Abstract
Bladder urothelium senses and communicates information about bladder fullness. However, the mechanoreceptors that respond to tissue stretch are poorly defined. Integrins are mechanotransducers in other tissues. Therefore, we eliminated β1-integrin selectively in urothelium of mice using Cre-LoxP targeted gene deletion. β1-Integrin localized to basal/intermediate urothelial cells by confocal microscopy. β1-Integrin conditional-knockout (β1-cKO) mice lacking urothelial β1-integrin exhibited down-regulation and mislocalization of α3- and α5-integrins by immunohistochemistry but, surprisingly, had normal morphology, permeability, and transepithelial resistance when compared with Cre-negative littermate controls. β1-cKO mice were incontinent, as judged by random urine leakage on filter paper (4-fold higher spotting, P<0.01; 2.5-fold higher urine area percentage, P<0.05). Urodynamic function assessed by cystometry revealed bladder overfilling with 80% longer intercontractile intervals (P<0.05) and detrusor hyperactivity (3-fold more prevoid contractions, P<0.05), but smooth muscle contractility remained intact. ATP secretion into the lumen was elevated (49 vs. 22 nM, P<0.05), indicating abnormal filling-induced purinergic signaling, and short-circuit currents (measured in Ussing chambers) revealed 2-fold higher stretch-activated ion channel conductances in response to hydrostatic pressure of 1 cmH2O (P<0.05). We conclude that loss of integrin signaling from urothelium results in incontinence and overactive bladder due to abnormal mechanotransduction; more broadly, our findings indicate that urothelium itself directly modulates voiding.—Kanasaki, K., Yu, W., von Bodungen, M., Larigakis, J. D., Kanasaki, M., Ayala de la Pena, F., Kalluri, R., Hill, W.G. Loss of β1-integrin from urothelium results in overactive bladder and incontinence in mice: a mechanosensory rather than structural phenotype.
Keywords: micturition, voiding dysfunction, mechanotransduction, urinary tract
Manifestations of lower urinary tract dysfunction, such as overactive bladder, urge incontinence, neurogenic bladder, and painful bladder syndrome, are the result of miscommunication at the cellular level between nerves, interstitial cells, smooth muscle, and urothelium (1, 2). In terms of relative importance, the urothelium has long been considered the least of these. However, an emerging recognition indicates that urothelium is more than just a barrier to urine and bacteria and is a dynamic, sensory tissue in its own right (3). The presence of mechanosensitive ion channels (4), transient receptor potential (TRP) channels (5), and many receptor families attest to the ability of urothelium to respond to external cues (6). Urothelial cells also can elaborate a complex array of signaling molecules (e.g., ATP, nitric oxide, acetylcholine), and these may act in either autocrine or paracrine fashion to modulate responses ultimately involving afferent nerve pathways (3, 7).
When the bladder fills, the 3-dimensional morphology of the urothelium changes from highly infolded to smooth and spherical and is subject to mechanical tension that is related to the radius, wall thickness, and intravesical pressure according to LaPlace's law. In vitro experiments in Ussing chambers, in which the urothelium is subjected to higher membrane tension on addition of a hydrostatic pressure, show markedly elevated secretion of ATP from both the basal and lumenal surfaces (8, 9). The amount of ATP released increases with membrane tension (10) and, since there are no ectonucleotidases on the apical membrane of umbrella cells (11), will persist in urine, with the potential to stimulate intracellular pathways downstream of P2 purinergic receptors (12). ATP released serosally, meanwhile, has immediate access to suburothelial neural afferents expressing P2X2/3 receptors and acts as a sensory neurotransmitter (13, 14).
As a result of these observations, we asked whether the urothelium, through its ability to communicate information related to bladder fullness, can regulate the voiding reflex. Normally, this function is ascribed to efferent nerve activity acting on detrusor smooth muscle to elicit contraction. However, studies of knockout and knock-in mice have suggested a more direct role for urothelium. In mice lacking either the P2X3 purinergic receptor (10) or TRPV1, the capsaicin-activated ion channel found in afferent nerves (15), bladder function was impaired. In mice lacking P2X3, urothelial ATP release was normal, but sensory nerve responses were not. TRPV1-deficient mice, in contrast, had impaired ATP release and abnormal urodynamics (15). Likewise, TRPV4-deficient mice exhibited bladder dysfunction characterized by abnormal cystometrograms and attenuated ATP release (16). These results indicated that urothelial ATP release helps coordinate membrane trafficking in urothelium in an autocrine fashion and suggested that urothelium might also help modulate reflex control of bladder filling and emptying (3). However, because these studies were performed in conventional knockout animals, which were therefore null for critical receptors in all cell types, including neurons and detrusor smooth muscle, they do not define the role of urothelium itself in regulating bladder function.
Integrins are transmembrane proteins with binding affinities for both extracellular matrix and intracellular actin. As a consequence, they physically link the cell to its extracellular environment and facilitate bidirectional signaling. They exist in all cells as heterodimers (one α and one β subunit) in the plasma membrane. They possess large extracellular domains, which bind to elements of the extracellular matrix (ECM), and on the inside, are linked to the actin cytoskeleton through a complex array of multiple adaptor proteins. As the main receptors that connect the cytoskeleton to the ECM, integrins are intimately involved in cellular sensing of force (17–20). We hypothesized, therefore, that integrins could be critical upstream regulators of the mechanosensory apparatus in the urothelium.
To determine the role of integrins in urothelial function, we used the Cre-LoxP targeted gene deletion system to knock out β1-integrin selectively in urothelium. As all of the major integrin heterodimers of urothelium are thought to contain β1 (mainly α2β1 and α3β1), this strategy theoretically creates an integrin-null transitional epithelium; except at the urothelial-stromal interface, where integrin α6β4 is thought to be important for substratum adhesion (21–24). We demonstrate that β1-integrin conditional-knockout (β1-cKO) mice lacking β1-containing integrins are incontinent and have mechanosensory defects but retain normal urothelial structure and barrier function. These results convincingly demonstrate that urothelium plays an important role in regulating voiding through mechanosensory signaling.
MATERIALS AND METHODS
Generating the uroplakin II (UPII)-Cre recombinase mouse
We generated a transgenic mouse that selectively expresses Cre recombinase in bladder epithelium by creating a construct in which the urothelial specific uroplakin II gene promoter drives expression of Cre. We cloned mouse UPII promoter (accession no. EF467361) from BAC clone RP24-308H8 by a PCR-based approach. During cloning, we found that a portion of the previously reported sequence of UPII promoter region (accession no. U14421) was miscloned, because 2 SacI restriction enzyme sites (from −1262 to −2805 from exon1) in the UPII promoter were oppositely inserted (25). UPII-Cre mice were generated by pronuclei microinjection of the indicated construct (see Fig. 1A). We obtained β1-integrin floxed/floxed (β1fl/fl) mice from the Jackson Laboratory (Bar Harbor, ME, USA; strain: B6;129-Itgb1tm1Efu/J). The β1-integrin urothelium-specific β1-cKO mouse was generated by crossing the UP2-Cre mice with the β1fl/fl mice and then interbreeding the offspring. The UPII-Cre; β1fl/fl mice were born in an expected mendelian ratio. Mice were maintained at the Beth Israel Deaconess Medical Center (BIDMC) animal facility under standard conditions. The male mice used in these experiments were between 8 and 12 wk of age. All animals used in these studies were homozygous for the floxed β1-integrin gene. The term “controls” refers to floxed mice not expressing Cre, i.e., Cre−, whereas “β1-cKO ” refers to those animals that do express Cre, i.e., Cre+. All animal studies and the experiments described were reviewed and approved by the institutional animal care and use committee of the BIDMC.
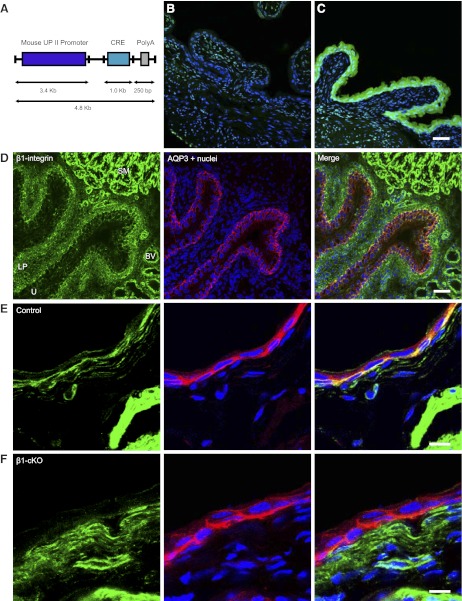
Figure 1.
UPII-Cre mice specifically excise flanking loxP sites in urothelium, and UPII-Cre/β1fl/fl mice lose expression in the basal cells of the urothelium. A) Schematic showing construct used to create UPII-Cre mice. B, C) Rosa-R26R-YFP floxed reporter mouse crossed with UPII-Cre mouse reveals specific expression of YFP in Cre-expressing urothelium (C) but not in Cre− littermate control (B). D) Immunofluorescent localization of β1-integrin in bladder shows modest expression in urothelium (delineated by AQP3 staining in red) located primarily along the basal surface (yellow in merged image). U, urothelium; LP, lamina propria; SM, smooth muscle; BV, blood vessel. E) Integrin expression in control mice (green) seen as yellow signal in urothelium (integrin colocalizing with red AQP3; Cre− littermates of β1-cKOs). F) Loss of integrin expression in urothelium of β1-cKOs. Scale bars = 50 μm (C, D); 10 μm (E, F).
Scanning laser confocal immunofluorescent microscopy
Bladder preparation and immunostaining for confocal immunofluorescent microscopy was performed essentially as described previously (26). Bladders were excised from euthanized animals, washed with PBS, and either opened and pinned flat on a silicone rubber mat for cryopreservation in 30% (w/v) sucrose/PBS (1–3 h at 4°C) or placed intact into a cryomold (Tissue-Tek, Torrance, CA, USA) containing optimal cutting temperature (OCT) compound and snap-frozen in liquid N2 vapor. Frozen blocks were either stored at −80°C or immediately cut into 4-μm-thick sections using a model CM1850 cryostat (Leica Microsystems, Nussloch, Germany). Sections on slides were allowed to dehydrate for 1 min and were then washed 3 times for 5 min with PBS before fixation in 100% methanol (−20°C) for 5 min. Slides were then washed 3 times with PBS, 3 times with block solution [PBS containing 0.5% (w/v) fish gelatin and 0.025% (w/v) saponin], then incubated in block solution for 30 min at room temperature. Sections were immunostained with rat anti-β1-integrin antibody (BD Pharmingen, San Jose, CA, USA; 1:100 in block solution) and goat anti-aquaporin 3 (AQP3; Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:100 in block solution) overnight at 4°C, and washed 3 times for 5 min with block solution prior to incubation with secondary antibodies and rabbit anti-rat and rabbit anti-goat IgG conjugated to Alexa 488 and Alexa 546, respectively (Invitrogen, Carlsbad, CA, USA; 1:100) and TO-PRO-3 iodide (Invitrogen; 1:1000) for 60 min. Slides were washed with block solution 3 times, then with PBS 3 times before addition of postfix solution [4% (w/v) paraformaldehyde and 100 mM sodium cacodylate, pH 7.4] for 10 min. A PBS wash was followed by quenching in PBS containing 18 mM glycine, 68 mM NH4Cl, and 0.1% Triton X-100 (pH 8.0) for 10 min, then requenching after another PBS wash, final washing with PBS, and addition of p-diaminobenzidine antifade mounting medium and coverslip. Microscopy was performed on an LSM 510 META confocal microscope (Carl Zeiss MicroImaging, New York, NY, USA) using either a ×20 or ×63 objective.
Immunoperoxidase staining
Deparaffinized (2 min, xylene, 4 times; 1 min, 100% ethanol twice; 30 s in 95% ethanol, 45 s 70% ethanol, 1 min in distilled water) or frozen bladder sections were labeled with anti-α3-integrin antibody (BD Pharmingen) or anti-α5-integrin antibody (Chemicon International, Temecula, CA, USA), respectively. Immunohistochemistry analysis was performed with the Vectastain ABC kit using DAB peroxidase substrate reagent (Vector Laboratories, Inc., Burlingame, CA, USA). Antigen retrieving in paraffin sections was performed with 10 mM citrate buffer. At least 3 mice/group were labeled and analyzed.
Hematoxylin and eosin (H&E) staining
H&E staining was performed using standard methods.
Immunoblotting
Bladders were excised and everted onto a pipette tip so that the urothelium was externalized. Urothelium was then lysed for 150 s in 200 μl RIPA buffer (150 mM NaCl, 50 mM Tris, 1% Igepal, 0.5% deoxycholic acid, and 0.1% SDS, pH 7.4) containing protease inhibitors (Complete, Mini; Roche Applied Science, Indianapolis, IN, USA). Proteins were resolved by SDS-PAGE and transferred to Immun-Blot PVDF (Bio-Rad, Hercules, CA, USA), and the blots were probed with antibodies to β1-integrin (Epitomics, Burlingame, CA, USA) and β-actin (Sigma, St. Louis, MO, USA) using standard methods. Bands were visualized with Amersham ECL reagent (Amersham, Arlington Heights, IL, USA); exposed and developed film was scanned, and the image contrast was corrected with Photoshop (Adobe Systems, San Jose, CA, USA). Images were imported into Adobe Illustrator CS2 (Adobe) for generation of figures.
Electron microscopy (EM)
EM was performed as described elsewhere (27). For transmission EM (TEM) and scanning EM (SEM), mouse bladders were placed in fixative containing 2.0% (v/v) glutaraldehyde and 2.0% (w/v) paraformaldehyde in 100 mM sodium cacodylate (pH 7.4), 1 mM CaCl2, and 0.5 mM MgCl2 for 60 min at room temperature. For TEM, the fixed tissue was washed for 15 min en bloc in 100 mM sodium cacodylate buffer (pH 7.4) and then treated with 1% (v/v) OsO4 in 100 mM sodium cacodylate buffer (pH 7.4) for 60 min on ice. After several water rinses, the samples were stained en bloc overnight with 0.5% (w/v) uranyl acetate in water. Samples were dehydrated in a graded series of ethanol [40, 50, 75, 80, 90, 95, and 100% (v/v) in water on ice] and then in 100% propylene oxide (EMS, Hatfield, PA, USA). Tissues were embedded in epoxy resin (LX-112; Ladd Research Industries, Burlington, VT, USA) and sectioned and imaged for TEM by the EM core facility at BIDMC. Toluidine blue staining was also performed by the BIDMC EM core facility. Samples for SEM had 1% potassium ferrocyanide included in osmium fixative. Following this step, they were dehydrated and prepared in a critical point drier for sputter coating and imaging by the SEM core facility at the Harvard School of Public Health.
Measurements of permeability and transepithelial resistance (TER)
TER and urea and water permeability were measured as described elsewhere (28, 29). Briefly, excised bladders were opened in the sagital plane from urethra to dome and carefully mounted on tissue rings that exposed 0.35 cm2 of surface area. Tissue rings were then mounted between the two halves of modified Ussing chambers and bathed in Krebs-Ringers buffer (110 mM NaCl, 5.8 mM KCl, 25 mM NaHCO3, 1.2 mM KH2PO4, 2.0 mM CaCl2, 1.2 mM MgSO4, and 11.1 mM glucose, pH 7.4; bubbled with 95% O2:5% CO2) and maintained at 37°C. Tritiated water (1 μCi/ml) and [14C]urea (0.25 μCi/ml) were added to the luminal chamber; two 100-μl aliquots were removed and the volume replaced from both the basolateral and apical chambers for scintillation counting at time 0. Repeated sampling was performed at 15 min intervals for 60 min, at which point 0.5% (v/v) Triton X-100 was added to the apical chamber to dissolve the apical membrane, and a further 60 min of sampling was performed. Removal of the apical membrane as the rate-limiting step for permeation allows calculation of the resistance offered by unstirred layers; hence, the permeability coefficient of the umbrella cell apical membrane can be derived (28, 29). TER was monitored by use of Ag-AgCl current and voltage electrodes connected to an epithelial voltage clamp (model EC825; Warner Instruments, Hamden, CT, USA), MacLab/8S (AD Instruments, Sydney, NSW, Australia), and an iMac computer (Apple, Cupertino, CA, USA) with LabChart software (AD Instruments).
Short-circuit current (Isc) measurements
Isc values were measured using bladders mounted as described above and with the voltage clamped to 0 mV in short-circuit mode. Prior to mounting bladders in the chamber, electrode voltage asymmetries were adjusted to 0, and fluid resistance compensation was performed. To induce stretch-activated ion conductances, warmed Krebs-Ringers buffer was added rapidly to the luminal chamber until a pressure head of 1 cm was achieved. An identical volume was removed at the end of the stretch stimulus. Typically, 4 stretch and release cycles were performed, and the responses were averaged.
Cystometry
Cystometry was performed with HEPES (10 mM) buffered saline infusion as described previously (30). Flame-flanged PE50 tubing, connected to a pressure transducer and coupled to a computerized World Precision Instruments Model DBA8000 CMG system (World Precision Instruments, Sarasota, FL, USA), was inserted through the apical dome of the bladder. Tubing was implanted under isoflurane anesthesia and secured in place with a nylon purse-string suture. Mice were placed into a Bollman mouse restrainer, and anesthesia was maintained throughout the experiment using s.c. urethane (100–125 μl of 250 mg/ml solution; 1.3 mg/g). The mice were stabilized in the restrainer for 45–60 min as buffered saline was infused at 20 μl/min by syringe pump (Braintree Scientific, Braintree, MA, USA). Voiding occurs naturally through the urethra. Following stabilization, the infusion rate was increased to 30 μl/min, and recordings of bladder activity were monitored continuously using LabChart software (AD Instruments). For ATP measurements in voided cystometric fluid, 6–8 individual voids/animal were collected and immediately frozen on dry ice. ATP was assayed by luciferase and luminometry using a bioluminescent assay kit from Sigma. Data from multiple voids were averaged, and the mean value was used for each animal.
Urine spot assay
Individual mice were placed in clean cages with filter paper taped to and covering the entire floor surface for 6 h in a quiet environment. Urine spots were subsequently visualized under UV light and digitally photographed. Images were thresholded to black and white, and quantitation was performed with ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA).
Statistics
Data are expressed as means ± sem. We made comparisons among data with Student's t test. In most cases, because we were predicting the directionality of effects, we used 1-tailed tests. Values of P < 0.05 were considered statistically significant.
RESULTS
Urothelial-specific UPII-Cre mouse
To investigate the function of integrins within the urothelium, we used the Cre-loxP conditional-knockout system. Uroplakins are expressed solely in urothelium (31); therefore, we utilized the UPII promoter and created a targeting construct as shown in Fig. 1A, which was injected into blastocysts to create a UPII-Cre mouse line described previously (25). To determine whether the construct produced the expected expression pattern, UPII-Cre mice were crossed with Rosa-R26R-yellow fluorescent protein (YFP) floxed reporter mice (Jackson Laboratory, strain B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J; ref. 32) as shown in Fig. 1B, C. Functional Cre excises a “stop cassette ” at 2 loxP sites, with subsequent expression of YFP driven by the ROSA promoter. YFP is not expressed in bladders of Cre− littermates (Fig. 1B), whereas Cre+ animals (Fig. 1C) exhibit abundant and highly specific expression of YFP in urothelium. YFP was not expressed in lamina propria or smooth muscle of the bladder, nor was it expressed in the kidneys or lungs (not shown), which confirms specificity of Cre recombinase expression in the cell type of interest.
Expression of β1-integrin in bladder
Initial experiments were performed to localize β1-integrin in bladder and urothelium (Fig. 1D, E). Low-power magnification of bladder cryosections coimmunostained with antibodies to β1-integrin and AQP3 (a urothelial marker) revealed highest expression of β1-integrin in smooth muscle, intermediate expression in lamina propria and blood vessels, and modest expression in urothelium (Fig. 1D). AQP3 staining, indicated in red in Fig. 1D, delineates the urothelial compartment and the merged image indicates a basal cell localization for β1-integrin, along the interface with the lamina propria (yellow colocalization). Higher-magnification imaging on an opened bladder confirms essentially basal cell expression of this integrin (Fig. 1E, merge).
Loss of β1-integrin from the urothelium
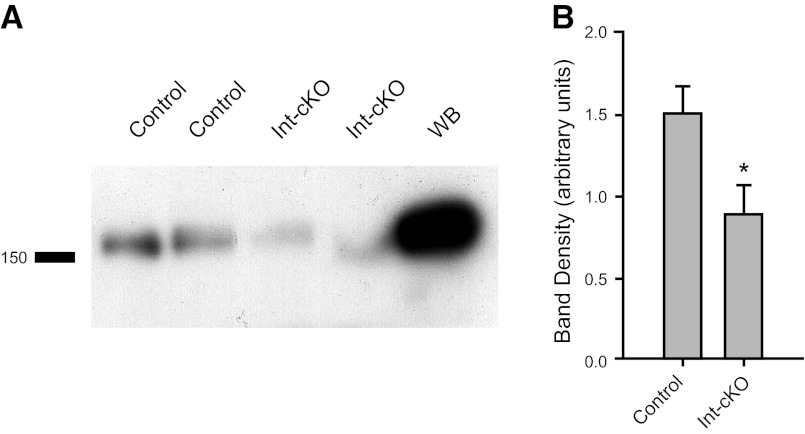
UPII-Cre+ mice were crossed with β1fl/fl mice obtained from Jackson Laboratories to obtain urothelial-specific knockout of the protein. To demonstrate urothelial-specific loss of β1-integrin, we used immunofluorescence, and a substantial loss of integrin in the urothelium (Fig. 1F) compared to controls (Fig. 1E) was evident, as judged by the lack of yellow signal in the merged image. Attempts to show knockdown by immunoblotting were only partially successful due to difficulties associated with contamination from the lamina propria. However, it can be seen in Fig. 2 that a significant loss of β1-integrin in β1-cKO mice from a urothelium-enriched detergent lysate occurred.
Figure 2.
Immunoblotting confirms a reduction in β1-integrin expression. A) Urothelial lysates (50 μg) from control and β1-cKO bladders were immunoblotted with β1-integrin rabbit monoclonal antibody. WB, whole bladder. B) Quantitation of β1-integrin expression by densitometry was normalized to β-actin signal (n=4 bladders/group, means ± sem). *P < 0.05.
β1-integrin knockdown leads to down-regulation and loss of localization of α3-integrin
Immunostaining of paraffin sections for α3-integrin, a β1-integrin binding partner in urothelium, revealed that loss of β1 results in diminished expression of α3 and a loss of localization to the basal and intermediate cell plasma membrane (Fig. 3C, D vs. A, B). Similar results were also seen for α5-integrin, with a complete loss of detectable antibody staining in the β1-cKO urothelium (not shown). Since functional integrins require both α and β subunits, normal heterodimer formation and trafficking to the cell surface is abrogated by selective knockdown of β1-integrin.
Figure 3.

Immunoperoxidase staining of α3-integrin reveals mislocalization and down-regulation of α3-integrin in β1-cKO bladders. A, B) Control bladders. C, D) β1-cKO bladders. U, urothelium; LP, lamina propria.
No evidence for altered morphology or loss of barrier function in β1-cKO mice
Integrins are cell-matrix and cell-cell adhesion molecules with important structural functions in many tissues. Targeted deletion of β1-integrin from podocytes, for example, caused gross abnormalities in the glomerular basement membrane (33). The question of whether similar basement membrane or connective tissue defects might be seen in bladder was therefore investigated. Examination of urothelial morphology by H&E staining, TEM, and SEM revealed no observable differences between β1-cKO mice and littermate controls (Fig. 4). At the level of light microscopy, urothelium had similar thickness and integrity, and no evidence indicated significant separation or derangement from the lamina propria (Fig. 4A, D). TEM did not detect abnormal umbrella cell morphology, and intact urothelium was found through several layers (Fig. 4B, E); whereas scanning EM of the luminal surface showed that umbrella cellss had intact tight junctions and the usual highly ridged surface morphology characteristic of the presence of uroplakin plaques (Fig. 4C, F).
Figure 4.
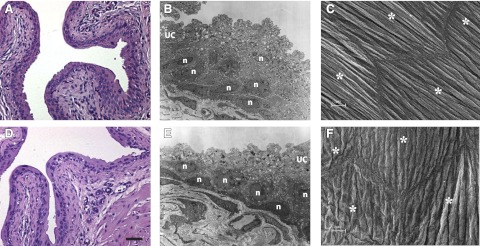
Bladder morphology looks normal for β1-cKO mice. Control (A, B, C) and β1-cKO mouse bladders (D, E, F) were examined by H&E staining (A, D), TEM (B, E), and SEM (C, F). Urothelial morphology and ultrastructure look normal in all groups. B, E) UC, umbrella cell; n, nuclei of intermediate and basal cells. C, F) Adjacent UCs shown by asterisks. No evidence indicates loss of surface cells, injury, or dedifferentiation. Tight junctions appear normal. Scale bars = 50 μm (A, D); = 10 μm (C–F).
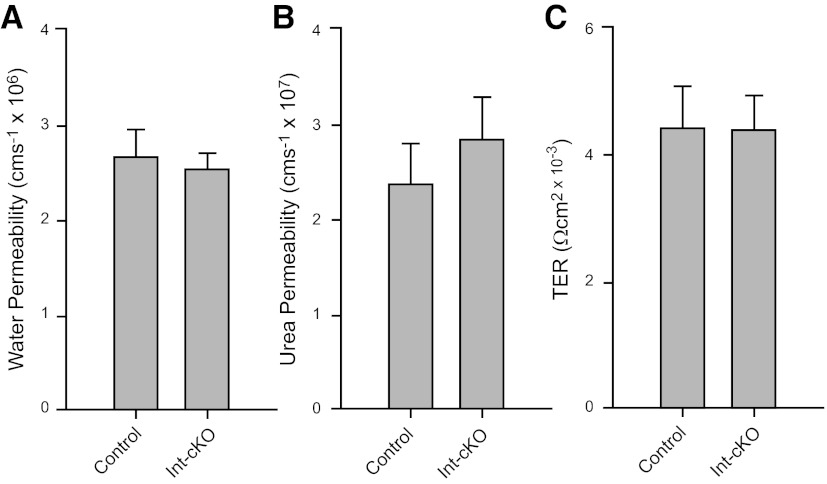
Urothelial integrity was assessed further by measuring water and urea permeability and TER (Fig. 5). No difference was found in any of these parameters, which suggested that barrier function was intact and that the urothelium had maintained one of its primary functional attributes.
Figure 5.
Barrier function is unimpaired in β1-cKO mice. Bladders from control mice and β1-cKO mice were mounted in modified Ussing chambers for measurements of permeability to isotopic water (A) and urea (B) and for transepithelial resistance (C). Permeability coefficients were calculated from the flux rates across the urothelium. Data are shown as means ± sem, with n = 5–8 mice/group. There were no significant differences in permeability or TER.
Mice without urothelial β1-integrin are incontinent
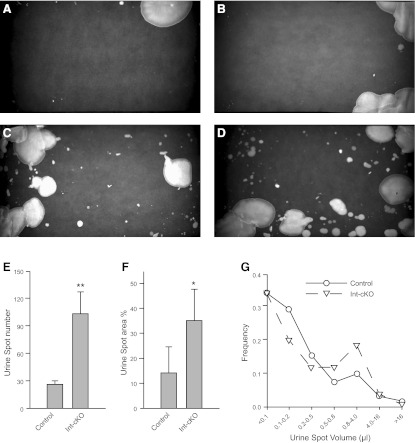
To examine micturition function, we placed mice individually into cages with filter paper on the floor for 6 h. Urine spots were visualized under UV light. Each mouse was tested in the same way on 3 consecutive days. Figure 6A–D shows representative urine spot patterns from 4 different mice. Control mice (Fig. 6A, B) demonstrated consistent “sanitary” voiding behavior, which manifested as urinating in a defined area, usually in a corner or along one or more edges of the cage. β1-cKO mice, however, exhibited a dramatic loss of voiding control, as evidenced by an inability to restrict voiding location and the presence of a distribution of spot sizes, which ranged from small to large, indicated random volume release (Fig. 6C, D). This behavior was quite reproducible and strongly suggested that β1-cKO mice were incontinent. Image analysis of the spot patterns allowed quantitation of the number of nonedge urine spots (Fig. 6E), the area fraction of the filter paper covered with urine (Fig. 6F), and the spot-size frequency distribution (Fig. 6G). The datum used for each mouse was the average of its urine filter papers analyzed over 3 d. The number of nonedge spots was significantly higher for the integrin-KO mice (P<0.01), compared to the littermate controls. The area fraction covered with urine was significantly higher also, and the spot-size frequency distribution revealed that frequency of spotting of very small volumes (0.1–0.5 μl) was lower in the β1-cKO mice but that larger volumes of 0.8–4.0 μl were deposited, consistent with loss of outlet control.
Figure 6.
β1-cKO mice are incontinent. Voiding behavior was analyzed by quantitation of urine spot patterns on filter paper. A–D) Representative urine spot patterns for control (A, B) and β1-cKO mice (C, D). Control mice void in a controlled manner in one or two areas, while β1-cKOs have uncontrolled random urine leakage. E–G) Summary data for 4 mice/group that were tested on 3 separate days. Urine spot number (E) and spot area (F) as a percentage of the filter paper area were significantly greater in the β1-cKO mice; frequency distribution of urine spot volumes (G) showed β1-cKO mice had a greater proportion of urine deposits that were moderately large (0.8–4 μl). *P < 0.05, **P < 0.01.
β1-cKO mice have overactive bladder and overfill
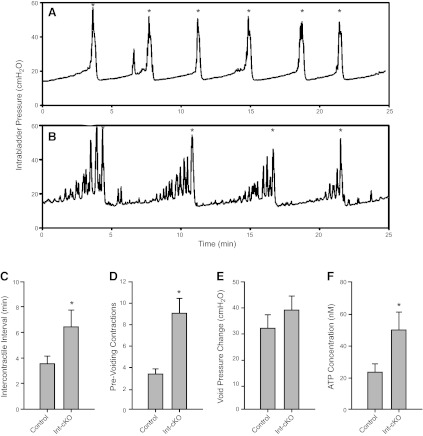
To investigate the urodynamic basis for the incontinent phenotype, we used cystometry to simulate bladder filling and voiding. This technique measures voiding intervals and contractile activity through continuous monitoring of intravesical pressure. The pressure profile for control animals shows a gradual increase over several minutes with rare pressure spikes during the filling phase, followed by rapid pressure increase that falls back to baseline as the bladder empties (Fig. 7A). Asterisks in Fig. 7A indicate the point of voiding (detrusor contraction), and the distance between asterisks is the intercontractile interval (ICI). The ICI provides a measure of fill volume required to initiate spontaneous voiding. Consistent with the urine spot data, β1-cKO males exhibit grossly abnormal urodynamics characterized by frequent prevoiding contractions (Fig. 7B, D) and significantly longer ICIs (Fig. 7C). Prevoiding contractions appear to increase in number and magnitude after the bladder is half-filled. The void pressure change for control and β1-cKO mice is shown in Fig. 7E and summarizes the peak to postvoid pressure difference. β1-cKO mice were not different from controls, indicating that detrusor contractile strength was not affected. The combined findings of incontinence with hyperactive bladder contractility suggest outlet control cannot be maintained in the face of rapid frequent smooth muscle contractions. Since the bladders also overfill, we conclude that inappropriate communication originates in the urothelium, which intensifies as the bladder reaches capacity and delays the micturition trigger until greater wall tension is reached.
Figure 7.
β1-cKO mice have overactive bladder and overfill. Cystometry was performed on anesthetized mice, and intrabladder pressure was monitored with a pressure transducer throughout multiple fill and void cycles. A, B) Representative cystometrograms of a control mouse (A) and a β1-cKO mouse (B). Asterisks indicate the point at which voiding occurs; time between voids is the intercontractile interval (ICI). C) ICI is greater for β1-cKO mice than for controls. *P < 0.05. D) Prevoiding contractions were much more frequent in β1-cKO mice than controls. *P < 0.01. E) Voiding pressure changes were not different. F) ATP concentrations in voided cystometry fluid were higher in β1-cKO mice. *P < 0.05. Data in panels C–F are shown as means ± sem. n = 6–7 mice/group (C–E); 4 mice/group (F).
It is well known that ATP is secreted by urothelium in response to hydrostatic pressure-induced stretch (8, 9), and it is thought to activate P2X and P2Y receptors to initiate intracellular signaling responses. We wanted to know whether purinergic signaling by β1-cKO mice would be abnormal in the face of bladder stretch. Accordingly, we collected multiple void samples from mice undergoing cystometry and measured ATP by luciferase assay. β1-cKO mice showed 2-fold higher concentrations of ATP in cystometric fluid than controls, indicating an inappropriate mechanosensory response during bladder filling leading to excess purinergic signaling (Fig. 7F).
β1-cKO mice have enhanced stretch-activated ion channel conductance
To provide further evidence of abnormal mechanosensory responses in β1-cKO mice, we measured the Isc of bladders mounted in Ussing chambers. Application of 1 cmH2O hydrostatic pressure to the luminal side of the bladder, simulating bladder filling, resulted in immediate increases in Isc, which fell back to baseline on removal of the pressure head (Fig. 8A, arrows). Comparisons of β1-cKO mice with littermate controls revealed that loss of β1-integrin resulted in significantly greater Isc, consistent with enhanced stimulation of stretch-activated ion channels (Fig. 8B).
Figure 8.
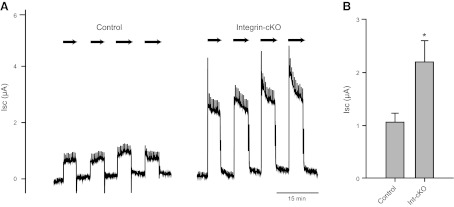
β1-cKO bladders exhibit higher stretch-activated ion conductances than controls: A) Bladders were mounted in Ussing chambers with continuous monitoring of Isc. On exposure to 1 cmH2O hydrostatic pressure on the luminal side (arrows), Isc increased dramatically. The magnitude of the increase was significantly greater for β1-cKO mouse bladders. B) Summary of Isc changes with pressure (n=4 mice/group). *P < 0.05.
DISCUSSION
The conditional deletion of β1-integrin in different kinds of epithelia has been shown to have diverse and often dramatic consequences. Ablation of β1-integrin in skin, for example, resulted in severe skin blistering concomitant with a massive failure of basement membrane assembly and hemidesmosomal instability (34). Deletion of β1-integrin in lens epithelia, meanwhile, appeared to predispose cells to epithelial-mesenchymal transition and resulted in a complete loss of the epithelial phenotype (35). In mammary epithelium, loss of β1-integrin resulted in multiple defects in branching morphogenesis and lack of milk production, implying defects in tissue patterning, differentiation, and responsiveness to cytokines (36); whereas, in intestine, overproliferation and dysplasia resulted in severe malnutrition and postnatal lethality (37). These disparate findings suggest that β1-integrin plays many complex roles, which are likely to depend on the α-integrin expression profile as well as the tissue-specific architecture, and overall, strongly emphasizes the context-dependent nature of integrin function.
To investigate the role of β1-integrin in urothelium, we created a conditional knockout using uroplakin promoter-driven expression of Cre recombinase, resulting in deletion of β1-integrin from the basal/intermediate cells of the urothelium. Deletion of this integrin led to a dramatic voiding phenotype closely resembling urge incontinence, as detrusor overactivity occurred in addition to conscious urine leakage. Somewhat surprisingly, given the role of integrins as cell and matrix adhesion molecules, we detected no evidence of abnormal urothelial morphology or of adhesion defects to the lamina propria. We further confirmed the maintenance of urothelial structural integrity by measuring permeability and electrical resistance and showed that bladders lacking urothelial integrins exhibited no apparent loss of barrier function. This finding led us to investigate whether integrins were fulfilling a sensory, rather than adhesion related function in urothelium.
The demonstration that total luminal ATP secretion (measured in fluid from multiple individual voiding cycles) is significantly elevated in β1-cKO mice pointed to an important link between mechanosensory integrin function and ATP secretory pathways within the urothelium. The understanding that urothelium secretes considerable ATP in response to stretch is now well established (8, 9); however, the mechanism is unknown. It appears, from our data, that integrin signaling as a response to bladder filling and increased wall tension plays at least some role in regulating the release of this purinergic agonist and that ATP secretion is partially regulated by integrin activation.
Cystometry allows us to study bladder function in vivo and to sample ATP release luminally (through capture of naturally voided fluid) but, unfortunately, does not allow us to draw conclusions about ATP release kinetics serosally. However, urothelial ATP secretion on stretch is known to be bidirectional; i.e., release occurs on both the serosal as well as luminal surfaces, and if excessive purinergic signaling in the β1-cKO mice is likewise bidirectional, inappropriate activation of P2X1 receptors on detrusor smooth muscle (12, 38) and/or P2X2/3 receptors on suburothelial sensory nerve fibers (14) might occur, leading to the symptoms of overactivity we observed.
We detected significantly higher Isc in β1-cKO mouse bladders exposed to a stretch stimulus of 1 cmH2O (Fig. 8). A recent report showed that urothelial ion transport is very sensitive to stretch and that transepithelial voltage, Isc, conductance, and capacitance responses are proportional to the stretch force magnitude and the speed at which stretch is applied (39). Interestingly, Isc changes were shown to parallel capacitance changes, the latter being interpreted as reflecting the trafficking of intracellular vesicles into the apical membrane of umbrella cells (39, 40). Since increased ion transport can occur by two pathways; either by an increase in number of channels (N), or by a change in the open probability (Po), an increase in membrane trafficking could result in more channels inserted (N) and therefore more current. Alternatively, higher currents could result from an increase in Po if mechanically gated channels become activated. Given the very rapid Isc responses noted, we tend to favor the latter explanation. Regulation of ion channel gating could occur through direct interactions between integrins and ion channels in macromolecular complexes (41, 42).
Membrane trafficking is actin dependent, and the actin cytoskeleton is a direct, internally linked effector system of integrins through focal adhesions and the many intracellular adaptor proteins that assemble and disassemble in response to tensional changes. Increased membrane trafficking with stretch in β1-cKO mice may also be a pathway for the exocytotic release of ATP, with subsequent stimulation of P2X receptor cation channels. It was shown recently that compensatory endocytosis of umbrella cell apical membrane following stretch is integrin regulated, with integrin-blocking antibodies able to partially block integrin-dependent endocytosis (43). This finding suggests that, in the cKO mice, the balance of stretch-induced trafficking is likely to be shifted toward greater exocytic and reduced endocytic activity. The signaling phenomenology is likely to be complex and to involve other known pathways, including possibly direct effects on mechanosensitive ion channels. Both ENaC and TRP channels have been suggested in this regard. It is also possible that loss of integrins permits an increase in exocytosis and ion transport at lower mechanical activation thresholds. For example, we note that in cystometric tracings on β1-cKO mice that the early filling phase is relatively quiescent (Fig. 7B) and that abnormal prevoiding contractions generally initiate during the latter stages of filling, when membrane stretch may reach a mechanotransductive threshold. The increased ion transport and trafficking of ATP in β1-cKO mice may thus reflect an increase in urothelial sensitivity to modest physical stimuli.
These experiments reveal an important sensory role for integrins within the urothelium as cell surface receptors that detect and signal to other structural elements in the bladder. The tissue specificity of the KO demonstrates directly for the first time that urothelial sensing of bladder filling regulates the voiding reflex. Loss of appropriate mechanosensation through mutations, disease, or injury to the urothelium could underlie some forms of incontinence or overactive bladder.
Acknowledgments
The project described was supported by U.S. National Institute of Diabetes and Digestive and Kidney Diseases grant DK083299 to W.G.H.
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the U.S. National Institutes of Health.
Footnotes
- β1-cKO
- β1-integrin conditional knockout
- β1fl/fl
- β1-integrin floxed/floxed
- AQP3
- aquaporin 3
- ECM
- extracellular matrix
- EM
- electron microscopy
- H&E
- hematoxylin and eosin
- ICI
- intercontractile interval
- Isc
- short-circuit current
- SEM
- scanning electron microscopy
- TEM
- transmission electron microscopy
- TER
- transepithelial resistance
- TRP
- transient receptor potential
- UPII
- uroplakin II
- YFP
- yellow fluorescent protein
REFERENCES
- 1. De Groat W. C. (2004) The urothelium in overactive bladder: passive bystander or active participant? Urology 64, 7–11 [DOI] [PubMed] [Google Scholar]
- 2. De Groat W. C., Yoshimura N. (2009) Afferent nerve regulation of bladder function in health and disease. Handb. Exp. Pharmacol. 91–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Apodaca G., Balestreire E., Birder L. A. (2007) The uroepithelial-associated sensory web. Kidney Int. 72, 1057–1064 [DOI] [PubMed] [Google Scholar]
- 4. Wang E. C., Lee J. M., Johnson J. P., Kleyman T. R., Bridges R., Apodaca G. (2003) Hydrostatic pressure-regulated ion transport in bladder uroepithelium. Am. J. Physiol. Renal Physiol. 285, F651–F663 [DOI] [PubMed] [Google Scholar]
- 5. Yu W., Hill W. G., Apodaca G., Zeidel M. L. (2011) Expression and distribution of transient receptor potential (TRP) channels in bladder epithelium. Am. J. Physiol. Renal Physiol. 300, F49–F59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Boer W. I., Schuller A. G., Vermey M., van der Kwast T. H. (1994) Expression of growth factors and receptors during specific phases in regenerating urothelium after acute injury in vivo. Am. J. Pathol. 145, 1199–1207 [PMC free article] [PubMed] [Google Scholar]
- 7. Birder L. A. (2010) Urothelial signaling. Auton Neurosci. 153, 33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferguson D. R., Kennedy I., Burton T. J. (1997) ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes—a possible sensory mechanism? J. Physiol. 505(Pt. 2), 503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lewis S. A., Lewis J. R. (2006) Kinetics of urothelial ATP release. Am. J. Physiol. Renal Physiol. 291, F332–F340 [DOI] [PubMed] [Google Scholar]
- 10. Vlaskovska M., Kasakov L., Rong W., Bodin P., Bardini M., Cockayne D. A., Ford A. P., Burnstock G. (2001) P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J. Neurosci. 21, 5670–5677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu W., Robson S. C., Hill W. G. (2011) Expression and distribution of ectonucleotidases in mouse urinary bladder. PLoS One 6, e18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee H. Y., Bardini M., Burnstock G. (2000) Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J. Urol. 163, 2002–2007 [PubMed] [Google Scholar]
- 13. Cockayne D. A., Hamilton S. G., Zhu Q. M., Dunn P. M., Zhong Y., Novakovic S., Malmberg A. B., Cain G., Berson A., Kassotakis L., Hedley L., Lachnit W. G., Burnstock G., McMahon S. B., Ford A. P. (2000) Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407, 1011–1015 [DOI] [PubMed] [Google Scholar]
- 14. Cockayne D. A., Dunn P. M., Zhong Y., Rong W., Hamilton S. G., Knight G. E., Ruan H. Z., Ma B., Yip P., Nunn P., McMahon S. B., Burnstock G., Ford A. P. (2005) P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J. Physiol. 567, 621–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Birder L. A., Nakamura Y., Kiss S., Nealen M. L., Barrick S., Kanai A. J., Wang E., Ruiz G., De Groat W. C., Apodaca G., Watkins S., Caterina M. J. (2002) Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat. Neurosci. 5, 856–860 [DOI] [PubMed] [Google Scholar]
- 16. Gevaert T., Vriens J., Segal A., Everaerts W., Roskams T., Talavera K., Owsianik G., Liedtke W., Daelemans D., Dewachter I., Van Leuven F., Voets T., De Ridder D., Nilius B. (2007) Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J. Clin. Invest. 117, 3453–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Puklin-Faucher E., Sheetz M. P. (2009) The mechanical integrin cycle. J. Cell Sci. 122, 179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alenghat F. J., Ingber D. E. (2002) Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci. STKE 2002, PE6. [DOI] [PubMed] [Google Scholar]
- 19. Katsumi A., Orr A. W., Tzima E., Schwartz M. A. (2004) Integrins in mechanotransduction. J. Biol. Chem. 279, 12001–12004 [DOI] [PubMed] [Google Scholar]
- 20. Katsumi A., Naoe T., Matsushita T., Kaibuchi K., Schwartz M. A. (2005) Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J. Biol. Chem. 280, 16546–16549 [DOI] [PubMed] [Google Scholar]
- 21. Liebert M., Washington R., Stein J., Wedemeyer G., Grossman H. B. (1994) Expression of the VLA beta 1 integrin family in bladder cancer. Am. J. Pathol. 144, 1016–1022 [PMC free article] [PubMed] [Google Scholar]
- 22. Southgate J., Kennedy W., Hutton K. A., Trejdosiewicz L. K. (1995) Expression and in vitro regulation of integrins by normal human urothelial cells. Cell Adhes. Commun. 3, 231–242 [DOI] [PubMed] [Google Scholar]
- 23. Wilson C. B., Leopard J., Cheresh D. A., Nakamura R. M. (1996) Extracellular matrix and integrin composition of the normal bladder wall. World J. Urol. 14(Suppl. 1), S30–S37 [DOI] [PubMed] [Google Scholar]
- 24. Liebert M., Washington R., Wedemeyer G., Carey T. E., Grossman H. B. (1994) Loss of co-localization of alpha 6 beta 4 integrin and collagen VII in bladder cancer. Am. J. Pathol. 144, 787–795 [PMC free article] [PubMed] [Google Scholar]
- 25. Ayala de la Pena F., Kanasaki K., Kanasaki M., Tangirala N., Maeda G., Kalluri R. (2011) Loss of p53 and acquisition of angiogenic microRNA profile are insufficient to facilitate progression of bladder urothelial carcinoma in situ to invasive carcinoma. J. Biol. Chem. 286, 20778–20787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Acharya P., Beckel J., Ruiz W. G., Wang E., Rojas R., Birder L., Apodaca G. (2004) Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am. J. Physiol. Renal Physiol. 287, F305–F318 [DOI] [PubMed] [Google Scholar]
- 27. Apodaca G., Kiss S., Ruiz W., Meyers S., Zeidel M., Birder L. (2003) Disruption of bladder epithelium barrier function after spinal cord injury. Am. J. Physiol. Renal Physiol. 284, F966–F976 [DOI] [PubMed] [Google Scholar]
- 28. Negrete H. O., Lavelle J. P., Berg J., Lewis S. A., Zeidel M. L. (1996) Permeability properties of the intact mammalian bladder epithelium. Am. J. Physiol. 271, F886–F894 [DOI] [PubMed] [Google Scholar]
- 29. Lavelle J., Meyers S., Ramage R., Bastacky S., Doty D., Apodaca G., Zeidel M. L. (2002) Bladder permeability barrier: recovery from selective injury of surface epithelial cells. Am. J. Physiol. Renal Physiol. 283, F242–F253 [DOI] [PubMed] [Google Scholar]
- 30. Hill W. G., Meyers S., von Bodungen M., Apodaca G., Dedman J. R., Kaetzel M. A., Zeidel M. L. (2008) Studies on localization and function of annexin A4a within urinary bladder epithelium using a mouse knockout model. Am. J. Physiol. Renal Physiol. 294, F919–F927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu X. R., Kong X. P., Pellicer A., Kreibich G., Sun T. T. (2009) Uroplakins in urothelial biology, function, and disease. Kidney Int. 75, 1153–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Srinivas S., Watanabe T., Lin C. S., William C. M., Tanabe Y., Jessell T. M., Costantini F. (2001) Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kanasaki K., Kanda Y., Palmsten K., Tanjore H., Lee S. B., Lebleu V. S., Gattone V. H., Jr., Kalluri R. (2008) Integrin beta1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev. Biol. 313, 584–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Raghavan S., Bauer C., Mundschau G., Li Q., Fuchs E. (2000) Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J. Cell Biol. 150, 1149–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Simirskii V. N., Wang Y., Duncan M. K. (2007) Conditional deletion of beta1-integrin from the developing lens leads to loss of the lens epithelial phenotype. Dev. Biol. 306, 658–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Naylor M. J., Li N., Cheung J., Lowe E. T., Lambert E., Marlow R., Wang P., Schatzmann F., Wintermantel T., Schuetz G., Clarke A. R., Mueller U., Hynes N. E., Streuli C. H. (2005) Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J. Cell Biol. 171, 717–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jones R. G., Li X., Gray P. D., Kuang J., Clayton F., Samowitz W. S., Madison B. B., Gumucio D. L., Kuwada S. K. (2006) Conditional deletion of beta1 integrins in the intestinal epithelium causes a loss of Hedgehog expression, intestinal hyperplasia, and early postnatal lethality. J. Cell Biol. 175, 505–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Elneil S., Skepper J. N., Kidd E. J., Williamson J. G., Ferguson D. R. (2001) Distribution of P2X(1) and P2X(3) receptors in the rat and human urinary bladder. Pharmacology 63, 120–128 [DOI] [PubMed] [Google Scholar]
- 39. Yu W., Khandelwal P., Apodaca G. (2009) Distinct apical and basolateral membrane requirements for stretch-induced membrane traffic at the apical surface of bladder umbrella cells. Mol. Biol. Cell 20, 282–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Truschel S. T., Wang E., Ruiz W. G., Leung S. M., Rojas R., Lavelle J., Zeidel M., Stoffer D., Apodaca G. (2002) Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol. Biol. Cell 13, 830–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arcangeli A., Becchetti A. (2006) Complex functional interaction between integrin receptors and ion channels. Trends Cell Biol. 16, 631–639 [DOI] [PubMed] [Google Scholar]
- 42. Becchetti A., Pillozzi S., Morini R., Nesti E., Arcangeli A. (2010) New insights into the regulation of ion channels by integrins. Inter. Rev. Cell. Mol. Biol. 279, 135–190 [DOI] [PubMed] [Google Scholar]
- 43. Khandelwal P., Ruiz W. G., Apodaca G. (2010) Compensatory endocytosis in bladder umbrella cells occurs through an integrin-regulated and RhoA- and dynamin-dependent pathway. EMBO J. 29, 1961–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]