumocystis carinii in Different Immunosuppressed and Immunocompetent Patient Groups. J Clin Microbiol. 2000;38(4):1461–7. doi: 10.1128/jcm.38.4.1461-1467.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azoulay E, Chevret S, Bergron A, et al. Polymerase Chain Reaction for Diagnosing Pneumocystis Pneumonia in Non-HIV Immunocompromised Patients With Pulmonary Infiltrates. Chest. 2009;135:655–61. doi: 10.1378/chest.08-1309. [DOI] [PubMed] [Google Scholar]
- 9.Medrano FJ, Montes-Cano M, Conde M, de la Horra C, Respaldiza N, Gasch A, et al. Pneumocystis jirovecii in General Population. Emerg Infect Dis. 2005;11(2):245–50. doi: 10.3201/eid1102.040487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maskell NA, Waine DJ, Lindley A, et al. Asymptomatic carriage of Pneumocystis jiroveci in subjects undergoing bronchoscopy: a prospective study. Thorax. 2003;58:594–7. doi: 10.1136/thorax.58.7.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid AB, Chen SCA, Worth LJ. Pneumocystis jirovecii pneumonia in non-HIV-infected patients: new risks and diagnostic tools. Curr Opin Infect Dis. 2011;24:534–44. doi: 10.1097/QCO.0b013e32834cac17. [DOI] [PubMed] [Google Scholar]
- 12.Post C, Dutz W, Nasarian I. Endemic Pneumocystis Carinii Pneumonia In South Iran. Arch Dis Childh. 1964;39:35–40. doi: 10.1136/adc.39.203.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohout E, Post C, Azadeh B, et al. Immunoglobulin levels in infantile pne-umocystosis. J Clin Path. 1972;25:135–40. doi: 10.1136/jcp.25.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharif M, Ziaei H, Daryani A, Nasrolahi M, Lackterashi B. Epidemiological survey of Pneumocystis Carinii in Rodents of Sari, Northern Iran. J Animal Vet Adv. 2006;5(5):390–4. [Google Scholar]
- 15.Mahmoodzadeh A, Hajia M, Rezaiemanesh MR, Morovati H. A Scoring System for Pneumocystis Pneumonia Based on Clinical Symptoms and Laboratory Findings in a Rat Model. Iran J Pathol. 2007;2(4):165–70. [Google Scholar]
- 16.Mahmoodzadeh A, Hajia M, Rezaiemanesh MR. Comparison of Gomori’s Methenamine Silver Method with PCR Technique on Oral Swab, Bronchoalveolar Lavage and lung Homogenate Specimens in Detection of Pneumocystis. Iranian J Parasitol. 2008;3(2):21–5. [Google Scholar]
- 17.Hajia M, Mahmoodzadeh A, Rezaiemanesh MR, Morovati H. Diagnosis of Pneumocystis pneumonia with two staining methods using various specimens collected from animal model. Internet J Microbiol. 2008;5(2) doi: 10.5580/1e40. [DOI] [Google Scholar]
- 18.Mahmoodzadeh A, Hajia M, Morovati H. Use of Razi Bovine Kidney Cell Line for Proliferation of Pneumocystis Carinii. Iran J Pathol. 2008;3(4):197–202. [Google Scholar]
- 19.Sharifi Mood B, Alavi Naini R, Salehi M, Hashemi M, Rakhshani F. Spectrum of clinical disease in a series of hospitalized HIV-infected patients from Southeast of Iran. Saudi Med J. 2006;27(9):1362–6. [PubMed] [Google Scholar]
- 20.Tabarsi P, Baghaei P, Karimi S, Alizadeh H, Mansoori SD, Masjedi MR, et al. Pneumocystis Pneumonia in Patients with Human Immunodeficiency Virus. Tanaffos. 2007;6(3):26–9. [Google Scholar]
- 21.Tabarsi P, Mirsaeidi M, Amiri M, Karimi S, Masjedi MR, Mansouri D. Inappropriate use of steroid and Pneumocystis jiroveci pneumonia: report of two cases. East Mediterr Health J. 2008;14(5):1217–1221. [PubMed] [Google Scholar]
- 22.Esmailpour N, Rasooli Nejad M, Badrfam R, Ghazi P, Zandifar A, Kheirandish P, et al. Fever in patients with HIV infection in a teaching hospital in Iran. Iranian J Clin Infect Dis. 2010;5(3):121–5. [Google Scholar]
- 23.Seyed Alinaghi SA, Vaghari B, Roham M, Moradmand Badie B, Jam S, Foroughi M, et al. Respiratory Complications in Iranian Hospitalized Patients with HIV/AIDS. Tanaffos. 2011;10(3):49–54. [PMC free article] [PubMed] [Google Scholar]
- 24.Pederiva MA, Wissmann G, Friaza V, Morilla R, de la Horra C, Montes-Cano M, et al. High prevalence of Pneumocystis jirovecii colonization in Brazilian cystic fibrosis patients. Med Mycol. 2012;50:556–60. doi: 10.3109/13693786.2011.645892. [DOI] [PubMed] [Google Scholar]
- 25.Nevez G, Jounieaux V, Linas MD, Guyot K, Leophonte P, et al. High frequency of Pneumocystis carinii sp.f. hominis colonization in HIV-negative patients. J Eukaryot Microbiol. 1997;44(6):36S. doi: 10.1111/j.1550-7408.1997.tb05760.x. [DOI] [PubMed] [Google Scholar]
- 26.Nevez G, Raccurt C, Vincent P, Jounieaux V, et al. Pulmonary colonization with Pneumocystis carinii in human immunodeficiency virus-negative patients: assessing risk with blood CD4_ T cell counts. Clin Infect Dis. 1999;29:1331–2. doi: 10.1086/313478. [DOI] [PubMed] [Google Scholar]
- 27.Vargas SL, Ponce CA, Sanchez CA, Ulloa AV, Bustamante R, et al. Pregnancy and asymptomatic carriage of Pneumocystis jiroveci. Emerg Infect Dis. 2003;9:605–6. doi: 10.3201/eid0905.020660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Probst M, Ries H, Schmidt-Wieland T, et al. Detection of Pneumocystis carinii DNA in patients with chronic lung diseases. Eur J Clin Microbiol Infect Dis. 2000;19:644–5. doi: 10.1007/s100960000329. [DOI] [PubMed] [Google Scholar]
- 29.Nevez G, Magois E, Duwat H, Gouilleux V, Jounieaux V, et al. Apparent absence of Pneumocystis jirovecii in healthy subjects. Clin Infect Dis. 2006;42(11):e99–101. doi: 10.1086/503908. [DOI] [PubMed] [Google Scholar]
- 30.Respaldiza N, Montes-Cano MA, Dapena FJ. Prevalence of colonisation and genotypic characterisation of Pneumocystis jirovecii among cystic fibrosis patients in Spain. Clin Microbiol Infect. 2005;11(12):1012–5. doi: 10.1111/j.1469-0691.2005.01276.x. [DOI] [PubMed] [Google Scholar]
- 31.Vidal S, de la Horra C, Martin J. Pneumocystis jirovecii colonisation in patients with interstitial lung disease. Clin Microbiol Infect. 2006;12(3):231–5. doi: 10.1111/j.1469-0691.2005.01337.x. [DOI] [PubMed] [Google Scholar]
- 32.Calderon EJ, Rivero L, Respaldiza N. Systemic inflammation in patients with chronic obstructive pulmonary disease who are colonized with Pneumocystis jiroveci. Clin Infect Dis. 2007;45:17–9. doi: 10.1086/518989. [DOI] [PubMed] [Google Scholar]
- 33.Morris A, Wei K, Afshar K, Huang L. Epidemiology and Clinical Significance of Pneumocystis Colonization. J Infect Dis. 2008;197:10–7. doi: 10.1086/523814. [DOI] [PubMed] [Google Scholar]