Abstract
Purpose: This study demonstrated invasive and reproducible hepatic artery catheterization in rats. A rigorously documented guide and pictorial essay describes the performance of an invasive hepatic artery catheterization technique suitable for the study of liver-targeted interventional procedures in rodent models of liver cancer. The goal was to produce a well-illustrated guide to hepatic artery catheterization under direct visualization via the gastroduodenal artery (GDA). Materials and Methods: 20 Sprague Dawley rats were inoculated with McA-RH7777 HCC cells in the left lateral liver lobe. Magnetic resonance imaging (MRI) was used to measure tumor growth. Catheter placement in the hepatic artery proper was performed by entry through the GDA under direct visualization after laparotomy. Digital subtraction angiography confirmed catheter placement in the hepatic artery proper. Antegrade blood flow to the liver was restored after catheter removal. Rats were euthanized after procedures; livers were harvested for hematoxylin and eosin (H&E) staining. Results: 85.0% of inoculated animals developed measurable tumors on MRI; average tumor size was 6.3 ± 2.3 mm × 4.3 ± 1.5 mm (mean ± SD). 94.1% of animals with tumors were successfully catheterized. H&E staining demonstrated tumor growth in all inoculated animals, including those with no measurable tumors on MRI. Conclusion: Invasive catheter placement in the hepatic artery of a rodent model of HCC can be performed reproducibly according to the techniques described in this tutorial. These catheterization techniques are ideal for a broad range of preclinical IR studies intending to evaluate the efficacy of intra-arterial therapies for the treatment of primary and metastatic liver tumors.
Keywords: Hepatocellular carcinoma (HCC), rodent model, McA-RH7777, intra-arterial (IA), catheterization
Introduction
Transcatheter arterial chemoembolization (TACE) and several other recently developed intra-arterial (IA) therapies are indispensable tools for the treatment of unresectable primary liver tumors such as hepatocellular carcinoma (HCC) [1-3]. IA delivery of therapeutic agents has the distinct advantage of permitting selective delivery to the targeted tumors [4], therefore reducing toxicity to adjacent normal liver parenchyma.
Several models have been used for the preclinical study of HCC therapies. With their inherently larger anatomies, rabbit, pig, and woodchuck models are more conducive to hepatic artery catheterization and transcatheter delivery of therapies; however, these models can be costly (in particular pigs and woodchucks) and require a significant amount of training and support staff. Rodent models can be more cost-effective, particularly for long-term therapeutic efficacy studies where relatively large sample sizes may be necessary to achieve requisite statistical power.
Invasive surgical catheterization of the hepatic artery via the gastroduodenal artery (GDA) has been used in a broad range of interventional studies investigating techniques such as TACE and virotherapy [5-7]. Less invasive methods have recently been described via carotid or femoral artery access, but these alternative approaches require custom microcatheter systems, specialized training, and involve a degree of procedural complexity that may limit their widespread utilization [8,9]. The former surgical GDA access route remains the most common approach for studies requiring catheter-directed access to the hepatic artery in rodent models; however, to our knowledge, the interventional radiology (IR) literature lacks a thorough tutorial describing and clearly illustrating this process [5-7,10,11].
The purpose of this illustrative study was to provide a rigorously documented guide and pictorial essay describing invasive hepatic artery catheterization techniques suitable for the study of liver-targeted interventional procedures in rodent models of liver cancer. We demonstrate that the hepatic artery can be invasively catheterized successfully and reproducibly under direct visualization.
Materials and methods
Animal model
All studies were approved by our institutional animal care and use committee and were performed in accordance with institutional guidelines. 20 male Sprague Dawley (SD) rats (Charles River Laboratories, Wilmington, MA) initially weighing 350 to 400 g were used for these studies.
For tumor inoculation, SD rats were anesthetized with an intraperitoneal (IP) injection of ketamine (75 – 100 mg/kg) and xylazine (2 – 6 mg/kg). Animals were monitored for depth of anesthesia throughout procedures by toe pinch and respiratory rates. After anesthesia, the abdominal area was shaved on each rat, and a laparotomy was performed extending roughly 5 cm caudally from the xiphoid process in order to access the liver. The left lateral liver lobe was exposed on a sterile compress by gentle lifting with cotton tipped applicators, and 1.0×106 McA-RH7777 HCC cells were injected by direct visualization under the hepatic capsule – successful inoculation was verified by a pale, whitish protrusion at the point of injection underneath the hepatic capsule (Figure 1) [12]. After slow withdrawal of the needle and placement of a small piece of hemostatic gauze (PRN Pharmacal, Pensacola, FL) at the injection site (Figure 1), gentle compression was applied for 15 seconds with a cotton tipped applicator in order to minimize bleeding and reflux of the inoculated cells out of the injection site. Abdominal incisions were closed by simple continuous suturing of the muscle layer and simple interrupted sutures for the skin layer followed by topical application of Vetbond tissue adhesive (3M, St. Paul, MN). Animals were allowed to recover after tumor implantation, and analgesia was administered as meloxicam (2 mg/kg) subcutaneously post-surgery and again 24 hours later. Animals were returned to storage facilities with periodic monitoring while tumors were allowed to grow for 8 days before imaging.
Figure 1.
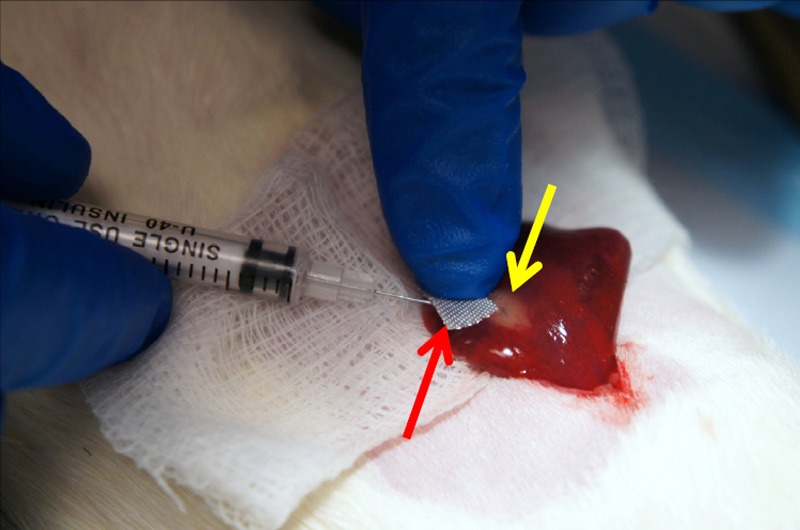
Subcapsular injection of tumor cells into rat liver. Inoculation site verified by whitish subcapsular protrusion (yellow arrow); hemostatic gauze placed at injection site (red arrow).
MRI
Magnetic resonance imaging (MRI) was performed to confirm inoculated tumor growth. Rats were anesthetized with isoflurane at an induction dose of 5% isoflurane, 2 L/min oxygen and maintained at a dose of 2% isoflurane, 2 L/min oxygen. A 7.0T 30 cm bore Bruker ClinScan MRI scanner (Bruker BioSpin, Billerica, MA) with Siemens Syngo clinical user interface and pulse sequences, 75 mm QuadTransceiver rat body coil (Bruker BioSpin, Billerica, MA), isoflurane anesthesia system, body temperature control, vital sign monitoring system, and small animal gating system (Small Animal Instruments, Inc., Stony Brook, NY) were used for free-breathing MRI acquisition. MRI scans were performed using a multi-slice T2-weighted turbo spin echo sequence (repetition time/echo time: 841 msec/28.00 msec; field of view: 70×70 mm2; matrix size: 128×128; imaging bandwidth: 200 Hz/pixel; slice thickness: 1.0 mm). Tumor size was measured in the coronal orientation by determining the longest in plane diameter in mm (per RECIST guidelines) and the width in mm perpendicular to the longest diameter [13].
Catheter placement
Rats were placed upon an angiography table and anesthetized via IP injection of ketamine/xylazine mixture as described previously. Table 1 summarizes the supplies used for the catheterization procedure. The abdominal area was shaved and cleaned with an alcohol wipe. A 5 cm long incision was made with a #10 scalpel blade extending caudally from the xiphoid process through the fascia and linea alba; atraumatic tissue forceps (Integra Miltex, Plainsboro, NJ) and iris scissors (Integra Miltex, Plainsboro, NJ) were used to carefully cut through residual connective tissue while avoiding damage to abdominal viscera. The left lateral lobe of the liver was externalized as previously described and retracted using a piece of saline-soaked gauze held back by needle holders (Figure 2). Cotton tipped applicators were then used to gently dissect through the mesentery to expose the portal triad after pushing the first loop of the duodenum caudally and to the left (Figure 3). Separation and dissection of the components of the portal triad as described in the following paragraphs was crucial for successful catheterization.
Table 1.
Catheterization supplies
| Quantity | Item |
|---|---|
| 1 | Small curved hemostat |
| 1 | Atraumatic tissue forceps |
| 1 | Iris scissors |
| 1 | Needle holder with suture scissors |
| 2 | Needle holder |
| 1 | #10 scalpel blade |
| Several | Cotton tipped applicator |
| 1 package | 4-0 suture |
| 1 roll | 2-0 suture |
| 1 | Polyurethane IV catheter (24G) |
| Several | 1 mL tuberculin slip tip syringe |
| 1 bottle | Iohexol contrast agent |
| 1 bottle | Heparin |
| 1 small bag | Sterile saline |
Figure 2.
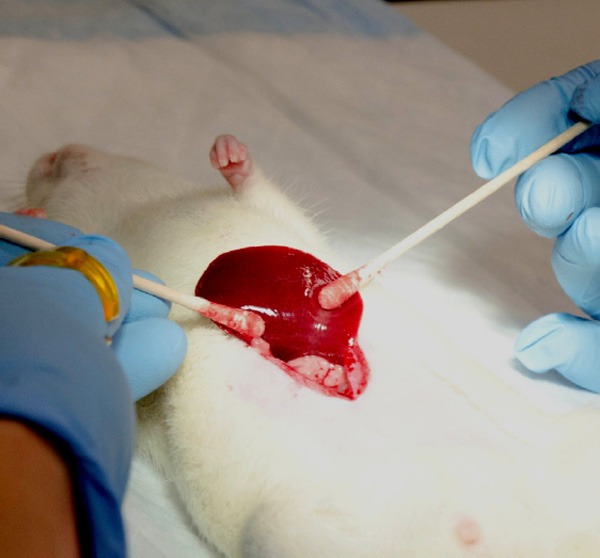
Externalized left lateral lobe of rat liver. Externalization of left lateral lobe with cotton tipped applicators.
Figure 3.
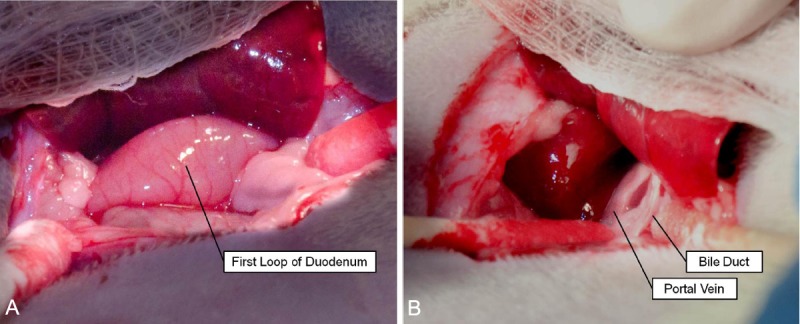
Exposure of the portal triad. A. First loop of duodenum visible deep to the externalized left lateral liver lobe. B. Bile duct identifiable as clear, whitish structure; portal vein distinguished by relatively large size and dark pink coloration.
A small curved hemostat was used to place an 8 cm piece of saline-soaked 2-0 silk braided non-absorbable suture (Stoelting Co., Wood Dale, IL) underneath the bile duct and adjacent mesenteric tissue to provide traction on the portal triad for dissection of its components (Figure 4). A clean cotton-tipped applicator was then used to clear the portal triad of excess connective tissue, exposing the common hepatic artery, hepatic artery proper, and GDA (Figure 5). The small curved hemostat was used to dissect underneath the common hepatic artery in order to place an 8 cm section of saline-soaked 2-0 silk braided non-absorbable suture that could be ligated permanently in the event of hemorrhage due to unexpected vessel puncture to temporarily stabilize the rat until euthanasia after imaging (Figure 6). During the catheterization procedure, a removable micro bulldog clamp (World Precision Instruments, Sarasota, FL) placed on the common hepatic artery prevented bleeding in the event of vessel puncture. The small curved hemostat was then used to isolate the GDA, placing both an 8 cm section of 4-0 Vetacryl absorbable polyglycolic acid suture (Webster Veterinary, Devens, MA) and an 8 cm section of saline-soaked 2-0 silk braided non-absorbable suture. The 4-0 suture was used to ligate the GDA distally to control retrograde bleeding during catheterization; this suture must be placed proximal to all GDA collaterals prior to ligation (Figure 7). The 2-0 suture initially placed underneath the bile duct was then removed after GDA ligation to avoid crowding the surgical field with excess suture.
Figure 4.
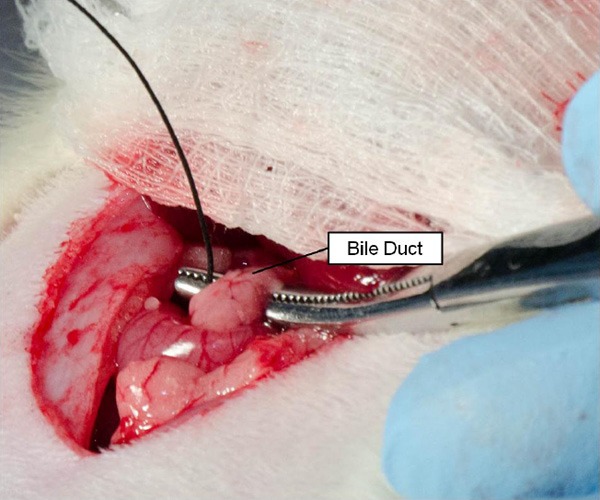
Placement of suture below the bile duct. A small curved hemostat was used to pull suture underneath the bile duct and associated connective tissue to provide tension on components of the portal triad for later dissection.
Figure 5.
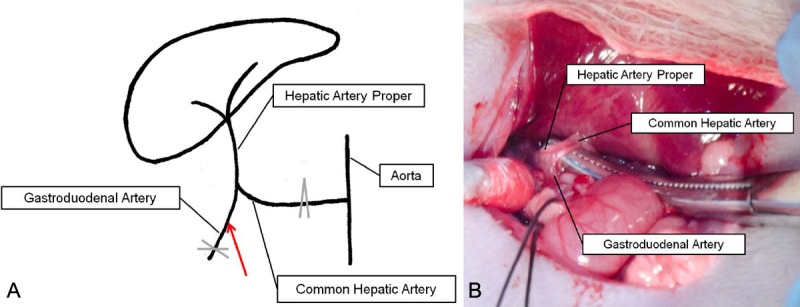
Vascular anatomy pertinent to hepatic artery catheterization. A. The common hepatic artery (CHA) arises from the aorta and bifurcates to yield the gastroduodenal artery (GDA) and hepatic artery proper. X indicates site of permanent GDA ligation; ^ indicates site of temporary CHA ligation; red arrow indicates catheter entry site. B. Vascular anatomy exposed after dissection. Note the characteristic bifurcation pattern in both the diagram and actual photograph.
Figure 6.
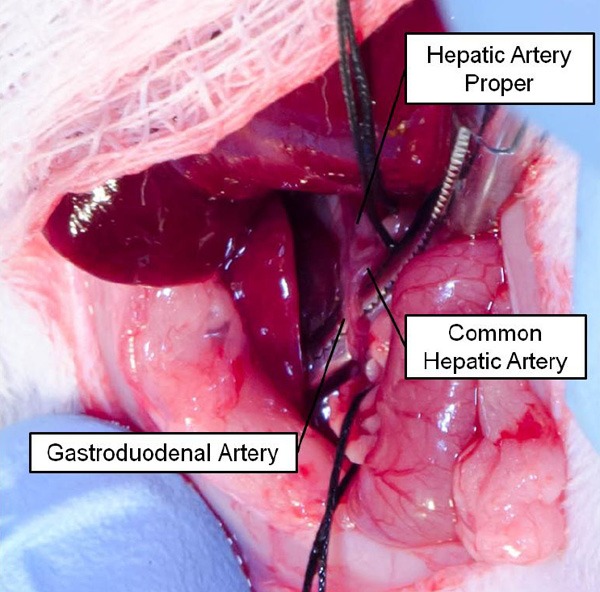
Temporary ligation of the common hepatic artery (CHA). Suture was placed around the common hepatic artery to prevent exsanguination if necessary. Micro bulldog clamp (not shown) was used for temporary intraprocedural ligation of the CHA. Note the characteristic bifurcation pattern of the vasculature. Yellow arrow indicates the entry point of the introducer and catheter.
Figure 7.
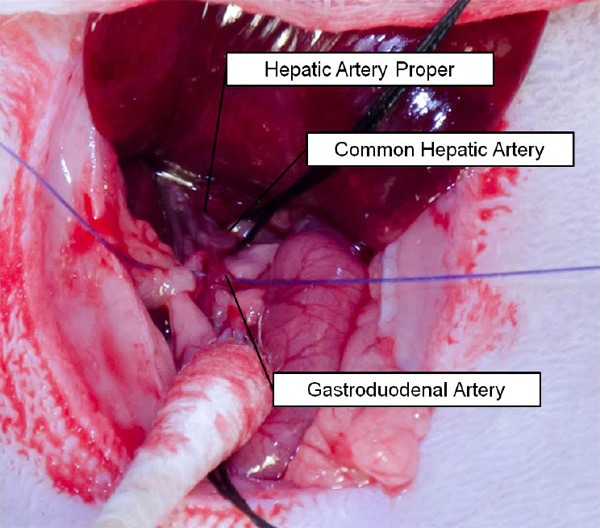
Ligation of the gastroduodenal artery (GDA). 4-0 suture was used to permanently ligate the GDA to prevent bleeding during catheterization; note the placement of the suture (purple) proximal to any collaterals arising from the vessel.
The 2-0 suture beneath the GDA was then used to place tension on the vessel during catheterization. The introducer of a 24G SurFlash polyurethane catheter (Terumo Medical Co., Somerset, NJ) was inserted into the GDA proximal to the ligation site but as distally to the branch point of the GDA from the common hepatic artery as possible. Successful placement of the introducer typically resulted in flashback of blood into the preview chamber (Figure 8); release of pressure on the common hepatic artery from the micro bulldog clamp assisted with confirmation of catheterization in the event that no flashback was seen after initial introducer placement. While maintaining tension on the 2-0 suture under the GDA, the introducer and catheter were advanced roughly 1 cm under direct visualization toward the hepatic artery proper. Using one hand, the introducer was then retracted with the index and middle fingers while the thumb continued to advance the catheter tip further toward the hepatic artery proper. After removal of the introducer, the catheter could be carefully advanced into the hepatic artery, placing the catheter tip in the common branch of the hepatic artery proper. The catheter was then fixed in place with the 2-0 suture; the knot was placed proximal to the insertion site to prevent bleeding and to hold the catheter tightly within the vessel wall without occluding the lumen. The previously externalized liver lobe was then returned to the abdominal cavity in order to restore hemodynamics. 0.1 mL of heparin lock flush (Hospira, Lake Forest, IL) was slowly injected with a tuberculin syringe (BD, Franklin Lakes, NJ) followed by 0.5 mL of saline solution; saline extravasation indicated poor catheter placement or vessel puncture. The catheter plug was used to prevent retrograde blood flow when the catheter was not being used.
Figure 8.
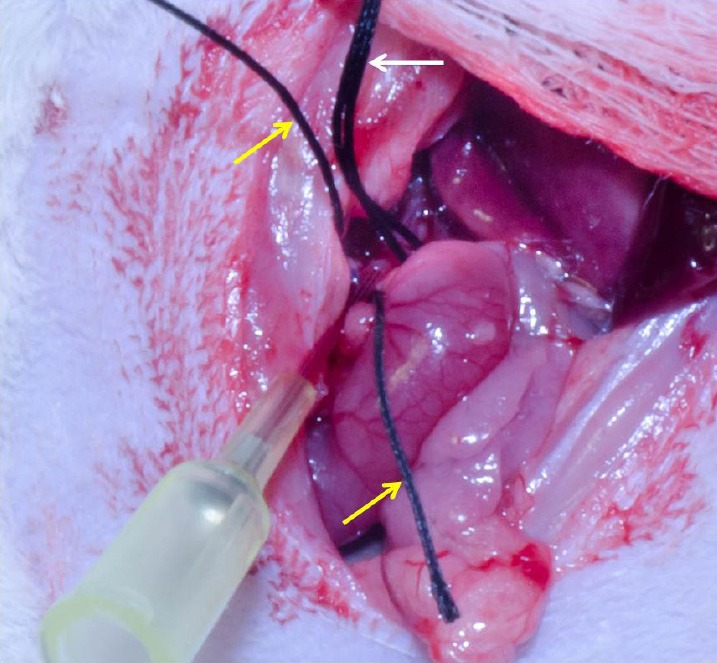
Successful catheterization of the hepatic artery proper. Intraluminal catheter placement without vessel puncture indicated by flashback of blood into the catheter. For anatomical reference: yellow arrows indicate suture around the gastroduodenal artery; white arrow indicates suture placed around the common hepatic artery.
Catheter removal after delivery of therapeutic agents or contrast was accomplished by slowly withdrawing the catheter and then ligating the GDA proximal to the catheter insertion site with the 2-0 suture. The micro bulldog clamp prevented exsanguination during catheter removal by maintaining pressure on the common hepatic artery. Antegrade blood flow to the liver via the common and proper hepatic artery was restored after catheter removal.
Hepatic angiography
Digital subtraction angiography (DSA) confirmed catheter placement in the common branch of the hepatic artery proper. 1 mL manual injections of Omnipaque (iohexol contrast agent) (GE Healthcare, Piscataway, NJ), were delivered through the catheter with a tuberculin syringe. The hepatic vasculature was visualized by a PowerMobile C-ARM angiographic system (Siemens Medical Solutions USA, Inc., Malvern, PA).
Histopathology
After MRI and angiography studies, each rat was euthanized with an intravenous [6] injection of 150 mg/kg Euthasol (Virbac Corporation, Fort Worth, TX) and bilateral thoracotomy. Livers were harvested and fixed overnight in formalin; sections bisecting the tumors were sampled and embedded in paraffin for hematoxylin and eosin (H&E) staining. Slides were later inspected visually with conventional light microscopy (10× and 20×).
Results
MRI
Of the 20 animals inoculated with McA-RH7777 HCC cells, 17 (85.0%) successfully developed measurable tumors observed upon follow-up MRI; these McA-RH7777 tumors were hyperintense relative to adjacent normal liver tissues in T2-weighted images (Figure 9). Average tumor size was (6.3 ± 2.3 mm) × (4.3 ± 1.5 mm) (mean ± SD).
Figure 9.
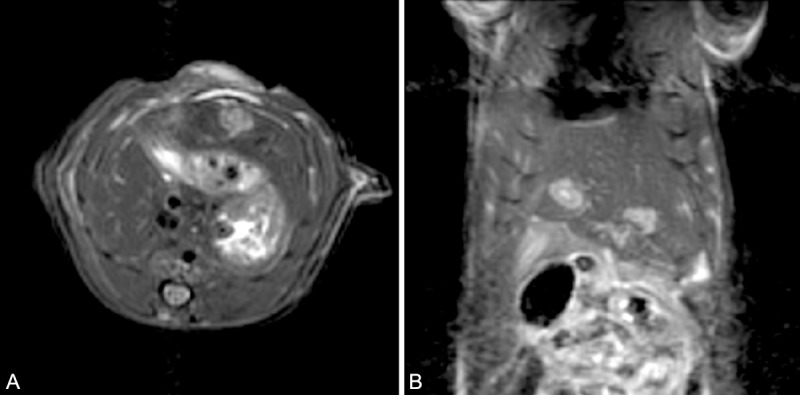
Turbo spin echo MRI of McA-RH7777 tumors in the rat. This animal had 1 tumor implanted in the left lateral lobe and 1 tumor implanted in the median lobe to demonstrate the ability to differentiate anatomic liver lobes. Tumors indicated by white arrows. A. transverse section. B. coronal section.
Hepatic angiography
Of the 17 animals that developed measurable tumors (observed during follow-up MRI), 16 (94.1%) were then successfully catheterized (evidenced by flashback of blood into the preview chamber, absence of extravasation of saline into the abdominal cavity, and hepatic artery enhancement on DSA) (Figure 10). One animal exsanguinated during catheterization and was euthanized as previously described.
Figure 10.
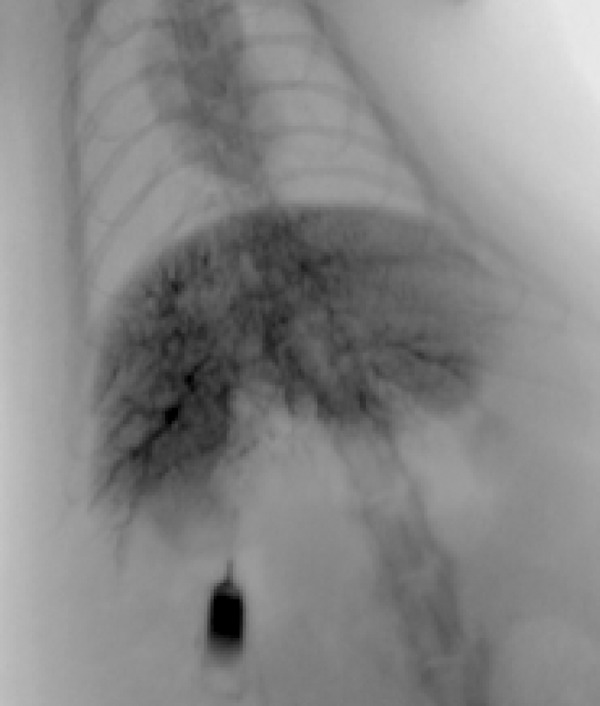
Hepatic angiography. Vasculature visible as darkened areas within the liver.
Histopathology
H&E staining demonstrated that McA-RH7777 hepatomas were solid masses with invasive borders. All 20 (100.0%) inoculated animals had histopathological evidence of tumor growth, including the 3 animals for which no tumors were detected during follow-up MRI (although tumors were limited in size even on histology). Tumors exhibited typical trabecular growth patterns (Figure 11). Tumor cells were epithelial in morphology with moderate cytological atypia.
Figure 11.
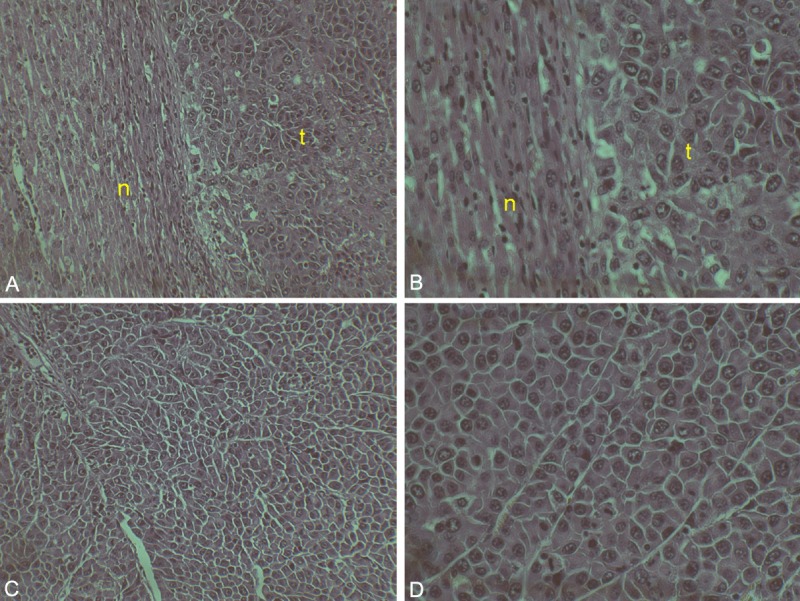
Histopathology of McA-RH7777 tumor. (a) 10× and (b) 20× view of normal liver (n) and tumor (t) border. (c) 10× and (d) 20× view of tumor core. Note the cytological atypia of the tumor cells.
Discussion
Liver cancer models permitting hepatic artery catheterization have been indispensable for preclinical investigations of liver-directed therapies. Rodent models can be particularly valuable because they can be more cost-effective, allow for accelerated studies due to rapid tumor inoculation, and require less specialized training in comparison to larger animal models such as rabbits and pigs. This study outlined the critical steps necessary for invasive hepatic artery catheterization in rodent models using surgical techniques and imaging methods (DSA and MRI) that can be readily adopted using skill sets already broadly available within most IR research laboratories.
Rodent models of HCC offer distinct advantages compared to other established models such as VX2 rabbit models and N-nitrosodiethylamine-induced pig models. The VX2 rabbit model is widely used due to its rapid growth rate and the larger vasculature of the rabbit in comparison to rodent models; however, the VX2 tumor cannot be maintained independently in vitro [14]. This limitation prevents in vitro studies with VX2 cells and requires the costly maintenance of donor animals for viable tumors [15]. Induced pig models have the advantage of even larger size compared to rabbits and similarity of liver anatomy to that of humans; however, the time duration required for tumor development can be highly variable with this drug-induced approach [15]. Rodent models of HCC address many of the limitations of rabbit and pig models, most importantly allowing for in vitro maintenance and experimentation with cell lines, rapid and predictable tumor growth, and lower costs compared to the larger animal models. These advantages allow rodent models to accommodate in vitro studies for validation of therapies before translation to more difficult and time-consuming animal experiments and facilitate larger sample sizes for increased sensitivity in experimental design – this combination provides a compelling model for applications to preclinical investigations of novel transcatheter therapies.
In this study, MRI was used for determination of tumor growth. 17 of the 20 inoculated animals developed measurable tumors on MRI, visualized as areas of increased signal intensity compared to normal liver. It was preferable to image rats prior to catheterization to prevent unnecessary surgery in animals with unsuccessful tumor inoculation or inadequate growth prior to the procedure. Due to the sensitivity of the MRI scanner to respiration artifact, isoflurane was preferred to the ketamine/xylazine mixture due to decreased respiratory depression and fewer variations in respiratory rate, allowing for regular respiratory gating during imaging and improved image quality [16]. In the event that catheterization was performed under anesthesia with ketamine/xylazine prior to imaging, our experience has shown that administration of a slow, steady stream of oxygen tends to minimize variability in respiratory rates.
DSA was used to verify successful catheterization of the hepatic artery. 16 of 17 animals were successfully catheterized. Finely branching vessels indicated successful catheterization, while patches of contrast were more indicative of catheter placement in a false lumen or intrahepatic vessel rupture.
One of the most important factors determining the likelihood of successful catheterization at the outset of the procedure was animal size. Larger animals are preferred to increase the likelihood of the animal having sufficiently large vascular diameters for manipulation and catheterization; however, for our purposes, rats exceeding 400 g could not be utilized because of the limited inner diameter of our rat body coil for MRI [17]. During the preparation steps leading up to the catheterization, several details can significantly improve the outcome of the procedure. The use of clean, dry cotton tipped applicators allows for efficient removal of connective tissue in order to expose the vascular anatomy. Throughout the catheterization procedure described in this study, the first loop of the duodenum was pushed caudally and to the left in order to facilitate the catheterization of the artery by a right-handed individual; however, with additional experience, a left-handed individual could mirror the steps if necessary. Additionally, the careful use of sutures can greatly reduce unintentional damage to adjacent structures and control bleeding. Presoaking sutures in saline before use minimizes abrasion and trauma to structures during manipulation. When strategically placing lengths of suture to allow for traction on vessels, allowing some excess connective tissue to remain between the suture and the vessel will serve to strengthen the vessel against possible breakage under tension. For example, the initial piece of suture placed beneath the bile duct receives a significant amount of tension during dissection of the portal triad; in our experience, the adjacent mesenteric connective tissue is crucial for preventing breakage of the bile duct. Maintaining the length of suture beneath the bile duct until ligation of the GDA is important because tension on the vascular tree exposes the anatomy and facilitates access to the GDA.
It is imperative to identify all collateral vessels branching from the GDA prior to ligation of the vessel in order to prevent excessive bleeding after puncture of the GDA with the catheter introducer. The GDA ligation with the 4-0 suture must take place as distally as possible from the origin of the GDA in order to preserve the full length of the GDA for catheter entry in the event that repeated attempts are required. Repeated attempts are possible as long as introducer punctures start distally to the branch point and move more proximal with subsequent attempts. Steady tension on the 2-0 suture is used to align the GDA for catheterization. The rationale for differing suture sizes is that the 4-0 suture used for GDA ligation results in a smaller knot that does not impede catheterization while the larger 2-0 suture is easier to manipulate manually during the actual insertion of the catheter. Retracting the introducer after catheter insertion into the GDA, slowly advancing the catheter through the GDA, and feeding the catheter carefully into the hepatic artery proper will decrease the risk of puncturing the delicate hepatic artery. Occasionally, vascular anatomy was encountered that was not conducive to catheterization of the hepatic artery; an acute angle between the GDA and the hepatic artery proper lead to puncture of the hepatic artery that accounted for the failed catheterization described in the results of the study. It is possible to be more selective than the common branch of the hepatic artery proper, feeding the catheter into the left branch that feeds the implanted tumor – DSA can be used to confirm desired catheter placement. However, this greatly increases the risk of puncturing the delicate vessels that taper in diameter as they enter the liver parenchyma, as encountered during our initial attempts at this technique.
The described invasive hepatic artery catheterization procedures are readily applicable for survival studies. Survival studies require that the puncture site in the GDA is isolated from the circulation by proximal and distal ligation to prevent excessive intra-abdominal bleeding. Additionally, the blood flow through the common hepatic artery must be restored by removal of the micro bulldog clamp and suture. Survival of the animal after delivery of therapeutic agents allows for studies evaluating the therapeutic efficacy of agents delivered via these transcatheter IA approaches.
The primary limitation of this approach is the inherent invasiveness of the procedure compared to other reported methods [9,17]. However, invasive catheterization for rodent models remains widely advocated because direct visualization of the vascular anatomy allows for high procedural success rates. Nonetheless, this invasive approach requires two laparotomy procedures for each study animal: the first for tumor implantation, and the second for catheterization and subsequent infusion of therapeutic materials. Undoubtedly, performing two procedures increases the potential for complications associated with wound infection and dehiscence. Recently described percutaneous liver tumor implantation techniques could conceivably mitigate complications associated with the latter limitation [18].
This illustrative study provides a rigorously documented guide and pictorial essay that describes invasive hepatic artery catheterization techniques suitable for the study of liver-targeted interventional procedures in rodent models of liver cancer. As demonstrated by our results, the hepatic artery is accessible in a reproducible fashion via the gastroduodenal artery, making the technique ideal for a broad range of preclinical IR studies intending to evaluate the efficacy of IA therapies for the treatment of primary and metastatic liver tumors.
Acknowledgments
Alexander Sheu is a Howard Hughes Medical Institute Medical Research Fellow. Angiography and MRI were performed at the Northwestern University Center for Translational Imaging. This work was supported by the Northwestern University Mouse Histology and Phenotyping Laboratory and a Cancer Center Support Grant (NCI CA060553). Microscopy work was performed at the Northwestern University Cell Imaging Facility generously supported by NCI CCSG P30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center. We thank Ray Chang who provided recording services for the photographs included in the manuscript. We also thank Weiguo Li who assisted with surgeries and procedures.
Conflicts of interest and financial disclosures
Reed A Omary: Founder of Interventional Oncology Research and Development (IORAD), LLC; Research consultant, DuNing, Inc, Research consultant, PhaseRx, Inc. Andrew C Larson: Founder of Interventional Oncology Research and Development (IORAD), LLC; Research consultant, DuNing, Inc; Research consultant, PhaseRx, Inc.
References
- 1.Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, Ibrahim SM, Sato KT, Baker T, Miller FH, Omary R, Abecassis M, Salem R. A Comparative Analysis of Transarterial Downstaging for Hepatocellular Carcinoma: Chemoembolization Versus Radioembolization. Am J Transplant. 2009;9:1920–1928. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, Rodés J, Bruix J. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 3.Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH, Sato KT, Wang E, Gupta R, Benson AB, Newman SB, Omary RA, Abecassis M, Kulik L. Radioembolization for Hepatocellular Carcinoma Using Yttrium-90 Microspheres: A Comprehensive Report of Long-term Outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Cabrera R, Nelson D. Review article: the management of hepatocellular carcinoma. Aliment Pharmacol Ther. 2010;31:461–476. doi: 10.1111/j.1365-2036.2009.04200.x. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Zheng CS, Feng GS, Zhuo CK, Zhao JG, Liu X. An implantable rat liver tumor model for experimental transarterial chemoembolization therapy and its imaging features. World J Gastroenterol. 2002;8:1035–1039. doi: 10.3748/wjg.v8.i6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinozaki K, Ebert O, Kournioti C, Tai YS, Woo SLC. Oncolysis of Multifocal Hepatocellular Carcinoma in the Rat Liver by Hepatic Artery Infusion of Vesicular Stomatitis Virus. Mol Ther. 2004;9:368–376. doi: 10.1016/j.ymthe.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Shinozaki K, Ebert O, Woo SL. Eradication of advanced hepatocellular carcinoma in rats via repeated hepatic arterial infusions of recombinant VSV. Hepatology. 2005;41:196–203. doi: 10.1002/hep.20536. [DOI] [PubMed] [Google Scholar]
- 8.Ju S, McLennan G, Bennett SL, Liang Y, Bonnac L, Pankiewicz KW, Jayaram HN. Technical aspects of imaging and transfemoral arterial treatment of N1-S1 tumors in rats: an appropriate model to test the biology and therapeutic response to transarterial treatments of liver cancers. J Vasc Interv Radiol. 2009;20:410–414. doi: 10.1016/j.jvir.2008.12.408. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Wang YX, Zhou X, Guan Y, Tang C. Catheterization of the Hepatic Artery Via the Left Common Carotid Artery in Rats. Cardiovasc Intervent Radiol. 2006;29:1073–1076. doi: 10.1007/s00270-005-8268-3. [DOI] [PubMed] [Google Scholar]
- 10.Lindell B, Aronsen KF, Rothman U. Repeated Arterial Embolization of Rat Livers by Degradable Microspheres. Eur Surg Res. 1977;9:347–356. doi: 10.1159/000127954. [DOI] [PubMed] [Google Scholar]
- 11.Garin E, Denizot B, Roux J, Noiret N, Lepareur N, Moreau M, Mesba H, Laurent JF, Herry JY, Bourguet P, Benoit JP, Lejeune JJ. Description and technical pitfalls of a hepatoma model and of intra-arterial injection of radiolabelled lipiodol in the rat. Lab Anim. 2005;39:314–320. doi: 10.1258/0023677054307051. [DOI] [PubMed] [Google Scholar]
- 12.Guo Y, Klein R, Omary RA, Yang GY, Larson AC. Highly malignant intra-hepatic metastatic hepatocellular carcinoma in rats. Am J Transl Res. 2010;3:114–120. [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Easty DM, Easty GC. Establishment of an in vitro cell line from the rabbit VX2 carcinoma. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;39:333–337. doi: 10.1007/BF02892859. [DOI] [PubMed] [Google Scholar]
- 15.Aravalli R, Golzarian J, Cressman E. Animal models of cancer in interventional radiology. Eur Radiol. 2009;19:1049–1053. doi: 10.1007/s00330-008-1263-8. [DOI] [PubMed] [Google Scholar]
- 16.Hanusch C, Hoeger S, Beck GC. Anaesthesia of small rodents during magnetic resonance imaging. Methods. 2007;43:68–78. doi: 10.1016/j.ymeth.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Ju S, McLennan G, Bennett SL, Liang Y, Bonnac L, Pankiewicz KW, Jayaram HN. Technical Aspects of Imaging and Transfemoral Arterial Treatment of N1-S1 Tumors in Rats: An Appropriate Model to Test the Biology and Therapeutic Response to Transarterial Treatments of Liver Cancers. J Vasc Interv Radiol. 2009;20:410–414. doi: 10.1016/j.jvir.2008.12.408. [DOI] [PubMed] [Google Scholar]
- 18.Chan HH, Chu TH, Chien HF, Sun CK, Wang EM, Pan HB, Kuo HM, Hu TH, Lai KH, Cheng JT, Tai MH. Rapid induction of orthotopic hepatocellular carcinoma in immune-competent rats by non-invasive ultrasound-guided cells implantation. BMC Gastroenterol. 2010;10:83. doi: 10.1186/1471-230X-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]


