Abstract
Mammalian target of rapamycin (mTOR) is a major regulator of cellular metabolism, proliferation, and survival that is implicated in various proliferative and metabolic diseases, including obesity, type 2 diabetes, hamartoma syndromes, and cancer. Emerging evidence suggests a potential critical role of mTOR signaling in pulmonary vascular remodeling. Remodeling of small pulmonary arteries due to increased proliferation, resistance to apoptosis, and altered metabolism of cells forming the pulmonary vascular wall is a key currently irreversible pathological feature of pulmonary hypertension, a progressive pulmonary vascular disorder with high morbidity and mortality. In addition to rare familial and idiopathic forms, pulmonary hypertension is also a life-threatening complication of several lung diseases associated with hypoxia. This review aims to summarize our current knowledge and recent advances in understanding the role of the mTOR pathway in pulmonary vascular remodeling, with a specific focus on the hypoxia component, a confirmed shared trigger of pulmonary hypertension in lung diseases. We also discuss the emerging role of mTOR as a promising therapeutic target and mTOR inhibitors as potential pharmacological approaches to treat pulmonary vascular remodeling in pulmonary hypertension.—Goncharova, E. A. mTOR and vascular remodeling in lung diseases: current challenges and therapeutic prospects.
Keywords: pulmonary hypertension, mTORC1, mTORC2, rapamycin
Mammalian target of rapamycin (mTOR) is a major integrator of environmental cues that controls cellular metabolism, growth, proliferation, and survival depending on mitogenic signals and nutrient and energy availability. It has now become clear that mTOR signaling plays a central role in regulating basic aspects of cell and organism behavior, and its deregulation is strongly associated with progression of numerous human proliferative and metabolic diseases, including cancer, obesity, type 2 diabetes, and hamartoma syndromes (1). In the past decade, mTOR has attracted broad scientific and clinical interest as a potential therapeutic target to treat diseases associated with proliferative and metabolic abnormalities. Emerging evidence suggests potential involvement of mTOR signaling in pulmonary vascular remodeling, a key pathological feature of pulmonary hypertension (PH), a progressive pulmonary vascular disorder with high morbidity and mortality. Our current knowledge about the role of mTOR in pulmonary vascular remodeling, however, is limited, and the mechanisms of activation and functions of mTOR in pulmonary vasculature remain to be elucidated.
PH is a panvasculopathy of elastic, muscular, and nonmuscular pulmonary arteries (PAs) and arterioles that manifests by increased right ventricular pressure, resulting in right heart overload, heart failure, and death (2). In addition to relatively rare idiopathic and heritable pulmonary arterial hypertension (PAH), PH is also a common complication of several lung diseases, including chronic obstructive pulmonary disease (COPD) and interstitial lung diseases (ILDs), which is strongly associated with decreased quality of life, increased morbidity, and reduced survival (3–5), and has recently been detected in pulmonary lymphangioleiomyomatosis (LAM) (6). Although the factors responsible for disease initiation may differ among the PH subtypes, all classes of PH share a currently irreversible pathological manifestation, vascular remodeling of small PAs. Pulmonary vascular remodeling is due to abnormal proliferation and resistance to apoptosis of cells forming the vascular wall, including pulmonary arterial vascular smooth muscle cells (PAVSMCs), endothelial cells (ECs), and pulmonary adventitial fibroblasts (7, 8). Recent studies also indicate that impaired oxygen sensing and/or exposure of pulmonary vascular cells to chronic hypoxia trigger a metabolic shift to glycolysis (8–10). Such metabolic abnormalities are required for pulmonary vascular remodeling in experimental PH (11) and have been proposed to play an important role in elevated vascular cell proliferation and survival in human disease (12).
While the role of mTOR in pulmonary vascular remodeling and its attractiveness as a potential molecular target for human PH are not clear, data from cellular and animal models indicate the importance of the mTOR signaling network for proliferation and survival of pulmonary vascular cells, and substantial progress has been made in understanding the role of mTOR in pulmonary vascular remodeling caused by chronic hypoxia, a recognized trigger of PH in lung diseases. This review will provide a brief overview of mTOR signaling, summarize current knowledge and recent advances in our understanding of the role of mTOR in pulmonary vascular remodeling, with a specific focus on the chronic hypoxia component, and discuss the potential role of mTOR signaling as a therapeutic target for PH.
BRIEF OVERVIEW OF mTOR SIGNALING
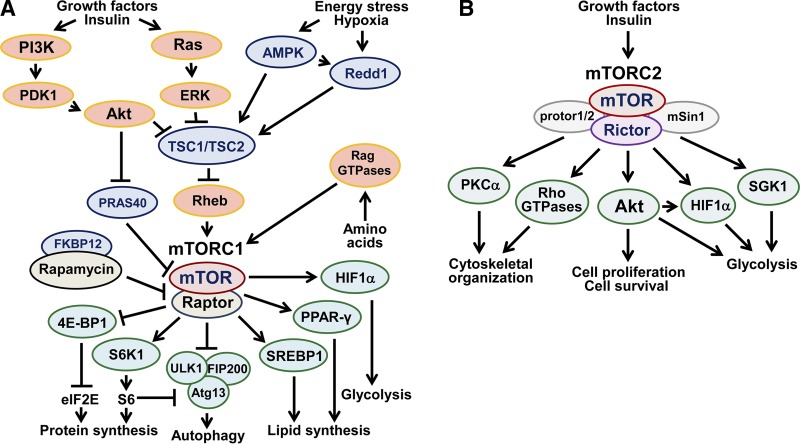
The mTOR is a well-conserved serine/threonine kinase that plays a central role in the signaling network controlling cell metabolism, growth, proliferation, and survival in response to various environmental factors (Fig. 1). mTOR forms a catalytic core of two distinct multiprotein complexes, mTOR complex 1 (mTORC1) and mTORC2. Both complexes share the catalytic subunit mTOR and mLST8/GβL, DEPTOR, and Tti1/Tel2 complex (13–16). mTORC1 and mTORC2 also have complex-specific proteins (raptor and PRAS40 for mTORC1 and rictor, mSin1, and protor1/2 for mTORC2) that regulate assembly, activity, and binding of complexes with specific substrates (reviewed in ref. 1).
Figure 1.
The mTOR signaling pathway. mTORC1 promotes mRNA translation, protein and lipid synthesis, and cell proliferation; up-regulates glycolysis; and inhibits autophagy by integrating growth factor signals, nutrient availability, energy levels, and various stress factors, including hypoxia. Growth factors activate mTORC1 via up-regulating PI3K/PDK1/Thr308-Akt and Ras/ERK1/2 signaling pathways. Akt and ERK, in turn, phosphorylate TSC2, thus inhibiting TSC1/TSC2 GAP activity toward Rheb. In contrast, energy stress, including hypoxia, activates AMPK and Redd1, which promote the assembly and activation of TSC1/TSC2, leading to mTORC1 inhibition. Amino acids activate mTORC1 via Rag GTPase-induced translocation to lysosomes, which enables mTORC1 to interact with Rheb. Activated mTORC1 phosphorylates S6K1 and 4E-BP1, leading to activation of ribosomal protein S6 and eIF2E, mRNA translation, and protein synthesis; stimulates lipid synthesis via activation of SREBP1, PPAR-γ, and C/EBP-α; promotes glycolysis potentially via expression of HIF1α; and inhibits autophagy directly via phosphorylation of ULK1-Atg13-FIP200 complex or indirectly via S6K. mTORC2 is activated by growth factors and insulin in a PI3K-dependent manner, and, in turn, activates Akt by phosphorylation at S473, SGK1, PKCα, and Rho GTPases (39, 40). Akt and SGK1 phosphorylate and inhibit FoxO1 and FoxO3a transcription factors that regulate stress resistance, metabolism, cell-cycle arrest, and apoptosis. mTORC2 also stimulates HIF1α expression and glycolysis by unknown mechanisms and modulates cytoskeletal organization via PKCα, paxillin, and small Rho GTPases.
mTORC1 and mTORC2 have different sensitivity to the antifungal macrolide rapamycin. mTORC1 is acutely rapamycin sensitive. Rapamycin forms a complex with intracellular 12-kDa FK506-binding protein (FKBP12) that specifically binds and inhibits mTORC1 via allosteric inhibition of mTOR kinase activity or complex destabilization (17–19). In contrast, mTORC2 is rapamycin resistant in a majority of cells or may be activated by rapamycin via inhibition of the mTORC1-dependent negative feedback loop to phosphatidylinositol 3 kinase (PI3K) (discussed below). However, prolonged (>6 h) rapamycin treatment inhibits the assembly of mTORC2, leading to a reduction of mTORC2 levels and down-regulation of mTORC2 signaling pathway in certain types of cancer cells, as well as in human aortic ECs and human umbilical vein ECs (HUVECs) (20–22), suggesting that sensitivity of mTORC2 to rapamycin is highly cell type specific.
mTORC1
The mTORC1 pathway accumulates multiple signaling inputs, including growth factor, energy, nutrient, and oxygen availability, and, in turn, regulates protein and lipid synthesis, cell growth, proliferation, and autophagy (Fig. 1A). Activation of mTORC1 requires its interaction with the GTP-bound form of small GTPase Ras homologue enriched in brain (Rheb). A major upstream negative regulator of mTORC1 is tuberous sclerosis complex 1 (TSC1)/TSC2, which acts as the GTPase-activating protein (GAP) for Rheb GTPase and inhibits mTORC1 activity by converting Rheb in an inactive GDP-bound form (23–24). Growth factors induce activation of mTORC1 via up-regulating PI3K-Akt and Ras/extracellular signal-regulated kinase (ERK)/ribosomal S6 kinase (Rsk) signaling pathways that suppress TSC1/TSC2 activity (25, 26). Akt also promotes mTORC1 activation by phosphorylation of PRAS40, a negative regulator of mTORC1. Amino acids activate mTORC1 in a TSC1/TSC2-independent manner through the Rag GTPases, which promote mTORC1 translocation to lysosomal membranes that allows its binding with Rheb (27–28). Energy stress, including hypoxia, inhibits mTORC1 activity via energy sensor AMP-activated protein kinase (AMPK) or AMPK-dependent hypoxic factor protein regulated in development and DNA damage response 1 (Redd1), which phosphorylate TSC2 and increase TSC1/TSC2-dependent mTORC1 inhibition (refs. 29–31 and Fig. 1A). In contrast, several types of cells forming pulmonary vascular wall respond to chronic hypoxic stress by activation of mTORC1 signaling and increase of proliferation by the mechanisms that are currently not well defined. The potential signaling events regulating such a unique response will be discussed below.
mTORC1 is a major activator of cell growth and proliferation. When activated, mTORC1 phosphorylates p70 S6 kinase 1 (S6K1) and 4E-binding protein 1 (4E-BP1) leading to activation of ribosomal protein S6 and eIF2E, protein synthesis, and cell proliferation (25, 32). Recent studies demonstrate that mTORC1 also induces lipid biogenesis by at least two different mechanisms. mTORC1 stimulates activation and nuclear translocation of transcription factor sterol regulatory element binding protein 1 (SREBP1), a positive regulator of the expression of genes required for cholesterol, fatty acid, triglyceride, and phospholipid synthesis. In adipose tissues, mTORC1 promotes adipogenesis via stimulating translation of mRNA encoding peroxisome proliferator-activated receptor-γ (PPAR-γ) and CCAAT/enhancer-binding protein-α (C/EBP-α), key components of the adipogenic program (reviewed in ref. 33). Further, mTORC1 contributes to expression of hypoxia-inducible factor 1 α (HIF1α), a confirmed activator of glycolysis (34–35), and rapamycin inhibits glycolysis in cells with disrupted TSC1/TSC2 complex (36), suggesting the role for mTORC1 as a positive regulator of glycolytic metabolism. In addition to promoting anabolic processes, mTORC1 is also shown to reduce catabolic pathways. mTORC1 suppresses autophagy directly through the phosphorylation of the Unc-51-like kinase 1 (ULK1)–autophagy-related gene 13 (Atg13)–focal adhesion kinase family interacting protein of 200 kDa (FIP200) complex or indirectly via S6K (reviewed in ref. 37; see Fig. 1A).
Interestingly, sustained mTORC1 activation blocks growth factor signaling via negative feedback loops that inhibit PI3K and Ras-ERK pathways (1, 38) that may serve as an additional cell-protective mechanism to balance growth factors-induced synthetic activity with available energy supply.
mTORC2
The knowledge about mTORC2 activation and functions is still very limited. mTORC2 is activated by growth factors and insulin and regulates cell survival, metabolism, proliferation, and cytoskeletal organization (reviewed in ref. 26).
The mechanisms of mTORC2 activation are not well characterized. Growth factors and insulin activate mTORC2 in a PI3K-dependent manner, but the exact mechanism is not clear. The known downstream effectors of mTORC2 are Akt, serum- and glucocorticoid-inducible kinase 1 (SGK1), protein kinase Cα (PKCα), and Rho GTPases (39–40). mTORC2 phosphorylates and activates Akt through specific phosphorylation at S473 (39).
Akt is also activated by PI3K downstream effector phosphoinositide-dependent kinase 1 (PDK1) via phosphorylation at T308, and phosphorylation on both sites is required for full Akt activation. Emerging evidence suggests that mTORC2 and PDK1 may phosphorylate and up-regulate Akt independently in response to distinct stimuli that lead to regulation of different Akt effectors. For example, findings from our group show that increased proliferation of PAVSMCs under chronic hypoxia requires activation of mTORC2- and mTORC2-specific S473-Akt phosphorylation, but not PDK1-dependent phosphorylation of Akt at T308 (41). Similarly, inhibition of mTORC2-specific S473-Akt phosphorylation in mice blocks phosphorylation of forkhead box protein O1 (FoxO1) and FoxO3a transcription factors that control the expression of genes involved in stress resistance, metabolism, cell-cycle arrest, and apoptosis, without affecting other Akt downstream effectors, TSC2 and glycogen synthase kinase 3 (GSK3) (13, 40). Recent findings clearly indicate involvement of mTORC2 in regulating glucose metabolism, cell proliferation, and apoptosis. mTORC2 is required for proliferation of human cancer cells (42–43) and for TSC2-null (41) and leukemic cell survival (44). mTORC2 is also implicated in the regulation of glycolysis in certain cell types independently of mTORC1. mTORC2, but not mTORC1, stimulates glycolysis in hepatocytes and promotes HIF1α expression and glycolytic shift in renal carcinoma cells (34, 45). Although the mechanisms of mTORC2 action are not well studied, it is highly possible that mTORC2 acts via Akt, a well-established positive regulator of cell metabolism, proliferation, and survival (reviewed in ref. 46), and SGK1 that is highly sensitive to mTORC2 inhibition and shares common FoxO1 and FoxO3a phosphorylation sites with Akt. In addition to an emerging role in glycolytic metabolism, proliferation, and survival, mTORC2 also modulates cytoskeletal organization via PKCα and small Rho GTPases (13, 47). The molecular mechanisms by which mTORC2 regulates these processes remain to be determined.
mTOR IN PULMONARY VASCULAR REMODELING: CURRENT KNOWLEDGE
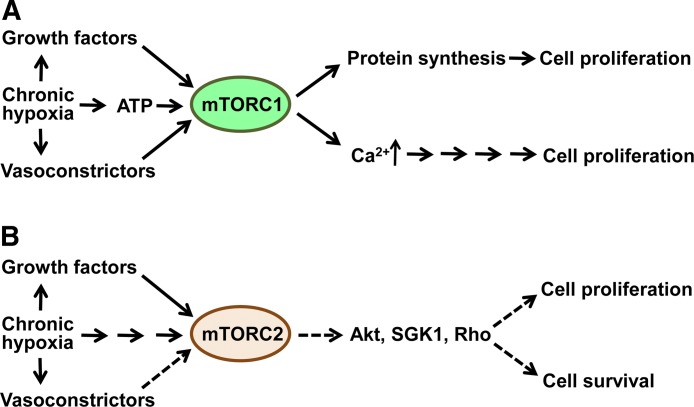
Currently, the role of mTOR in pulmonary vascular remodeling in human PH is not known. Several lines of evidence, however, indicate the importance of mTOR in signaling events regulating proliferation and survival of pulmonary vascular cells and in development of pulmonary vascular remodeling in experimental PH (Fig. 2). To note, most of the existing data came from experimental models based on the use of chronic hypoxia, a recognized trigger of pulmonary vascular remodeling in lung diseases that strongly suggest involvement of mTOR in COPD- and ILD-associated PH. The current knowledge and recent advances in our understanding of the role of mTOR signaling in pulmonary vascular remodeling are summarized below.
Figure 2.
Schematic representation of the roles of mTORC1 and mTORC2 in pulmonary vascular remodeling. A) Increased levels of growth factors and vasoconstrictors and chronic hypoxia activate mTORC1, which, in turn, promotes cell growth and proliferation via S6K1-S6 signaling pathway. mTORC1 may also stimulate proliferation via inducing Ca2+ release (shown only for PAVSMCs). B) Growth factors, vasoconstrictors, and chronic hypoxia activate mTORC2, which stimulates PAVSMC proliferation and resistance to apoptosis by unknown mechanisms.
mTOR in growth factor-induced vascular cell proliferation
Increased levels of growth factors, confirmed activators of mTOR signaling, are detected in different forms of human PH, and their contribution to pulmonary vascular remodeling is well recognized. Basic fibroblast growth factor (FGF) levels are shown to be elevated in plasma and urine from patients with PAH (48); EC-derived FGF2 is overproduced in remodeled vascular endothelium of lungs from patients with idiopathic PAH and stimulates PAVSMC proliferation and pulmonary vascular remodeling in rats (49); and increased expression of vascular endothelial growth factor (VEGF) and VEGF receptor 2 have been detected in ECs in the plexiform lesions of subjects with PAH and in PAs of patients with COPD (50, 51). Transforming growth factor-α (TGF-α), platelet-derived growth factor (PDGF), and epidermal growth factor (EGF) have also been implicated in PH pathogenesis, and inhibition of EGF and PDGF receptors showed beneficial effects on pulmonary vascular remodeling in human and experimental PH (52–53).
We have demonstrated that PDGF- and TGF-α-induced proliferation of human PAVSMCs requires activation of PI3K/mTORC1/S6K1 signaling pathway and is abrogated by mTORC1 inhibitor rapamycin (54). Our findings are in good agreement with recent work from Bentley and colleagues (55) showing requirement of mTORC1 downstream effectors, S6K1 and ribosomal protein S6, for TGF-β-induced human PAVSMC proliferation and suggest an importance of mTORC1 signaling in growth factor-dependent pulmonary vascular remodeling. Given that, in the majority of cells, growth factors also act as activators of mTORC2 signaling pathway, and that known downstream effectors of mTORC2 (i.e., Rho GTPases, Akt, and SGK1) play important role in vasoconstriction, pulmonary vascular remodeling and/or cell survival, analysis of mTORC2 functions in growth factor-dependent pulmonary vascular cell proliferation may highly benefit our knowledge about the mechanisms of pulmonary vascular remodeling in PH.
mTOR and vasoactive agents
Deregulation of vasoactive agents in PAH and in PH associated with lung diseases, which initially has been implicated only in increased vasoconstriction (2, 56), also appeared to promote pulmonary vascular cell proliferation and, as a consequence, is hypothesized to have a role in pulmonary vascular remodeling. Potent vasoconstrictors endothelin-1 (ET-1) and 5-hydroxytryptamine (5-HT), the levels of which are elevated in the lungs of subjects with PH, act as proproliferative agents for PAVSMCs and pulmonary arterial fibroblasts (reviewed in refs. 2, 57). In contrast, known vasodilators, nitric oxide (NO) and prostacyclin, act as antimitogenic factors, and their decrease in human and experimental PH (57) may also have an impact on vascular cell proliferation. Elegant work from Adnot and colleagues (58) demonstrated that 5-HT secreted by ECs from subjects with idiopathic PAH promotes proliferation of nondiseased human PAVSMCs, confirming that endothelial dysfunction in PH may trigger PAVSMC remodeling via secretion of mitogenic stimuli. Two independent groups reported that PAVSMC proliferation induced by 5-HT and ET-1 requires activation of PI3K/mTORC1 signaling and, accordingly, is sensitive to rapamycin and pan-PI3K/mTOR inhibitors (55, 59), which demonstrates the importance of mTORC1 for vasoconstrictor-induced PAVSMC proliferative response. Interestingly, 5-HT also induced phosphorylation of Akt at S473, suggesting potential involvement of mTORC2 in 5-HT-induced proliferation, but the mechanisms of mTORC2 action in PAVSMCs remain to be determined.
mTOR signaling and Ca2+ channels
The Ca2+ channels that had been initially linked to changes in pulmonary vascular tone are also involved in dysregulation of cellular homeostasis and PAVSMC proliferation. Ca2+-activated transient receptor potential (TRPC) 3 and TRPC6 ion channels are up-regulated in PAVSMCs in idiopathic PAH that, at least in part, contributes to increased PAVSMC proliferation and pulmonary vascular medial hypertrophy (60). Interestingly, Yuan and colleagues (61) demonstrated that inhibition of mTORC1 by rapamycin attenuates store-operated Ca2+ entry in PAVSMCs from patients with chronic thromboembolic PH while having a lesser effect on control cells. Similar data were obtained in portal vein VSMCs (62), suggesting that, in addition to regulating protein synthesis, mTORC1 may also promote VSMC proliferation via Ca2+ release.
mTOR and chronic hypoxia
Chronic hypoxia is one of the recognized triggers of PH associated with lung diseases and is also implicated as a secondary factor in the pathogenesis of idiopathic and heritable PAH (63). Chronic hypoxia is an energy stress condition that increases intracellular AMP/ATP ratio and induces activation of AMPK. In the majority of adult differentiated cells, including human airway SMCs and lung fibroblasts, chronic hypoxia inhibits cell proliferation via AMPK-dependent suppression of mTORC1 and protein synthesis (41, 64–66). In contrast, chronic hypoxia promotes proliferation of PAVSMCs, ECs, and pulmonary adventitial fibroblasts and induces pulmonary vascular remodeling in animal models (2, 22, 25). While the molecular and cellular mechanisms modulating such a unique response of pulmonary vasculature to chronic hypoxia are not clear, studies from several research groups, including ours, demonstrate the requirement of mTORC1 activation for chronic hypoxia-induced vascular cell proliferation and pulmonary vascular remodeling and suggest potential mechanisms of mTORC1 up-regulation under hypoxic conditions.
It is well accepted that chronic hypoxia promotes pulmonary vascular remodeling via modulating behavior of pulmonary vascular ECs. Early in vitro observations showed that production of PDGF and ET-1 is increased in chronic hypoxia-exposed umbilical vein ECs (67, 68). These data, coupled with findings that PAVSMCs under chronic hypoxia have increased sensitivity to certain mitogens (69), led to the hypothesis that chronic hypoxia induces the release of promitogenic factors from ECs, leading to a pathological PAVSMC proliferative response. Later studies showed that chronic hypoxia induces changes in EC plasma membrane composition, increases permeability, and stimulates expression of inflammatory cell markers and production of matrix proteins that may stimulate proliferation of underlying PAVSMCs and adventitial fibroblasts (reviewed in refs. 70, 71).
Recent studies of Stenmark et al. (72) demonstrated that chronic hypoxia may also stimulate pulmonary vascular remodeling via activation of adventitial fibroblasts. The researchers found that adventitial fibroblasts under chronic hypoxia have increased proliferation, motility, and expression of proinflammatory cytokines, chemokines, and adhesion molecules that, together with recruitment of leukocytes and progenitor cells in adventitia, may contribute to an increase in myofibroblast mass and PAVSMC and EC proliferation (reviewed in ref. 72).
It has also been shown that chronic hypoxia can directly promote proliferation of PAVSMCs via several different mechanisms, such as stimulation of bFGF release (73), increase of the cytoplasmic Ca2+ levels via up-regulating store- and receptor-operated Ca2+ channels, and/or RhoA GTPase-dependent Ca2+ sensitization (74, 75) and PAVSMC dedifferentiation (76).
Notably, studies from several independent laboratories demonstrate that chronic hypoxia-induced proliferation requires activation of the mTORC1 signaling pathway (Fig. 3A). We (41) and the Battegay group (77) reported that chronic hypoxia-induced proliferation of human PAVSMCs and rat aortic SMCs requires persistent activation of the mTORC1 and showed the antiproliferative effects of the mTORC1 inhibitor rapamycin on VSMC proliferation under hypoxia. Stenmark and colleagues (78) detected transient up-regulation of PI3K activity and mTORC1 signaling in bovine PA adventitial fibroblasts at the early stages of hypoxia exposure and provided evidence that the pan-PIK/mTOR kinase inhibitor LY294002 and the mTORC1 inhibitor rapamycin reduce chronic hypoxia-induced adventitial fibroblast proliferation. Last, Humar and colleagues (22, 77) demonstrated the contribution of mTORC1 to rat aortic EC proliferation caused by hypoxia. These in vitro data are well supported by preclinical pharmacological studies on chronic hypoxia animal models showing that systemic administration of rapamycin completely blocks chronic hypoxia-induced proliferative activity in small PAs and attenuates neomuscularization of distal PAs in mice (79). Further, the antidiabetic agent metformin, which inhibits mTORC1 directly or via activation of AMPK (27), also reduced pulmonary arterial cell proliferation, improved endothelial function, and prevented pulmonary vascular remodeling and PH in chronic hypoxia-exposed rats (80).
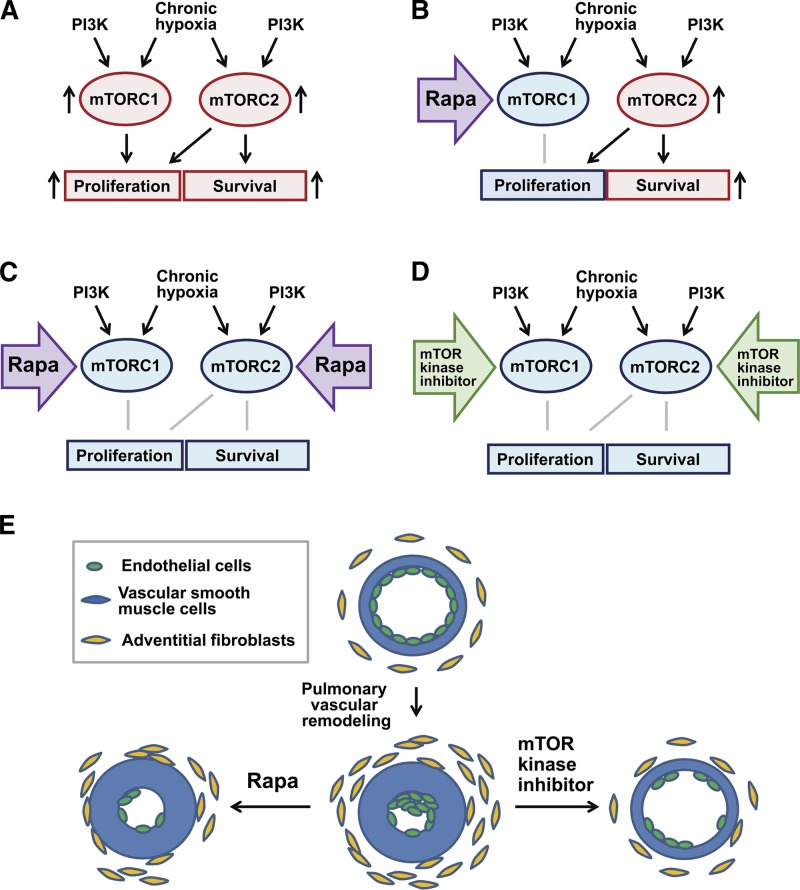
Figure 3.
Potential effects of therapeutic inhibition of mTOR signaling on pulmonary vascular remodeling. A) mTOR signaling in the absence of inhibitors. B, C) Therapeutic inhibition of mTOR by prolonged rapamycin treatment of cells with rapamycin (rapa)-resistant (B) and rapamycin-sensitive mTORC2 (C). D) Therapeutic inhibition of mTOR by mTOR kinase inhibitors. E) Potential effects of prolonged rapamycin treatment and mTOR kinase inhibitors on existing pulmonary vascular remodeling.
The mechanisms of mTORC1 activation under chronic hypoxia are not well understood. A variety of mitogenic stimuli activate PI3K/PDK1/T308-Akt and Ras-ERK/Rsk signaling pathways that inhibit TSC1/TSC2 and suppress or counterbalance AMPK activity allowing activation of mTORC1 and protein synthesis (reviewed in refs. 1, 25), and it is logical to assume that chronic hypoxia activates mTORC1 via PI3K and Ras signaling cascades. Recent findings from our group, however, show that sustained mTORC1 activation under chronic hypoxia cannot be fully explained by increased mitogen levels. We found that PAVSMCs exposed to chronic hypoxia in vivo and in vitro have elevated proliferation and increased mTORC1 activity without mitogenic stimuli and/or PI3K and ERK activation (41), suggesting alternative mechanisms of mTORC1 up-regulation by hypoxia. Studies from the Archer laboratory (81) demonstrated that hypoxia deregulates mitochondrial function that triggers the metabolic shift to glycolysis in PAVSMCs, and our unpublished data show that PAVSMCs exposed to chronic hypoxia have increased cellular energy levels due to glycolytic metabolism. mTORC1 is a homeostatic energy sensor and may be activated by ATP directly or via down-regulation of AMPK in a PI3K- and ERK1/2-independent manner (82). Taken together, published studies and our unpublished observations suggest that mTORC1 activation and increased vascular cell proliferation under chronic hypoxia may be explained, at least in part, by elevated cellular energy levels due to the metabolic shift to glycolytic energy generation.
Compared to current knowledge about mTORC1, our understanding of the role of mTORC2 signaling in chronic hypoxia-induced pulmonary vascular remodeling is very limited and based mostly on indirect evidence. Studies performed by our group revealed that mTORC2 signaling is up-regulated in chronic hypoxia-exposed PAVSMCs and is required for hypoxia-induced PAVSMC proliferation (41). Elevation of mTORC2-specific S473-Akt phosphorylation under chronic hypoxia has also been observed in bovine PA adventitial fibroblasts and rat aortic ECs (22, 78). The potential involvement of mTORC2 in chronic hypoxia-induced pulmonary vascular remodeling is also supported by the fact that the confirmed downstream effector of mTORC2, RhoA GTPase, is playing an important role in chronic hypoxia-induced vasoconstriction and pulmonary vascular remodeling, and RhoA/Rho kinase signaling is considered a potential therapeutic target for COPD-associated PH (83–84). Collectively, these data suggest the potential importance of mTORC2 signaling for chronic hypoxia-induced vascular cell proliferation and pulmonary vascular remodeling. More studies, however, are needed to determine the mechanisms of mTORC2 regulation and functions in pulmonary vasculature under hypoxia exposure (Fig. 3A).
mTOR and apoptosis
In addition to increased proliferation, pulmonary vascular cells in PH have resistance to apoptosis that is also considered as an important pathological component of pulmonary vascular remodeling (7, 8). Reduced apoptosis has been detected in PAVSMCs and ECs from subjects with idiopathic PAH (8) and is implicated in the development and maintenance of severe PH (85). Currently, pharmacological agents that simultaneously reduce proliferation and promote apoptosis in pulmonary vasculature are considered potentially attractive therapeutic approaches for human PH.
The studies with the mTORC1 inhibitor rapamycin have demonstrated that inhibition of mTORC1 in experimental PH models reduces PAVSMC proliferation but does not promote apoptosis. Treatment with rapamycin, while significantly inhibiting VSMC proliferation and preventing pulmonary vascular remodeling, failed to reverse existing PH in rats and had antiapoptotic effects on VSMCs in a rat carotid model of vascular injury (86, 87). Consistent with this study, our unpublished observations show that either molecular or pharmacological inhibition of mTORC1, while suppressing proliferation, does not promote apoptosis in PAVSMCs from chronic hypoxia-exposed rats and from subjects with idiopathic PAH, suggesting that mTORC1 is critical for PAVSMC proliferation, but not survival.
Although the role of mTORC2 in regulating pulmonary vascular cell survival in pulmonary hypertensive conditions is not defined, unpublished observations from our group strongly suggest a critical role for mTORC2 in maintenance of the apoptosis-resistant PAVSMC phenotype in human and experimental hypoxia PH. We found that the mTORC2 signaling pathway is activated in small remodeled PAs and in subcultured PAVSMCs from idiopathic PAH lungs and that inhibition of mTORC2 promotes marked apoptosis in idiopathic PAH PAVSMCs. Notably, similar data were obtained on PAVSMCs from rats with chronic hypoxia-induced PH, suggesting importance of mTORC2 in PAVSMC survival under chronic hypoxia. While no data exist about the role of mTORC2 in pulmonary vascular EC survival in human or experimental PH, inhibition of mTORC2-Akt signaling by prolonged rapamycin treatment leads to significant apoptosis and necrosis of nontransformed HUVECs and primary aortic ECs (20–22). Of note, confirmed downstream effectors of mTORC2, Akt and Rho GTPases, act as prosurvival molecules in a variety of human cancers (46, 88), further supporting involvement of mTORC2 signaling in regulating cell survival in diseased conditions.
mTOR IN PULMONARY VASCULAR REMODELING: AVENUES TO EXPLORE
Despite the lack of knowledge about the role of mTOR in pulmonary vascular remodeling in humans, emerging evidence suggests that the mTOR signaling network plays an important role in a number of pathological processes that are critical for development of pulmonary vascular remodeling in PAH and PH associated with lung diseases, such as pulmonary vascular cell proliferation induced by growth factors, vasoconstrictors, and chronic hypoxia; deregulation of Ca2+ levels; and PAVSMC survival. The role of mTOR in other increasingly recognized pathogenic components of pulmonary vascular remodeling, including inflammatory processes, vascular cell metabolism, and autophagy (2), however, remains to be established.
Compelling evidence demonstrates that inflammation plays an important role in the pathogenesis of idiopathic PAH, COPD- and ILD-associated PH, and PH related to connective tissue diseases, human immunodeficiency virus, and other viral etiologies. Recruitment of inflammatory cells to PAs and its involvement in increased PAVSMC and EC proliferation is well documented in human and experimental PH (89). There is no existing evidence linking mTOR and PH-related inflammation. It is important to consider, however, that mTORC1 acts as an anti-inflammatory agent and immunoregulator in many pathological conditions and that the mTORC1 inhibitor rapamycin (sirolimus) is approved by the U.S. Food and Drug Administration (FDA) as an immunosuppressor drug for the prevention of kidney allograft transplant rejection (90), suggesting a potential role for mTORC1 signaling in the regulation of the inflammatory component of pulmonary vascular remodeling.
Recent studies strongly suggest that alterations in energy metabolism of pulmonary vascular cells play a critical role in pulmonary vascular remodeling. PAVSMCs and ECs in idiopathic PAH have alterations in mitochondrial function leading to impaired oxygen sensing and the metabolic shift to glycolysis (8) that may underlie the resistance to apoptosis and increased vascular cell proliferation (12). Notably, similar metabolic changes have been detected in experimental hypoxia models of PH (81), and our unpublished data support the requirement of the metabolic glycolytic shift to increased proliferation and survival of chronic hypoxia-exposed human PAVSMCs. Recent work from the Michelakis laboratory (11) indicated that alterations in lipid metabolism also affect glycolytic metabolic shift and pulmonary vascular remodeling in mice. The similarities of metabolic abnormalities found in pulmonary vascular cells in human and experimental hypoxic PH with those detected in human cancers (the Warburg effect) suggest that regulatory mechanisms may be shared. Given the function of mTOR as a key regulator of glycolytic and lipid metabolism and its confirmed involvement in human cancer pathogenesis (1), evaluating the role of mTOR signaling in altered metabolism of vascular cells should be considered.
Another pathological condition that may be shared between cancer and pulmonary vascular cells in PH is deregulated autophagy. Autophagy is a tightly regulated pathway involving the lysosomal degradation of cytoplasmic organelles or cytosolic components that provides metabolic adaptation to multiple forms of cellular stress, including hypoxia, deregulated reactive oxygen species (ROS) generation, inflammation, and endothelial damage. Autophagy may be important in the pathogenesis of cancer by allowing prolonged survival of tumor cells with defects in apoptosis, providing a protective function to limit tumor necrosis and inflammation, and mitigating genome damage in tumor cells in response to metabolic stress (91). Although the functional significance of autophagy in human PH remains unclear, recent studies suggest that, similar to cancer cells, autophagy may represent an adaptive mechanism of the pulmonary vasculature during disease pathogenesis. The activation of autophagic markers in remodeled PAs has been observed in human and experimental PH and appears to contribute to increased vascular cell proliferation caused by chronic hypoxia (92). Paradoxically, mTORC1, a confirmed positive regulator of vascular cell proliferation in experimental hypoxia models of PH, acts as an inhibitor of autophagy in many cell types, and inhibition of mTORC1 by rapamycin suppresses cell proliferation while inducing an autophagic response (1). Such controversies give rise to the possibility of alternative mechanisms of mTORC1-dependent regulation of autophagy in pulmonary hypertensive conditions, the understanding of which may improve our knowledge of the role of autophagy in PH pathogenesis.
TARGETING mTOR SIGNALING: PROMISING THERAPEUTIC STRATEGY TO TREAT PULMONARY VASCULAR REMODELING?
Because of its central role in regulating cellular metabolism, proliferation, and survival, mTOR signaling is considered an attractive therapeutic target for a variety of diseases associated with proliferative and metabolic abnormalities. Given that increased proliferation, resistance to apoptosis, and deregulated metabolism are three major pathological manifestations of vascular remodeling in idiopathic PAH and chronic hypoxia-induced PH, targeting mTOR signaling may represent a potential therapeutic strategy for human PH associated with severe pulmonary vascular remodeling.
Currently, the only FDA-approved mTOR inhibitors are rapamycin and its analogs that inhibit predominantly mTORC1 (Fig. 3B). Rapamycin is in clinical use as an immunosuppressor to prevent transplant rejection and as an antiproliferative agent applied to coronary stents to reduce local restenosis (93), which allows a potential for the rapid translation to human PH. Rapamycin inhibits proliferation of all three types of PA vascular cells via suppression of mTORC1. It is also shown that prolonged (≥24 h) rapamycin treatment induces apoptosis in two different types of primary human ECs, HUVECs and aortic ECs, via inhibition of mTORC2 (20–22) that allows proposing a potential role of rapamycin as a proapoptotic agent for human pulmonary vascular ECs (Fig. 3C). Preclinical studies and our pilot data demonstrate, however, that either acute or chronic rapamycin treatment, while having strong antiproliferative properties and preventing the development of pulmonary vascular remodeling, is not sufficient to induce apoptosis in PAVSMCs from subjects with idiopathic PAH and in chronic hypoxia-exposed normal human PAVSMCs, failed to reverse established pulmonary vascular remodeling, and improves VSMC survival in a rat carotid model of vascular injury (41, 86, 87), suggesting that in VSMC rapamycin acts predominantly as cytostatic agent (Fig. 3B).
Whether prolonged rapamycin treatment induces apoptosis in pulmonary microvascular ECs in human PAH is not yet determined, and whether selective induction of apoptosis in ECs, but not PAVSMCs, would benefit pulmonary vascular function in PAH is not clear. At the early stages of pulmonary vascular remodeling, various factors, including shear stress, ROS, toxins, bone morphogenic protein receptor II dysfunction, and deregulated autoimmune response, promote apoptosis or reprogramming of normal pulmonary microvascular ECs, leading to development of phenotypically altered hyperproliferative apoptosis-resistant ECs (reviewed in ref. 94), in which sensitivity to proapoptotic rapamycin action may be diminished compared to nondiseased cells. If sensitive to prolonged rapamycin treatment, rapamycin-induced EC apoptosis coupled with suppression of proliferation may decrease volume and/or number of EC-containing plexiform lesions, as well as EC-dependent secretion of FGF2 and VEGF, reduce PA lumen obliteration, and therefore partially improve pulmonary vascular circulation. It should be considered, however, that rapamycin plays cytoprotective role in SMCs and that release of mitogenic stimuli from apoptotic ECs (94) may further augment survival and migratory potential of underlying PAVSMCs (and potentially adventitial fibroblasts) via activation of rapamycin-insensitive PI3K and Ras-ERK1/2 signaling pathways (25, 54, 94) resulting in maintenance of medial and, at least in part, intimal PA thickening (Fig. 3E). In either scenario, the combination of rapamycin with additional proapoptotic drugs might be beneficial to induce apoptosis in all three types of pulmonary vascular cells, including ECs, PAVSMCs, and adventitial fibroblasts and attenuate and/or reverse pulmonary vascular remodeling in PAH.
Although the role of mTORC2 in regulating PAVSMC survival is not well known, our unpublished observations show that inhibition of mTORC2 signaling not only suppresses proliferation, but also promotes marked apoptosis in PAVSMCs exposed to chronic hypoxia in vitro, as well as in PAVSMCs from subjects with idiopathic PAH and chronic hypoxia-maintained rats. Coupled with prosurvival function of mTORC2 in ECs, these data suggest the potential attractiveness of mTORC2 as a molecular target to attenuate and/or reverse pulmonary vascular remodeling in idiopathic and hypoxia-related PH. Currently, there are no pharmacological agents available to selectively target rapamycin-resistant mTORC2. Recently, however, small-molecule ATP-competitive inhibitors were generated that directly block mTOR catalytic activity and inhibit both mTORC1 and mTORC2 signaling pathways, allowing simultaneous inhibition of proliferation and induction of apoptosis in cells with either rapamycin-sensitive or rapamycin-resistant mTORC2 (Fig. 3D, E). Indeed, testing of mTOR kinase inhibitors PP242 and AZD8055 on several animal models of cancer demonstrated an improved therapeutic response compared to rapamycin (95–97) that is in good agreement with our pilot studies on PAVSMCs from rats with chronic hypoxia-induced pulmonary vascular remodeling and from subjects with idiopathic PAH. Another class of novel mTOR catalytic inhibitors that may be considered a treatment option for PH is dual PI3K/mTOR kinase inhibitors that have already demonstrated promising results in mouse and rat cancer models (98–100). PI3K/mTOR kinase inhibitors suppress mTORC1, mTORC2, and PI3K activities, which may be beneficial to account for PI3K-specific mTOR-independent outcomes of mitogenic stimuli. The mTOR kinase inhibitors PP242 and AZD8055 and the PI3K/mTOR kinase inhibitor NVP-BEZ235 are currently undergoing phase I clinical trials as anticancer agents and, therefore, may be considered for preclinical testing on animal models as a potential pharmacological approach to inhibit pulmonary vascular cell proliferation, promote apoptosis, and attenuate pulmonary vascular remodeling in PH.
Acknowledgments
The author thanks Dmitry Goncharov (University of Pennsylvania) for helpful discussion and preparation of figures and Mary McNichol (University of Pennsylvania) for critical reading of the manuscript.
This work is supported by U.S. National Institutes of Health grant 1R01HL113178 and American Lung Association grant RG196551 to E.A.G.
Footnotes
- 4E-BP1
- 4E-binding protein 1
- 5-HT
- 5-hydroxytryptamine
- AMPK
- AMP-activated protein kinase
- Atg13
- autophagy-related gene 13
- C/EBP-α
- CCAAT/enhancer-binding protein-α
- COPD
- chronic obstructive pulmonary disease
- EC
- endothelial cell
- EGF
- epidermal growth factor
- ERK
- extracellular-signal regulated kinase
- ET-1
- endothelin-1
- FGF
- fibroblast growth factor
- FIP200
- focal adhesion kinase family interacting protein of 200 kDa
- FKBP12
- 12-kDa FK506-binding protein
- FoxO
- forkhead box protein O
- GAP
- GTPase-activating protein
- HIF1α
- hypoxia-inducible factor α
- HUVEC
- human umbilical vein endothelial cell
- ILD
- interstitial lung disease
- mTOR
- mammalian target of rapamycin
- mTORC1
- mammalian target of rapamycin complex 1
- mTORC2
- mammalian target of rapamycin complex 2
- PA
- pulmonary artery
- PAH
- pulmonary arterial hypertension
- PAVSMC
- pulmonary arterial vascular smooth muscle cell
- PDGF
- platelet-derived growth factor
- PDK1
- phosphoinositide-dependent kinase 1
- PH
- pulmonary hypertension
- PI3K
- phosphatidylinositol 3 kinase
- PKCα
- protein kinase Cα
- PPAR-γ
- peroxisome proliferator-activated receptor-γ
- Redd1
- hypoxic factor protein regulated in development and DNA damage response 1
- Rheb
- GTPase Ras homolog enriched in brain
- Rsk
- ribosomal S6 kinase
- ROS
- reactive oxygen species
- S6K1
- p70 S6 kinase 1
- SGK1
- serum- and glucocorticoid-inducible kinase 1
- SMC
- smooth muscle cell
- SREBP1
- sterol regulatory element binding protein 1
- TGF-α
- transforming-growth factor-α
- TRPC
- transient receptor potential channel
- TSC1
- tuberous sclerosis complex 1
- TSC2
- tuberous sclerosis complex 2
- ULK1
- Unc-51-like kinase 1
- VEGF
- vascular endothelial growth factor
- VSMC
- vascular smooth muscle cell
REFERENCES
- 1. Laplante M., Sabatini D. M. (2012) mTOR signaling in growth control and disease. Cell 149, 274–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morrell N. W., Adnot S., Archer S. L., Dupuis J., Jones P. L., MacLean M. R., McMurtry I. F., Stenmark K. R., Thistlethwaite P. A., Weissmann N., Yuan J. X. J., Weir E. K. (2009) Cellular and molecular basis of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 54, S20–S31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simonneau G., Robbins I. M., Beghetti M., Channick R. N., Delcroix M., Denton C. P., Elliott C. G., Gaine S. P., Gladwin M. T., Jing Z. C., Krowka M. J., Langleben D., Nakanishi N., Souza R. (2009) Updated clinical classification of pulmonary hypertension, J. Am. Coll. Cardiol. 54, S43–S54 [DOI] [PubMed] [Google Scholar]
- 4. Behr J., Ryu J. H. (2008) Pulmonary hypertension in interstitial lung disease. Eur. Respir. J. 31, 1357–1367 [DOI] [PubMed] [Google Scholar]
- 5. Barbera J. A., Peinado V. I., Santos S. (2003) Pulmonary hypertension in chronic obstructive pulmonary disease, Eur. Respir. J. 21, 892–905 [DOI] [PubMed] [Google Scholar]
- 6. Cottin V., Harari S., Humbert M., Mal H., Dorfmüller P., Jaïs X., Reynaud-Gaubert M., Prevot G., Lazor R., Taillé C., Lacronique J., Zeghmar S., Simonneau G., Cordier J.F., and Groupe d'Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM“O”P) (2012) Pulmonary hypertension in lymphangioleiomyomatosis: characteristics in 20 patients. Eur. Respir. J. 40, 630–640 [DOI] [PubMed] [Google Scholar]
- 7. Tuder R. M., Marecki J. C., Richter A., Fijalkowska I., Flores S. (2007) Pathology of pulmonary hypertension. Clin. Chest Med. 28, 23–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Archer S. L., Gomberg-Maitland M., Maitland M. L., Rich S., Garcia J. G. N., Weir E. K. (2008) Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1α-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am. J. Physiol. Heart Circ. Physiol. 294, H570–H578 [DOI] [PubMed] [Google Scholar]
- 9. Xu W., Koeck T., Lara A. R., Neumann D., DiFilippo F. P., Koo M., Janocha A. J., Masri F. A., Arroliga A. C., Jennings C., Dweik R. A., Tuder R. M., Stuehr D. J., Erzurum S. C. (2007) Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 104, 1342–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fijalkowska I., Xu W., Comhair S. A., Janocha A. J., Mavrakis L. A., Krishnamachary B., Zhen L., Mao T., Richter A., Erzurum S. C., Tuder R. M. (2010) Hypoxia-inducible-factor1α regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am. J. Pathol. 176, 1130–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sutendra G., Bonnet S., Rochefort G., Haromy A., Folmes K. D., Lopaschuk G. D., Dyck J. R. B., Michelakis E. D. (2010) Fatty acid oxidation and malonyl-CoA decarboxylase in the vascular remodeling of pulmonary hypertension. Sci. Transl. Med. 2, 44ra58. [DOI] [PubMed] [Google Scholar]
- 12. Tuder R. M., Davis L. A., Graham B. B. (2012) Targeting energetic metabolism. Am. J. Respir. Crit. Care Med. 185, 260–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacinto E., Loewith R., Schmidt A., Lin S., Ruegg M. A., Hall A., Hall M. N. (2004) Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 6, 1122–1128 [DOI] [PubMed] [Google Scholar]
- 14. Kim D. H., Sarbassov D. D., Ali S. M., Latek R. R., Guntur K. V. P., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2003) GβL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol. Cell 11, 895–904 [DOI] [PubMed] [Google Scholar]
- 15. Peterson T. R., Laplante M., Thoreen C. C., Sancak Y., Kang S. A., Kuehl W. M., Gray N. S., Sabatini D. M. (2009) DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 137, 873–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaizuka T., Hara T., Oshiro N., Kikkawa U., Yonezawa K., Takehana K., Iemura S., Natsume T., Mizushima N. (2010) Tti1 and Tel2 are critical factors in mammalian target of rapamycin complex assembly. J. Biol. Chem. 285, 20109–20116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown E. J., Beal P. A., Keith C. T., Chen J., Shin T. B., Schreiber S. L. (1995) Control of p70 S6 kinase by kinase activity of FRAP in vivo. Nature 377, 441–446 [DOI] [PubMed] [Google Scholar]
- 18. Brunn G. J., Hudson C. C., Sekulić A., Williams J. M., Hosoi H., Houghton P. J., Lawrence J.C., Jr., Abraham R. T. (1997) Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science 277, 99–101 [DOI] [PubMed] [Google Scholar]
- 19. Kim D. H., Sarbassov D. D., Ali S. M., King J. E., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175 [DOI] [PubMed] [Google Scholar]
- 20. Sarbassov D. D., Ali S. M., Sengupta S., Sheen J. H., Hsu P. P., Bagley A. F., Markhard A. L., Sabatini D. M. (2006) Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 22, 159–168 [DOI] [PubMed] [Google Scholar]
- 21. Barilli A., Visigalli R., Sala R., Gazzola G. C., Parolari A., Tremoli E., Bonomini S., Simon A., Closs E. I., Dall'Asta V., Bussolati O. (2008) In human endothelial cells rapamycin causes mTORC2 inhibition and impairs cell viability and function. Cardiovasc. Res. 78, 563–571 [DOI] [PubMed] [Google Scholar]
- 22. Li W., Petrimpol M., Molle K. D., Hall M. N., Battegay E. J., Humar R. (2007) Hypoxia-induced endothelial proliferation requires both mTORC1 and mTORC2. Circ. Res. 100, 79–87 [DOI] [PubMed] [Google Scholar]
- 23. Inoki K., Li Y., Xu T., Guan K. L. (2003) Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 17, 1829–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tee A. R., Manning B. D., Roux P. P., Cantley L. C., Blenis J. (2003) Tuberous sclerosis complex gene products, tuberin and hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr. Biol. 13, 1259–1268 [DOI] [PubMed] [Google Scholar]
- 25. Krymskaya V. P., Goncharova E. A. (2009) PI3K/mTORC1 Activation in hamartoma syndromes: therapeutic prospects. Cell Cycle 8, 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laplante M., Sabatini D. M. (2009) mTOR signaling at a glance. J. Cell Sci. 122, 3589–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kalender A., Selvaraj A., Kim S. Y., Gulati P., Brûlé S., Viollet B., Kemp B. E., Bardeesy N., Dennis P. B., Schlager J. J., Marette A., Kozma S. C., Thomas G. (2010) Metformin, independent of AMPK, inhibits mTORC1 in a Rag GTPase-dependent manner. Cell Metab. 11, 390–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sancak Y., Peterson T. R., Shaul Y. D., Lindquist R. A., Thoreen C. C., Bar-Peled L., Sabatini D. M. (2008) The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320, 1496–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shaw R. J. (2009) LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol. 196, 65–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schneider A., Younis R. H., Gutkind J. S. (2008) Hypoxia-induced energy stress inhibits the mTOR pathway by activating an AMPK/REDD1 signaling axis in head and neck squamous cell carcinoma. Neoplasia 10, 1295–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brugarolas J., Lei K., Hurley R. L., Manning B. D., Reiling J. H., Hafen E., Witters L. A., Ellisen L. W., Kaelin W. G., Jr. (2004) Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 18, 2893–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ma X. M., Blenis J. (2009) Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 10, 307–318 [DOI] [PubMed] [Google Scholar]
- 33. Laplante M., Sabatini D. M. (2009) An emerging role of mTOR in lipid biosynthesis. Curr. Biol. 19, R1046–R1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Toschi A., Lee E., Gadir N., Ohh M., Foster D. A. (2008) Differential dependence of hypoxia-inducible factors 1α and 2α on mTORC1 and mTORC2. J. Biol. Chem. 283, 34495–34499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marin-Hernandez A., Gallardo-Perez J. C., Ralph S. J., Rodriguez-Enriquez S., Moreno-Sanchez R. (2009) HIF-1α modulates energy metabolism in cancer cells by inducing overexpression of specific glycolytic isoforms. Mini Rev. Med. Chem. 9, 1084–1101 [DOI] [PubMed] [Google Scholar]
- 36. Düvel K., Yecies J. L., Menon S., Raman P., Lipovsky A. I., Souza A. L., Triantafellow E., Ma Q., Gorski R., Cleaver S., Vander Heiden M. G., MacKeigan J. P., Finan P. M., Clish C. B., Murphy L. O., Manning B. D. (2010) Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 39, 171–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hands S. L., Proud C. G., Wyttenbach A. (2009) mTOR's role in ageing: protein synthesis or autophagy? Aging 1, 586–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carracedo A., Ma L., Teruya-Feldstein J., Rojo F., Salmena L., Alimonti A., Egia A., Sasaki A. T., Thomas G., Kozma S. C., Papa A., Nardella C., Cantley L. C., Baselga J., Pandolfi P. P. (2008) Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J. Clin. Invest. 118, 3065–3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Phosphorylation and regulation of Akt/PKB by the Rictor-mTOR complex. Science 307, 1098–1101 [DOI] [PubMed] [Google Scholar]
- 40. Guertin D. A., Stevens D. M., Thoreen C. C., Burds A. A., Kalaany N. Y., Moffat J., Brown M., Fitzgerald K. J., Sabatini D. M. (2006) Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but Not S6K1. Dev. Cell 11, 859–871 [DOI] [PubMed] [Google Scholar]
- 41. Krymskaya V. P., Snow J., Cesarone G., Khavin I., Goncharov D. A., Lim P. N., Veasey S. C., Ihida-Stansbury K., Jones P. L., Goncharova E. A. (2011) mTOR is required for pulmonary arterial vascular smooth muscle cell proliferation under chronic hypoxia. FASEB J. 25, 1922–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roulin D., Cerantola Y., Dormond-Meuwly A., Demartines N., Dormond O. (2010) Targeting mTORC2 inhibits colon cancer cell proliferation in vitro and tumor formation in vivo. Mol. Cancer 9, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gulati N., Karsy M., Albert L., Murali R., Jhanwar-Uniyal M. (2009) Involvement of mTORC1 and mTORC2 in regulation of glioblastoma multiforme growth and motility. Int. J. Oncol. 35, 731–740 [DOI] [PubMed] [Google Scholar]
- 44. Carayol N., Vakana E., Sassano A., Kaur S., Goussetis D. J., Glaser H., Druker B. J., Donato N. J., Altman J. K., Barr S., Platanias L. C. (2010) Critical roles for mTORC2- and rapamycin-insensitive mTORC1-complexes in growth and survival of BCR-ABL-expressing leukemic cells. Proc. Natl. Acad. Sci. U. S. A. 107, 12469–12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hsu P. P., Sabatini D. M. (2008) Cancer cell metabolism: Warburg and beyond. Cell 134, 703–707 [DOI] [PubMed] [Google Scholar]
- 46. Manning B. D., Cantley L. C. (2007) AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sarbassov D. D., Ali S. M., Kim D. H., Guertin D. A., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2004) Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and Raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302 [DOI] [PubMed] [Google Scholar]
- 48. Benisty J. I., McLaughlin V. V., Landzberg M. J., Rich J. D., Newburger J. W., Rich S., Folkman J. (2004) Elevated basic fibroblast growth factor levels in patients with pulmonary arterial hypertension. Chest 126, 1255–1261 [DOI] [PubMed] [Google Scholar]
- 49. Izikki M., Guignabert C., Fadel E., Humbert M., Tu L., Zadigue P., Dartevelle P., Simonneau G., Adnot S., Maitre B., Raffestin B., Eddahibi S. (2009) Endothelial-derived FGF2 contributes to the progression of pulmonary hypertension in humans and rodents. J. Clin. Invest. 119, 512–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Geiger R., Berger R. M. F., Hess J., Bogers A. J. J. C., Sharma H. S., Mooi W. J. (2000) Enhanced expression of vascular endothelial growth factor in pulmonary plexogenic arteriopathy due to congenital heart disease. J. Pathol. 191, 202–207 [DOI] [PubMed] [Google Scholar]
- 51. Tuder R. M., Chacon M., Alger L., Wang J., Taraseviciene-Stewart L., Kasahara Y., Cool C. D., Bishop A. E., Geraci M. W., Semenza G. L., Yacoub M., Polak J. M., Voelkel N. F. (2001) Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J. Pathol. 195, 367–374 [DOI] [PubMed] [Google Scholar]
- 52. Schermuly R. T., Dony E., Ghofrani H. A., Pullamsetti S., Savai R., Roth M., Sydykov A., Lai Y. J., Weissmann N., Seeger W., Grimminger F. (2005) Reversal of experimental pulmonary hypertension by PDGF inhibition. J. Clin. Invest. 115, 2811–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Merklinger S. L., Jones P. L., Martinez E. C., Rabinovitch M. (2005) Epidermal growth factor receptor blockade mediates smooth muscle cell apoptosis and improves survival in rats with pulmonary hypertension. Circulation 112, 423–431 [DOI] [PubMed] [Google Scholar]
- 54. Goncharova E. A., Ammit A. J., Irani C., Carroll R. G., Eszterhas A. J., Panettieri R. A., Krymskaya V. P. (2002) PI3K is required for proliferation and migration of human pulmonary vascular smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 283, L354–L363 [DOI] [PubMed] [Google Scholar]
- 55. Deng H., Hershenson M. B., Lei J., Anyanwu A. C., Pinsky D. J., Bentley J. K. (2010) Pulmonary artery smooth muscle hypertrophy: roles of glycogen synthase kinase-3β and p70 ribosomal S6 kinase, Am. J. Physiol. Lung Cell. Mol. Physiol. 298, L793–L803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hida W., Tun Y. E., Kikuchi Y., Okabe S., Shirato K. (2002) Pulmonary hypertension in patients with chronic obstructive pulmonary disease: recent advances in pathophysiology and management. Respirology 7, 3–13 [DOI] [PubMed] [Google Scholar]
- 57. Wilkins M. R. (2012) Pulmonary hypertension: the science behind the disease spectrum. Eur. Respir. Rev. 21, 19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Eddahibi S., Guignabert C., Barlier-Mur A. M., Dewachter L., Fadel E., Dartevelle P., Humbert M., Simonneau G., Hanoun N., Saurini F., Hamon M., Adnot S. (2006) Cross talk between endothelial and smooth muscle cells in pulmonary hypertension. Circulation 113, 1857–1864 [DOI] [PubMed] [Google Scholar]
- 59. Liu Y., Fanburg B. L. (2006) Serotonin-induced growth of pulmonary artery smooth muscle requires activation of phosphatidylinositol 3-kinase/serine-threonine protein kinase B/mammalian target of rapamycin/p70 ribosomal S6 kinase 1. Am. J. Respir. Cell Mol. Biol. 34, 182–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yu Y., Fantozzi I., Remillard C. V., Landsberg J. W., Kunichika N., Platoshyn O., Tigno D. D., Thistlethwaite P. A., Rubin L. J., Yuan J. X. J. (2004) Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc. Natl. Acad. Sci. U. S. A. 101, 13861–13866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ogawa A., Firth A. L., Yao W., Madani M. M., Kerr K. M., Auger W. R., Jamieson S. W., Thistlethwaite P. A., Yuan J. X. J. (2009) Inhibition of mTOR attenuates store-operated Ca2+ entry in cells from endarterectomized tissues of patients with chronic thromboembolic pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L666–L676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. MacMillan D., McCarron J. G. (2009) Regulation by FK506 and rapamycin of Ca2+ release from the sarcoplasmic reticulum in vascular smooth muscle: the role of FK506 binding proteins and mTOR. Br. J. Pharmacol. 158, 1112–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ghofrani H. A., Voswinckel R., Reichenberger F., Weissmann N., Schermuly R. T., Seeger W., Grimminger F. (2006) Hypoxia- and non-hypoxia-related pulmonary hypertension—established and new therapies. Cardiovasc. Res. 72, 30–40 [DOI] [PubMed] [Google Scholar]
- 64. Arsham A. M., Howell J. J., Simon M. C. (2003) A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J. Biol. Chem. 278, 29655–29660 [DOI] [PubMed] [Google Scholar]
- 65. Liu L., Cash T. P., Jones R. G., Keith B., Thompson C. B., Simon M. C. (2006) Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol. Cell 21, 521–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wouters B. G., Koritzinsky M. (2008) Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat. Rev. Cancer 8, 851–864 [DOI] [PubMed] [Google Scholar]
- 67. Kourembanas S., Marsden P. A., McQuillan L. P., Faller D. V. (1991) Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J. Clin. Invest. 88, 1054–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kourembanas S., Hannan R. L., Faller D. V. (1990) Oxygen tension regulates the expression of the platelet-derived growth factor-B chain gene in human endothelial cells. J. Clin. Invest. 86, 670–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Eddahibi S., Fabre V., Boni C., Martres M. P., Raffestin B., Hamon M., Adnot S. (1999) Induction of serotonin transporter by hypoxia in pulmonary vascular smooth muscle cells: relationship with the mitogenic action of serotonin. Circ. Res. 84, 329–336 [DOI] [PubMed] [Google Scholar]
- 70. Rabinovitch M. (2001) Pathobiology of pulmonary hypertension: extracellular matrix. Clin. Chest Med. 22, 433–449 [DOI] [PubMed] [Google Scholar]
- 71. Stenmark K. R., Fagan K. A., Frid M. G. (2006) Hypoxia-induced pulmonary vascular remodeling. Circ. Res. 99, 675–691 [DOI] [PubMed] [Google Scholar]
- 72. Stenmark K. R., Frid M. G., Yeager M., Li M., Riddle S., McKinsey T., El Kasmi K. C. (2012) Targeting the adventitial microenvironment in pulmonary hypertension: a potential approach to therapy that considers epigenetic change. Pulm. Circ. 2, 3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ambalavanan N., Bulger A., Philips J. B., III (1999) Hypoxia-induced release of peptide growth factors from neonatal porcine pulmonary artery smooth muscle cells. Neonatology 76, 311–319 [DOI] [PubMed] [Google Scholar]
- 74. Lin M. J., Leung G. P. H., Zhang W. M., Yang X. R., Yip K. P., Tse C. M., Sham J. S. K. (2004) Chronic hypoxia–induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells. Circ. Res. 95, 496–505 [DOI] [PubMed] [Google Scholar]
- 75. Broughton B. R. S., Jernigan N. L., Norton C. E., Walker B. R., Resta T. C. (2010) Chronic hypoxia augments depolarization-induced Ca2+ sensitization in pulmonary vascular smooth muscle through superoxide-dependent stimulation of RhoA. Am. J. Physiol. Lung Cell. Mol. Physiol. 298, L232–L242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhou W., Dasgupta C., Negash S., Raj J. U. (2007) Modulation of pulmonary vascular smooth muscle cell phenotype in hypoxia: role of cGMP-dependent protein kinase. Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L1459–L1466 [DOI] [PubMed] [Google Scholar]
- 77. Humar R., Kiefer F. N., Berns H., Resink T. J., Battegay E. J. (2002) Hypoxia enhances vascular cell proliferation and angiogenesis in vitro via rapamycin (mTOR)-dependent signaling, FASEB J. 16, 771–780 [DOI] [PubMed] [Google Scholar]
- 78. Gerasimovskaya E. V., Tucker D. A., Stenmark K. R. (2005) Activation of phosphatidylinositol 3-kinase, Akt, and mammalian target of rapamycin is necessary for hypoxia-induced pulmonary artery adventitial fibroblast proliferation. J. Appl. Physiol. 98, 722–731 [DOI] [PubMed] [Google Scholar]
- 79. Paddenberg R., Stieger P., von Lilien A. L., Faulhammer P., Goldenberg A., Tillmanns H., Kummer W., Braun-Dullaeus R. C. (2007) Rapamycin attenuates hypoxia-induced pulmonary vascular remodeling and right ventricular hypertrophy in mice. Resp. Res. 8, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Agard C., Rolli-Derkinderen M., Dumas-de-La-Roque E., Rio M., Sagan C., Savineau J. P., Loirand G., Pacaud P. (2009) Protective role of the antidiabetic drug metformin against chronic experimental pulmonary hypertension. Br. J. Pharmacol. 158, 1285–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Archer S. L., Weir E. K., Wilkins M. R. (2010) Basic science of pulmonary arterial hypertension for clinicians. Circulation 121, 2045–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dennis P. B., Jaeschke A., Saitoh M., Fowler B., Kozma S. C., Thomas G. (2001) Mammalian TOR: a homeostatic ATP sensor. Science 294, 1102–1105 [DOI] [PubMed] [Google Scholar]
- 83. Fagan K. A., Oka M., Bauer N. R., Gebb S. A., Ivy D. D., Morris K. G., McMurtry I. F. (2004) Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase, Am. J. Physiol. Lung Cell. Mol. Physiol. 287, L656–L664 [DOI] [PubMed] [Google Scholar]
- 84. Hyvelin J. M., Howell K., Nichol A., Costello C. M., Preston R. J., McLoughlin P. (2005) Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circ. Res. 97, 185–191 [DOI] [PubMed] [Google Scholar]
- 85. Zhang S., Fantozzi I., Tigno D. D., Yi E. S., Platoshyn O., Thistlethwaite P. A., Kriett J. M., Yung G., Rubin L. J., Yuan J. X. J. (2003) Bone morphogenetic proteins induce apoptosis in human pulmonary vascular smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 285, L740–L754 [DOI] [PubMed] [Google Scholar]
- 86. McMurtry M. S., Bonnet S., Michelakis E. D., Bonnet S., Haromy A., Archer S. L. (2007) Statin therapy, alone or with rapamycin, does not reverse monocrotaline pulmonary arterial hypertension: the rapamcyin-atorvastatin-simvastatin study. Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L933–L940 [DOI] [PubMed] [Google Scholar]
- 87. Reddy M. K., Vasir J. K., Sahoo S. K., Jain T. K., Yallapu M. M., Labhasetwar V. (2008) Inhibition of apoptosis through localized delivery of rapamycin-loaded nanoparticles prevented neointimal hyperplasia and reendothelialized injured artery/clinical perspective. Circ. Cardiovasc. Interv. 1, 209–216 [DOI] [PubMed] [Google Scholar]
- 88. Sahai E., Marshall C. J. (2002) Rho-GTPases and cancer. Nat. Rev. Cancer 2, 133–142 [DOI] [PubMed] [Google Scholar]
- 89. Hassoun P. M., Mouthon L., Barberà J.A., Eddahibi S., Flores S. C., Grimminger F., Jones P. L., Maitland M. L., Michelakis E. D., Morrell N. W., Newman J. H., Rabinovitch M., Schermuly R. T., Stenmark K. R., Voelkel N. F., Yuan J. X. J., Humbert M. (2009) Inflammation, growth factors, and pulmonary vascular remodeling, J. Am. Coll. Cardiol. 54, S10–S19 [DOI] [PubMed] [Google Scholar]
- 90. Weichhart T., Säemann M. D. (2009) The multiple facets of mTOR in immunity. Trends Immunol. 30, 218–226 [DOI] [PubMed] [Google Scholar]
- 91. Mathew R., Karantza-Wadsworth V., White E. (2007) Role of autophagy in cancer. Nat. Rev. Cancer 7, 961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Patel A.S., Morse D., Choi A. M. K. (2013) Regulation and functional significance of autophagy in respiratory cell biology and disease. Am. J. Respir. Cell Mol. Biol. 48, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Morice M. C., Serruys P. W., Sousa J. E., Fajadet J., Ban Hayashi E., Perin M., Colombo A., Schuler G., Barragan P., Guagliumi G., Molnàr F., Falotico R. (2002) A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. New. Engl. J. Med. 346, 1773–1780 [DOI] [PubMed] [Google Scholar]
- 94. Sakao S., Tatsumi K., Voelkel N. F. (2009) Endothelial cells and pulmonary arterial hypertension: apoptosis, proliferation, interaction and transdifferentiation. Resp. Res. 10, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Janes M. R., Limon J. J., So L., Chen J., Lim R. J., Chavez M. A., Vu C., Lilly M. B., Mallya S., Ong S. T., Konopleva M., Martin M. B., Ren P., Liu Y., Rommel C., Fruman D. A. (2010) Effective and selective targeting of Leukemia cells using a TORC1/2 kinase inhibitor. Nat. Med. 16, 205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hoang B., Frost P., Shi Y., Belanger E., Benavides A., Pezeshkpour G., Cappia S., Guglielmelli T., Gera J., Lichtenstein A. (2010) Targeting TORC2 in multiple myeloma with a new mTOR kinase inhibitor. Blood 116, 4560–4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chresta C. M., Davies B. R., Hickson I., Harding T., Cosulich S., Critchlow S. E., Vincent J. P., Ellston R., Jones D., Sini P., James D., Howard Z., Dudley P., Hughes G., Smith L., Maguire S., Hummersone M., Malagu K., Menear K., Jenkins R., Jacobsen M., Smith G. C. M., Guichard S., Pass M. (2010) AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 70, 288–298 [DOI] [PubMed] [Google Scholar]
- 98. Engelman J. A., Chen L., Tan X., Crosby K., Guimaraes A. R., Upadhyay R., Maira M., McNamara K., Perera S. A., Song Y., Chirieac L. R., Kaur R., Lightbown A., Simendinger J., Li T., Padera R. F., Garcia-Echeverria C., Weissleder R., Mahmood U., Cantley L. C., Wong K. K. (2008) Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat. Med. 14, 1351–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cho D. C., Cohen M. B., Panka D. J., Collins M., Ghebremichael M., Atkins M. B., Signoretti S., Mier J. W. (2010) The efficacy of the novel dual PI3-kinase/mTOR inhibitor NVP-BEZ235 compared with rapamycin in renal cell carcinoma. Clin. Cancer Res. 16, 3628–3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Serra V., Markman B., Scaltriti M., Eichhorn P. J. A., Valero V., Guzman M., Botero M. L., Llonch E., Atzori F., Di Cosimo S., Maira M., Garcia E. C., Parra J. L., Arribas J., Baselga J. (2008) NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 68, 8022–8030 [DOI] [PubMed] [Google Scholar]