Abstract
Tendons are often injured and heal poorly. Whether this is caused by a slow tissue turnover is unknown, since existing data provide diverging estimates of tendon protein half-life that range from 2 mo to 200 yr. With the purpose of determining life-long turnover of human tendon tissue, we used the 14C bomb-pulse method. This method takes advantage of the dramatic increase in atmospheric levels of 14C, produced by nuclear bomb tests in 1955–1963, which is reflected in all living organisms. Levels of 14C were measured in 28 forensic samples of Achilles tendon core and 4 skeletal muscle samples (donor birth years 1945–1983) with accelerator mass spectrometry (AMS) and compared to known atmospheric levels to estimate tissue turnover. We found that Achilles tendon tissue retained levels of 14C corresponding to atmospheric levels several decades before tissue sampling, demonstrating a very limited tissue turnover. The tendon concentrations of 14C approximately reflected the atmospheric levels present during the first 17 yr of life, indicating that the tendon core is formed during height growth and is essentially not renewed thereafter. In contrast, 14C levels in muscle indicated continuous turnover. Our observation provides a fundamental premise for understanding tendon function and pathology, and likely explains the poor regenerative capacity of tendon tissue.—Heinemeier, K. M., Schjerling, P., Heinemeier, J., Magnusson, S. P., Kjaer, M. Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb 14C.
Keywords: collagen fibril, turnover, synthesis rate, skeletal muscle, carbon-14
The prevalence of tendon injuries associated with sports and leisure activity is quite considerable, and often injuries are accompanied by incomplete healing, chronic tendon pain, and degenerative changes within the tendon (1, 2). This indicates a limited regenerative capacity of tendons, which may be due to a slow tissue turnover. The rate of tendon tissue turnover has been largely unknown, although, based on determination of aspartic acid racemization in horses, a half-life of tendon collagen protein approaching 200 yr has been suggested (3). In distinct contrast to this, indirect determination of collagen turnover in human tendon tissue, by sampling of procollagen peptides from the peritendinous tissue using microdialysis, suggests that relatively high rates of collagen protein synthesis take place in tendon (4). Furthermore, biopsy data on incorporation of stable isotope-labeled amino acids into human tendon indicated collagen synthesis rates that correspond to ∼1%/d (equivalent to a half-life of ∼2 mo), which is close to that of skeletal muscle (5, 6). Moreover, mechanical loading seems to increase collagen synthesis (4, 6), and physical training appears to induce hypertrophy of human tendons (7, 8). These findings indicate that human tendons are mechano-sensitive and respond to loading by up-regulating collagen protein synthesis. In contrast to this, data from core tendon tissue in humans did not show any loading-induced stimulation of collagen mRNA expression (9, 10), and the poor regenerative capacity of human tendon in general, challenges suggestions of high levels of turnover in this tissue (5, 6). To answer the fundamental question of how fast human tendon tissue is replaced, we have studied life-long Achilles tendon turnover with use of the 14C bomb-pulse method (11, 12) (explained below).
The amount of 14C in atmospheric CO2 was relatively constant until 1955, when above ground nuclear bomb tests caused it to rise dramatically to about twice the natural level (ref. 13 and Fig. 1). When testing was stopped after the Test Ban Treaty in 1963, the 14C levels in the atmosphere dropped exponentially, primarily due to diffusion into the oceans. Atmospheric 14C, normally generated only by cosmic radiation, reacts with oxygen and forms carbon dioxide (14CO2). This 14CO2 is incorporated into plants during photosynthesis and passed directly into humans via plant foods, and indirectly via meat from animals, in amounts that closely parallel the atmospheric concentration. Most tissues in living organisms are gradually replaced over weeks or months and therefore have a content of 14C corresponding to the current level in the atmosphere (12). However, tissues with absent or very slow turnover will contain 14C levels resembling the levels in the atmosphere at the time of tissue formation (11, 12). Thus, by measuring the content of 14C in tissue from people who have lived during and after the bomb pulse peak, the replacement rate of a particular tissue or tissue component can be estimated accurately (11, 12, 14–16). At present, no consensus exists with regard to the replacement rate of human tendon tissue, but some findings indicate that the tissue turnover could be relatively slow (3, 9, 10). Thus, in the present study we have taken advantage of the 14C bomb pulse method to study the degree of Achilles tendon tissue renewal during life using skeletal muscle as a reference.
Figure 1.
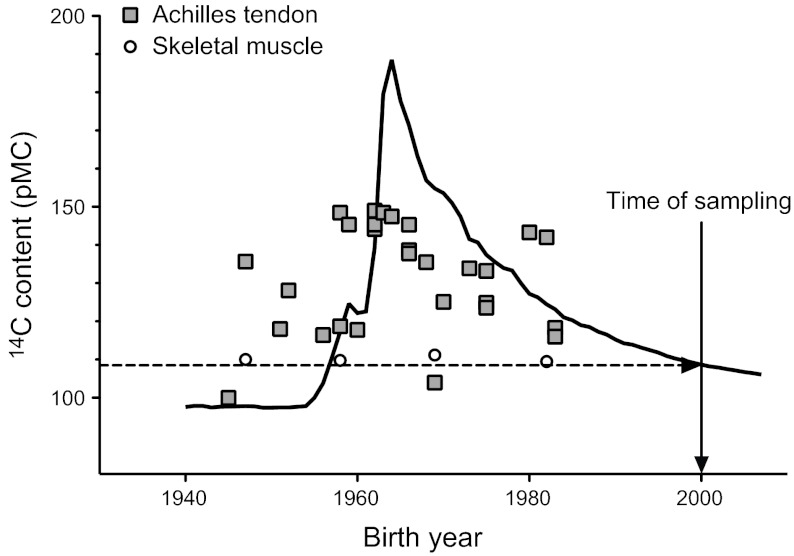
14C bomb-pulse curve. Concentration of 14C in atmospheric CO2 (black line), shown as pMC (26). Testing of nuclear bombs from 1955 to 1963 nearly doubled the atmospheric levels of 14C, which was followed by an exponential decrease after the Test Ban Treaty in 1963. Measured levels of 14C are shown in relation to birth year for samples of human Achilles tendon (gray squares) and skeletal muscle (open circles) taken in year 2000. The levels of 14C found in the tendon tissue generally correspond to the atmospheric levels many years before sampling, thus indicating a very slow rate of tissue replacement. Skeletal muscle levels of 14C correspond to atmospheric levels ∼2 yr before the time of sampling (dashed arrow), thus indicating continuous tissue turnover, as essentially no memory of lifetime exposure to the bomb pulse remains in the muscle tissue.
MATERIALS AND METHODS
Tendon and muscle samples
Achilles tendon samples (n=28) were obtained in March 2000 during routine forensic autopsies with permission at the Arkansas State Crime Laboratory (Little Rock, AR, USA). Samples with a diameter of ∼4 mm were obtained from the Achilles tendon core 2–5 cm from the most proximal part of the calcaneus and kept at −80°C until further analyses. Skeletal muscle samples (M. psoas major) were also obtained from 4 of the donors. The birth years of the donors ranged from 1945 to 1983 (see anthropometric details for all donors in Table 1).
Table 1.
Characteristics of tissue donors, 14C concentration, and stable isotope data (δ13C and δ15N) for tissue samples
| Sample | AMS ID, AAR- | Tissue | Sex | Height (cm) | Weight (kg) | Birth year | [14C] (pMC) | Bomb-pulse calibration year | δ13C, VPDB | δ15N, air | Carbon fraction | Nitrogen fraction | C/N ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 15880 | Tendon | M | 174 | 99 | 1945 | 99.95 ± 0.29 | 1955 | −15.27 ± 0.10 | 13.66 ± 0.16 | 0.46 ± 0.02 | 0.165 ± 0.006 | 3.24 ± 0.16 |
| 24 | 16243 | Tendon | M | 174 | 97 | 1947 | 135.71 ± 0.35 | 1962/1976 | −14.35 ± 0.14 | 11.83 ± 0.12 | 0.43 ± 0.01 | 0.156 ± 0.002 | 3.23 ± 0.06 |
| 24 | 17279 | Muscle | M | 174 | 97 | 1947 | 109.96 ± 0.34 | 1957/1998 | −18.90 ± 0.10 | 8.51 ± 0.23 | 0.46 ± 0.01 | 0.139 ± 0.003 | 3.88 ± 0.09 |
| 20 | 16306 | Tendon | M | 164 | 65 | 1951 | 118.02 ± 0.36 | 1958/1987 | −15.44 ± 0.22 | 11.81 ± 0.16 | 0.32 ± 0.08 | 0.111 ± 0.030 | 3.35 ± 1.21 |
| 6 | 16295 | Tendon | M | 169 | 64 | 1952 | 128.10 ± 0.30 | 1959/1980 | −15.66 ± 0.25 | 12.09 ± 0.10 | 0.48 ± 0.07 | 0.127 ± 0.003 | 3.77 ± 0.93 |
| 21 | 16307 | Tendon | M | 169 | 67 | 1956 | 116.37 ± 0.35 | 1958/1989 | −15.38 ± 0.15 | 11.92 ± 0.12 | 0.46 ± 0.05 | 0.160 ± 0.021 | 3.32 ± 0.59 |
| 26 | 16246 | Tendon | F | 169 | 49 | 1958 | 148.48 ± 0.41 | 1962/1972 | −14.33 ± 0.14 | 11.75 ± 0.12 | 0.39 ± 0.01 | 0.143 ± 0.002 | 3.17 ± 0.06 |
| 26 | 17281 | Muscle | F | 169 | 49 | 1958 | 109.79 ± 0.34 | 1998 | −17.92 ± 0.10 | 8.95 ± 0.23 | 0.47 ± 0.01 | 0.132 ± 0.003 | 4.10 ± 0.11 |
| 29 | 16251 | Tendon | F | 157 | 68 | 1958 | 118.69 ± 0.32 | 1987 | −15.82 ± 0.14 | 11.89 ± 0.12 | 0.46 ± 0.01 | 0.156 ± 0.002 | 3.46 ± 0.06 |
| 5 | 16294 | Tendon | M | 174 | 87 | 1959 | 145.42 ± 0.33 | 1962/1972 | −14.77 ± 0.22 | 11.90 ± 0.16 | 0.45 ± 0.07 | 0.163 ± 0.028 | 3.20 ± 0.74 |
| 31 | 16311 | Tendon | F | 159 | 71 | 1960 | 117.85 ± 0.32 | 1988 | −15.78 ± 0.15 | 11.93 ± 0.12 | 0.46 ± 0.05 | 0.155 ± 0.020 | 3.48 ± 0.59 |
| 18 | 16304 | Tendon | M | 174 | 70 | 1962 | 144.21 ± 0.35 | 1973 | −15.67 ± 0.10 | 12.08 ± 0.11 | 0.45 ± 0.02 | 0.160 ± 0.007 | 3.30 ± 0.19 |
| 23 | 16309 | Tendon | F | 154 | 54 | 1962 | 148.99 ± 0.37 | 1972 | −13.90 ± 0.22 | 12.53 ± 0.16 | 0.45 ± 0.08 | 0.157 ± 0.032 | 3.36 ± 0.90 |
| 27 | 16310 | Tendon | F | 172 | 57 | 1962 | 145.44 ± 0.39 | 1972 | −14.63 ± 0.22 | 11.70 ± 0.16 | 0.46 ± 0.08 | 0.162 ± 0.032 | 3.33 ± 0.87 |
| 14 | 16301 | Tendon | F | 162 | 72 | 1963 | 148.49 ± 0.39 | 1972 | −15.31 ± 0.22 | 11.05 ± 0.16 | 0.46 ± 0.07 | 0.151 ± 0.026 | 3.58 ± 0.81 |
| 22 | 16308 | Tendon | F | 149 | 52 | 1964 | 147.52 ± 0.35 | 1972 | −14.38 ± 0.22 | 12.60 ± 0.16 | 0.47 ± 0.07 | 0.155 ± 0.029 | 3.54 ± 0.87 |
| 7 | 16296 | Tendon | M | 181 | 86 | 1966 | 138.72 ± 0.33 | 1975 | −14.31 ± 0.15 | 11.57 ± 0.12 | 0.43 ± 0.05 | 0.152 ± 0.020 | 3.31 ± 0.59 |
| 16 | 16303 | Tendon | F | 167 | 75 | 1966 | 137.78 ± 0.37 | 1975 | −16.29 ± 0.19 | 11.57 ± 0.10 | 0.44 ± 0.01 | 0.144 ± 0.003 | 3.58 ± 0.08 |
| 19 | 16305 | Tendon | M | 176 | 72 | 1966 | 145.27 ± 0.38 | 1972 | −15.70 ± 0.22 | 12.27 ± 0.16 | 0.46 ± 0.08 | 0.158 ± 0.032 | 3.40 ± 0.91 |
| 4 | 16293 | Tendon | M | 176 | 86 | 1968 | 135.47 ± 0.34 | 1976 | −14.78 ± 0.10 | 11.78 ± 0.10 | 0.45 ± 0.02 | 0.122 ± 0.002 | 3.49 ± 0.19 |
| 25 | 15882.1 | Tendon | M | 176 | 70 | 1969 | 105.56 ± 0.33 | OL | −15.55 ± 0.10 | 11.51 ± 0.16 | 0.46 ± 0.02 | 0.164 ± 0.007 | 3.26 ± 0.19 |
| 25a | 15882.2 | Tendon | M | 176 | 70 | 1969 | 104.93 ± 0.26 | OL | −15.46 ± 0.10 | 11.80 ± 0.16 | 0.45 ± 0.02 | 0.159 ± 0.007 | 3.26 ± 0.20 |
| 25 | 17280 | Muscle | M | 176 | 70 | 1969 | 111.17 ± 0.35 | 1996 | −17.43 ± 0.10 | 8.80 ± 0.13 | 0.55 ± 0.02 | 0.099 ± 0.008 | 6.37 ± 0.59 |
| 11 | 15881 | Tendon | M | 189 | 100 | 1970 | 125.09 ± 0.33 | 1982 | −15.60 ± 0.10 | 11.07 ± 0.16 | 0.46 ± 0.02 | 0.160 ± 0.007 | 3.35 ± 0.19 |
| 12 | 16299 | Tendon | F | 164 | 109 | 1973 | 133.86 ± 0.39 | 1977 | −14.75 ± 0.22 | 11.48 ± 0.16 | 0.45 ± 0.08 | 0.157 ± 0.032 | 3.34 ± 0.90 |
| 3 | 16292 | Tendon | M | 162 | 73 | 1975 | 124.92 ± 0.29 | 1982 | −14.58 ± 0.15 | 11.18 ± 0.12 | 0.44 ± 0.06 | 0.158 ± 0.023 | 3.27 ± 0.64 |
| 10 | 16298 | Tendon | F | 194 | 112 | 1975 | 123.58 ± 0.33 | 1983 | −14.90 ± 0.22 | 11.34 ± 0.16 | 0.45 ± 0.07 | 0.161 ± 0.027 | 3.25 ± 0.74 |
| 13 | 16300 | Tendon | F | 169 | 89 | 1975 | 133.18 ± 0.36 | 1978 | −14.66 ± 0.22 | 12.07 ± 0.16 | 0.29 ± 0.05 | 0.105 ± 0.020 | 3.26 ± 0.85 |
| 30 | 16252 | Tendon | F | 167 | 76 | 1980 | 143.34 ± 0.37 | OL | −14.69 ± 0.14 | 11.91 ± 0.12 | 0.46 ± 0.01 | 0.163 ± 0.002 | 3.26 ± 0.06 |
| 28 | 15883 | Tendon | F | 162 | 77 | 1982 | 142.28 ± 0.37 | OL | −14.92 ± 0.10 | 11.71 ± 0.16 | 0.46 ± 0.02 | 0.161 ± 0.008 | 3.32 ± 0.23 |
| 28a | 16247 | Tendon | F | 162 | 77 | 1982 | 144.59 ± 0.42 | OL | −14.69 ± 0.14 | 11.85 ± 0.12 | 0.46 ± 0.01 | 0.160 ± 0.002 | 3.33 ± 0.06 |
| 28 | 17283 | Muscle | F | 162 | 77 | 1982 | 109.48 ± 0.34 | 1998 | −18.01 ± 0.10 | 8.51 ± 0.23 | 0.46 ± 0.01 | 0.130 ± 0.003 | 4.15 ± 0.11 |
| 9 | 16297 | Tendon | W | 152 | 84 | 1983 | 118.28 ± 0.34 | 1987 | −14.78 ± 0.22 | 11.92 ± 0.16 | 0.44 ± 0.06 | 0.162 ± 0.025 | 3.19 ± 0.67 |
| 15 | 16302 | Tendon | M | 169 | 66 | 1983 | 115.95 ± 0.33 | 1989 | −15.16 ± 0.15 | 10.97 ± 0.12 | 0.45 ± 0.05 | 0.156 ± 0.019 | 3.39 ± 0.54 |
Bomb-pulse calibration year represents the year or years (after donor birth) at which the atmospheric level corresponded to the measured 14C concentration (pMC) of the tissue sample. For most donors born before or in the beginning of the bomb-pulse peak, 2 possible corresponding years are given, which represent the ascending and descending part of the bomb-pulse curve. Samples 25, 28, and 30 contained levels of 14C that do not correspond to atmospheric levels at any time after the annotated birth years of the donors, and thus these samples are clear outliers (OL). Tissue δ13C values are discussed in Supplemental Data.
Second analysis.
Sample preparation and isotope analyses
Prior to analysis, ∼0.5 mm of the outer layer of the tendon and muscle samples was discarded to eliminate any risk of contamination with modern carbon. Samples were freeze-dried, and subsamples of a few milligrams were taken for analysis at the AMS 14C Dating Centre, Aarhus University. Samples for accelerator mass spectrometry (AMS) were combusted with CuO in sealed combustion tubes at 950°C, and, due to permanent damage to the Centre's own EN tandem accelerator, the resulting CO2 was submitted for graphitization and AMS analysis at the Accelerator Mass Spectrometry Laboratory, Accium Biosciences (Seattle, WA, USA) under the direction of Ugo Zoppi (17). The radiocarbon dating results are reported according to international convention (18), and 14C content are given as percentage modern carbon (pMC) based on the measured 14C/13C ratio corrected for the natural isotopic fractionation by normalizing the result to the standard δ13C value of −25‰ Vienna Pee Dee Belemnite (VPDB; δ13C calibration standard). Stable isotope values of δ13C, δ15N, carbon and nitrogen fraction (by weight) and carbon/nitrogen (C/N) atomic ratios were measured at the Aarhus AMS Centre by continuous-flow mass spectrometry and are shown for all subjects in Table 1.
RESULTS
The measured concentrations of 14C in tendons from donors who were born during and after the bomb-pulse peak clearly exceeded the level that was present at the time of sampling (Fig. 1 and Table 1). In fact, for the majority of the samples, 14C levels corresponded to the atmospheric levels several decades before sampling, showing that many years had passed with minimal or no tendon tissue renewal (see values for bomb-pulse calibration in Table 1).
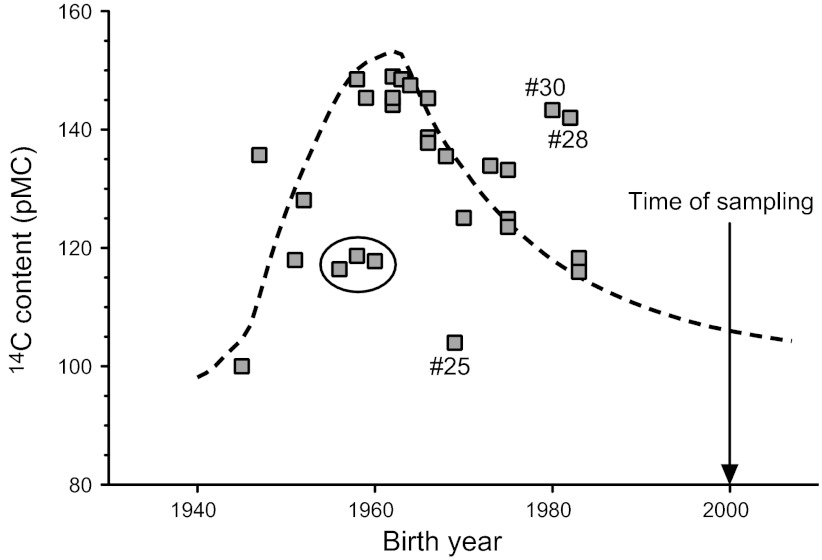
To estimate tissue formation and turnover, it is preferable to study persons born at a time close to the peak of the bomb pulse or later. This period offers a good time resolution because of the high atmospheric 14C level at birth and subsequent gradual decline during the growing years of the donors. The present study included 19 persons born during this period (1962–1985), and the 14C content in their tendons corresponded to the atmospheric level present 8 yr (median) after birth (Fig. 1 and Table 1). Based on this observation, and considering that height growth continues to an average of 17 yr in humans (19), we hypothesized that tendon tissue is formed during the first ca. 17 yr of life and is thereafter practically inert. According to this theory, the tendon tissue would be expected to contain approximately an average of the atmospheric 14C level present during the first 17 yr of life. We have tested this proposed model for tendon formation/turnover by comparing the 14C tendon data to a 0–17 yr moving average of the bomb-pulse curve (Fig. 2). The data fit this curve relatively well and support the hypothesis that human tendons are formed primarily during height growth and that the rate of tissue renewal is very limited thereafter (Fig. 2).
Figure 2.
Model for human tendon tissue formation and turnover. Dotted line shows the 0–17 yr moving average of the 14C level in the atmosphere, representing the relation between calendar year and the average atmospheric 14C level during the subsequent 17 yr (e.g., value in 1960 corresponds to average atmospheric 14C between 1960 and 1977). The curve reproduces relatively well the observed levels of 14C in Achilles tendons samples (gray squares), suggesting that the tendon tissue 14C was incorporated during height growth (0–17 yr) and that tissue replacement during adulthood was very limited. The large black circle indicates three tendon samples with deviating 14C concentrations, potentially due to abnormally high turnover during later periods in life with low atmospheric 14C. The dataset contained three clear outliers, indicated as #25, #28, and #30.
Clearly, in contrast to tendon tissue, samples of skeletal muscle had retained essentially no memory of the bomb pulse, as they contained levels of 14C corresponding to atmospheric levels shortly before death (1998: samples 24, 26, and 28; 1996: sample 25). Thus, these data demonstrate that skeletal muscle is continuously replaced (Fig. 1 and Table 1).
DISCUSSION
The difficulties in resolving tendon overuse injuries (2) may relate to a limited turnover of tendon tissue. However, existing data on tendon tissue turnover rates are highly diverging (3, 5, 6) and no consensus exists with regard to how rapidly tendon tissue is replaced during life. Taking advantage of the bomb-pulse method, which has previously been used to demonstrate extremely low turnover rates of human eye lens (11) and dental enamel (14), we show that human Achilles tendon core tissue is practically inert during adult life.
Tendon tissue turnover and adaptability
Previous reports based on incorporation of stable isotopes in adult human tendon indicate that tendon tissue has a high turnover rate, which is comparable to that of skeletal muscle (5, 6). However, the present data clearly show that rates of tissue turnover are distinctly different in tendon and muscle, and that core tendon tissue is not continuously renewed like muscle. The high rates of tendon protein synthesis, estimated in previous studies using stable isotopes (5, 6), may be explained by the fact that all new synthesis of protein is detected when measuring incorporation of stable isotopes into tissue. Thus, a potentially large production of excess protein, which is broken down relatively quickly and never incorporated into the more permanent tissue structures (i.e., collagen fibrils), will contribute to the measured protein synthesis rate. Thus, previous studies of tendon protein synthesis rates (5, 6) may have given a false impression of rapid tissue replacement. In this context, the 14C bomb-pulse technique has a clear advantage because it allows determination of actual tissue replacement over a long time span. Another potential explanation for at least part of the divergence in estimated tendon turnover could be that heterogeneity in tendon tissue turnover exists between the outer and inner regions of the tendon. Possibly the core tendon tissue, which was measured in the present study, could have a lower turnover than the outer part of the tendon, which was included in samples analyzed in previous stable isotope studies (5, 6). A higher level of adaptability in the tendon periphery is also compatible with observations made in microdialysis studies (4). Here collagen synthesis markers (e.g., carboxy-terminal propeptide of type I procollagen) were measured in the peritendinous tissue (just ventral to the Achilles tendon), and shown to increase in response to loading/training (4, 20). Thus, data from stable isotope and microdialysis studies suggest that human tendon tissue is relatively adaptable, at least in the peripheral region. In further support of some degree of tendon adaptability is the fact that tendon hypertrophy can be induced by long-term training (8, 21). These observations, combined with the present finding of negligible tendon core turnover, could indicate that a large degree of tendon adaptation happens in the outer region of the tendon.
Individual variation in tendon tissue turnover
We found relatively large differences in 14C content between individuals from the same birth year and considering previous observations in bone this is perhaps not surprising (22). Thus, 14C levels measured in femoral bone collagen were highly variable between individuals; this was attributed to biological variation in collagen turnover rates (22). In addition, tendons are likely to have different loading histories during early years, as opposed to more protected tissues, such as the eye lens, that showed a very close association between birth year and 14C content (11). Albeit speculative, loading-induced overuse injury (of which we have no donor history) may also have increased the tendon tissue turnover in some individuals. Even a short period with high turnover during adulthood could markedly influence the 14C level in the tendon tissue at sampling time, and this could be a potential explanation for the deviation of some samples from the 0–17 yr moving average (e.g., 3 samples marked with a circle in Fig. 2). It should be noted that the data set contains 3 clear outliers (samples 25, 28, and 30), which cannot be explained by variation in tendon tissue turnover, since they have 14C contents that are incompatible with the birth year of the donors. Thus, samples 28 and 30 have higher 14C levels than the atmospheric levels at any point in their lifetimes, while sample 25 is lower. Samples 25 and 28 were remeasured, to ensure that the unexpected 14C values were not related to technical errors, and no difference was found in the second measurement (Table 1). Thus, the outliers are not readily explained but could relate to contamination due to, e.g., medical diagnostic use of 14C for donors 28 and 30. Also, erroneous birth year registration at the time of sampling cannot be excluded, and understated biological age could explain the results for all three samples.
Tissue preparation for AMS analysis
It may be speculated that the noncollagen components of tendon tissue have higher turnover than the collagen component and, since the collagen fibrils are the major bearing element of the tendon (23, 24), it would be desirable to separately analyze the 14C content of the collagen fraction. However, to avoid the risk of contamination with modern carbon, we did not attempt to separate collagen from other constituents of the tendon. This decision was based on the following considerations. Collagen constitutes at least 65–80% of tendon dry mass of tendon tissue (25), and the additional components, including sugar-containing proteoglycans, make up a minor part of the tissue (25). A C/N ratio of 3.36 ± 0.14 (mean ± sd) was measured in the Achilles tendon tissue samples (Table 1). Compared to a theoretical C/N ratio of 3.10 for type I and 3.09 for type III collagen (the main collagens of tendon tissue), this indicates a limited presence of components with higher carbon contents, such as sugars and fat, and is in agreement with a high collagen content (calculation of the theoretical C/N ratios for collagens are explained in Supplemental Data). It is important to note that even if a minor portion of the tendon protein has normal turnover, this would have little effect on the 14C readings. For example 10% carbon from a high-turnover pool would only reduce a completely inert collagen 14C content of, e.g., 140 pMC to a reading of ∼137 (140 × 90% + 109 × 10%) due to dilution with carbon from the sampling year 2000 (109 pMC atmospheric value, Fig. 1). So, although most tendon protein does have very low turnover during adult life, it is quite possible (and very likely) that a subpool of the proteins do have a more normal turnover, i.e., proteins within cells and perhaps between the fibrils.
CONCLUSIONS
Despite a relatively large variation between individuals, it is evident that the Achilles tendon tissue samples had retained levels of 14C corresponding to the atmospheric levels several decades before sampling. Based on this observation, it can be concluded that renewal of adult core tendon tissue is extremely limited. The lack of tissue turnover likely explains the relatively poor regenerative capacity of tendon tissue and provides a fundamental basis for future investigation of tendon disease and treatment.
Supplementary Material
Acknowledgments
The authors are thankful for the reliable and prompt service from Dr. Ugo Zoppi (Accium Biosciences, Seattle, WA, USA) with 14C measurements and for help from Patrick Hanson with tissue sampling and Professor Niels Lynnerup with grant applications.
Financial support from the Danish Medical Research Council (11-107595), Danish Rheumatism Association (A1698), Weimann Foundation, Lundbeck Foundation, Novo Nordisk Foundation, Nordea Foundation (Healthy Ageing grant) and the Danish Research Council for Natural Sciences is greatly appreciated.
The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- AMS
- accelerator mass spectrometry
- pMC
- percentage modern carbon
- VPDB
- Vienna Pee Dee Belemnite
REFERENCES
- 1. Kujala U.M., Sarna S., Kaprio J. (2005) Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin. J. Sport Med. 15, 133–135 [DOI] [PubMed] [Google Scholar]
- 2. Riley G. (2008) Tendinopathy–from basic science to treatment. Nat. Clin. Pract. Rheumatol. 4, 82–89 [DOI] [PubMed] [Google Scholar]
- 3. Thorpe C. T., Streeter I., Pinchbeck G. L., Goodship A. E., Clegg P. D., Birch H. L. (2010) Aspartic acid racemization and collagen degradation markers reveal an accumulation of damage in tendon collagen that is enhanced with aging. J. Biol. Chem. 285, 15674–15681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Langberg H., Skovgaard D., Petersen L. J., Bulow J., Kjaer M. (1999) Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J. Physiol. 521, 299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Babraj J. A., Cuthbertson D. J., Smith K., Langberg H., Miller B., Krogsgaard M. R., Kjaer M., Rennie M. J. (2005) Collagen synthesis in human musculoskeletal tissues and skin. Am. J. Physiol. Endocrinol. Metab. 289, E864–E869 [DOI] [PubMed] [Google Scholar]
- 6. Miller B. F., Olesen J. L., Hansen M., Dossing S., Crameri R. M., Welling R. J., Langberg H., Flyvbjerg A., Kjaer M., Babraj J. A., Smith K., Rennie M. J. (2005) Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J. Physiol. 567, 1021–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kongsgaard M., Reitelseder S., Pedersen T. G., Holm L., Aagaard P., Kjaer M., Magnusson S. P. (2007) Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiol. (Oxf.) 191, 111–121 [DOI] [PubMed] [Google Scholar]
- 8. Couppe C., Kongsgaard M., Aagaard P., Hansen P., Bojsen-Moller J., Kjaer M., Magnusson S. P. (2008) Habitual loading results in tendon hypertrophy and increased stiffness of the human patellar tendon. J. Appl. Physiol. 105, 805–810 [DOI] [PubMed] [Google Scholar]
- 9. Heinemeier K. M., Bjerrum S. S., Schjerling P., Kjaer M. (2011) Expression of extracellular matrix components and related growth factors in human tendon and muscle after acute exercise. [E-pub ahead of print] Scand. J. Med. Sci. Sports doi: 10.1111/j.1600-0838.2011.01414.x [DOI] [PubMed] [Google Scholar]
- 10. Sullivan B. E., Carroll C. C., Jemiolo B., Trappe S. W., Magnusson S. P., Dossing S., Kjaer M., Trappe T. A. (2009) Effect of acute resistance exercise and sex on human patellar tendon structural and regulatory mRNA expression. J. Appl. Physiol. 106, 468–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lynnerup N., Kjeldsen H., Heegaard S., Jacobsen C., Heinemeier J. (2008) Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout life. PLoS ONE 3, e1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Libby W. F., Berger R., Mead J. F., Alexander G. V., Ross J. F. (1964) Replacement rates for human tissue from atmospheric radiocarbon. Science 146, 1170–1172 [DOI] [PubMed] [Google Scholar]
- 13. Goodsite M. E., Rom W., Heinemeier J., Lange T., Ooi S., Appleby P. G., Shotyk W., van der Knaap W. O., Lohse C., Hansen T. S. (2001) High-resolution AMS C-14 dating of post-bomb peat archives of atmospheric pollutants. Radiocarbon 43, 495–515 [Google Scholar]
- 14. Spalding K. L., Buchholz B. A., Bergman L. E., Druid H., Frisen J. (2005) Forensics: age written in teeth by nuclear tests. Nature 437, 333–334 [DOI] [PubMed] [Google Scholar]
- 15. Spalding K. L., Bhardwaj R. D., Buchholz B. A., Druid H., Frisen J. (2005) Retrospective birth dating of cells in humans. Cell 122, 133–143 [DOI] [PubMed] [Google Scholar]
- 16. Bergmann O., Liebl J., Bernard S., Alkass K., Yeung M. S., Steier P., Kutschera W., Johnson L., Landen M., Druid H., Spalding K. L., Frisen J. (2012) The age of olfactory bulb neurons in humans. Neuron 74, 634–639 [DOI] [PubMed] [Google Scholar]
- 17. Zoppi U., Crye J., Song Q., Arjomand A. (2007) Performance evaluation of the new ams system at accium biosciences. Radiocarbon 49, 173–182 [Google Scholar]
- 18. Stuiver M., Polach H. A. (1977) Reporting of C-14 data–discussion. Radiocarbon 19, 355–363 [Google Scholar]
- 19. Department of Health and Human Services. (2000) 2000 CDC growth charts for the United States: methods and development. Vital. Health Stat. 11, 1–178 [PubMed] [Google Scholar]
- 20. Langberg H., Rosendal L., Kjaer M. (2001) Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J. Physiol. 534, 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosager S., Aagaard P., Dyhre-Poulsen P., Neergaard K., Kjaer M., Magnusson S. P. (2002) Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand. J. Med. Sci. Sports 12, 90–98 [DOI] [PubMed] [Google Scholar]
- 22. Hedges R. E., Clement J. G., Thomas C. D., O'Connell T. C. (2007) Collagen turnover in the adult femoral mid-shaft: modeled from anthropogenic radiocarbon tracer measurements. Am. J. Phys. Anthropol. 133, 808–816 [DOI] [PubMed] [Google Scholar]
- 23. Svensson R. B., Hassenkam T., Hansen P., Kjaer M., Magnusson S. P. (2011) Tensile force transmission in human patellar tendon fascicles is not mediated by glycosaminoglycans. Connect Tissue Res. 52, 415–421 [DOI] [PubMed] [Google Scholar]
- 24. Provenzano P. P., Vanderby R., Jr. (2006) Collagen fibril morphology and organization: implications for force transmission in ligament and tendon. Matrix Biol. 25, 71–84 [DOI] [PubMed] [Google Scholar]
- 25. Kannus P. (2000) Structure of the tendon connective tissue. Scand. J. Med. Sci. Sports 10, 312–320 [DOI] [PubMed] [Google Scholar]
- 26. Kueppers L. M., Southon J., Baer P., Harte J. (2004) Dead wood biomass and turnover time, measured by radiocarbon, along a subalpine elevation gradient. Oecologia 141, 641–651 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.