Abstract
Previous studies suggested that β-blockers with adjunctive α1-blocking activities warrant renoprotective function other than the therapeutic effect on hypertension. The current report is designed to dissect the role of TJ0711, a novel β-blocker with a 1:1 ratio for the β1/α1 blocking activities, in renoprotection in SHR rats. It was noted that TJ0711 possesses similar potency for control of blood pressure as that of Carvedilol. However, TJ0711 is much more potent in terms of protecting SHR rats against hypertension induced renal injury. Specifically, SHR rats treated with 20mg/kg/day of TJ0711 manifested significantly lower levels for urine albumin and total protein. In line with these result, TJ0711 treated rats displayed much less severe pathological changes in the kidneys. Mechanistic studies revealed that TJ0711 improves kidney perfusion during the course of hypertensive insult by enhancing eNOS expression through suppressing inflammatory cytokine secretion. TJ0711 also attenuates Vasohibin-1 expression to prevent HIF-1α from signal-induced degradation, and by which it promotes HO-1 expression to protect SHR rats against oxidative stress induced by hypertension in the kidneys. Together, our data suggest that TJ0711 possesses higher potency for renoprotection while manifesting the similar effect on hypertension therapy as Carvedilol.
Keywords: TJ0711, carvedilol, hypertension, renal injury, renoprotection
Introduction
Hypertension is a leading risk factor for cardiovascular disease and a significant cause of morbidity and mortality throughout the world [1]. Given that hypertension leads to various pathological changes within the kidneys, including vascular, glomerular, and tubulointerstitial injuries, the incidence of chronic kidney disease (CKD) in the general population has increased enormously over the past several decades, which also becomes a significant burden for the healthcare system in the current society. As a result, therapeutic strategies aimed at treating hypertension efficiently while ultimately protecting kidneys from hypertension induced injuries are wanting.
Since the introduction of propranolol in 1965, β-blockers have become the first-line antihypertensive drugs. Although β-blockers are potent antihypertensive agents by inhibiting β-adrenergic receptors in the heart and kidneys, they are different in terms of hemodynamic effect on renal function. The cardioselective β-blockers such as Atenolol and Metoprolol are known to retard the progression of renal diseases, but to a lesser degree compared with blockers of the renin-angiotensin-aldosterone system. Interestingly, the newer vasodilating β-blockers such as Carvedilol and Nebivolol possess stronger potency on renoprotection primarily because of their greater adjunctive α1-blocking activities [2]. For example, Carvedilol has been found to prevent the reduction of glomerular filtration rate (GFR) by decreasing renal vascular resistance in patients with hypertension [3]. Given that TJ0711 acts as a novel β-blocker with much higher adjunctive α1-blocking activities [4], we thus assumed that TJ0711 may possess higher potency for protecting kidneys against hypertension induced injury while manifesting similar effect on hypertension therapy as Carvedilol. To test this assumption, we conducted studies in spontaneously hypertensive (SHR) rats and demonstrated that TJ0711 is more effective to protect SHR rats against hypertension induced renal injury as compared with that of Carvedilol. As expected, TJ0711 manifested similar therapeutic effect as Carvedilol on hypertension. Together, our data support that TJ0711 has the great potential to be a better antihypertensive therapy in the settings of patients with impaired renal function.
Materials and methods
Animals
Spontaneously hypertensive (SHR) male rats (12wk-old, 200-300g) were directly purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd. The animals were housed and acclimatized in Tongji Hospital Animal Care Unit in a temperature (22°C) and light-controlled (12h/12h light/dark) environment for a week, and then randomly divided into following experimental groups with each containing 7 rats: TJ0711 10 (the rats were treated with 10mg/kg/day of TJ0711), TJ0711 20 (the rats were received 20mg/kg/day of TJ0711), TJ0711 40 (40mg/kg/day of TJ0711 were administered), Carvedilol (the rats were treated with 20mg/kg/day of Carvedilol), and controls (the rats were treated with same volume of saline). The animals were subjected to drug administration as indicated above via oral gavage for 7 weeks. Tail artery systolic blood pressure before and after drug administration was monitored along with 24h urine collection on a weekly basis. All rats were euthanized at 20wk-old of age. Serum samples were collected for biochemical assays, and kidneys were removed for immunohistochemical analysis and protein extraction. All experimental procedures were conducted in accordance with NIH guidelines and were approved by the Animal Care and Use Committee (ACUC) in Tongji Hospital.
Blood pressure measurement
Systolic blood pressure (SBP) measurements were carried out using a Rat Tail Cuff Blood Pressure Systems (IITC Life Science Inc., CA, USA) according to the manufacturer’s instruction. Briefly, the rats were first anesthetized with sodium pentobarbital (50mg/kg, I.P.) and then placed on a heating pad. After exposing the left carotid artery, a short microbore teflon (0.75mm) was cannulated to connect with a low-volume displacement pressure transducer (P23Gb, Gould Inc., Cleveland, OH). Once the intra-arterial tracing became stable, the tail cuff and pulse sensor were placed on the tail to measure blood pressure. The signals from the pulse and pressure sensors were analyzed by the software provided by the vendor.
Immunostaining and histological analysis
Renal sections (3-um) were first incubated with 3% H2O2 for 10min, followed by BSA for 30min at room temperature. After washes, the slides were incubated with primary antibodies against either HO-1 (1:100, Millipore, MA, USA), or HIF-1α (1:80, Bioworld Technology, Shanghai, China), or Vasohibin-1 (1:20, Santa Cruz, CA, USA) at 4°C overnight. The slides were next incubated with a goat anti-rabbit IgG for 30min followed by staining with diaminobenzidine. HE staining and Masson staining of renal sections were carried out as previously reported [5]. Images under a light microscope were captured and analyzed by the Image-Pro Plus software (Rockville, USA).
Western blot analysis
Renal tissues were homogenized in RIPA lysis buffer containing cocktail for protease inhibitors using the established techniques [6]. Total protein concentrations were determined using a BCA assay kit (Pierce, IL, USA) as instructed. Total proteins (100ug) were separated by SDS-PAGE, and the separated proteins were transferred onto PVDF membranes. The membranes were first blocked with 5% nonfat milk in TBS with 0.1% Tween-20 for 1h at 37°C, and then probed with antibodies against HO-1 (1:1000), HIF-1α (1:100), Vasohibin-1 (1:100), eNOS (1:100, Millipore, MA, USA), GAPDH (1:4000, Santa Cruz, CA, USA), and β-actin (1:1000, Santa Cruz, CA, USA) at 4°C overnight, respectively. After washes, the blots were incubated with an HRP-conjugated anti-IgG, and the target bands were visualized with ECL plus reagents (Pierce, Rockford, IL) as instructed. The intensity of target bands was analyzed by densitometry and normalized by GAPDH or β-actin using the Quantity One software (BioRad, CA, USA).
Measurement of cytokines by ELISA
Serum cytokines for IL-1β, TNF-α and IL-10 as well as Adiponectin were measured using ELISA kits purchased from eBioscience (San Diego, USA). In brief, cytokine standards and serum samples were diluted according to the manufacture’s instruction and then applied to the ELISA plates. The plates were incubated at room temperature for 2h, followed by probing with the detection antibody for 1h. After washes, Avidin-HRP was added into the plates and incubated for 30min. The plates were next developed by addition of substrate solution, followed by addition of TMB stop solution to stop the reaction. The results were read under a microplate reader (Thermo Fisher Scientific, Waltham, USA) as previously reported [7]. Each sample was assayed in triplicates.
Biochemical profiles
Serum levels for urea nitrogen (BUN), creatinine (Scr), cystatin C and C-reactive protein (CRP), as well as 24h urine protein and albumin were examined by Sysmex CHEMIX-180 automatic biochemical analyzer (Japan) as previously reported [8].
Statistical analysis
All data are expressed as mean ± S.D. Comparisons between groups for blood pressure, renal parameters such as BUN and creatinine, and cytokine levels were accomplished by one-way ANOVA. Student-Newman-Keuls post hoc test was employed for multiple comparisons. All data were analyzed using SPSS 13.0 software for windows. In all cases, p < 0.05 was considered with statistical significance.
Results
TJ0711 possesses similar efficiency as carvedilol for control of blood pressure
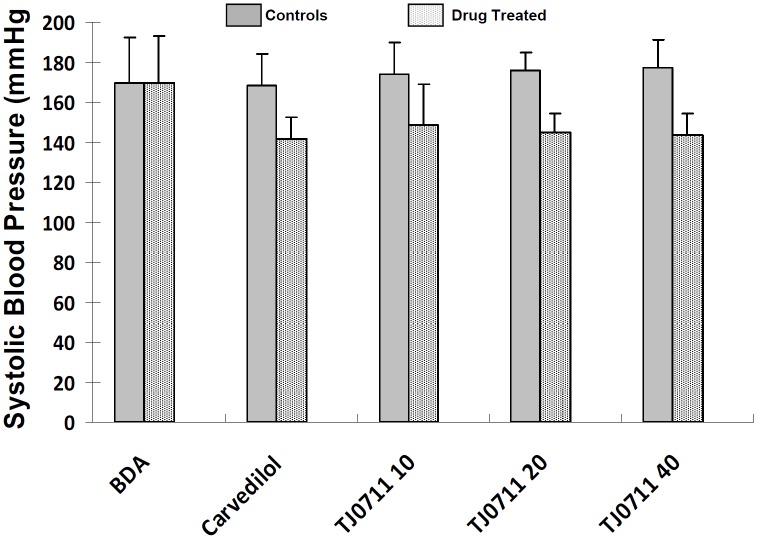
We first sought to examine the effect of TJ0711 on the control of blood pressure. To this end, SHR rats in each study group were subjected to measurement of systolic blood pressure (SBP) using a Rat Tail Cuff Blood Pressure Systems as described. TJ0711 or Carvedilol with indicated doses were directly administered into the stomach of rats via oral gavage 40 minutes before the measurements. As shown in Figure 1, before drug administration (BDA), both control and experimental rats displayed similar SBP, while administration of both TJ0711 and Carvedilol significantly decreased SBP in all rats examined. Particularly, TJ0711 showed similar potency for lowering blood pressure as that of Carvedilol. However, 10mg/kg/day of TJ0711 was enough to efficiently reduce SBP in SHR rats, while higher doses of TJ0711 (e.g., 20mg/kg/day or 40mg/kg/day) failed to show a dose-dependent effect on blood pressure.
Figure 1.
Systolic blood pressure (SBP) in rats before and after drug administration. Each group contains 7 rats. BDA, before drug administration.
TJ0711 is more potent to protect SHR rats against hypertension induced renal injury
Next, we intend to define the impact of TJ0711 on hypertension induced renal injury, a condition prone to the development of chronic kidney disease (CKD). For this purpose, all rats at 20wk old of age were sacrificed, and serum/urine samples were collected for biochemical analysis of parameters relevant to renal function. Surprisingly, rats treated with 20mg/kg/day of TJ0711 displayed significantly lower urine albumin as compared with that of animals either in the control group (0.05±0.01 vs. 0.11±0.09, p < 0.05, Table 1) or in the Carvedilol group (0.05±0.01 vs. 0.08±0.04, p < 0.05, Table 1). In line with this result, the amount of total urine protein in animals from TJ0711 20 group was significantly lower than that of animals from control group (6.63±2.55 vs. 9.34±2.24, p < 0.05, Table 1). However, we failed to detect a perceptible impact for both TJ0711 and Carvedilol on the serum levels for BUN, Scr, Cystatin C and CRP (Table 1). In contrast, rats treated with 40mg/kg/day of TJ0711 showed higher levels of Scr than that of control rats (33.20±2.28 vs. 26.40±5.77, Table 1), which was most likely caused by the toxicity of high-dose of TJ0711. In support of this notion, therapeutic dose of TJ0711 (10mg/kg/day) failed to show a perceptible protection on renal injury. Taken together, these results suggest that both TJ0771 and Carvedilol provided protection for SHR rats against hypertension induced early renal injury, but a more potent effect was noted for TJ0711 under the dose of 20mg/kg/day.
Table 1.
Biochemical results for analysis of renal function
| Biochemical parameters | Control | Carvedilol | TJ0711 10 | TJ0711 20 | TJ0711 40 |
|---|---|---|---|---|---|
| BUN (mmol/L) | 7.34±1.84 | 8.11±2.35 | 8.15±1.35 | 6.70±0.35 | 9.90±2.30 |
| Scr (umol/L) | 26.40±5.77 | 26.50±8.27 | 30.00±2.70 | 26.75±3.86 | 33.20±2.28* |
| Cystain C (mg/L) | 0.04±0.00 | 0.05±001 | 0.06±0.02 | 0.05±0.01 | 0.05±0.01 |
| CRP (mg/L) | 0.10±0.00 | 0.13±0.05. | 0.10±0.00 | 0.10±0.00 | 0.14±0.05 |
| 24h Urine protein (mg) | 9.34±2.24 | 7.56±3.69 | 9.18±5.40 | 6.63±2.55* | 8.81±2.91 |
| 24h Urine albumin (mg) | 0.11±0.09 | 0.08±0.04 | 0.09±0.36 | 0.05±0.01*,# | 0.08±0.04 |
p < 0.05 as compared with that of rats in the control group,
p < 0.05 as compared with that of rats in the Carvedilol group (n=7).
To further confirm the above results, we then performed histological analysis. PAS staining of renal sections revealed the presence of hyaline arteriolosclerosis in the arterioles (Figure 2A, indicated by arrows), and partial of the glomerulus was noted with ischemia (Figure 2B). HE staining further demonstrated the appearance of scattered interstitial infiltration of lymphocytes (Figure 2C, indicated by arrows), which was more evident when the sections assessed in higher amplifications under a microscope (Figure 2D, higher resolution for the inset area in Figure 2C). However, no obvious glomerulosclerosis or intertubular fibrosis was noted in all animals examined as manifested by the Masson trichrome staining (data not shown). Of note, both Carvedilol and TJ0711 significantly attenuated these pathological changes, and animals treated with 20mg/kg/day of TJ0711 manifested the least pathological changes. Collectively, our data provided convincing evidence that TJ0711 protects SHR rats against hypertension induced renal injury.
Figure 2.
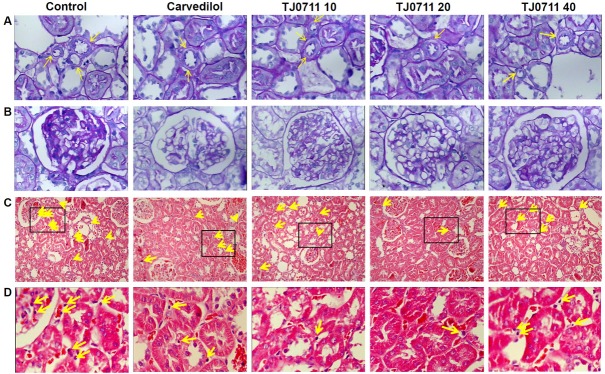
Histological analysis of renal sections. A. PAS staining results showing hyaline arteriolosclerosis (indicated by yellow arrows, magnification ×400). B. PAS staining results showing partial glomerular ischemia (magnification ×400). C. HE staining results showing scattered interstitial infiltration of lymphocytes. D. Higher magnification of inset area in Figure 2C (magnification ×400). Infiltrated lymphocytes are indicated by yellow arrows.
TJ0711 regulates the secretion of inflammatory cytokines
To dissect the molecular mechanisms by which TJ0711 protects SHR rats against hypertension induced renal injury, we first examined the impact of TJ0711 administration on the production of inflammatory cytokines, as chronic inflammatory response is a well known factor implicated in the pathogenesis of renal injury. Remarkably, administration of TJ0711 at the dose of 20mg/kg/day significantly attenuated the concentration of IL-1β in the serum as compared with that of rats treated with control vehicle (Figure 3A). A similar trend was also noted for TNF-α, although the results did not reach a statistical significance. Similar as TJ0711, treatment of rats with Carvedilol also significantly decreased serum IL-1β levels, but its potency was lower than that of TJ0711 (Figure 3A). In sharp contrast, significantly higher levels of serum IL-10 was noted in rats treated with either TJ0711 or Carvedilol as compared with that of control rats, and importantly, the concentration for serum IL-10 was even higher in 20mg/kg/day of TJ0711 treated rats as compared with that of Carvedilol treated rats (Figure 3A). We further examined Adiponectin, an adipocyte-secreted protein that circulates in high concentrations in the serum and plays an important role in the regulation of renal inflammation [9]. In consistent with the results for IL-10, both TJ0711 and Carvedilol enhanced Adiponectin secretion as manifested by the detection of significantly higher levels of serum Adiponectin, and furthermore, much higher potency was noted for 20mg/kg/day of TJ0711 as compared with that of Carvedilol (Figure 3B). All together, our data suggest that both TJ0711 and Carvedilol possess the properties to attenuate chronic inflammatory response during the course of hypertension induced renal injury, but the potency is much higher for TJ0711.
Figure 3.
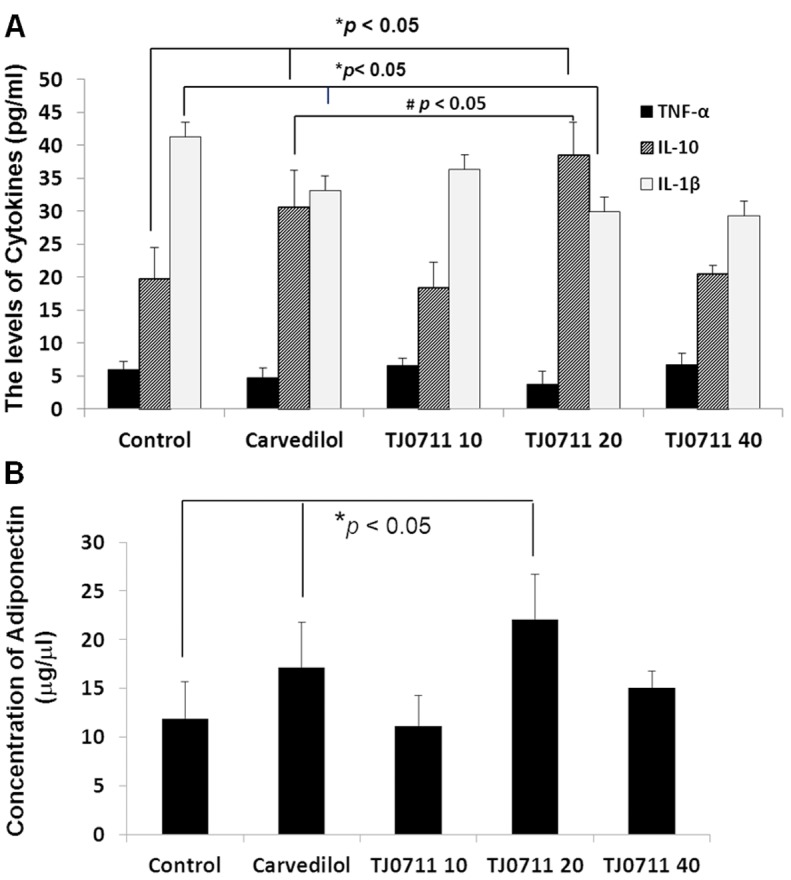
ELISA results for serum cytokines. A. Serum levels for TNF-α, IL-1β, and IL-10. B. Serum levels for Adiponection. *, p < 0.05 as compared with that of control rats.
TJ0711 promotes the expressions for eNOS and HO-1 in the kidney
There is compelling evidence that attenuated eNOS expression induces the loss of glomerular and peritubular capillary endothelium and exacerbate renal injury in hypertensive subjects [10], while IL-10 is capable of for the restoration of eNOS expression [11]. The above results, therefore, prompted us to examine the impact of TJ0711 on eNOS expression. Renal lysates from each group of rats were prepared and then subjected to Western blot analysis. Remarkably, significantly higher levels of eNOS expressions were detected in animals treated with either TJ0711 or Carvedilol as compared with that of control animals. Specifically, animals treated with 20mg/kg/day of TJ0711 showed 2.3 fold higher levels of eNOS than that of control animals. More importantly, a 70% higher eNOS expression was noted in animals treated with 20mg/kg/day of TJ0711 as compared with that of animals treated with Carvedilol (Figure 4), indicating that TJ0711 possesses much higher capacity for induction of eNOS expression in the kidney.
Figure 4.
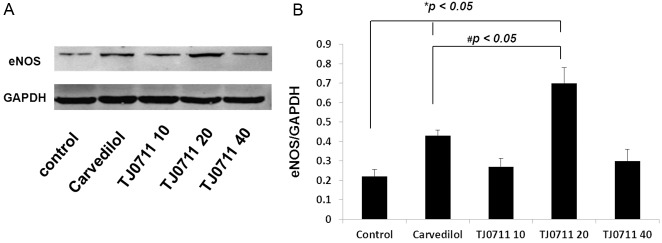
Western blot analysis of eNOS expression levels. A. A representative results for Western blot analysis. B. A bar graphic figure showing the relative eNOS expression levels normalized by GAPDH. Four rats were analyzed for each group. *, p < 0.05 as compared with that of control rats; #, p < 0.05 as compared with that of rats in the Carvedilol group.
Given that heme oxygenase-1 (HO-1) is downstream of Adiponectin signaling [12], and acts as a well known antioxidant factor during the course of ischemic renal injury, we next examined HO-1 expressions. In line with the above results, administration of TJ0711 and Carvedilol increased the levels for HO-1 expression significantly in the kidneys, and similarly, higher potency for TJ0711 (20mg/kg/day) was noted as compared with that of Carvedilol (Figure 5A). To further confirm these results, we performed immunostaining of renal sections. As shown in Figure 5B, high levels of HO-1 expressions were readily detected in sections derived from TJ0711 or Carvedilol treated animals, and the highest HO-1 expression was noted in animals treated with 20mg/kg/day of TJ0711.
Figure 5.
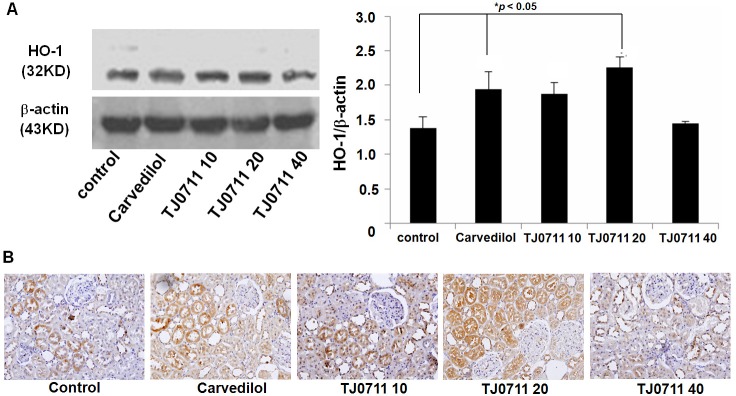
Results for HO-1 expression analysis. A. Western blot analysis of HO-1 expression levels. Left: A representative Western blot results. Right: A bar graphic figure showing the average results of all rats analyzed (n = 4). *, p < 0.05 as compared with that of control rats. B. HO-1 immunostaining of renal sections.
TJ0711 enhances the transcriptional activity of HIF-1α in the kidney
To further address the mechanisms underlying TJ0711 regulation of HO-1 expression, we examined its impact on HIF-1α, an essential transcriptional factor responsible for HO-1 expression during the course of ischemic renal injury [13]. We first performed Western blot analysis of renal lysates and found that administration of TJ0711 at the dose of 20mg/kg/day resulted in a 1.4 fold increase for the HIF-1α expression as compared with that of controls. Similarly, TJ0711 (20mg/kg/day) induced 86% higher HIF-1α expression than that of Carvedilol (Figure 6A). Indeed, immunostaining of renal sections confirmed that the highest levels of HIF-1α expression were detected in rats treated with 20mg/kg/day of TJ0711 (Figure 6B). Given that Vasohibin-1 acts a negative regulator for HIF-1α expression [14], we then examined the impact of TJ0711 on Vasohibin-1 expression. Interestingly, Western blot analysis revealed that TJ0711 at 20mg/kg/day of dose attenuated Vasohibin-1 expression by almost 100%, and a dose-dependent inhibitory effect was also noted. Unexpectedly, Carvedilol failed to show a discernable effect on suppressing Vasohibin-1 expression (Figure 7A), and consistent results were obtained by immunostaining of renal sections (Figure 7B). All together, our data support that TJ0711 suppresses Vasohibin-1 expression to prevent HIF-1α from degradation, and by which it enhances HO-1 expression to protect rats against hypertension induced oxidative stress.
Figure 6.
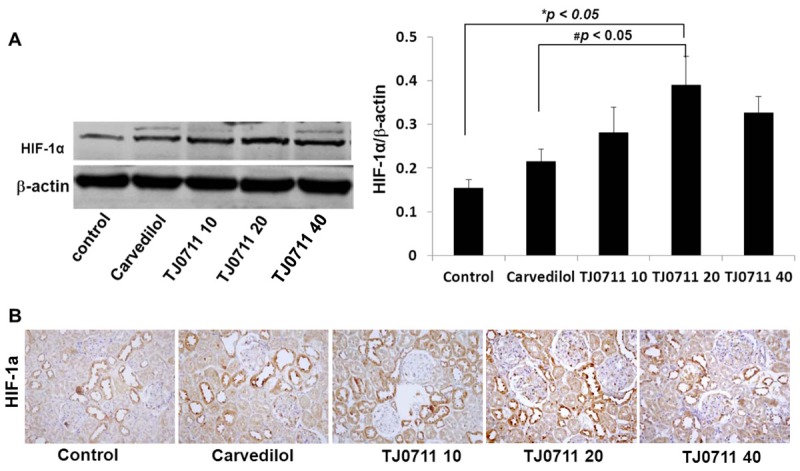
Analysis of HIF-1α expression in the kidney. A. Results for Western blot analysis. Relative levels for HIF-1α expression were normalized by β-actin and presented by a bar graphic figure (n = 4). *, p < 0.05 as compared with that of control rats. B. Immunostaining of renal sections for HIF-1α expression.
Figure 7.
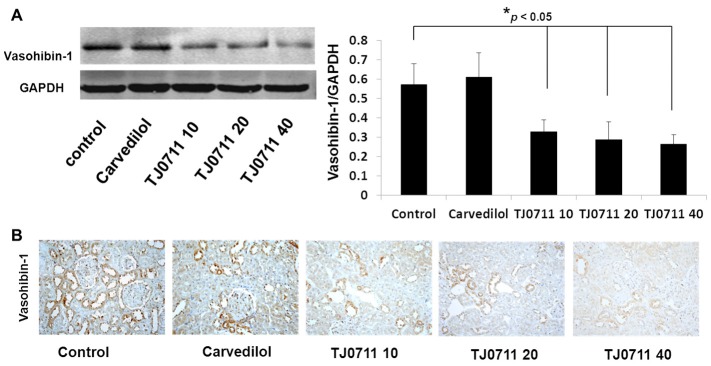
Expression analysis of Vasohibin-1 in the kidney. A. Western blot analysis of Vasohibin-1 expression in each group, and the bar graphs display the average levels of Vasohibin-1 normalized by GAPDH (n = 4). *, p < 0.05 as compared with that of rats within the control group. B. Immunostaining of Vasohibin-1 in the renal sections.
Discussion
Kidney is not only a target organ prone to hypertension induced injury, but also implicated in exacerbating the development of hypertension. Therefore, antihypertensive drugs with renal protective effect would have a wider range of clinical applications. For example, the angiotensin receptor blockers (ARB drugs) and the angiotensin converting enzyme inhibitors (ACEI) have been well demonstrated with independent renoprotective effect other than their antihypertensive therapy. In sharp contrast, although β-adrenergic blockers serve as the first-line of antihypertensive therapies in the clinical settings, the exact impact for most of those drugs on renoprotection, however, remained controversial [15]. Carvedilol is the third generation of β-blockers with partial (7:1) overlap for α-blockades [2], and is thus far the few β-blockers demonstrated with anti-oxidative stress and antiapoptotic effect other than lowering blood pressure [16-19], and therefore, it has been recommended as an alternative choice for treatment of patients with CKD [3].
TJ0711 is a novel β-blocker manifested by that it contains an aminopropanol group, a characteristic structure for most β-adrenergic blocking agents [20,21]. Our previous studies revealed that TJ0711 hydrochloride actually functions as an α1/β1 receptor-blocking agent (C.N. Patent.101,508,652) as it blocks α1-adrenoceptor similar as that of β1-adrenoceptor (1:1 ratio), which renders TJ0711 with a relatively stronger vasodilator property than other known vasodilating β-blockers such as Carvedilol [22]. Given that TJ0711 executes its effect much quicker and without perceptible accumulation in the target organs, it has the great potential to serve as a better therapeutic reagent in treatment of hypertension and the related complications. We thus in the current report, conducted studies to demonstrate the impact of TJ0711 on renoprotection and the related molecular mechanisms using SHR rats as a model.
It was interestingly noted that TJ0711 possesses similar potency for lowering blood pressure as that of Carvedilol, and TJ0711 at the dose of 10mg/kg/day is sufficient to control blood pressure to the levels similar as that of Carvedilol at the dose of 20mg/kg/day, while increase of TJ0711 dose (e.g., 2mg/kg/day or 40mg.kg/day) does not further decrease blood pressure (Figure 1). However, it is quite evident that TJ0711 is much more potent in protecting kidneys from hypertension induced injury as manifested by the observation of significantly lower levels of urine albumin in rats treated with 20mg/kg/day of TJ0711 than that of rats treated with either Carvedilol or control vehicle, and similar trends were noted for 24h urine protein (Table 1). However, unlike the treatment of high blood pressure, 10mg/kg/day of TJ0711 failed to show a perceptible protective effect on renal function, and it is likely that 20mg/kg/day of TJ0711 is the optimal dose for this purpose, because higher dose of TJ0711 such as 40mg/kg/day did not reach the similar protective effect. It is worthy of note that administration of either TJ0711 or Carvedilol did not result in a discernable change for serum BUN, creatinine, cystatin C and CRP, which is likely due to that the animals were still in the early stage of hypertensive renal injury. Indeed, histological analysis and immunostaining only suggested the appearance of ischemia in partial of the glomerulus (Figure 2B), and scattered interstitial infiltration of lymphocytes (Figure 2C and 2D). Nevertheless, both histological and immunostaining data further confirmed the notion that TJ0711 is more effective in terms of protecting rats against hypertension induced renal injury.
Given that inflammation is not only implicated in the pathogenesis of hypertension [23], but also an important risk factor contributing to the development of chronic kidney disease [24,25], we therefore first examined the impact of TJ0711 on serum cytokines for our mechanistic studies. Remarkably, TJ0711 significantly attenuated serum concentrations for IL-1β, but enhanced the levels for IL-10 and Adiponectin (Figure 3A and 3B). More importantly, significantly higher levels of IL-10 and Adiponectin were noted in TJ0711 treated rats as compared with that of Carvedilol treated rats, demonstrating that TJ0711 protects rats against hypertension induced renal injury probably by attenuating chronic inflammatory response.
It has been well known that chronic inflammation along with oxidative stress serves as the major mechanism contributing to renal injuries. We thus next examined the impact of TJ0711 on renal eNOS expression, as IL-10 signaling has been suggested to promote eNOS expression in the endothelium [11]. In line with this notion, a 2.3 fold higher eNOS expression was noted in rats treated with 20mg/kg/day of TJ0711 than that of control rats (Figure 4). Indeed, eNOS expression has been found to play a pivotal role in limiting local cellular inflammatory response, as evidenced by the increased leukocyte infiltration and enhanced MPO activities in eNOS-KO mice [26].
Hypertension provides a chronic ischemic microenvironment in the kidneys because of pathological changes in the arteries. The next important question is how TJ0711 regulates oxidative stress during the course of hypertension induced renal injury. Since Adiponectin has been noted to induce the expression of HO-1, a well known antioxidant factor, we thus first examined HO-1 expression. As expected, TJ0711 promoted higher levels of HO-1 expression in the kidneys as manifested by Western blot analysis (Figure 5A) and immunostaining (Figure 5B). To address the mechanisms underlying increased HO-1 expression in TJ0711 treated rats, we then check the effect of TJ0711 on HIF-1α, the essential transcription factor responsible for HO-1 expressions. Similar as above, Western blot analysis and immunostaining consistently demonstrated significantly higher levels of HIF-1α expression in rats treated with 20mg/kg/day of TJ0711 as compared with that of either Carvedilol treated rats or control rats (Figure 6A and 6B). In line with our data, HIF-1α has been well demonstrated to function as a master regulator of oxygen homeostasis. It regulates the expressions of proteins to increase oxygen delivery for cells being able to survive in oxygen-deficient conditions [27]. Recent studies have consistently demonstrated that upregulation of HIF-1α expression is beneficial to hypoxic ischemic diseases and that HIF-1α may serve as a novel target for treatment of CKD [28]. For example, activated HIF-1α cis-regulates VEGF transcription to improve tissue ischemic hypoxia [29].
The above results further prompted us to examine the expression of Vasohibin-1, a negative regulator for HIF-1α activity. Interestingly, both Western blot analysis and immunostaining consistently demonstrated that TJ0711 significantly attenuated the expression of Vasohibin-1 in the kidneys (Figure 7A and 7B). Indeed, Vasohibin-1 was found to promote the degradation of HIF-1α [14] and it was identified to be the first secretory antiangiogenic factor induced by VEGF in endothelial cells (ECs) [30]. We thus speculated that suppression of Vasohibin-1 might be helpful to promote perfusion for improving oxygen delivery during the course of hypertension induced injury. Of importantly note, we failed to detect a perceptible impact for Carvedilol on Vasohibin-1 expression, suggesting that other than the above shared pathways Carvedilol may involve additional pathways that are distinct from that of TJ0711. Taken together, our data support that TJ0711 relieves renal oxidative stress by attenuating the expression of Vasohibin-1 to prevent HIF-1α from degradation, which then transcribes high levels of HO-1 expression to protect animals against hypertension-induced oxidative stress in the kidneys.
Apparently, TJ0711 protects SHR rats from hypertension induced renal injury independent of its impact on blood pressure, as blood pressure was almost the same between all drug treated rats, particularly between those rats treated with either 10mg/kg/day or 20mg/kg/day of TJ0711. Given that Carvedilol has been suggested to exert its effect against oxidative stress and inflammatory response by blocking α1-adrenoceptor [31,32], it is plausible to assume that the protective effect observed in TJ0711 is also independent of its impact on β1-adrenoceptor, but is rather relevant to its effect on α1-adrenoceptor. In support of this notion, TJ0711 has been demonstrated with a 1:1 ratio for its impact on β1-adrenoceptor versus α1-adrenoceptor [4], and in contrast, the ratio for Carvedilol is only 7:1 [2].
In summary, we have demonstrated evidence supporting that TJ0711 is more effective to protect SHR rats against hypertension induced renal injury as compared with that of Carvedilol, and this protective effect is likely relevant to its impact on blocking α1-adrenoceptor. Mechanistic studies revealed that TJ0711 suppresses Vasohibin-1 expression to prevent HIF-1α from signal-induced degradation, which then transcribes high levels of HO-1 expression, and by which, TJ0711 protects SHR rats against oxidative stress induced by hypertension in the kidneys. We also obtained evidence suggesting that TJ0711 suppresses inflammatory response by enhancing IL-10 and suppressing IL-1β in the serum, and through which, TJ0711 promotes eNOS expression to improve perfusion during the course of hypertensive insult in the kidneys. Together, our data support that TJ0711 could serve as a potent antihypertensive drug along with better renoprotective effect.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (81170686, 30800383 and 81130014) and by a grant from the Ministry of Education (311028).
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Yoshikawa T, Port JD, Asano K, Chidiak P, Bouvier M, Dutcher D, Roden RL, Minobe W, Tremmel KD, Bristow MR. Cardiac adrenergic receptor effects of carvedilol. Eur Heart J. 1996;17(Suppl B):8–16. doi: 10.1093/eurheartj/17.suppl_b.8. [DOI] [PubMed] [Google Scholar]
- 3.Hart PD, Bakris GL. Should beta-blockers be used to control hypertension in people with chronic kidney disease? Semin Nephrol. 2007;27:555–564. doi: 10.1016/j.semnephrol.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Sun S, Si L, Fan Z, Qiu J, Li G. Stereoselective HPLC assay of TJ0711 enantiomers by precolumn derivatization with GITC using UV detection and its application in pharmacokinetics in rats. J Huazhong Univ Sci Technolog Med Sci. 2009;29:427–430. doi: 10.1007/s11596-009-0407-7. [DOI] [PubMed] [Google Scholar]
- 5.Zhang S, Lv JW, Yang P, Yu Q, Pang J, Wang Z, Guo H, Liu S, Hu J, Li J, Leng J, Huang Y, Ye Z, Wang CY. Loss of dicer exacerbates cyclophosphamide-induced bladder overactivity by enhancing purinergic signaling. Am J Pathol. 2012;181:937–946. doi: 10.1016/j.ajpath.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 6.Rao X, Zhong J, Zhang S, Zhang Y, Yu Q, Yang P, Wang MH, Fulton DJ, Shi H, Dong Z, Wang D, Wang CY. Loss of methyl-CpG-binding domain protein 2 enhances endothelial angiogenesis and protects mice against hind-limb ischemic injury. Circulation. 2011;123:2964–2974. doi: 10.1161/CIRCULATIONAHA.110.966408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong J, Yang P, Muta K, Dong R, Marrero M, Gong F, Wang CY. Loss of Jak2 selectively suppresses DC-mediated innate immune response and protects mice from lethal dose of LPS-induced septic shock. PLoS One. 2010;5:e9593. doi: 10.1371/journal.pone.0009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miao Z, Yan S, Wang J, Wang B, Li Y, Xing X, Yuan Y, Meng D, Wang L, Gu J, Zhang S, Li C, Wang CY. Insulin resistance acts as an independent risk factor exacerbating high-purine diet induced renal injury and knee joint gouty lesions. Inflamm Res. 2009;58:659–668. doi: 10.1007/s00011-009-0031-9. [DOI] [PubMed] [Google Scholar]
- 9.Gavrila A, Chan JL, Yiannakouris N, Kontogianni M, Miller LC, Orlova C, Mantzoros CS. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab. 2003;88:4823–4831. doi: 10.1210/jc.2003-030214. [DOI] [PubMed] [Google Scholar]
- 10.Savard S, Lavoie P, Villeneuve C, Agharazii M, Lebel M, Lariviere R. eNOS gene delivery prevents hypertension and reduces renal failure and injury in rats with reduced renal mass. Nephrol Dial Transplant. 2012;27:2182–2190. doi: 10.1093/ndt/gfr641. [DOI] [PubMed] [Google Scholar]
- 11.Zemse SM, Chiao CW, Hilgers RH, Webb RC. Interleukin-10 inhibits the in vivo and in vitro adverse effects of TNF-alpha on the endothelium of murine aorta. Am J Physiol Heart Circ Physiol. 2010;299:H1160–1167. doi: 10.1152/ajpheart.00763.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin H, Yu CH, Jen CY, Cheng CF, Chou Y, Chang CC, Juan SH. Adiponectin-mediated heme oxygenase-1 induction protects against iron-induced liver injury via a PPARalpha dependent mechanism. Am J Pathol. 2010;177:1697–1709. doi: 10.2353/ajpath.2010.090789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL, Choi AM. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem. 1997;272:5375–5381. [PubMed] [Google Scholar]
- 14.Kozako T, Matsumoto N, Kuramoto Y, Sakata A, Motonagare R, Aikawa A, Imoto M, Toda A, Honda S, Shimeno H, Soeda S. Vasohibin induces prolyl hydroxylase-mediated degradation of hypoxia-inducible factor-1alpha in human umbilical vein endothelial cells. FEBS Lett. 2012;586:1067–1072. doi: 10.1016/j.febslet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Ritz E, Rump LC. Do beta-blockers combined with RAS inhibitors make sense after all to protect against renal injury? Curr Hypertens Rep. 2007;9:409–414. doi: 10.1007/s11906-007-0075-6. [DOI] [PubMed] [Google Scholar]
- 16.Jovanovic D, Jovovic D, Mihailovic-Stanojevic N, Miloradovic Z, Naumovic R, Dimitrijevic J, Maksic N, Djukanovic L. Effect of carvedilol on pulse pressure and left ventricular hypertrophy in spontaneously hypertensive rats with adriamycin nephropathy. Biomed Pharmacother. 2009;63:571–576. doi: 10.1016/j.biopha.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues MA, Rodrigues JL, Martins NM, Barbosa F, Curti C, Santos NA, Santos AC. Carvedilol protects against cisplatin-induced oxidative stress, redox state unbalance and apoptosis in rat kidney mitochondria. Chem Biol Interact. 2011;189:45–51. doi: 10.1016/j.cbi.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Carvalho Rodrigues MA, Gobe G, Santos NA, Santos AC. Carvedilol protects against apoptotic cell death induced by cisplatin in renal tubular epithelial cells. J Toxicol Environ Health A. 2012;75:981–990. doi: 10.1080/15287394.2012.696512. [DOI] [PubMed] [Google Scholar]
- 19.Singh D, Chander V, Chopra K. Carvedilol attenuates ischemia-reperfusion-induced oxidative renal injury in rats. Fundam Clin Pharmacol. 2004;18:627–634. doi: 10.1111/j.1472-8206.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- 20.Groszek G, Nowak-Krol A, Wdowik T, Swierczynski D, Bednarski M, Otto M, Walczak M, Filipek B. Synthesis and adrenolytic activity of 1-(1H-indol-4-yloxy)-3-(2-(2-methoxy phenoxy) ethylamino)propan-2-ol analogs and its enantiomers. Part 2. Eur J Med Chem. 2009;44:5103–5111. doi: 10.1016/j.ejmech.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Chiu CC, Lin YT, Tsai CH, Liang JC, Chiang LC, Wu JR, Chen IJ, Yeh JL. Pharmacological effects of an aldehyde type alpha/beta-adrenoceptor blocking agent with vasodilating properties. Gen Pharmacol. 2000;34:391–400. doi: 10.1016/s0306-3623(01)00076-3. [DOI] [PubMed] [Google Scholar]
- 22.Takara K, Sakaeda T, Okumura K. Carvedilol: a new candidate for reversal of MDR1/P-glycoprotein-mediated multidrug resistance. Anticancer Drugs. 2004;15:303–309. doi: 10.1097/00001813-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vianna HR, Soares CM, Tavares MS, Teixeira MM, Silva AC. [Inflammation in chronic kidney disease: the role of cytokines] . J Bras Nefrol. 2011;33:351–364. doi: 10.1590/s0101-28002011000300012. [DOI] [PubMed] [Google Scholar]
- 25.Miyamoto T, Carrero JJ, Stenvinkel P. Inflammation as a risk factor and target for therapy in chronic kidney disease. Curr Opin Nephrol Hypertens. 2011;20:662–668. doi: 10.1097/MNH.0b013e32834ad504. [DOI] [PubMed] [Google Scholar]
- 26.Kaminski A, Pohl CB, Sponholz C, Ma N, Stamm C, Vollmar B, Steinhoff G. Up-regulation of endothelial nitric oxide synthase inhibits pulmonary leukocyte migration following lung ischemia-reperfusion in mice. Am J Pathol. 2004;164:2241–2249. doi: 10.1016/S0002-9440(10)63780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JW, Chun YS, Kim MS. Hypoxia-inducible factor 1-related diseases and prospective therapeutic tools. J Pharmacol Sci. 2004;94:221–232. doi: 10.1254/jphs.94.221. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka T, Nangaku M. Drug discovery for overcoming chronic kidney disease (CKD): prolyl-hydroxylase inhibitors to activate hypoxia-inducible factor (HIF) as a novel therapeutic approach in CKD. J Pharmacol Sci. 2009;109:24–31. doi: 10.1254/jphs.08r09fm. [DOI] [PubMed] [Google Scholar]
- 29.Katavetin P, Inagi R, Miyata T, Tanaka T, Sassa R, Ingelfinger JR, Fujita T, Nangaku M. Albumin suppresses vascular endothelial growth factor via alteration of hypoxia-inducible factor/hypoxia-responsive element pathway. Biochem Biophys Res Commun. 2008;367:305–310. doi: 10.1016/j.bbrc.2007.12.086. [DOI] [PubMed] [Google Scholar]
- 30.Sato Y, Sonoda H. The vasohibin family: a negative regulatory system of angiogenesis genetically programmed in endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27:37–41. doi: 10.1161/01.ATV.0000252062.48280.61. [DOI] [PubMed] [Google Scholar]
- 31.Dandona P, Ghanim H, Brooks DP. Antioxidant activity of carvedilol in cardiovascular disease. J Hypertens. 2007;25:731–741. doi: 10.1097/HJH.0b013e3280127948. [DOI] [PubMed] [Google Scholar]
- 32.Calo LA, Semplicini A, Davis PA. Antioxidant and antiinflammatory effect of carvedilol in mononuclear cells of hypertensive patients. Am J Med. 2005;118:201–202. doi: 10.1016/j.amjmed.2004.05.030. [DOI] [PubMed] [Google Scholar]