Abstract
Bladder dysfunction characterized by abnormal bladder smooth muscle (BSM) contractions is pivotal to the disease process in overactive bladder, urge incontinence, and spinal cord injury. Purinergic signaling comprises one key pathway in modulating BSM contractility, but molecular mechanisms remain unclear. Here we demonstrate, using myography, that activation of P2Y6 by either UDP or a specific agonist (MRS 2693) induced a sustained increase in BSM tone (up to 2 mN) in a concentration-dependent manner. Notably, activation of P2Y6 enhanced ATP-mediated BSM contractile force by up to 45%, indicating synergistic interactions between P2X and P2Y signaling. P2Y6-activated responses were abolished by phospholipase C (PLC) and inositol trisphosphate (IP3) receptor antagonists U73122 and xestospongin C, demonstrating involvement of the PLC/IP3 signal pathway. Mice null for Entpd1, an ectonucleotidase on BSM, demonstrated increased force generation on P2Y6 activation (150%). Thus, in vivo perturbations to purinergic signaling resulted in altered P2Y6 activity and bladder contractility. We conclude that UDP, acting on P2Y6, regulates BSM tone and in doing so selectively maximizes P2X1-mediated contraction forces. This novel neurotransmitter pathway may play an important role in urinary voiding disorders characterized by abnormal bladder motility.—Yu, W., Sun, X., Robson, S. C., Hill, W. G. Extracellular UDP enhances P2X-mediated bladder smooth muscle contractility via P2Y6 activation of the phospholipase C/inositol trisphosphate pathway.
Keywords: detrusor, uridine nucleotides, urinary tract, micturition, myography
Urinary bladder dysfunction is often characterized by inappropriate contraction of the bladder smooth muscle (BSM), leading to hyperactive or hypoactive motility. Millions have disorders of this nature, which include overactive bladder, urge incontinence, frequency, and nocturia. Treatment options are few and of limited efficacy, in no small part because the underlying mechanisms are unknown. Indeed, basic elements of efferent neurotransmitter signaling to BSM still remain unclear.
Purinergic signaling by extracellular nucleotides and nucleosides regulates many aspects of bladder function, including urine volume sensation, neural signal transduction, and voiding contraction (1–3). Dysregulation of purinergic signaling, through the abnormal production and release of ATP or altered expression of various P2X receptors, is a common feature of many pathologies and has been associated with interstitial cystitis (4–8), detrusor overactivity (9–12), outlet obstruction (13–17), inflammation (18, 19), neurogenic bladder (9, 17, 20), spinal cord-injured bladder (21, 22), and aging bladder (23). Despite these associations, there is very limited understanding of both the molecular mechanisms involved in purinergic signaling and the etiology of urological disease.
The urinary bladder undergoes repetitive cycles of filling and emptying as a result of coordinated BSM relaxation and contraction. During the expulsive phase, BSM exhibits biphasic contractility in response to parasympathetic release of 2 neurotransmitters: ATP and acetylcholine. This manifests as an initial rapid purinergic contraction followed by sustained acetylcholine-mediated cholinergic contraction (24). The relative importance of cholinergic contraction compared with that of purinergic contraction varies greatly, depending on the species, with rodents such as rats, mice, and guinea pigs showing robust purinergic contractions accounting for up to 50% of the BSM-generated force (24, 25). In humans, the evidence for significant purinergic neurotransmission to BSM is less clear, but it is generally felt to be a minor contributor compared with cholinergic stimulation. More widely accepted, however, is the idea that purinergic signaling becomes more important in disease and in aging (23, 26, 27) and may come to play a dominant role, accounting for up to 65% of the total force (28).
The relative contributions of purinergic and cholinergic pathways have been examined using potent receptor inhibition strategies. Atropine blocks muscarinic acetylcholine receptors, leaving purinergic responses intact, and, conversely, α,β-methylene-ATP (α,β-meATP) induces rapid activation and subsequent desensitization of P2X1, thereby leaving cholinergic signaling intact. Simultaneous inhibition of both receptors, however, does not completely abrogate contractility and leaves a residual component that is usually described as nonspecific or an electrical field stimulation (EFS) artifact (25). We hypothesized that this noncholinergic, non-ATP (NCNA) contractility is physiologically relevant and could reflect the activity of other NCNA receptors.
In addition to P2X-mediated BSM contractility, indirect pharmacological evidence has led some to speculate that P2Y receptors may be present in BSM and could mediate relaxation (29–31). However, there has been no direct evidence to support this, and no P2Y receptors have been identified in BSM to date. We were intrigued, however, by observations of P2Y6 expression in vascular smooth muscle in isolated arteries (32) and the loss of UDP-induced contractile function in the aorta of P2Y6-knockout mice (33). P2Y6 is activated by UDP in vivo, and we undertook an investigation of whether it plays a role in BSM contraction. Functional experiments indicate that it increases BSM muscle tone in response to UDP and thereby significantly enhances P2X-mediated BSM contraction by activation of the phospholipase C (PLC)/inositol trisphosphate (IP3) signal pathway. These data reveal a new tension-inducing neurotransmitter, probably released from efferent parasympathetic neurons, as well as novel synergistic molecular pathways involving P2X and P2Y crosstalk, which regulate BSM contractility and bladder function.
MATERIALS AND METHODS
Materials
Unless otherwise specified, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA) and were of reagent grade or better.
Antibodies
P2Y6 antibodies tested and found to lack specificity in immunofluorescence and immunoblotting applications were APR-011 (Alomone Labs, Jerusalem, Israel), sc-15215 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and MC-27264 (MBL International, Woburn, MA, USA).
Animals
C57BL/6J mice (3–4 mo old) were used in this study. Mice were euthanized by inhalation of 100% CO2. After euthanasia and thoracotomy, the bladders were rapidly excised and processed as described below. All animal studies were performed in adherence to U.S. National Institutes of Health guidelines for animal care and use and with the approval of the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee. Entpd1−/− transgenic mice were described in detail previously (34). Bladders from P2Y6-knockout and control mice were kindly provided by Dr. Bernard Robaye (Universite Libre de Bruxelles, Brussels, Belgium).
Agonists and antagonists
Except for atropine (Sigma-Aldrich), all other agonists and antagonists including MRS 2693 (5-iodouridine-5′-O-diphosphate trisodium salt) and MRS 2578 (N,N″-1,4-butanediylbis[N′-(3-isothiocyanatophenyl)thiourea]) were purchased from Tocris Bioscience (Bristol, UK).
Myography
Bladders were pinned on a small Sylgard block, and muscle was dissected free of the urothelium and suburothelium. BSM strips were then cut longitudinally (2–3 mm wide and 5–7 mm long) and mounted in an SI-MB4 tissue bath system (World Precision Instruments, Sarasota, FL, USA). Force sensors were connected to a TBM 4M transbridge (World Precision Instruments), and the signal was amplified by PowerLab (AD Instruments, Colorado Springs, CO, USA) and monitored through Chart software (AD Instruments). Contraction force was monitored dynamically with a sampling rate of 2000/s. BSM strips were gently prestretched to get optimized force and equilibrated for ≥1 h before any experiments. All experiments were conducted at 37°C in physiological saline solution, with continuous bubbling of 95% O2 and 5% CO2.
EFS
EFS was performed with an S48 field stimulator (Grass Technologies, Quincy, RI, USA) using standard protocols described previously (25).
Statistical analysis
The number of samples for all experiments was ≥4. All data are expressed as means ± sd. To determine significance for simple treatment effects, paired or unpaired Student's t tests were performed. For multiple comparisons, analysis of variance was first performed, and if the P value was <0.05, the Bonferroni t test was applied. Tests were considered significant at P ≤ 0.05.
RESULTS
Inhibition of cholinergic and ATP-dependent purinergic signaling demonstrates a residual EFS force component
We used EFS and specific receptor blockers to isolate the contribution of different neurotransmitters to BSM contractility. EFS induces rapid neuronal release of neurotransmitters to cause BSM contraction, thereby mimicking in vivo neuromuscular innervation (25). We demonstrated a significant reduction in contraction force by application of 0.5 μM atropine to block cholinergic receptors and then further significant reduction in the same tissue with 10 μM α,β-meATP, which desensitizes P2X receptors (Fig. 1A).
Figure 1.
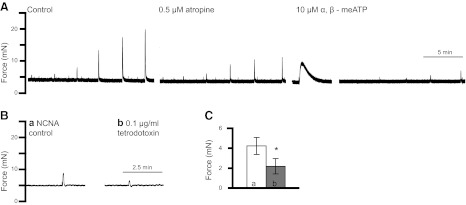
BSM contraction with EFS. BSM strips were subjected to 1, 2, 5, 10, 20, and 50 Hz EFS, which stimulates neurotransmitter release and causes BSM to contract with greater force at higher frequencies. A) Majority of the contraction force is blocked by 0.5 μM atropine followed by 10 μM α,β-meATP. A small amount of noncholinergic and NCNA contraction force remains. B) This NCNA force (at 20 Hz; trace a) can be partially blocked by tetrodotoxin (trace b), indicating that other neurotransmitters are present. C) Summary of 4 experiments showing ∼50% of remaining NCNA contraction force (bar a) can still be inhibited by tetrodotoxin (bar b). *P < 0.05.
The remaining contractile response is termed NCNA force. This residual has often been considered to be nonspecific; however, it can be further reduced 48% by treatment of bladder strips with tetrodotoxin, which functions to block action potentials and eliminate nerve firing (Fig. 1B, C). This result confirms that, in addition to acetylcholine and ATP, there are other neurotransmitters causing BSM contraction. It further implied the presence of other receptors, and so we explored the possibility that a P2Y receptor might be expressed in BSM.
UDP modulates BSM tone in a concentration-dependent and sustained manner
To test whether P2Y6 is involved in modulating BSM function, UDP, a P2Y6 agonist, was applied to mounted BSM strips, and tension responses were recorded by myography. UDP modulated muscle tone with a relatively quick increase that took about a minute to fully develop, followed by a slow reduction (Fig. 2A). This response was concentration dependent with increasing concentrations of UDP eliciting increased BSM tone (Fig. 2B). Application of MRS 2693, a selective P2Y6 receptor agonist with no activity on other P2Y subtypes (35), induced a similar response on BSM, indicating a specific functional role of P2Y6 in modulating BSM muscle tone (Fig. 2C). When pretreated with MRS 2578, a selective P2Y6 antagonist (35), the MRS 2693-induced response was fully abolished (Fig. 2C, D).
Figure 2.
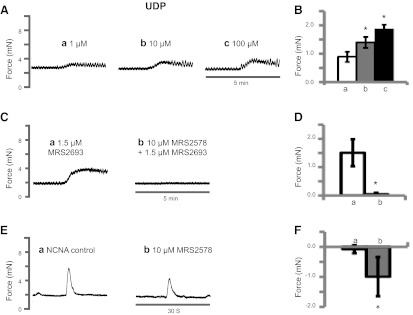
UDP modulates BSM muscle tone. A) Representative traces of BSM responses to 1 μM (trace a), 10 μM (trace b), and 100 μM UDP (trace c). There is a 30-min interval between each addition. B) Summary of force change (peak force minus baseline) with increasing UDP concentration. Bars a–c correspond to traces a–c, respectively, in panel A. C) Representative traces of BSM responses to P2Y6 agonist 1.5 μM MRS 2693 without (trace a) or with (trace b) 15-min pretreatment of antagonist 10 μM MRS 2578. D) Summary of force changes shown in panel C. E) a) Representative trace of BSM strips in response to 20 Hz EFS after treatment with 0.5 μM atropine and 10 μM α,β-meATP for 15 min, which is the remaining NCNA response. b) Treatment with MRS 2578 (15 min) reduced the NCNA response, revealing the presence of neurotransmitters capable of stimulating P2Y6. F) Summary of force changes shown in panel E. a) Peak force change between two consecutive NCNA stimuli. b) Peak force change between NCNA control and subsequent MRS 2578 treatment. *P < 0.05; n = 4–10 BSM strips for all summary data.
Similarly, application of the P2Y6 antagonist MRS 2578 to tissue before EFS-induced contractions also resulted in a further reduction in contractile force. Figure 2E shows a representative NCNA response to EFS on the left and the reduced response on incubation with MRS 2578. The average change in force without the drug (Fig. 2F, bar a) or with the drug (Fig. 2F, bar b) confirms that there is an endogenous, UDP-mediated regulation of BSM contractility.
Activation of P2Y6 enhances P2X1-mediated BSM contraction force
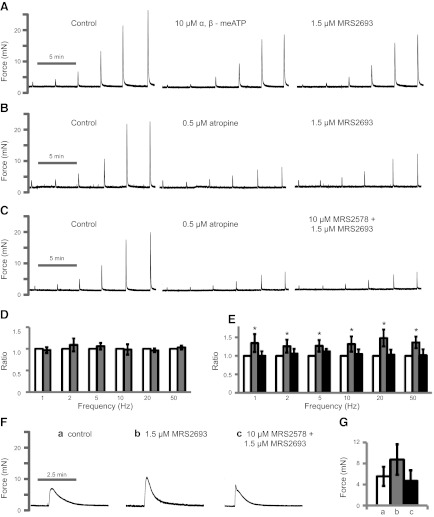
Our data indicate that P2Y6 activation results in an increase in BSM muscle tone, and it is known that muscle tone plays a key role in the generation of maximal contraction force amplitude. However, the underlying mechanism is unknown (36). We hypothesized that P2Y6 could be involved in this phenomenon. On application of α,β-meATP to abolish purinergic contraction but spare cholinergic contraction, addition of the P2Y6 agonist MRS 2693 did not cause any change to EFS-induced cholinergic contractile force (Fig. 3A, D),indicating that P2Y6 receptor-mediated signaling does not interact with the cholinergic signaling pathway. Notably, when cholinergic contractions are eliminated, sparing purinergic contractions, MRS 2693 significantly increased EFS-induced purinergic contraction force by up to 45%, and this force potentiation was fully cancelled by pretreatment with MRS 2578 (Fig. 3B, C, E). For example, EFS at 50 Hz yields ∼4 mN of greater purinergic contractile force compared with that in the absence of the P2Y6 agonist MRS 2693. This result suggests that P2Y6 “preconditions” the muscle for greater force generation by a small increase in muscle tone. MRS 2693 activation of P2Y6 for 10 min also significantly enhanced α,β-meATP-induced BSM contraction force by 56% (Fig. 3F, G). In sum, these data indicate that activation of P2Y6 significantly enhances BSM purinergic contraction force but has no effect on cholinergic contraction force.
Figure 3.
Activation of P2Y6 enhances P2X1-mediated BSM contraction force. A−C) Representative EFS traces (1–50 Hz) under control conditions and after blockade of purinergic transmission with 10 μM α,β-meATP (A) or blockade of cholinergic transmission with 0.5 μM atropine (B, C), followed by subsequent activation of P2Y6 with MRS 2693 with (C) or without (B) antagonist MRS 2578. Agonists/antagonists were added 10–15 min before EFS. D) Summary of cholinergic force change with P2Y6 activation. Shaded bars, MRS 2693. E) Summary of purinergic force changes after activation and then inhibition of P2Y6. Shaded bars, MRS 2693; solid bars, MRS 2578 + MRS 2693. Significance testing was performed on paired force measurements, not ratios. F, G) Representative traces of BSM responses to 10 μM α,β-meATP (control, a) after pretreatment with P2Y6 activator, MRS 2693, with (c) or without (b) the inhibitor MRS 2578 (F) and summary of force amplitudes (G). In panel G, significance was established by analysis of variance but not by Bonferroni t test. *P < 0.05; n = 4–8 BSM strips for all summary data.
P2Y6 signals through a PLC/IP3 pathway
Although it has been reported that P2Y6 can activate Gα12/13 (37), this receptor is usually coupled to Gq protein, which, in turn, activates PLC. PLC hydrolyzes phosphatidylinositol 4,5-bisphosphate into diacylglycerol and IP3, which binds IP3 receptors in endoplasmic reticulum and induces the release of calcium (38). We observed that both U73122 (PLC inhibitor) and xestospongin C (IP3 receptor inhibitor) consistently abolished the effect of MRS 2693 in increasing BSM muscle tone (Fig. 4A). Both inhibitors also consistently cancel the potentiating effect of MRS 2693 on EFS-stimulated purinergic contraction and even diminish purinergic contraction at high concentrations of U73122 (Fig. 4B, C). Activation of P2Y6 therefore results in intracellular signaling via the PLC/IP3 pathway to regulate BSM tone and further modulation of purinergic contractility. The potentiating effect of P2Y6 activation in response to α,β-meATP was also eliminated by shutting down PLC/IP3 signaling (Fig. 4D).
Figure 4.
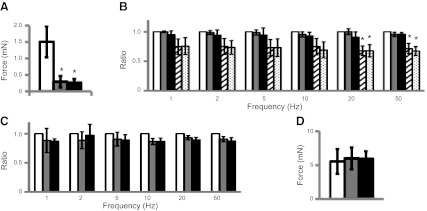
P2Y6 signaling through the PLC/IP3 pathway. A) MRS 2693-induced force responses are inhibited by pretreatment with 50 μM U73122 (PLC inhibitor; shaded bar) and 1 μM xestospongin C (IP3 inhibitor; solid bar). B) U73122 inhibits MRS 2693-induced potentiation on EFS-stimulated purinergic contraction. BSM strips were treated with 0.5 μM atropine, and then EFS responses were monitored after each treatment with 0 μM (open bar), 0.5 μM (shaded bar), 5 μM (solid bar), and 50 μM (cross-hatched bar; ref. 13) U73122 and then 1.5 μM MRS 2693 (dotted bar) sequentially. Data are normalized to control (open bar). C) Xestospongin C inhibits MRS 2693-induced potentiation on EFS-stimulated purinergic contraction. BSM strips were treated with 0.5 μM atropine and then EFS responses monitored after each treatment with 0 μM xestospongin C (open bar), 1 μM xestospongin C (shaded bar), and then MRS 2693 (solid bar) sequentially. Data are normalized to control (open bar). D) U73122 (shaded bar) and xestospongin C (solid bar) inhibit the potentiation of MRS 2693 on α,β-meATP-induced BSM purinergic contraction (open bar). *P < 0.05; n = 4 BSM strips for all summary data.
Deletion of ectonucleoside triphosphate diphosphohydrolase 1 (Entpd1) in BSM increases P2Y6 contractility
Levels of extracellular nucleotides are tightly regulated by a group of enzymes called ectonucleotidases, which rapidly convert them and thereby regulate purine availability for corresponding receptors (39). We recently demonstrated that Entpd1 is expressed on mouse BSM (40). Entpd1 rapidly converts both ATP and UTP to the respective nucleoside di- and monophosphates. Therefore, its loss would result in longer exposure times to ATP and UTP, with concomitant reductions in concentrations of downstream metabolites, such as UDP/UMP. To test the hypothesis that in vivo disruptions to normal purinergic signaling would alter P2Y6 function and bladder contractile responses, we examined bladder strips from Entpd1-null mice. Application of MRS 2693 to Entpd1−/− bladder strips induced a significantly larger response in BSM tone compared with that of the controls (Fig. 5A). The potentiating effects of MRS 2693 on EFS-induced BSM purinergic contraction in these knockout mice appeared to be diminished (Fig. 5B). Interpretation of this result is complicated by multiple possible effects, including the likelihood of P2X1 down-regulation in the face of excess ATP. Examination of just α,β-meATP-stimulated contractions revealed significantly reduced force responses in Entpd−/− mice (Fig. 5C, shaded bar), supporting our hypothesis that P2X1 has been down-regulated. However, potentiation of BSM contractility via the P2Y6 receptor with MRS 2693 remained intact and indeed was more robust with a 3-fold stimulation (Fig. 5C, solid bar) compared with that of normal mice, which respond with a 1.5-fold increase (Fig. 3G, bar b vs. bar a). However, it can be seen that the enhanced contraction force in these knockout mice (Fig. 5C, solid bar) is still slightly smaller than the control force (Fig. 5C, open bar) and much less than enhanced contraction force in normal mice (Fig. 3G, bar b), indicating an overall decrease in BSM contractility in these mice.
Figure 5.
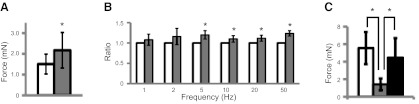
Disruption of UTP/UDP metabolism in BSM from Entpd−/− mice alters P2Y6 responses. A) MRS 2693 stimulates increased force response in BSM from Entpd1−/− mice (shaded bar). B) MRS 2693 potentiation of EFS-induced purinergic contractions (0.5 μM atropine pretreatment) in BSM from Entpd1−/− mice (shaded bar). C) α,β-meATP-induced contractions on control (open bar), Entpd1−/− (shaded bar), and Entpd1−/− mice pretreated with MRS 2693 (solid bar). *P < 0.05; n = 4–12 BSM strips for all summary data.
DISCUSSION
Atropine-resistant (i.e., noncholinergic) contractions of BSM had been noted as early as 1972 (41, 42), and it is now known that ATP is the neurotransmitter released by parasympathetic nerves to cause noncholinergic BSM contraction. P2X1 is believed to be the primary receptor for ATP activation, based on several lines of evidence including quantitative polymerase chain reaction (43), P2X1-knockout mice (44), and recently available selective antagonists of purinergic subtypes, including P2X1 (45).
P2Y receptors, to our knowledge, have not been shown or localized within BSM. However, their presence has been inferred. Notably, several groups have proposed that P2Y receptors may be involved in the relaxation of BSM after ATP stimulation, based on experiments involving nonspecific purinergic agonists with a preference for P2Y receptors (29–31). However, other explanations for these observations are possible, including P2X1 internalization after stimulation, as well as the production of smooth muscle relaxants such as adenosine, from the coordinated activity of Entpd1 and CD73/Nt5e (40).
Unlike ATP, little is known about UTP/UDP release within the bladder wall. The observation that BSM NCNA contractions can be further blocked by tetrodotoxin indicates unequivocally that other neurotransmitters are released by nerve firing (Fig. 1), and the effect of MRS 2578 in partially antagonizing NCNA contractions demonstrates that either UTP or UDP is endogenously released (Fig. 2E, F). At this point, it is not certain which of these related nucleotides is actually being secreted, because the latter could be generated from the former.
P2Y6 is the chief UDP receptor in the P2 receptor family, and our pharmacological studies clearly indicate its presence in urothelium-free BSM. Contractile effects of UDP and generation of Ca2+ waves in rat BSM strips were also noted in a recent study (46). However, the location of the receptor is unclear, because antibodies against P2Y6 have proven to be of dubious specificity. Using confocal immunofluorescent microscopy, we tested 3 commercially available antibodies (see Materials and Methods for details), all of which stained BSM strongly. However, all 3 stained bladders from P2Y6-knockout mice, thereby failing to exhibit the necessary specificity. The antibodies were also unhelpful in identifying P2Y6−/− bladder tissue by Western blotting, thus highlighting a problem in identifying the cellular location and expression of this receptor. Given what we know about the cellular architecture of BSM, P2Y6 may be present on myocytes, interstitial cells of Cajal, or even myofibroblasts in close apposition to smooth muscle. However, because our functional data show that activation of P2Y6 specifically enhances P2X-mediated BSM contraction force and because Entpd1 is located on BSM, we believe it probably has a smooth muscle localization.
The finding that P2Y6 modulates BSM muscle tone is likely to be quite important for bladder function. Stretch of the bladder wall during filling normally induces an increase in BSM tone, which constantly adjusts through compliance adaptation. Regulation of BSM muscle tone might play a key role in the mechanism of urine accommodation and may participate in mechanosensory mechanisms that signal the degree of fullness.
Stretch or tonic regulation is also a key determinant for BSM to maximize force generation (36). This phenomenon has been known for decades; however, the underlying mechanism is poorly understood. Our data clearly indicate that activation of P2Y6 signaling modulates BSM tone and synergistically enhances P2X-mediated BSM contraction, indicating P2X-P2Y pathway crosstalk. It has previously been noted that there is crosstalk between muscarinic and purinergic signaling in BSM, mediated by the IP3 and ryanodine receptors (24, 47). Acetylcholine binding to muscarinic receptors stimulates an increase in the second messenger IP3 that leads to intracellular release of Ca2+ through endoplasmic reticulum-localized IP3 receptor. Sensitized IP3 receptors then function together with the ryanodine receptor 2, which is rapidly activated by ATP-stimulated Ca2+ influx through P2X1 to generate a Ca2+ wave and BSM contraction (47). In fact, studies using P2X1−/− mice reveal that there is rapid engagement of P2X1 to increase action potentials (seen as Ca2+ flashes) and a delayed increase in excitability on muscarinic activation. The two pathways cooperate in shaping the time course of force transients, with purinergic activation actually reducing the magnitude of muscarinic transients (24). Our data add further complexity to this coordinated muscarinic/purinergic axis with P2Y6 stimulation leading to activation of PLC and subsequent IP3 signal pathway involvement to enhance the P2X1-mediated contraction (see Fig. 6 for a proposed model).
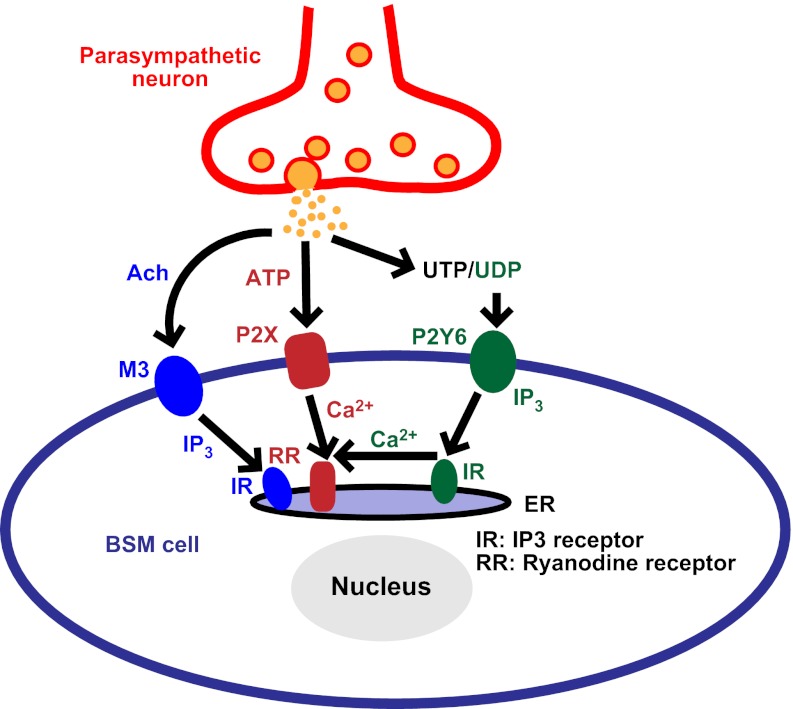
Figure 6.
Proposed model of P2Y6 potentiation on BSM purinergic contraction. UTP/UDP coreleased with ATP and acetylcholine (Ach) from parasympathetic efferents. UDP binds to BSM cell surface P2Y6 receptors, which then sequentially activate Gq protein and PLC. PLC further hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylglycerol and IP3, which binds IP3 receptors in endoplasmic reticulum (ER) and induces the release of calcium. By an unknown mechanism, released calcium could interact with the ATP-P2X1-ryanodine receptor signal pathway and enhance this ATP-mediated BSM contraction.
With regard to effects of the PLC inhibitor, U73122, it is noteworthy that in bladder, cholinergic signaling through the M3 receptor mobilizes IP3 but through a PLC-independent mechanism, contrasting with prototypical M3 signaling in other types of smooth muscle. It has been shown in mice, rats, and humans that U73122 has no effect on cholinergic contractions in BSM (48). Thus, IP3 generation in response to stimulation of M3 as shown in Fig. 6 should not be considered to be a consequence of M3-mediated effects on PLC activity. P2Y6 activation in response to extracellular UDP, however, does activate PLC, with subsequent generation of IP3.
The functional importance of uridine nucleotides to BSM contractility was seen in Entpd1−/− mice, which have disrupted nucleotide metabolism. In response to the deletion of Entpd1, mice exhibited apparent increases in P2Y6 expression as a consequence of perturbed signaling, and diminished contractility. Our data reveal a finely tuned, sensitive, and dynamic uridine metabolic pathway responsible for force potentiation in BSM. They also imply an important regulatory role for ectonucleotidase enzymes, which can clearly modulate the spatial/temporal availability of nucleotides (39).
The discovery of a new muscle tone-regulating purinergic receptor in BSM has important implications for research into human bladder disease. Although we do not know at this stage whether P2Y6 plays any prominent role in overactive bladder or painful bladder syndromes, dysregulation of purinergic signaling at ATP receptors of the P2X family has been widely observed. Changes in receptor density have been noted in interstitial cystitis (49), outlet obstruction (15, 16, 50), urge incontinence (10, 11), and detrusor overactivity (51). Because P2Y6 modulates P2X1 responses, there is likely to be important crosstalk in pathological settings. It will be necessary in the first instance to determine whether P2Y6 plays a role in human bladder qualitatively similar to that in the mouse and, if so, to quantitate the magnitude of its contribution to force generation. If confirmed, this will motivate studies to begin looking at P2Y6 expression levels in patient samples as well as to investigate in greater detail the physiology of uridine nucleotides as neurotransmitters in the bladder.
In summary, we have shown that P2Y6 is functionally expressed in BSM strips. The activation of P2Y6 by UDP modulates BSM muscle tone, which, in turn, synergistically enhances BSM purinergic contraction. Disturbing this pathway results in altered BSM motility.
Acknowledgments
The authors thank Dr. Bernard Robaye (Université Libre de Bruxelles, Brussels, Belgium) for providing bladders from P2Y6-knockout mice.
The project described was supported by the U.S. National Institute of Diabetes and Digestive and Kidney Diseases (grant DK083299 to W.G.H.) and the U.S. National Heart, Lung, and Blood Institute (grants P01-HL076540 and R01-HL094400 to S.C.R.).
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the U.S. National Institutes of Health.
Footnotes
- α,β-meATP
- α,β-methylene-ATP
- BSM
- bladder smooth muscle
- EFS
- electrical field stimulation
- ENTPD1
- ectonucleoside triphosphate diphosphohydrolase 1
- IP3
- inositol trisphosphate
- NCNA
- noncholinergic non-ATP
- PLC
- phospholipase C
REFERENCES
- 1. Burnstock G. (2011) Therapeutic potential of purinergic signalling for diseases of the urinary tract. BJU Int. 107, 192–204 [DOI] [PubMed] [Google Scholar]
- 2. Ford A. P., Cockayne D. A. (2011) ATP and P2X purinoceptors in urinary tract disorders. Handb. Exp. Pharmacol. 202, 485–526 [DOI] [PubMed] [Google Scholar]
- 3. Sun Y., Chai T. C. (2010) Role of purinergic signaling in voiding dysfunction. Curr. Bladder Dysfunct. Rep. 5, 219–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Birder L. A., Barrick S. R., Roppolo J. R., Kanai A. J., de Groat W. C., Kiss S., Buffington C. A. (2003) Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am. J. Physiol. Renal Physiol. 285, F423–F429 [DOI] [PubMed] [Google Scholar]
- 5. Sun Y., Chai T. C. (2002) Effects of dimethyl sulphoxide and heparin on stretch-activated ATP release by bladder urothelial cells from patients with interstitial cystitis. BJU Int. 90, 381–385 [DOI] [PubMed] [Google Scholar]
- 6. Sun Y., Chai T. C. (2004) Up-regulation of P2X3 receptor during stretch of bladder urothelial cells from patients with interstitial cystitis. J. Urol. 171, 448–452 [DOI] [PubMed] [Google Scholar]
- 7. Sun Y., Chai T. C. (2006) Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. Am. J. Physiol. Cell Physiol. 290, C27–C34 [DOI] [PubMed] [Google Scholar]
- 8. Tempest H. V., Dixon A. K., Turner W. H., Elneil S., Sellers L. A., Ferguson D. R. (2004) P2X and P2X receptor expression in human bladder urothelium and changes in interstitial cystitis. BJU Int. 93, 1344–1348 [DOI] [PubMed] [Google Scholar]
- 9. Kumar V., Chapple C. R., Rosario D., Tophill P. R., Chess-Williams R. (2010) In vitro release of adenosine triphosphate from the urothelium of human bladders with detrusor overactivity, both neurogenic and idiopathic. Eur. Urol. 57, 1087–1092 [DOI] [PubMed] [Google Scholar]
- 10. Moore K. H., Ray F. R., Barden J. A. (2001) Loss of purinergic P2X3 and P2X5 receptor innervation in human detrusor from adults with urge incontinence. J. Neurosci. 21, RC166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ray F. R., Moore K. H., Hansen M. A., Barden J. A. (2003) Loss of purinergic P2X receptor innervation in human detrusor and subepithelium from adults with sensory urgency. Cell Tissue Res. 314, 351–359 [DOI] [PubMed] [Google Scholar]
- 12. Sugaya K., Nishijima S., Kadekawa K., Miyazato M., Mukouyama H. (2009) Relationship between lower urinary tract symptoms and urinary ATP in patients with benign prostatic hyperplasia or overactive bladder. Biomed. Res. 30, 287–294 [DOI] [PubMed] [Google Scholar]
- 13. Bayliss M., Wu C., Newgreen D., Mundy A. R., Fry C. H. (1999) A quantitative study of atropine-resistant contractile responses in human detrusor smooth muscle, from stable, unstable and obstructed bladders. J. Urol. 162, 1833–1839 [PubMed] [Google Scholar]
- 14. Calvert R. C., Thompson C. S., Khan M. A., Mikhailidis D. P., Morgan R. J., Burnstock G. (2001) Alterations in cholinergic and purinergic signaling in a model of the obstructed bladder. J. Urol. 166, 1530–1533 [PubMed] [Google Scholar]
- 15. Kim J. C., Yoo J. S., Park E. Y., Hong S. H., Seo S. I., Hwang T. K. (2008) Muscarinic and purinergic receptor expression in the urothelium of rats with detrusor overactivity induced by bladder outlet obstruction. BJU Int. 101, 371–375 [DOI] [PubMed] [Google Scholar]
- 16. O'Reilly B. A., Kosaka A. H., Chang T. K., Ford A. P., Popert R., McMahon S. B. (2001) A quantitative analysis of purinoceptor expression in the bladders of patients with symptomatic outlet obstruction. BJU Int. 87, 617–622 [DOI] [PubMed] [Google Scholar]
- 17. Pinna C., Sanvito P., Puglisi L. (2006) Altered neurogenic and mechanical responses to acetylcholine, ATP and substance P in detrusor from rat with outlet obstruction. Life Sci. 79, 1301–1306 [DOI] [PubMed] [Google Scholar]
- 18. Kageyama A., Fujino T., Taki Y., Kato Y., Nozawa Y., Ito Y., Yamada S. (2008) Alteration of muscarinic and purinergic receptors in urinary bladder of rats with cyclophosphamide-induced interstitial cystitis. Neurosci. Lett. 436, 81–84 [DOI] [PubMed] [Google Scholar]
- 19. Smith C. P., Vemulakonda V. M., Kiss S., Boone T. B., Somogyi G. T. (2005) Enhanced ATP release from rat bladder urothelium during chronic bladder inflammation: effect of botulinum toxin A. Neurochem. Int. 47, 291–297 [DOI] [PubMed] [Google Scholar]
- 20. Brady C. M., Apostolidis A., Yiangou Y., Baecker P. A., Ford A. P., Freeman A., Jacques T. S., Fowler C. J., Anand P. (2004) P2X3-immunoreactive nerve fibres in neurogenic detrusor overactivity and the effect of intravesical resiniferatoxin. Eur. Urol. 46, 247–253 [DOI] [PubMed] [Google Scholar]
- 21. Smith C. P., Gangitano D. A., Munoz A., Salas N. A., Boone T. B., Aoki K. R., Francis J., Somogyi G. T. (2008) Botulinum toxin type A normalizes alterations in urothelial ATP and NO release induced by chronic spinal cord injury. Neurochem. Int. 52, 1068–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Groat W. C., Yoshimura N. (2010) Changes in afferent activity after spinal cord injury. Neurourol. Urodyn. 29, 63–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoshida M., Miyamae K., Iwashita H., Otani M., Inadome A. (2004) Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology 63, 17–23 [DOI] [PubMed] [Google Scholar]
- 24. Heppner T. J., Werner M. E., Nausch B., Vial C., Evans R. J., Nelson M. T. (2009) Nerve-evoked purinergic signalling suppresses action potentials, Ca2+ flashes and contractility evoked by muscarinic receptor activation in mouse urinary bladder smooth muscle. J. Physiol. 587, 5275–5288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sibley G. N. (1984) A comparison of spontaneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. J. Physiol. 354, 431–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoshida M., Homma Y., Inadome A., Yono M., Seshita H., Miyamoto Y., Murakami S., Kawabe K., Ueda S. (2001) Age-related changes in cholinergic and purinergic neurotransmission in human isolated bladder smooth muscles. Exp. Gerontol. 36, 99–109 [DOI] [PubMed] [Google Scholar]
- 27. Sjogren C., Andersson K. E., Husted S., Mattiasson A., Moller-Madsen B. (1982) Atropine resistance of transmurally stimulated isolated human bladder muscle. J. Urol. 128, 1368–1371 [DOI] [PubMed] [Google Scholar]
- 28. Andersson K. E., Arner A. (2004) Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol. Rev. 84, 935–986 [DOI] [PubMed] [Google Scholar]
- 29. Boland B., Himpens B., Paques C., Casteels R., Gillis J. M. (1993) ATP induced-relaxation in the mouse bladder smooth muscle. Br. J. Pharmacol. 108, 749–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bolego C., Pinna C., Abbracchio M. P., Cattabeni F., Puglisi L. (1995) The biphasic response of rat vesical smooth muscle to ATP. Br. J. Pharmacol. 114, 1557–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tong Y. C., Hung Y. C., Cheng J. T. (1997) Evidence of P2Y-purinoceptor mediated bladder neck smooth muscle post-contractile relaxation in the male mini-pig. Neurosci. Lett. 225, 181–184 [DOI] [PubMed] [Google Scholar]
- 32. Rayment S. J., Latif M. L., Ralevic V., Alexander S. P. (2007) Evidence for the expression of multiple uracil nucleotide-stimulated P2 receptors coupled to smooth muscle contraction in porcine isolated arteries. Br. J. Pharmacol. 150, 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bar I., Guns P. J., Metallo J., Cammarata D., Wilkin F., Boeynams J. M., Bult H., Robaye B. (2008) Knockout mice reveal a role for P2Y6 receptor in macrophages, endothelial cells, and vascular smooth muscle cells. Mol. Pharmacol. 74, 777–784 [DOI] [PubMed] [Google Scholar]
- 34. Enjyoji K., Sevigny J., Lin Y., Frenette P. S., Christie P. D., Esch J. S., 2nd, Imai M., Edelberg J. M., Rayburn H., Lech M., Beeler D. L., Csizmadia E., Wagner D. D., Robson S. C., Rosenberg R. D. (1999) Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat. Med. 5, 1010–1017 [DOI] [PubMed] [Google Scholar]
- 35. Jacobson K. A., Ivanov A. A., de Castro S., Harden T. K., Ko H. (2009) Development of selective agonists and antagonists of P2Y receptors. Purinergic Signal. 5, 75–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Finkbeiner A. E., Bissada N. K. (1980) Effect of detrusor muscle length and tension on its response to pharmacologic and electrical stimulation. Part II. In vitro study. Urology 16, 650–655 [DOI] [PubMed] [Google Scholar]
- 37. Nishida M., Sato Y., Uemura A., Narita Y., Tozaki-Saitoh H., Nakaya M., Ide T., Suzuki K., Inoue K., Nagao T., Kurose H. (2008) P2Y6 receptor-Gα12/13 signalling in cardiomyocytes triggers pressure overload-induced cardiac fibrosis. EMBO J. 27, 3104–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koizumi S., Shigemoto-Mogami Y., Nasu-Tada K., Shinozaki Y., Ohsawa K., Tsuda M., Joshi B. V., Jacobson K. A., Kohsaka S., Inoue K. (2007) UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 446, 1091–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Robson S. C., Sevigny J., Zimmermann H. (2006) The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2, 409–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yu W., Robson S. C., Hill W. G. (2011) Expression and distribution of ectonucleotidases in mouse urinary bladder. PLoS One 6, e18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Burnstock G. (1972) Purinergic nerves. Pharmacol. Rev. 24, 509–581 [PubMed] [Google Scholar]
- 42. Burnstock G., Satchell D. G., Smythe A. (1972) A comparison of the excitatory and inhibitory effects of non-adrenergic, non-cholinergic nerve stimulation and exogenously applied ATP on a variety of smooth muscle preparations from different vertebrate species. Br. J. Pharmacol. 46, 234–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O'Reilly B. A., Kosaka A. H., Chang T. K., Ford A. P., Popert R., Rymer J. M., McMahon S. B. (2001) A quantitative analysis of purinoceptor expression in human fetal and adult bladders. J. Urol. 165, 1730–1734 [PubMed] [Google Scholar]
- 44. Vial C., Evans R. J. (2000) P2X receptor expression in mouse urinary bladder and the requirement of P2X1 receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br. J. Pharmacol. 131, 1489–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. King B. F., Knowles I. D., Burnstock G., Ramage A. G. (2004) Investigation of the effects of P2 purinoceptor ligands on the micturition reflex in female urethane-anaesthetized rats. Br. J. Pharmacol. 142, 519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fry C. H., Young J. S., Jabr R. I., McCarthy C., Ikeda Y., Kanai A. J. (2012) Modulation of spontaneous activity in the overactive bladder—the role of P2Y agonists. Am. J. Physiol. Renal Physiol. 302, F1447–F1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Berridge M. J. (2008) Smooth muscle cell calcium activation mechanisms. J. Physiol. 586, 5047–5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Frazier E. P., Peters S. L., Braverman A. S., Ruggieri M. R., Sr., Michel M. C. (2008) Signal transduction underlying the control of urinary bladder smooth muscle tone by muscarinic receptors and β-adrenoceptors. Naunyn Schmiedebergs Arch. Pharmacol. 377, 449–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Birder L. A., Ruan H. Z., Chopra B., Xiang Z., Barrick S., Buffington C. A., Roppolo J. R., Ford A. P., de Groat W. C., Burnstock G. (2004) Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am. J. Physiol. Renal Physiol. 287, F1084–F1091 [DOI] [PubMed] [Google Scholar]
- 50. Chua W. C., Liu L., Mansfield K. J., Vaux K. J., Moore K. H., Millard R. J., Burcher E. (2007) Age-related changes of P2X1 receptor mRNA in the bladder detrusor from men with and without bladder outlet obstruction. Exp. Gerontol. 42, 686–692 [DOI] [PubMed] [Google Scholar]
- 51. Kumar V., Chapple C. R., Rosario D., Tophill P. R., Chess-Williams R. (2010) In vitro release of adenosine triphosphate from the urothelium of human bladders with detrusor overactivity, both neurogenic and idiopathic. Eur. Urol. 57, 1087–1092 [DOI] [PubMed] [Google Scholar]