Abstract
Pneumocystis jirovecii is an important opportunistic pathogen associated with AIDS and other immunodeficient conditions. Currently, very little is known about its nuclear and mitochondrial genomes. In this study, we sequenced the complete mitochondrial genome (mtDNA) of this organism and its closely related species Pneumocystis carinii and Pneumocystis murina by a combination of sequencing technologies. Our study shows that P. carinii and P. murina mtDNA share a nearly identical number and order of genes in a linear configuration, whereas P. jirovecii has a circular mtDNA containing nearly the same set of genes but in a different order. Detailed studies of the mtDNA terminal structures of P. murina and P. carinii suggest a unique replication mechanism for linear mtDNA. Phylogenetic analysis supports a close association of Pneumocystis species with Taphrina, Saitoella, and Schizosaccharomyces, and divergence within Pneumocystis species, with P. murina and P. carinii being more closely related to each other than either is to P. jirovecii. Comparative analysis of four complete P. jirovecii mtDNA sequences in this study and previously reported mtDNA sequences for diagnosing and genotyping suggests that the current diagnostic and typing methods can be improved using the complete mtDNA data. The availability of the complete P. jirovecii mtDNA also opens the possibility of identifying new therapeutic targets.—Ma, L., Huang, D. W., Cuomo, C. A., Sykes, S., Fantoni, G., Das, B., Sherman, B. T., Yang, J., Huber, C., Xia, Y., Davey, E., Kutty, G., Bishop, L., Sassi, M., Lempicki, R. A., Kovacs, J. A. Sequencing and characterization of the complete mitochondrial genomes of three Pneumocystis species provide new insights into divergence between human and rodent Pneumocystis.
Keywords: inverted repeats, DNA replication, pneumonia
Pneumocystis is an opportunistic fungal pathogen found in the lungs of humans and other animals. Pneumocystis organisms obtained from different host species are genetically and antigenically distinct from but closely related to each other. The species that infect humans, rats, and mice are Pneumocystis jirovecii, Pneumocystis carinii, and Pneumocystis murina, respectively (1). P. jirovecii causes Pneumocystis pneumonia (PCP) in humans, which is a major cause of morbidity and mortality in patients with impaired immunity, especially those with HIV infection (2). The diagnosis of PCP can be challenging, and therapeutic options are limited. Many aspects of the basic biology, genomics, and epidemiology of this pathogen are still not well understood. The slow progress in Pneumocystis research is, in part, due to the lack of a reliable in vitro culture system.
To date, only about a dozen genes of P. jirovecii have been identified, including the genes encoding mitochondrial (mt) cytochrome b (cob) and the mt large subunit ribosomal RNA (mt LSU rRNA; rnl) and mt small subunit rRNA (mt SSU rRNA; rns). The mt LSU rRNA gene has served as a major target for polymerase chain reaction (PCR)-based detection and typing of P. jirovecii (3–5), while the cob protein is the target of atovaquone, one of the few drugs available to treat PCP (6, 7). Identification of additional genes of P. jirovecii may lead to new therapeutic targets and provide new diagnostic methods. Research on Pneumocystis in animal models (particularly P. carinii and P. murina), including a project to sequence the P. carinii genome (http://pgp.cchmc.org), has provided important information relevant to the biology of P. jirovecii.
Although Pneumocystis organisms were originally classified as protozoan, they are now recognized as members of the fungal kingdom (1, 8). Phylogenetic analysis using multiple genes consistently place Pneumocystis within the fungal phylum Ascomycota. A recent phylogenomic analysis, combining larger numbers of nuclear and mitochondrial genes, strongly supports the grouping of P. carinii with Schizosaccharomyces, Taphrina, and Saitoella, under the subphylum Taphrinomycotina as a sister group to Saccharomycotina and Pezizomycotina (9). However, the relationships among the members of Taphrinomycotina remain to be resolved. Additional data from Pneumocystis mt genomes are expected to improve tree resolutions.
In the present study, we determined the complete mtDNA sequences of P. jirovecii, P. carinii, and P. murina, compared their sequence organizations to each other and to those described in other fungal species, and examined the evolutionary relationship of Pneumocystis. We also proposed a possible replication model for P. carinii and P. murina mtDNA. Furthermore, we explored possible implications of the P. jirovecii mtDNA data in the development of new diagnostic tools and therapeutic agents for PCP.
MATERIALS AND METHODS
Pneumocystis organisms and DNA preparation
For P. jirovecii, 2 autopsy lung samples and 2 induced sputum samples obtained from patients with PCP (1 sample/patient) were used for DNA extraction. P. carinii-infected lung samples were obtained from immunosuppressed rats and partially purified by Ficoll-Hypaque density gradient centrifugations (10) before DNA extraction. P. murina-infected lung samples were obtained from CD40 ligand-knockout mice (11). P. murina organisms were partially purified by sequential treatment with trypsin, collagenase, DNase, and sodium dodecyl sulfate before DNA extraction. Genomic DNA extraction was performed using the MasterPure yeast DNA purification kit (Epicenter, Madison, WI, USA). The guidelines of the U.S. Department of Health and Human Services and the U.S. National Institutes of Health were followed in the conduct of these studies.
All DNA samples were quantitated by the Nanodrop 1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA), followed by a real-time quantitative PCR (qPCR) assay targeting the dihydrofolate reductase (Dhfr) gene of P. murina (12) or P. carinii (13), as well as a qPCR assay targeting a highly conserved region of the single-copy polycystic kidney disease 1 (Pkd1) gene in vertebrates (14). The Pkd1-based qPCR assay involved 2 primers (PKD.F3 and PKD.R6, Table 1) and 2 fluorescence-resonance energy transfer probes (PKD.P1 and PKD.P2, Table 1). The purity of each sample was estimated by the ratio of Pneumocystis Dhfr/host Pkd1 copy numbers and was based on an estimated genome size of 8.2 Mbp for both P. carinii and P. murina (15). DNA samples extracted from partially purified P. murina and P. carinii organisms contained 44 and 98% Pneumocystis DNA, respectively. DNA samples extracted directly from lung tissues contained <1% Pneumocystis DNA.
Table 1.
Oligonucleotide primers and probes used in this study
| Primer | Sequence, 5′→3′ |
|---|---|
| PKD.F3 | GAGCTTTTCCTGCGTAG |
| PKD.R6 | AGACCCAGGGTGTGTC |
| PKD.P1 | CGTACCCACCTCCTTGACCTT-Fluorescein |
| PKD.P2 | Red640-GAAGCCCATCCAGAGCCGAA-phosphate |
| R2 | TGGGTCGAAAAGATTCGA |
| F6 | CTCACGGTACTCTTCACTA |
| C1 | TAGATTGTACGAATTATTCTAGA |
| C2 | TCTAGAATAATTCGTACAATCTA |
| m1 | TGGTTAACTTAACTAGTTCATC |
| m2 | GATGAACTAGTTAAGTTAACCA |
| cox1.f1 | GCWGCWSTGAAATAWGCTC |
| cox1.f2 | TCTTGGAAAGGCCATATCTG |
| cox1.r2 | GTTATTTAGGTATGGTTTATGC |
| cob.f2 | GATAATGAACCATAATTCCA |
| cob.r2 | GCAATCTTGTGATCTATTTC |
| mit.f8 | TAGTCCGATTTGTATTTCAC |
| mit.r3 | GTCACAGAAATTTGAGTTCTG |
| mit.r8 | GTGAAATACAAATCGGACTA |
| mit.r11 | TGAAGACAAGTCCTCATGAC |
| nad5.r1 | TTACCTCAWGCNATGGARGGTCC |
| nad5.f2 | CAAGTGGAATAAGCAATAATTC |
| rnl.f1 | TWAACCCAACTCACG |
| rnl.r2 | CACCTCGATGTCGACTCA |
| rns.f2 | CATTTCACAACACGAACTAA |
The P. jirovecii mtDNA was first obtained by amplifying 7 overlapping fragments with primer pairs rns.f2-cob.r2, cob.f2-nad5.r1, nad5.f2-cox1.r2, cox1.f1-mit.r3, cox1.f2-rnl.r2, rnl.f1-mit.r8, and mit.f8-mit.r11. The complete assembly was reamplified into 6 overlapping fragments of 3–10 kb each, using DNA from different patients and new primer pairs. Sequence letters other than A, G, C, and T represent degenerative codes.
DNA sequencing of mtDNA
Partially purified P. murina DNA (8 μg) and partially purified P. carinii DNA (0.5 μg) were used to construct shotgun libraries following the 454 sequencing protocols. Sequencing was performed in a 454 GS FLX Titanium Sequencer (Roche Applied Science, Indianapolis, IN, USA) at Science Applications International Corp. (SAIC)–Frederick (Frederick, MD, USA), according to the 454 standard shotgun sequencing protocols. The image, base-calling, and contig assembly were processed by software interfaced with the sequencer. Purified P. murina DNA was also subjected to Illumina sequencing (Illumina, Inc., San Diego, CA, USA)at the Broad Institute (Cambridge, MA, USA) as a part of the P. murina genome sequencing project [U.S. National Center for Biotechnology Information (NCBI; Bethesda, MD, USA) BioProject accession no. PRJNA70803 and http://www.broadinstitute.org/annotation/genome/Pneumocystis_group.2/MultiHome.html]. Seven different DNA preps were used to construct small insert libraries; each was sequenced, and the library with the least amount of mouse sequences was used for assembly. From this library, a total of 34 × 106 paired 101-base Illumina reads with a mean insert size of 153 bp were generated. A second larger insert library with mean insert size of 1247 bases was prepared from a different sample, and a total of 83 × 106 101-base Illumina reads were generated. To assemble the mitochondrial genome, mitochondrial reads were identified as Basic Local Alignment Search Tool (BLAST; NCBI) hits to the P. carinii (16) and other fungal mitochondrial genomes. These reads were assembled with Allpaths version R37380 (17). The resulting contigs were screened against an in-house mt database using BLAST. Those contigs that showed similarity to fungal mitochondria, but not mouse mitochondria, were retained. All gaps in sequence assemblies were closed by PCR.
To clone the P. jirovecii mt genome, we designed PCR primers (Table 1) from conserved regions based on an alignment of P. murina and P. carinii mtDNAs and known P. jirovecii mtDNA sequences, including mt LSU rRNA, mt SSU rRNA, and cob.
The mtDNA sequences for P. murina and P. jirovecii were confirmed by resequencing the entire mt genome using overlapping fragments that were amplified by PCR using new sets of primers. For P. carinii, the newly identified regions were resequenced in a similar manner; in addition, 2 independent P. carinii isolates were utilized to confirm the presence of these newly identified regions by PCR amplification and sequencing.
PCR was performed in a 50-μl volume containing ∼50 ng genomic DNA, 0.25 μM of each primer, and 25 μl of LongAmp Hot Start Taq 2X Master Mix (New England Biolabs, Ipswich, MA, USA) using the following conditions: 94°C for 30 s; 10 cycles of 94°C for 30 s, 65°C for 60 s with 2°C decrease per cycle, and 65°C for 2 to 20 min; and 35 cycles of 94°C for 15 s, 45°C for 30 s, and 65°C for 2 to 20 min. To amplify highly AT-rich regions, including both ends of P. murina and P. carinii mtDNAs and the long noncoding region of P. jirovecii mtDNA, the extension temperature was changed to 60°C. PCR products were purified with the QuickStep2 PCR Purification Kit (Edge BioSystems, Gaithersburg, MD, USA) and sequenced commercially by Macrogen (Rockville, MD, USA) using the dideoxy chain termination method (referred to as Sanger sequencing).
Sequence analysis
Alignment and mapping of 454 sequence reads was performed using SeqMan software (DNASTAR, Madison, WI, USA). Sequence translation and codon usage were determined using MacVector 12.6 (MacVector, Cary, NC, USA). Transfer RNA (tRNA) genes were predicted using tRNAscan-SE (18) and compared to known tRNAs in the GenBank database (NCBI; http://www.ncbi.nlm.nih.gov/genbank/). Tandem repeats were identified using the program Perfect Microsatellite Repeat Finder (http://sgdp.iop.kcl.ac.uk/nikammar/repeatfinder.html).
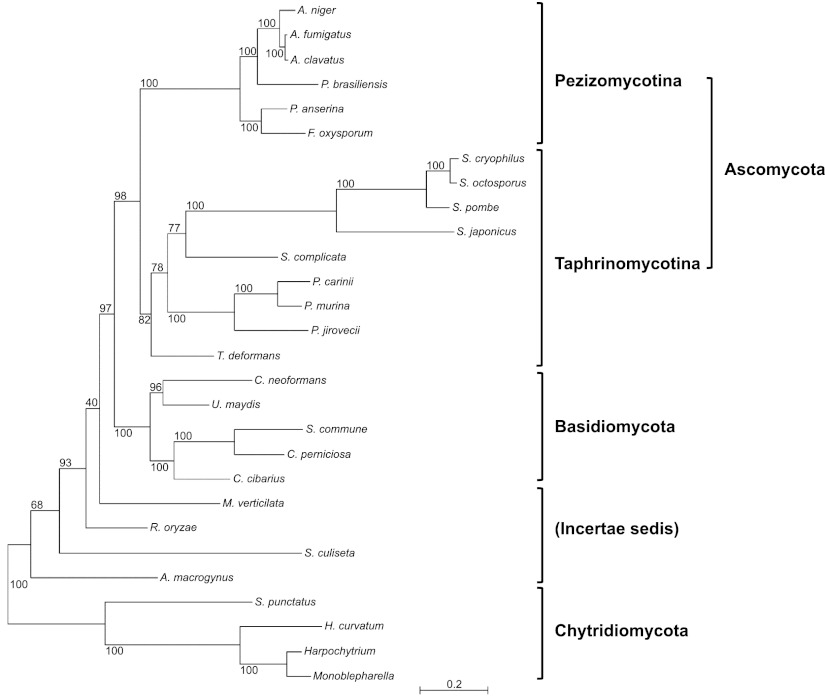
For phylogenetic analysis, we constructed a data set containing 13 well-conserved proteins encoded by mtDNA genes from 28 fungal species of different taxa, including Taphrinomycotina, Pezizomycotina, Basidiomycota, Zygomycota, and Chytridiomycota (9). Deduced protein sequences were concatenated in the order of cytochrome c oxidase (cox) subnits cox1–cox3; cob; ATP synthase (atp) subunits atp6, atp9; NADH dehydrogenase (nad) subunits nad1–nad4, nad4L, nad5, nad6. Sequence alignment was performed with Muscle (19), and ambiguous regions were removed with Gblocks (20). Phylogenetic trees were inferred by maximum likelihood (ML) using RAxML 7.3.3 with substitution matrix MTZOA (21). Bootstrap values were determined from 1000 replicates. (For the fungal species and GenBank accession numbers, see Fig. 3.)
Figure 3.
Phylogeny based on 13 mtDNA-encoded proteins from 28 fungal species. Fungal species and GenBank accession numbers are as follows: Aspergillus clavatus, JQ354999; Aspergillus fumigatus, NC_017016; Allomyces macrogynus, U41288; Aspergillus niger, DQ207726; Cryptococcus neoformans, NC_004336; Crinipellis perniciosa, AY376688; Fusarium oxysporum, AY945289; Harpochytrium sp., AY182006; Hyaloraphidium curvatum, AF402142; Monoblepharella sp., AY182007; Podospora anserina, NC_001329; P. jirovecii, JX499143; P. carinii, JX499145; P. murina, JX499144; S. pombe, X54421; Schizosaccharomyces octosporus, AF275271); Schizosaccharomyces japonicus, AF547983; S. commune, NC_003049; Smittium culisetae, AY863213; Spizellomyces punctatus, AF404303; and Ustilago maydis, NC_008368. Rhizopus oryzae and Schizosaccharomyces cryophilus mtDNA sequences were downloaded from the Broad Institute genome database (http://www.broadinstitute.org). Cantharellus cibarius, Mortierella verticillata, Saitoella complicata, Taphrina deformans, and Paracoccidioides brasiliensis protein sequences were downloaded from the Fungal Mitochondrial Genome database (http://www.bch.umontreal.ca/People/lang/FMGP/FMGP.html).
Southern blot analysis of P. carinii mtDNA
Purified P. carinii genomic DNA was treated with restriction endonuclease AfeI, HindIII, NdeI, NruI, XbaI, or XhoI, or exonuclease BAL-31 following manufacturer's instructions (New England Biolabs). Digested DNA was separated by 0.8% agarose gel electrophoresis (580 ng DNA/well) and transferred to a Nytran membrane (Schleicher & Schuell, Keene, NH, USA). All probes were labeled using the PCR DIG Probe Synthesis Kit (Roche Applied Science). (See Fig. 2 for the location of the probe sequences.) Blots were hybridized with probes and detected using the DIG Probe Hybridization system (Roche Applied Science) as described previously (22).
Figure 2.
Southern blot analysis of P. carinii mtDNA. A) Hybridization with probe p1, specific for the right terminus (tail). B) Hybridization with probe p2, specific for the left terminus (head). C) Hybridization with probe p3, with a sequence shared between 2 TIRs. D) Hybridization with probe p4, specific for an internal fragment (3566 bp with NdeI). Panels A–D use the same DNA blot containing P. carinii genomic DNA digested with restriction enzymes (indicated above each lane) or undigested P. carinii DNA as a control (lane U). The blot was stripped following each hybridization. Solid and open triangles indicate the major and minor bands (for example, detected in HindIII digest in panels A and C), representing the right terminal fragment and tail-to-tail junction, respectively. Solid and open chevrons indicate the major and minor bands (for example, detected in HindIII digest in panels B–D), representing the left terminal fragment and head-to-head junction, respectively. There is no cross-hybridization of probe p1 with probe p2 or p4, indicating no head-to-tail junction. Probe p3, whose sequence is present at both ends, shows cross-hybridization with both probes p1 and p2, as expected. E) Hybridization with the right terminus-specific probe p1 and a blot containing P. carinii genomic DNA digested with either XbaI or HindIII alone, or both enzymes (lane XH). The higher band (potential concatemer) seen with HindIII digestion alone is lost following codigestion with XbaI, which has a restriction site very near each terminus. In the last 4 lanes, P. carinii genomic DNA was first treated with BAL 31 nuclease for 1 to 30 min as indicated, followed by digestion with HindIII alone. A decrease in size and intensity of the HindIII fragment is seen with time. Similar patterns were also obtained in hybridization with probe p2 (not shown). In panels A–E, lane M contains size markers, with DNA sizes (kb) given on the left of panels A and E. F) Schematic diagram of the architecture of P. carinii mtDNA. Arrows represent inverted repeats; vertical bars at both ends indicate SSL sequences. Asterisks indicate the location of the probe sequence used in hybridization. Nucleotide positions of each probe in the P. carinii mtDNA (GenBank JX499145) are as follows: p1, 23,289–23,625; p2, 2400–2731; p3, 1333–1564 or 24,575–24,788; p4, 3641–4536. G) Restriction map of P. carinii mtDNA for the enzymes used in panels A–E. Numbers inside boxes indicate the size for each fragment. The two shortest fragments with HindIII are 267 bp (gray box) and 424 bp (a). The shortest fragments with XbaI are indicated by letters b (122 bp), c (283 bp), d (485 bp), and e (264 bp).
GenBank accession numbers
The mtDNA sequences described in this article have been deposited in the GenBank under accession numbers: JX499144 (P. murina), JX499145 (P. carinii), and JX499143 and JX855936–JX855938 (P. jirovecii), and are also available from the DAVID Bioinformatics Resources (http://david.abcc.ncifcrf.gov/). All the sequences reported here are from the heavy (H) strand instead of the light (L) strand as previously reported (23).
RESULTS
mtDNA sequencing and assembly
The P. murina mtDNA was sequenced with both 454 and Illumina technology. We initially used 454 sequencing and obtained 56,791 reads (mean read length 236 bp) that had significant homology to the known P. carinii mtDNA (23). These reads were assembled into 7 contigs totaling 19,438 bp. These contigs were oriented using the known P. carinii mtDNA as the reference, allowing all gaps to be closed by PCR, resulting in a single supercontig of 19,470 bp. Subsequently, we used Illumina sequencing and obtained a total of 83 × 106 101-bp reads. From these reads, we assembled 12 contigs related to P. murina mtDNA. Alignment of these 12 contigs with the supercontig above generated a single sequence assembly of 20,135 bp. Both ends of the assembly were extended by reassembling with 454 reads, followed by PCR and Sanger sequencing. The final assembly was 24,608 bp in length. The complete assembly was further verified by PCR and Sanger sequencing of 3 overlapping regions (2753, 19,129, and 3271 bp, respectively), which covered the full length.
The P. carinii mtDNA was sequenced with 454 technology. A total of 125,238 reads (mean read length 272 bp) were obtained after removal of the contaminating host DNA sequences. There were 13,965 reads that could be mapped to the published P. carinii mtDNA (GenBank GU133622) over its full length except for the first 31 bp and the last 27 bp. When using primers from the 31- and 27-bp regions in combination with other primers to amplify P. carinii genomic DNA, we did not obtain any products, while PCR with primers from other regions was successful, suggesting the possibility of misassembly in the two ends of the published mtDNA sequence. Removal of both ends and reassembly of the truncated sequence with 454 reads resulted in an extension at both ends, giving rise to a final complete assembly of 26,119 bp. All extended sequences were confirmed by PCR and Sanger sequencing. The final assembly could be realigned with 15,019 raw reads of the P. carinii 454 sequence library, without any gaps. Aside from both ends, this genome assembly is identical to the published mtDNA (23) except for 8 nucleotide changes scattered throughout the overlapping regions of the two genomes, which may represent allelic variation or possibly sequencing errors. Furthermore, the terminal repeat sequences identified in our study (described below) but not present in the published mtDNA were identified by PCR amplification and sequencing of DNA from 2 additional P. carinii isolates, and were also found in the P. carinii genome database (http://pgp.cchmc.org), suggesting that the published P. carinii mtDNA, which was assembled using sequences from the same database, is incomplete.
The P. jirovecii mtDNA was amplified in 7 fragments using primers from regions conserved between P. murina and P. carinii mtDNAs. All gaps were then closed by PCR. The whole assembly was reamplified in 6 overlapping fragments of 3–10 kb using genomic DNA from 4 unrelated isolates. The complete genome size was 33,690, 35,517, 35,634, and 35,626 bp, respectively.
Genome content and gene order
In a previous study (23), the P. carinii mtDNA was found to be 22,898 bp, and encoded 41 genes, including atp6, atp8, atp9; cox1–cox3; nad1–nad4, nad4L, nad5, nad6; cob; RNase P RNA (rnpB); rnl, rns; 20 tRNA species; and 4 putative open reading frames (orfs). In the present study, we identified a complete genome that is 26,119 bp, encoding the same set of genes except that one of the 4 orfs (orf 106) was ambiguous, while a second copy of orf 101 and 5 additional tRNA genes were identified (Table 2). Orf 106 was reported to be located between the tRNA-Asn and tRNA-Tyr genes of P. carinii mtDNA (23) but is not identified in the current GenBank record (accession no. GU133622). From this region, the longest identifiable orf was 159 bp (including 46 bp of the 86 bp for the tRNA-Tyr gene), which encodes a protein of 53 aa with unknown function. It is uncertain whether this is the orf indicated in the previous report (23). The complete P. carinii mtDNA has a 29.8% content of GC, with a coding density of 73.3%. All genes are encoded on the H strand except for 2 orfs (orf 144 and one of the 2 orf 101 copies), which are encoded on the L strand. This is in contrast to the current annotation of the P. carinii mtDNA sequence (GenBank GU133622), in which all RNA genes are assigned on the L strand while all protein-coding genes except for orf 101 and orf 144 are on the H strand.
Table 2.
Comparison of the mtDNAs of P. carinii, P. murina, and P. jirovecii
| Characteristic | P. carinii | P. murina | P. jirovecii |
|---|---|---|---|
| Size (bp) | 26,119 | 24,608 | 33,690–35,634a |
| Structure | Linear | Linear | Circular |
| G+C content (%) | 29.8 | 29.8 | 25.7b |
| Noncoding region (%) | 26.7 | 20.8 | 55.2b |
| Protein-coding genes (n) | 14 | 14 | 14 |
| rRNA genes (n) | 2 | 2 | 2 |
| tRNA genes (n) | 25 | 28 | 25 |
| rnpB RNA genes (n) | 1 | 1 | 1 |
| Orfs (n)c | 4 | 2 | 1 |
Four strains with 33,690, 35,517, 35,626, and 35,634 bp, respectively.
Average of 4 genomes.
Putative orfs with unknown functions.
The complete P. murina mtDNA is 24,608 bp in length, with a coding density of 79.2% and a GC content of 29.8%. The only observed difference in gene content between P. murina and P. carinii mtDNAs is the absence of the orf 101 gene and the duplication of 3 tRNA genes in P. murina. Aside from these 4 genes, the gene order and transcription direction are the same for P. murina and P. carinii mtDNAs.
The complete mtDNA of P. jirovecii is 33,690–35,634 bp in length, ∼10 kb longer than that of P. carinii and P. murina. Both the GC content (25.7%) and coding density (44.8%) of this genome are lower than that of P. carinii and P. murina. All genes that are found in the P. carinii and P. murina mtDNAs are present in the P. jirovecii mtDNA except for orf 101 and orf 144, and all are on the H strand. However, the gene order in P. jirovecii mtDNA is very different from that observed in P. carinii and P. murina (Fig. 1).
Figure 1.
Architecture of the complete mtDNA sequences of P. carinii, P. murina, and P. jirovecii. Thick arrows represent the terminal inverted repeats at both ends that are in reverse-complement orientation to each other. Boxes at both ends represent the single-stranded loop sequence. Locations of primers (m1, m2, F6, R2, C1, and C2) used to amplify the left and right termini are indicated by thin black arrows. Numbers inside bars indicate the length (bp) for each region. Slanted dotted lines indicate the borders of the regions encoding all genes. tRNA genes are indicated by the one-letter code of the amino acid they represent. The number and order of genes are identical between P. carinii and P. murina mtDNA except for the extra copies of 3 tRNA genes and the absence of orf 101 in P. murina. P. jirovecii mtDNA contains the same set of genes but in a different order compared to P. carinii mtDNA, with the rearrangement of the order of some gene blocks highlighted by different degrees of gray shading.
While we identified the same 3 orfs (348–588 bp in size) in the P. carinii mtDNA that have been described previously (23), only one (orf 195) is also present in both P. murina and P. jirovecii mtDNAs. In addition, only this orf is encoded on the H strand, as are all other genes in all 3 species. The conservation of this orf and its transcription direction across species suggests that this orf does encode a protein whose function is unknown. BLAST analysis of the GenBank database identified only weak homology with ribosomal protein S3 in other species. The other 2 orfs (101 and 144) were found only in P. carinii or P. murina, and showed no significant homology to any proteins in the GenBank database. In addition, orf 144 and one of the 2 orf 101 genes are encoded on the L strand, different from all other genes. These observations suggest that these orfs may not encode a functional protein.
Genetic code and tRNA genes
Pneumocystis mtDNA sequences were translated utilizing genetic code 4 (mold, protozoan, and coelenterate mitochondrial code). In all 3 Pneumocystis species, all genes start with the initiation codon AUG and end with the stop codon UAA or UAG. Excluding orf 144 and one of the duplicated orf 101 genes, which are encoded on the L strand, all genes are encoded on the H strand and separated from each other except for an overlap of 7–14 bp between nad3 and cox2 in both P. carinii and P. murina, and between nad6 and nad1 in both P. murina and P. jirovecii.
As shown in Supplemental Table S1, the codon usage of P. carinii and P. murina are clearly more similar to each other than to that of P. jirovecii. Of the 64 standard codons, UUA (encoding Leu) is the most frequently used codon, followed by AUU (Ile), UAU (Tyr), UUU (Phe), AUA (Ile) UCU (Ser), and GGA (Gly) in all 3 species. These 7 codons account for 31–38% of all codons, and all of them except for UCU and GGA consist exclusively of A and U, consistent with the high A+T content of Pneumocystis mtDNA. Six codons, AAC, GAC, CGC, CGG, AGG, and GGC, are not used at all in P. jirovecii and are underrepresented in P. carinii and P. murina, being used from 1 to 13 times each. The UGA codon (Trp) is found in all 3 species.
There are 25 to 28 tRNA genes identified in each Pneumocystis mtDNA, which correspond to all 20 standard amino acids. In P. carinii, there is only one tRNA for each of the 20 standard amino acids except for Leu, Arg, and Ser, each of which has 2 tRNA isoacceptors, and Met, which has 3 tRNA isoacceptors (including one initiator tRNA-Met and 2 elongator tRNA-Mets). These 25 tRNAs appear to be highly homologous in sequence to the 25 tRNAs in the Schizosaccharomyces pombe mtDNA (24). The only difference in the tRNA set between these two species is that S. pombe has one elongator tRNA-Met and two tRNA isoacceptors for Ile (with anticodons GAU and CAU), while P. carinii has two elongator tRNA-Met and one tRNA-Ile (with anticodon GAU). P. murina has homologues of all the P. carinii tRNA genes, but three of them (for Gly, Asp, and Ser) are duplicated in the inverted repeats at the two ends (Fig. 1). P. jirovecii has the same set of tRNAs as in P. carinii except for the presence of only one tRNA-Leu and an extra tRNA-Thr (with anticodon UAG). These tRNA genes are present as 6–7 clusters in each mtDNA.
Amino acid Trp encoded by UGA is found in protein-coding genes of all 3 genomes, but there is no tRNA gene containing the corresponding anticodon UCA. It is possible that the C in the wobble position of the tRNA-Trp with anticodon CCA present in all 3 genomes is modified to recognize the UGA codon, as has been proposed from studies of S. pombe and Schizophyllum commune (24).
Sequence diversity and repetitive elements
Although P. carinii and P. murina mtDNAs share a nearly identical number and order of genes, there is 27% difference in the complete mtDNA sequence and an average difference of 19 and 18%, respectively, in the nucleotide and deduced amino acid sequences of all 14 protein-coding genes. P. jirovecii shows an average difference of 30–31% and 31–33%, respectively, in the nucleotide and deduced amino acid sequences of the 14 protein-coding genes, compared to P. carinii and P. murina. Noncoding sequences account for 55.2% of the P. jirovecii mtDNA, in contrast to the 26.7 and 20.8% of the P. carinii and P. murina mtDNA, respectively.
A striking feature of the P. carinii and P. murina mtDNA is the presence of identical terminal inverted repeats (TIRs) of ∼2 kb at the two ends. There is no significant homology in the TIRs between these species. In addition to the TIRs, short tandem repeats were identified in many loci of both genomes. Including loci with ≥5 copies of the repeat units, P. carinii has dinucleotide repeats (AT, TA, and CT) in 27 loci, predominantly in TIR regions, with only 3 in the noncoding region near the center of the genome. P. murina has dinucleotide (AT and TA), trinucleotide (TTA and TAT) and tetranucleotide (ATTT, ATAA, GAGT) repeats in 13 loci, predominantly in TIR regions, with only 2 in the coding regions of 2 genes (rnl and cox1). We did not observe any variation in the copy number for these repeats during PCR and sequencing of a limited number of Pneumocystis isolates. Also, no variation was noted in the 454 sequence reads generated from a DNA sample pooled from 2 mice or rats. By PCR and sequencing of both ends (∼2.7 and ∼3 kb, respectively) from 2 additional P. carinii isolates, one isolate showed identical sequences and the other showed 6 scattered nucleotide changes compared to the P. carinii mtDNA assembly. The 24-bp tandem repeat unit previously described in the P. carinii mtDNA (23) was present in 454 reads, with the repeat copy number varying from 1 to 6. P. murina mtDNA does not have a homologue to this repeat, but instead has 3 copies of a 27-bp tandem repeat unit present in the TIR regions. Again, there was no variability in the copy number based on sequencing or search of the 454 sequence library.
We sequenced the complete P. jirovecii mtDNA from 4 unrelated isolates. The gene number and order are identical among the 4 genomes. The overall nucleotide differences among the 4 genomes are 1–7%. In the 14 protein-coding genes, there is <1% difference in the nucleotide and deduced amino acid sequences among the 4 genomes. However, the 12-kb noncoding region is highly variable in size and sequence (Supplemental Fig. S1). P. jirovecii mtDNA does not contain any long inverted repeats or direct repeats but is rich in short tandem repeats. Comparison of the 4 complete genomes identified 40 loci containing variable numbers of short tandem repeats, including di-, tri-, tetra-, penta-, and decanucleotide repeats, all in noncoding regions (Supplemental Table S2).
Terminal structure of mtDNA
Both P. carinii and P. murina mtDNAs contain a long TIR of ∼2 kb, followed by a relatively short repeat sequence (200 bp in P. carinii and 496 bp in P. murina) at each end (referred to as the left end or head, and the right end or tail, respectively, Fig. 1). The short repeat sequence is a putative single-stranded loop (SSL) sequence. It can be in either of the two orientations, as supported by the following observations. First, in the P. murina and P. carinii mtDNA 454 sequence libraries, there are at least 84 and 100 raw reads, respectively, which cover the SSL-TIR junction. All these reads contain an identical TIR segment linked to an SSL sequence oriented in either direction (reverse and complementary to each other), with approximately equal frequency (35:49 in P. murina and 45:55 in P. carinii). Second, when PCR was performed with P. murina and P. carinii genomic DNA using a primer from the nonrepeat region internal to TIR at each end (primers R2 and F6 in Fig. 1) coupled with a primer from the SSL region or its reverse-complementary primer (primers m1, m2, C1, and C2 in Fig. 1), both primers from the SSL region yielded strong bands for each end. Sequencing verified that each PCR product contained a part of the nonrepeat region and the entire TIR followed by an SSL fragment with an orientation specified by the corresponding primer.
The features of these TIRs and SSLs resemble those in the mtDNA of two yeast species, Pichia pijperi and P. jadinii (25), in which the SSL sequence was identified as a single-stranded sequence to serve as a linker to join the TIRs through head-to-head, tail-to-tail, and head-to-tail junctions. To examine this in P. carinii, we performed Southern blot analysis. Genomic DNA was digested with 5 restriction enzymes that do not cut the TIR-SSL termini but have at least one cut within the nonrepeat region adjacent to TIR. Digested DNA was separated by gel electrophoresis followed by Southern blotting. The blot was then hybridized to 4 different probes (p1 to p4; Fig. 2A–D) located near either end of the genome, with stripping of the blot between hybridizations. In all the blots, the strongest hybridization occurred with a band of a size predicted by digestion of a linear mtDNA. For fragments that were smaller than 5 kb, less intense hybridization was seen with a second band approximately twice the size of the major band for those enzymes that cut internal to the probe. In the latter situation, the strong band appears to represent the left or right terminal fragment, each containing one copy of the entire SSL-TIR sequence, plus its adjacent nonrepeat region, while the weaker band potentially represents concatemers of two terminal fragments resulting from head-to-head or tail-to-tail junction. This was confirmed by subsequent hybridization with P. carinii DNA digested with XbaI that cuts once within the SSL region (Fig. 2E), which corresponds to the central, double-stranded region of the dimer concatemers (see Fig. 4B). These data suggest that most of the P. carinii mtDNA is present in a linear nonconcatemerized form, while a minority is present as concatemers. There was no hybridization with the same band when using probes specific for the head (p2 and p4) and tail (p1) ends of the genome, which suggests no head-to-tail junction, and thus no circular form of mtDNA and no head-to-tail concatemers. In addition, searching of the P. carinii 454 sequence data identified multiple reads suggesting head-to-head and tail-to-tail junctions, but no reads suggesting a head-to-tail junction.
Figure 4.
Possible replication model for P. carinii and P. murina mtDNAs. A) Replication scheme with nicking at both ends, producing two new monomer mtDNAs. B) Replication scheme with nicking at only one end, producing a concatemer. Shown is an example of nicking at the left end. Dashed lines represent newly synthesized daughter strands. At the end of replication, nicking is required to separate the concatemer. Red bars represent TIRs. Blue bars represent the SSL sequence. Triangles indicate site-specific nicking.
To further confirm the linearity of the P. carinii mtDNA, Southern blot analysis was performed using P. carinii genomic DNA treated with exonuclease BAL 31 for 1 min to 30 min, followed by HindIII digestion. Following hybridization with a probe specific for either the right terminus (p1, Fig. 2E) or left terminus (data not shown), the major band (representing the terminal fragment of monomer mtDNA, as described above) progressively decreased in size and intensity. While the minor band (representing the head-to-head or tail-to-tail concatemer as described above) is expected to remain unchanged after BAL31 treatment, it was not clearly visible in hybridization with either probes, presumably due to its low quantity or nonspecific digestion by BAL 31. Furthermore, we were unable to join the left and right termini by PCR using multiple primer pairs (data not shown). These findings support a linear form for the P. carinii mtDNA, consistent with previous observations from contour-clamped homogeneous electric field (CHEF) analysis of BAL 31-treated DNA (23).
Southern blot analysis of P. murina mtDNA was unsuccessful. Nevertheless, a search of the P. murina 454 sequence data identified multiple reads suggesting head-to-head and tail-to-tail junctions, but no reads suggesting a head-to-tail junction. Given the high similarity to P. carinii mtDNA in gene content and terminal sequence structure (including TIRs and SSLs), it is likely that the P. murina mtDNA has the same configuration as P. carinii mtDNA.
The mtDNA of all 4 P. jirovecii isolates was consistently amplified in 6 overlapping fragments, without any gaps. Although each of them contains a 10- to 12-kb highly AT-rich (84%) noncoding region, none of them has long inverted repeats (>20 bp) or direct repeats (>10 bp) as are usually found in the termini of a linear mtDNA (26–29). In addition, this region could be consistently amplified using primers from its flanking coding regions in all 4 P. jirovecii isolates, suggesting a lack of putative ends in this region, as would be seen with a linear mtDNA. These observations suggest that P. jirovecii mtDNA is circular though the possibility exists that the amplified regions represent only the head-to-tail concatemer of a linear mtDNA. We attempted to define the structure further by Southern blot analysis, without success.
Phylogenetic relationships
A recent study combining multiple nuclear- and mtDNA-encoded proteins from P. carinii and other fungi has grouped Pneumocystis with Schizosaccharomyces under the monophyly of the Taphrinomycotina, as a sister group to Saccharomycotina and Pezizomycotina within Ascomycota (9). We constructed a data set containing 13 concatenated mtDNA-encoded proteins from 28 fungal species, similar to the mtDNA data set utilized in the previous report (9). Saccharomycotina group was excluded in this data set to avoid the possibility of long-branch attraction artifact that causes erroneous grouping together of long branches (fast-evolving lineages), such as those of yeast (9, 30). In our phylogenetic analysis (Fig. 3), the three Pneumocystis species group with all Schizosaccharomyces species, S. complicata and T. deforman, with a bootstrap value of 82%, supporting the monophyly of the Taphrinomycotina (9, 31). This phylogeny also illustrates that the mtDNA of three Pneumocystis species appears to be highly diverged as each is separated by long branches, with P. murina and P. carinii being more closely related to each other than either is to P. jirovecii.
DISCUSSION
In the present study, we first sequenced the complete mtDNA of P. carinii and P. murina primarily using next-generation sequencing technologies, and subsequently determined the complete mtDNA sequence of P. jirovecii by PCR using primers based on conserved regions of P. carinii and P. murina mtDNAs. The availability of the complete mtDNA from three Pneumocystis species has enabled us to do a comprehensive comparative analysis of mtDNA sequence and structure.
P. carinii and P. murina mtDNAs are closely related; they share a nearly identical number and order of genes in a linear configuration. In contrast, P. jirovecii likely has a circular mtDNA structure containing nearly the same set of genes, but in a different order from the former two. Although at present, we cannot definitely exclude the possibility that the primary structure of P. jirovecii mtDNA is linear, a circular form of mtDNA is found in most fungal species, either as a primary structure or as a secondary structure resulting from head-to-tail fusion of a linear mtDNA molecule. Linear mtDNA molecules usually possess special structures at their ends, such as long inverted repeats or tandem repeats (26–29), which we did not find in P. jirovecii mtDNA. The differences in organization and structure of the mitochondrial genomes of these species highlight that P. jirovecii can have substantial, potentially biologically relevant, differences from the species commonly used in models to study Pneumocystis infection.
The terminal structure of P. carinii and P. murina mtDNAs is similar to that for P. pijperi and P. jadinii (25), in which the two DNA strands at each end are covalently joined by a single-stranded sequence to form a closed-loop structure. High-throughput 454 sequencing and/or Southern blot analysis of P. carinii and P. murina mtDNAs confirms the presence of head-to-head and tail-to-tail junctions, but the absence of a head-to-tail junction, suggesting that these Pichia and Pneumocystis species have different replication mechanisms for mtDNA. On the basis of these observations, we proposed a self-priming replication model as shown in Fig. 4, which was adapted from the model described for vaccinia virus genome (32). This model involves a site-specific nicking in the TIR region to open the closed loop and produce a free 3′ OH end, which then acts as a primer for DNA replication. If the nick occurs at both ends, the replication starts on both strands in opposite directions, generating two new mtDNA molecules with a closed-loop structure, which correspond to the major bands detected in Southern blot analysis. If the nick occurs at only one end, the replication goes through the closed hairpin of the other end, generating a double-stranded head-to-head or tail-to-tail concatemer, which correspond to the minor bands detected in Southern blot analysis. This model is further supported by a previous report (23) in which two discrete bands were consistently detected at ∼30 and ∼62 kb on CHEF gels of P. carinii, likely representing the monomer mtDNA and the concatemer, respectively. The closed-loop structure may also serve to protect the ends of the linear mtDNA. In this model, a head-to-tail junction or circular forms are not expected to appear, consistent with the sequence and experimental data on P. carinii and P. murina mtDNAs. This model requires further validation and may have important implications in studying other linear DNA genomes. It is unclear why P. carinii and P. murina evolved a linear structure for mtDNA while P. jirovecii mtDNA is apparently circular, and whether one offers a selective advantage over the other (33).
Our phylogenetic study based on mtDNA-encoded proteins clearly shows that P. carinii, P. murina, and P. jirovecii form a monophyletic group, with P. carinii and P. murina being more closely related to each other than to P. jirovecii, consistent not only with the difference in mtDNA gene content and terminal structure in these species, but also with previous phylogenetic studies based on nuclear-encoded genes (15, 34). Our study also supports the grouping of Pneumocystis, Taphrina, and Saitoella together with Schizosaccharomyces to form a monophyletic Taphrinomycotina, as described previously, based on multigene analysis (9, 15, 31).
The availability of the complete mtDNA from 4 isolates of P. jirovecii may facilitate the development of molecular diagnostic and typing methods for PCP. There have been many reports on the use of the mt LSU rRNA and mt SSU rRNA genes in PCR for PCP diagnosis (5, 35–38). Primers used in these reports have been designed based on the only two known short fragments of the P. jirovecii mtDNA without taking into account the sequence variation in large patient populations. We compared the sequences of the 9 reported primers (5 pairs) for the 2 mt genes with the 4 complete P. jirovecii mtDNA sequences obtained in this study, and found that 6 of the 9 primers contain 1–4 variable nucleotides (Supplemental Table S3). The mismatches between the primers and the DNA template likely affect the efficiency of PCR amplification, potentially giving rise to weak signals or false negative results. The availability of multiple complete P. jirovecii mtDNA sequences could help develop optimized diagnostic PCR assays that target regions of the genome that are highly conserved among multiple isolates.
In addition to its use in diagnostic PCR, the mt LSU rRNA gene has been used alone or in combination with other nuclear genomic loci for P. jirovecii typing (3, 39, 40). However, there have been only 3 variable positions observed at this locus (∼350 bp; ref. 3). Although there are other more variable loci available from chromosomes, particularly the internal transcribed spacer (ITS) regions of the nuclear rRNA operon (3, 40, 41), the discriminative power of these targets is insufficient to study evolutionary relatedness among clinical isolates. By comparing the 4 complete P. jirovecii mtDNA sequences obtained in this study, we found that ∼1-kb fragment within the 12-kb noncoding region is highly variable (Supplemental Fig. S1). Preliminary analysis of this region in 23 unrelated P. jirovecii isolates (data not shown) identified 20 unique genotypes, suggesting its potential as a new target for strain typing. Other potential regions for genotyping include loci containing highly variable short tandem repeats, which could be useful for the development of a multilocus variable number of tandem repeats method (42).
The availability of the complete P. jirovecii mtDNA sequence also has the potential to facilitate the development of new drugs to treat PCP. Atovaquone, which targets the cob gene of mtDNA, has been widely used as an important second-line therapy for PCP prophylaxis and treatment. This highlights the importance of mitochondrial function to Pneumocystis viability, and validates mitochondrial genes as therapeutic targets. However, there have been reports of mutations in the P. jirovecii cob gene associated with atovaquone resistance (6, 7). More concerning is the emergence of potential resistance to the first-line anti-PCP drug trimethoprim-sulfamethoxazole (2). Therefore, there is a clear need to discover new agents against PCP. Recently, mitochondria have been recognized as a potentially important drug target in fungi (43) and as important contributors to the virulence and drug tolerance of human fungal pathogens (44). The availability of the complete mtDNA sequence should allow identification of differences between P. jirovecii and human mitochondria in structure, replication mechanisms, and function, which may facilitate identification of new therapeutic targets. Recombinant expression of mitochondrial-encoded proteins could, for example, be used to determine their 3-dimensional structure or to develop in vitro screening assays of potential therapeutic agents. Alternatively, given the lack of an in vitro culture system for P. jirovecii and the similarities of P. jirovecii to yeast, another approach would be to utilize yeast expressing P. jirovecii genes (6).
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the U.S. National Institutes of Health Clinical Center; the National Institute of Allergy and Infectious Diseases (contract NOI-CO-56000); and the National Human Genome Research Institute (grant U54HG003067). Y.X. was supported by a scholarship from Chongqing Medical University (Chongqing, China).
The authors declare no conflicts of interests. The authors thank Rene Costello and Howard Mostowski for their support of the animal studies.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- atp
- ATP synthase
- cob
- cytochrome b
- cox
- cytochrome c oxidase
- Dhfr
- dihydrofolate reductase
- HIV
- human immunodeficiency virus
- mt
- mitochondrial
- mtDNA
- mitochondrial DNA
- mt LSU rRNA
- mitochondrial large subunit ribosomal RNA (rnl)
- mt SSU rRNA
- mitochondrial small subunit ribosomal RNA (rns)
- nad
- NADH dehydrogenase
- orf
- open reading frame
- PCP
- Pneumocystis pneumonia
- Pkd1
- polycystic kidney disease 1
- PCR
- polymerase chain reaction
- qPCR
- quantitative polymerase chain reaction
- rnl
- mitochondrial large subunit ribosomal RNA (mt LSU rRNA)
- rns
- mitochondrial small subunit ribosomal RNA (mt SSU rRNA)
- rRNA
- ribosomal RNA
- SSL
- single-stranded loop
- TIR
- terminal inverted repeat
- tRNA
- transfer RNA
REFERENCES
- 1. Redhead S. A., Cushion M. T., Frenkel J. K., Stringer J. R. (2006) Pneumocystis and Trypanosoma cruzi: nomenclature and typifications. J. Eukaryot. Microbiol. 53, 2–11 [DOI] [PubMed] [Google Scholar]
- 2. Kovacs J. A., Masur H. (2009) Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment. JAMA 301, 2578–2585 [DOI] [PubMed] [Google Scholar]
- 3. Beard C. B., Roux P., Nevez G., Hauser P. M., Kovacs J. A., Unnasch T. R., Lundgren B. (2004) Strain typing methods and molecular epidemiology of Pneumocystis pneumonia. Emerg. Infect. Dis. 10, 1729–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wakefield A. E. (1996) DNA sequences identical to Pneumocystis carinii f. sp. carinii and Pneumocystis carinii f. sp. hominis in samples of air spora. J. Clin. Microbiol. 34, 1754–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wakefield A. E., Pixley F. J., Banerji S., Sinclair K., Miller R. F., Moxon E. R., Hopkin J. M. (1990) Detection of Pneumocystis carinii with DNA amplification. Lancet 336, 451–453 [DOI] [PubMed] [Google Scholar]
- 6. Kessl J. J., Hill P., Lange B. B., Meshnick S. R., Meunier B., Trumpower B. L. (2004) Molecular basis for atovaquone resistance in Pneumocystis jirovecii modeled in the cytochrome bc(1) complex of Saccharomyces cerevisiae. J. Biol. Chem. 279, 2817–2824 [DOI] [PubMed] [Google Scholar]
- 7. Walker D. J., Wakefield A. E., Dohn M. N., Miller R. F., Baughman R. P., Hossler P. A., Bartlett M. S., Smith J. W., Kazanjian P., Meshnick S. R. (1998) Sequence polymorphisms in the Pneumocystis carinii cytochrome b gene and their association with atovaquone prophylaxis failure. J. Infect. Dis. 178, 1767–1775 [DOI] [PubMed] [Google Scholar]
- 8. Edman J. C., Kovacs J. A., Masur H., Santi D. V., Elwood H. J., Sogin M. L. (1988) Ribosomal RNA sequence shows Pneumocystis carinii to be a member of the fungi. Nature 334, 519–522 [DOI] [PubMed] [Google Scholar]
- 9. Liu Y., Leigh J. W., Brinkmann H., Cushion M. T., Rodriguez-Ezpeleta N., Philippe H., Lang B. F. (2009) Phylogenomic analyses support the monophyly of Taphrinomycotina, including Schizosaccharomyces fission yeasts. Mol. Biol. Evol. 26, 27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kovacs J. A., Halpern J. L., Swan J. C., Moss J., Parrillo J. E., Masur H. (1988) Identification of antigens and antibodies specific for Pneumocystis carinii. J. Immunol. 140, 2023–2031 [PubMed] [Google Scholar]
- 11. Bishop L. R., Helman D., Kovacs J. A. (2012) Discordant antibody and cellular responses to Pneumocystis major surface glycoprotein variants in mice. BMC Immunol. 13, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vestereng V. H., Bishop L. R., Hernandez B., Kutty G., Larsen H. H., Kovacs J. A. (2004) Quantitative real-time polymerase chain-reaction assay allows characterization of Pneumocystis infection in immunocompetent mice. J. Infect. Dis. 189, 1540–1544 [DOI] [PubMed] [Google Scholar]
- 13. Larsen H. H., Kovacs J. A., Stock F., Vestereng V. H., Lundgren B., Fischer S. H., Gill V. J. (2002) Development of a rapid real-time PCR assay for quantitation of Pneumocystis carinii f. sp. carinii. J. Clin. Microbiol. 40, 2989–2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodova M., Islam M. R., Peterson K. R., Calvet J. P. (2003) Remarkable sequence conservation of the last intron in the PKD1 gene. Mol. Biol. Evol. 20, 1669–1674 [DOI] [PubMed] [Google Scholar]
- 15. Keely S. P., Fischer J. M., Cushion M. T., Stringer J. R. (2004) Phylogenetic identification of Pneumocystis murina sp. nov., a new species in laboratory mice. Microbiology 150, 1153–1165 [DOI] [PubMed] [Google Scholar]
- 16. Xu Z., Lance B., Vargas C., Arpinar B., Bhandarkar S., Kraemer E., Kochut K. J., Miller J. A., Wagner J. R., Weise M. J., Wunderlich J. K., Stringer J., Smulian G., Cushion M. T., Arnold J. (2003) Mapping by sequencing the Pneumocystis genome using the ordering DNA sequences V3 tool. Genetics 163, 1299–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maccallum I., Przybylski D., Gnerre S., Burton J., Shlyakhter I., Gnirke A., Malek J., McKernan K., Ranade S., Shea T. P., Williams L., Young S., Nusbaum C., Jaffe D. B. (2009) ALLPATHS 2: small genomes assembled accurately and with high continuity from short paired reads. Genome Biol. 10, R103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schattner P., Brooks A. N., Lowe T. M. (2005) The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 33, W686–W689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Edgar R. C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Talavera G., Castresana J. (2007) Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 56, 564–577 [DOI] [PubMed] [Google Scholar]
- 21. Stamatakis A. (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690 [DOI] [PubMed] [Google Scholar]
- 22. Ma L., Kutty G., Jia Q., Kovacs J. A. (2003) Characterization of variants of the gene encoding the p55 antigen in Pneumocystis from rats and mice. J. Med. Microbiol. 52, 955–960 [DOI] [PubMed] [Google Scholar]
- 23. Sesterhenn T. M., Slaven B. E., Keely S. P., Smulian A. G., Lang B. F., Cushion M. T. (2010) Sequence and structure of the linear mitochondrial genome of Pneumocystis carinii. Mol. Genet. Genomics. 283, 63–72 [DOI] [PubMed] [Google Scholar]
- 24. Bullerwell C. E., Leigh J., Forget L., Lang B. F. (2003) A comparison of three fission yeast mitochondrial genomes. Nucleic Acids Res. 31, 759–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dinouel N., Drissi R., Miyakawa I., Sor F., Rousset S., Fukuhara H. (1993) Linear mitochondrial DNAs of yeasts: closed-loop structure of the termini and possible linear-circular conversion mechanisms. Mol. Cell. Biol. 13, 2315–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morin G. B., Cech T. R. (1986) The telomeres of the linear mitochondrial DNA of Tetrahymena thermophila consist of 53 bp tandem repeats. Cell 46, 873–883 [DOI] [PubMed] [Google Scholar]
- 27. Nosek J., Tomaska L., Fukuhara H., Suyama Y., Kovac L. (1998) Linear mitochondrial genomes: 30 years down the line. Trends Genet. 14, 184–188 [DOI] [PubMed] [Google Scholar]
- 28. Perez-Brocal V., Shahar-Golan R., Clark C. G. (2010) A linear molecule with two large inverted repeats: the mitochondrial genome of the stramenopile Proteromonas lacertae. Genome Biol. Evol. 2, 257–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Valach M., Farkas Z., Fricova D., Kovac J., Brejova B., Vinar T., Pfeiffer I., Kucsera J., Tomaska L., Lang B. F., Nosek J. (2011) Evolution of linear chromosomes and multipartite genomes in yeast mitochondria. Nucleic Acids Res. 39, 4202–4219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stiller J. W., Hall B. D. (1999) Long-branch attraction and the rDNA model of early eukaryotic evolution. Mol. Biol. Evol. 16, 1270–1279 [DOI] [PubMed] [Google Scholar]
- 31. James T. Y., Kauff F., Schoch C. L., Matheny P. B., Hofstetter V., Cox C. J., Celio G., Gueidan C., Fraker E., Miadlikowska J., Lumbsch H. T., Rauhut A., Reeb V., Arnold A. E., Amtoft A., Stajich J. E., Hosaka K., Sung G. H., Johnson D., O'Rourke B., Crockett M., Binder M., Curtis J. M., Slot J. C., Wang Z., Wilson A. W., Schussler A., Longcore J. E., O'Donnell K., Mozley-Standridge S., Porter D., Letcher P. M., Powell M. J., Taylor J. W., White M. M., Griffith G. W., Davies D. R., Humber R. A., Morton J. B., Sugiyama J., Rossman A. Y., Rogers J. D., Pfister D. H., Hewitt D., Hansen K., Hambleton S., Shoemaker R. A., Kohlmeyer J., Volkmann-Kohlmeyer B., Spotts R. A., Serdani M., Crous P. W., Hughes K. W., Matsuura K., Langer E., Langer G., Untereiner W. A., Lucking R., Budel B., Geiser D. M., Aptroot A., Diederich P., Schmitt I., Schultz M., Yahr R., Hibbett D. S., Lutzoni F., McLaughlin D. J., Spatafora J. W., Vilgalys R. (2006) Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 443, 818–822 [DOI] [PubMed] [Google Scholar]
- 32. Baroudy B. M., Venkatesan S., Moss B. (1982) Incompletely base-paired flip-flop terminal loops link the two DNA strands of the vaccinia virus genome into one uninterrupted polynucleotide chain. Cell 28, 315–324 [DOI] [PubMed] [Google Scholar]
- 33. Kosa P., Valach M., Tomaska L., Wolfe K. H., Nosek J. (2006) Complete DNA sequences of the mitochondrial genomes of the pathogenic yeasts Candida orthopsilosis and Candida metapsilosis: insight into the evolution of linear DNA genomes from mitochondrial telomere mutants. Nucleic Acids Res. 34, 2472–2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ma L., Imamichi H., Sukura A., Kovacs J. A. (2001) Genetic divergence of the dihydrofolate reductase and dihydropteroate synthase genes in Pneumocystis carinii from 7 different host species. J. Infect. Dis. 184, 1358–1362 [DOI] [PubMed] [Google Scholar]
- 35. Helweg-Larsen J., Jensen J. S., Dohn B., Benfield T. L., Lundgren B. (2002) Detection of Pneumocystis DNA in samples from patients suspected of bacterial pneumonia—a case-control study. BMC Infect. Dis. 2, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hunter J. A., Wakefield A. E. (1996) Genetic divergence at the mitochondrial small subunit ribosomal RNA gene among isolates of Pneumocystis carinii from five mammalian host species. J. Eukaryot. Microbiol. 43, 24S–25S [DOI] [PubMed] [Google Scholar]
- 37. Maskell N. A., Waine D. J., Lindley A., Pepperell J. C., Wakefield A. E., Miller R. F., Davies R. J. (2003) Asymptomatic carriage of Pneumocystis jirovecii in subjects undergoing bronchoscopy: a prospective study. Thorax 58, 594–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsolaki A. G., Beckers P., Wakefield A. E. (1998) Pre-AIDS era isolates of Pneumocystis carinii f. sp. hominis: high genotype similarity with contemporary isolates. J. Clin. Microbiol. 36, 90–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gianella S., Haeberli L., Joos B., Ledergerber B., Wuthrich R. P., Weber R., Kuster H., Hauser P. M., Fehr T., Mueller N. J. (2010) Molecular evidence of interhuman transmission in an outbreak of Pneumocystis jirovecii pneumonia among renal transplant recipients. Transpl. Infect. Dis. 12, 1–10 [DOI] [PubMed] [Google Scholar]
- 40. Ma L., Kutty G., Jia Q., Imamichi H., Huang L., Atzori C., Beckers P., Groner G., Beard C. B., Kovacs J. A. (2002) Analysis of variation in tandem repeats in the intron of the major surface glycoprotein expression site of the human form of Pneumocystis carinii. J. Infect. Dis. 186, 1647–1654 [DOI] [PubMed] [Google Scholar]
- 41. Lee C. H., Helweg-Larsen J., Tang X., Jin S., Li B., Bartlett M. S., Lu J. J., Lundgren B., Lundgren J. D., Olsson M., Lucas S. B., Roux P., Cargnel A., Atzori C., Matos O., Smith J. W. (1998) Update on Pneumocystis carinii f. sp. hominis typing based on nucleotide sequence variations in internal transcribed spacer regions of rRNA genes. J. Clin. Microbiol. 36, 734–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weniger T., Krawczyk J., Supply P., Niemann S., Harmsen D. (2010) MIRU-VNTRplus: a web tool for polyphasic genotyping of Mycobacterium tuberculosis complex bacteria. Nucleic Acids Res. 38, W326–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martins Vde P., Dinamarco T. M., Curti C., Uyemura S. A. (2011) Classical and alternative components of the mitochondrial respiratory chain in pathogenic fungi as potential therapeutic targets. J. Bioenerg. Biomembr. 43, 81–88 [DOI] [PubMed] [Google Scholar]
- 44. Shingu-Vazquez M., Traven A. (2011) Mitochondria and fungal pathogenesis: drug tolerance, virulence, and potential for antifungal therapy. Eukaryot. Cell 10, 1376–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.