Abstract
Aim: The present study describes the assessment of true formation of type III collagen in different pathologies using a neo-epitope specific competitive Enzyme-linked immunosorbent assay (ELISA) towards the N-terminal propeptide of type III collagen (PRO-C3). Methods: The monoclonal antibody was raised against the N-protease mediated cleavage site of the N-terminal propeptide of type III collagen and a competitive ELISA was developed using the selected antibody. The assay was evaluated in relation to neo-epitope specificity, technical performance, and as a marker for liver fibrosis and muscle mass using the rat carbon tetrachloride (CCl4) model and a study of immobilization induced muscle loss in humans, respectively. Results: The ELISA was neo-epitope specific, technically stable and can be assessed in serum and plasma samples. In the CCl4 liver fibrosis model it was observed that serum PRO-C3 were significantly elevated in rats with liver fibrosis as seen by histology (56% elevated in the highest quartile of total hepatic collagen compared to control rats, p<0.001) and correlated significantly to total hepatic collagen in the diseased rats (r=0.46, p<0.01) and not in control rats, suggesting the pathological origin of the epitope. Human plasma PRO-C3 correlated significantly to muscle mass at baseline (R2=0.44, p=0.036). Conclusion: The developed neo-epitope specific serum ELISA for type III procollagen (PRO-C3) reflects true formation as it is specific for the propeptide cleaved off the intact collagen molecule. In a clinical and in a rodent study we showed that this marker was highly related to liver fibrosis and muscle mass.
Keywords: Biochemical markers, type III collagen, formation, neo-epitope, liver fibrosis, muscle mass
Introduction
Together with type I collagen, type III collagen constitutes the major structural proteins in the human body, in which type III collagen is crucial for type I collagen fibrillogenesis except in bones, which almost exclusively consist of type I collagen [1,2]. During fibrillar assembly the N-terminal propeptide of type III collagen is cleaved off by specific N-proteases prior to incorporation of the mature collagen in the extracellular matrix (ECM), thus released in the ECM and into circulation. The propeptide molecule consists of three identical α-chains with a total molecular weight of 42 kDa. The removal of the propeptide is sometimes incomplete leaving the propeptide attached to the molecule resulting in thin fibrils with abnormally cross-links and thereby prone to rapid metabolic turnover [3,4], thus PIIINP can both be a marker of formation and degradation. We hypothesized that by targeting the N-protease cleavage site of the procollagen with a monoclonal antibody we might be able to assess true formation of type III collagen. The current commercial available assays for quantification of the N-terminal propeptide of type III collagen either employs polyclonal antibodies or monoclonal antibodies targeting internal sequences of the propeptide [5,6].
Remodeling of tissue integrity plays an important role in the pathogenesis of various diseases as altered components and non-coded modifications of the ECM leads to tissue stiffness and changes in signaling potential of the intact ECM and fragments thereof. ECM remodeling is an important prerequisite for tissue function and repair, and is tightly controlled by modifying enzymes responsible for synthesis and degradation of the ECM. During pathological situations such as in fibrotic diseases, the balance between formation and degradation is disturbed leading to an altered composition of the ECM and tissue function [7,8]. PIIINP has been suggested as a biomarker for several pathologies such as lung injury [9], viral and non-viral hepatitis [10], systemic sclerosis [11], vascular remodeling [12], and kidney diseases [13].
Limited attention has been given to the ECM remodeling in skeletal muscle tissue. In rat models increased collagen gene expression and biosynthesis have been demonstrated in quadriceps femoris and tibialis anterior muscles after exercise [14,15], whereas increased serum levels of PIIINP has been demonstrated in clinical studies after exercise [16]. Therefore, as the skeletal muscle proteins remodels increased levels of PIIINP in the circulation may serve as a biomarker for early muscle anabolism. Serum levels of PIIINP have previously been suggested as a biomarker of response to testosterone [17], recombinant human growth hormone [18] or the combination thereof [19,20].
In liver fibrosis the fibrillar collagens type I and III are highly up-regulated [21,22]. Type III collagen is dominant in the early stages of fibrosis, while up-regulation of type I collagen is related to the later stages of fibrosis. Therefore, the N-terminal propeptide of type III collagen (PIIINP) is one of the best studied markers for fibrogenesis [23-25]. Through the years several radioimmunoassays have been developed for quantification of PIIINP with a sensitivity of up to 94% and specificity up to 81% for the detection of cirrhosis [5,6], however none of the previous assays are neo-epitope specific.
The aim of the present study was to generate a neo-epitope specific competitive Enzyme-linked immunosorbent assay (ELISA) towards the N-terminal propeptide of type III collagen (PRO-C3) to assess true formation by development of monoclonal antibody towards the N-protease generated neo-epitope of N-terminal propeptide of type III collagen. The biological relevance of this assay was evaluated in a carbon tetrachloride (CCl4) inhalation rat liver model as well as in a human study of immobilization induced muscle loss.
Materials and methods
Reagents
All reagents used in the experiments were high-standard chemicals from companies such as Merk (Whitehouse Station, NJ, USA) and Sigma Aldrich (St. Louis, MO, USA). The synthetic peptides used for monoclonal antibody production and - validation were 1) Immunogenic peptide: Ovalbumine (OVA)-CGG-CPTGPQNYSP, 2) Screening peptide: Biotin-CGG- CPTGPQNYSP, and 3) Selection peptide: CPTGPQNYSP. All synthetic peptides were purchased from the Chinese Peptide Company, Beijing, China.
Monoclonal antibodies
The sequence for the N-terminal propeptide of type III collagen was aligned between human, rat and mouse species and selected from homology between the species and uniqueness among other ECM proteins by protein blasting. The amino acid sequence 145’-CPTGPQNYSP-’153 in the α1 chain PRO-C3 is 100% homologues between human and rat (Figure 1). Generation of monoclonal antibodies was initiated by subcutaneous immunization of 4-5 week old Balb/C mice with 200 µl emulsified antigen and 50 µg PRO-C3 (OVA-CGG-CPTGPQNYSP) using Freund’s incomplete adjuvant. The immunizations were repeated every 2 weeks until stable serum titer levels were reached. The mouse with the highest serum titer was selected for fusion. The mouse rested for a month and then boosted intravenously with 50 µg PRO-C3 in 100 µl 0.9% NaCl solution three days before isolation of the spleen. The spleen cells were fused with SP2/0 myeloma cells to produce hybridoma as described by [26], and cloned in culture dishes using the semi-medium method. The clones were plated into 96-well microtiter plates for further growth employing the limited dilution method to secure monoclonal growth. The supernatants were screened for reactivity against calibrator peptide and native material in an indirect ELISA using streptavidin-coated plates. Biotin-CGG-PTGPQNYSP was used as screening peptide, while the free peptide PTGPQNYSP was used as calibrator to test for further specificity of clones.
Figure 1.

Alignment of the targeted PRO-C3 α1 chain sequence in human, and rat species (red box). Position of the corresponding human PRO-C3 (▪) and rat PRO-C3 (~) sequences within the alpha 1 chain of the N-terminal pro-peptide of type III collagen. The alignment was performed using the NLP CLUSTALW software.
Clone characterization
Native reactivity and affinity of the peptide were assessed using different biological materials such as urine, serum, and amniotic fluid (AF) from both humans and rats in a preliminary ELISA using 2 ng/ml biotinylated peptide on streptavidin-coated microtiter plates and the supernatants from growing monoclonal hybridoma cells. Human AF was obtained from 30 women undergoing elective lower segment Caesarean sections at the Beijing Obstetrics Gynecology Hospital over a 2 month period. 100-200 ml AF was collected directly after incision and the fluid was stored at -20°C until use. The local ethical board had approved the study and all women provided written consent prior to collection. Rat AF was drawn from the uterus of pregnant Wistar rats two days prior to expected birth. Antibody specificity was tested in a preliminary assay using deselection and elongated peptides (i.e. calibrator peptide with ten amino acid substitutions and calibrator peptide with one additional amino acid at the cleavage site, respectively). The isotype of the monoclonal antibodies was determined using the Clonotyping System-HRP kit, cat. 5300-05 (Southern Biotech, Birmingham, AL, USA).
Antibody characterization
Prior to Western Blotting, the total protein concentration of human and rat AF was measured using Bicinchoninic acid (BCA) Protein Assay according to manufacturer’s instruction. Briefly, BCA was diluted 2-fold in phosphate-buffered saline (PBS) from 2 mg/ml to produce a standard row for calculation of the samples. Samples were diluted 1:4 in 1x PBS and 25 µl sample was added to a microtiter plate along with 200 µl working reagent (Reagent A and B mixed in the ratio 50:1). The content was mixed on a plate shaker for 30 seconds followed by incubation for 30 minutes at 37°C. After ended incubation the plate was cooled to room temperature and the absorbance was measured in the ELISA reader at 562 nm (Molecular Devices, SpectraMax M, CA, USA). Hereafter, rat or human AF was mixed with sample buffer (x2) and reducing agent (x10), heated at 70°C for 10 minutes, loaded on a 4-20% tris-glycein sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-page), and run for 1 hour at 180V. Protein bands were blotted onto a nitrocellulose membrane using the Invitrogen i-Blot gel transfer system according to manufacturer’s instruction. The membrane was blocked in blocking buffer (5% skimmed milk in Tris-buffered saline with Tween (TBST) overnight at 4°C and incubated with 1 µg/ml horseradish peroxidase (HRP)-conjugated PRO-C3 monoclonal antibody NB61N-62 for 2 hours. Specificity of the PRO-C3 monoclonal antibody was investigated by addition of excess PRO-C3 calibrator peptide and antibody in the ratio 10:1 and allowed to pre-incubate for 1 hour before it was added to the membrane for overnight incubation. After incubation the membranes was washed 4x10 minutes in TBST, incubated with 4 ml chemiluminescence detection kit (ECL), and developed using Amersham Hyperfilm.
PRO-C3 ELISA protocol
Supernatant from antibody producing hybridoma was collected and the monoclonal antibody was purified using HiTrap affinity columns (GE Healthcare Life Science, Little Chalfont, Buckinghamshire, UK) and labeled with HRP using Lightning-Link™ HRP Conjugation Kit (Innova Biosciences, Babraham, Cambridge, UK), according to the manufacturer’s instructions.
The PRO-C3 competitive ELISA procedure was as follows: A 96-well streptavidin-coated ELISA plate from Roche, cat. 11940279, was coated with the biotinylated peptide Biotin-CGG- PTGPQNYSP dissolved in coater buffer (50 mM PBS-BTE + 10% sorbitol, pH 7.4), incubated for 30 min at 20°C in the dark and subsequently washed in washing buffer (20 mM Tris, 50 mM NaCl, pH 7.2). Thereafter 20 µl of peptide calibrator or sample were added to appropriate wells, followed by 100 µl of HRP-conjugated monoclonal antibody NB61N-62 dissolved in incubation buffer (50 mM PBS-BTB + 10% LiquidII (Roche), pH 7.4) and the plate was incubated for 20 hours at 4°C and washed. Finally, 100 µl tetramethylbenzinidine (TMB) (Kem-En-Tec cat.: 438OH) was added, the plate was incubated for 15 min at 20°C in the dark and in order to stop the reaction, 100 µl of stopping solution (1% H2SO4) was added and the plate was analyzed in the ELISA reader at 450 nm with 650 nm as the reference (Molecular Devices, SpectraMax M, CA, USA). A calibration curve was plotted using a 4-parametric mathematical fit model.
Technical evaluation
A 2-fold dilution of healthy serum and plasma samples from human and rats were used to determine linearity and calculated as percentage of recovery of the 100% sample. Antibody specificity was calculated as percentage of recovery of the 100% calibrator peptide (PTGPQNYSP), elongated peptide (PTGPQNYSPQ), and non-sense peptide (GSPGKDGVRG). Lower limit of detection (LLOD) was calculated as the mean + 3xStandard Deviation (SD) of the blank from 21 determinations of standard K (i.e. buffer). Upper limit of detection (ULOD) was determined as the mean – 3xSD of 10 measurements of Standard A. Lower limit of quantification (LLOQ) was determined as the lowest concentration reproducibly measured with a precision lower than 30%. The intra- and inter-assay variation was determined by 10 independent runs of 8 QC samples, with each run consisting of double determination of the samples. Accuracy of the samples was measured in healthy human serum samples spiked with standard curve or human amniotic fluid at significant concentrations and calculated as the percentage recovery of the theoretical amount of serum. Interference was measured in healthy human serum spiked with hemoglobin, lipemia, and biotin at significant concentrations and calculated as the percentage recovery of the theoretical amount of serum.
Analyte and reagent stability
The analyte stability was determined for three healthy human serum samples for four times freeze and thaw cycles and calculated as the percentage recovery of the first freeze/thaw cycle. The analyte stability was further tested by incubating three healthy serum samples for 2 hours, 4 hours, 24 hours, and 48 hours at 4°C and 20°C and tested against non-stressed analytes. Reagent stability was determined for incubation at 4°C, 20°C and 37°C for up to 7 days and tested against non-stresses reagents. All sample tests were run as double determinations.
Comparison of PRO-C3 and UniQ PIIINP assays
To test for novelty and uniqueness, 20 randomly selected healthy human serum samples were assessed in the PRO-C3 ELISA and in the UniQ PIIINP RIA (Orion Diagnostica, Espoo, Finland) according to manufacturer’s instructions. The subjects were originally included in a prospective study to evaluate the normal changes in bone mineral density as described by Warming et al [27].
Rat CCl4 liver fibrosis model
Serum levels of PRO-C3 were assessed in a CCl4 inhalation rat model of liver fibrosis. Complete details of the study are described elsewhere [28]. The study included 52 male Wistar rats treated with CCl4 and 28 male Wistar vehicle rats (Charles-River, Saint Aubin les Elseuf, France). Induction of liver fibrosis was performed as previously described by others [29]. Briefly, CCl4 was administered by inhalation twice a week, starting with 0.5 minutes per exposure. The duration of exposure was increased by one minute after every three session until it reached five minutes, which was used until the end of the investigation. Phenobarbital (0.3 g/l) was added to the drinking water and vehicle rats received phenobarbital only. Animals were stratified into groups receiving 8, 12, 16, or 20 weeks of CCl4 or vehicle treatment (n=13 for CCl4; n=7 vehicle for each group). The study was performed according to the criteria of the Investigation and Ethics Committee of the Hospital Clinic Universitari (Barcelona, Spain), approval #B-NNP-233/09. Four animals from the CCl4 groups died during the study. Blood was collected at termination and allowed to stand at room temperature for 20 min to clot before centrifugation at 2500 rpm for ten minutes. Samples were stored at −80°C prior to biomarker assessment.
Muscle loss
PRO-C3 was measured in plasma samples from 11 young men (n=11, age: 24.4 ± 0.5 y, height: 181.4 ± 1.8 cm, weight: 72.2 ± 2.3 kg) that were subjected to two weeks of unilateral leg immobilization (through full leg casting) followed by four weeks of resistance training remobilization. Subjects were sampled for venous blood, leg muscle volume and strength at baseline (PRE), after immobilization (2W) and after remobilization (4W). During immobilization the subjects lost approximately 9 and 20%, of muscle size (MRI-determined quadriceps muscle volume) and strength (knee extensor force measured by maximal voluntary contraction in KinCom device) respectively, as previous reported [30]. Subjects were not fasted or placed on custom diets prior to testing and sampling. Samples were stored at -80°C prior to biomarker assessment.
Statistical analyses
Comparisons of serum PRO-C3 levels in the CCl4 and vehicle rats at each time point and quartile were performed using the unpaired t-test applying a Welch correction. Results are shown as Mean ± Standard Error of Mean (SEM). All correlations were tested using a nonparametric Spearman correlation. All statistical analyses were performed in GraphPad Prism v.5 (Graph Pad Software, La Jolla, CA, USA). Differences were considered statistically significant if p<0.05. Asterisks indicate the following: *: p<0.05; **: p<0.01; ***: p<0.001; ns=non-significant difference.
Results
Clone selection and characterization
The subtype was determined to be an IgG1 subtype. From the Western Blot analysis it was seen that the PRO-C3 monoclonal antibody NB61N-62 recognized two bands with molecular sizes around 52-60 kDa in rat amniotic fluid, while only one band around 52 kDa was detected in human amniotic fluid. In addition, the signal could be partly inhibited by the specific peptide in the rat, and inhibited in human (Figure 2). Native reactivity was observed using the NB61N-62 antibody in the ELISA. Native reactivity was seen towards human serum, plasma, and AF as well as against rodent serum, plasma, and AF (Figure 3A-C). The signal was slightly less inhibited against mouse serum and plasma. The signal of the competitive ELISA was inhibited using from 1:2 to 1:16, undiluted to 1:8, or undiluted to 1:4 in human, rodent, and mouse native material, respectively. Dilution of the native material approximately followed the same dilution pattern as the calibrator curve for all three species. Human AF inhibited the signal up to 100%; 80% for rat AF; 70% for human serum and plasma and rat serum; 44% for rat plasma, and 35% for mouse serum and plasma. No inhibition was observed using the elongated peptide and non-sense peptide (Figure 3D).
Figure 2.
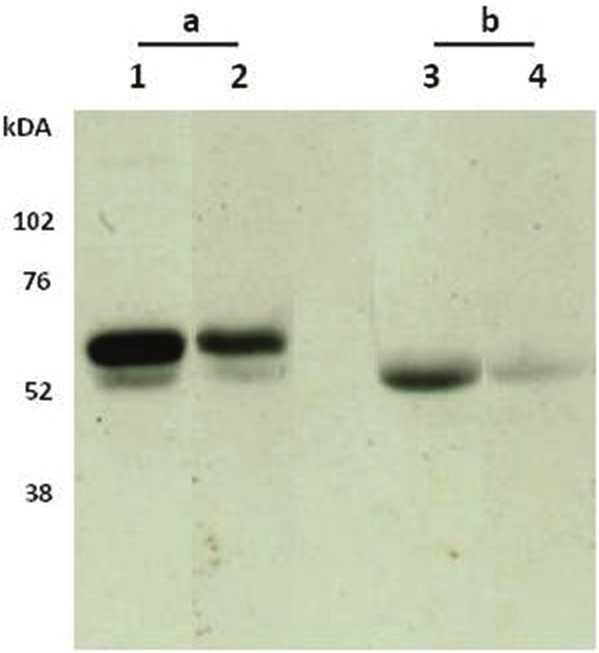
Western Blot showing the specific bands of N-terminal propeptide of type III collagen in Amniotic fluid from a) rat and b) human recognized by the PRO-C3 monoclonal antibody NB61N62 (lane 1 and 3) and NB61N62 + specific peptide (lane 2+4). Two bands around 52-60 kDA was observed for the rat, whereas one band was observed for human. Addition of specific peptide resulted in weakness of band intensity for both rat and human.
Figure 3.
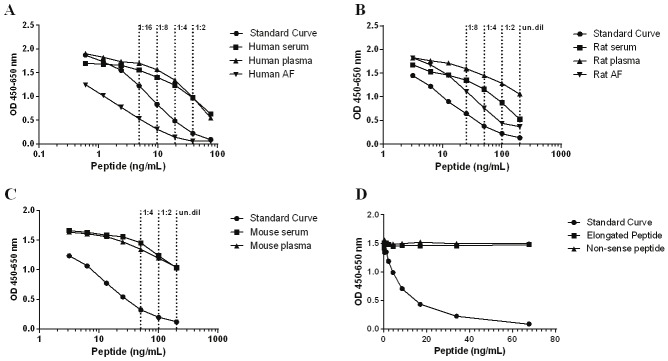
PRO-C3 ELISA runs showing typical calibration curves and native reactivity against human, rodent, and mouse material. A. Calibration curve and inhibition of the competitive ELISA using healthy human serum, plasma, and amniotic fluid (AF). The calibrator curve was diluted in 2-fold from 76.31 ng/mL, whereas native material was run diluted 1:2 to 1:16 as indicated (--), B. Calibration curve and inhibition of the competitive ELISA using healthy rat serum, plasma, and AF. The calibrator curve was diluted in 2-fold from 200 ng/mL, whereas native material was run undiluted to 1:8 as indicated (--), C. Calibration curve and inhibition of the competitive ELISA using healthy mouse serum and plasma. The calibrator curve was diluted in 2-fold from 200 ng/mL, whereas native material was run undiluted to 1:4 as indicated (--), D. Neo-epitope specificity of the PRO-C3 antibody using elongated peptide, i.e. peptide sequence of calibration peptide with one additional amino acid in the C-terminal end. The calibration curve, elongated peptide, and non-sense peptide were diluted in 2-fold from 76.31 ng/mL. The signal is seen as the optical density at 450 nm, subtracting the background at 650 nm, as a function of peptide concentration.
Technical evaluation
The measurement range of the human PRO-C3 ELISA was determined by calculating ULOD and LLOQ providing a range from 0.867-60.1 ng/ml with a LLOD of 0.606 ng/ml. The technical performance of the PRO-C3 ELISA showed acceptable inter- and intra assay variation of mean 11.03% and 4.11% (Table 1), with acceptance range below 15% and 10%, respectively. Dilution recovery was performed using healthy serum and plasma samples from humans, rat and mouse. The dilution recovery was within the acceptable 100±20% recovery (Table 2). Further dilution resulted in measurements below LLOQ. Spiking of calibrator peptide in serum or plasma resulted in a mean recovery of 56% and 55%, respectively (Table 3). However, spiking of human AF in 2-fold dilution starting from 1:2 into healthy human serum or plasma resulted in mean recovery of 100% and 111%, respectively. No interference was observed in serum spiked with different concentrations of hemoglobin, biotin, and lipemia (Table 4). The stability of the analyte was acceptable up to four freeze/thaw cycles with 100±20% recovery compared to 1 freeze/thaw cycle (Table 5).
Table 1.
Inter- and intra-assay variation for the PRO-C3 assay using human serum quality control samples # 1-8 (HS1-HS8). The variation was calculated as the mean variation between 10 individual determinations of each sample
| PRO-C3 Sample | Value (ng/mL) | Intra-assay variability % | Inter-assay variability % |
|---|---|---|---|
| HS1 | 24.24 | 2.28 | 5.94 |
| HS2 | 11.62 | 2.90 | 6.45 |
| HS3 | 8.40 | 5.31 | 11.99 |
| HS4 | 6.54 | 4.46 | 11.31 |
| HS5 | 6.36 | 3.88 | 13.09 |
| HS6 | 5.23 | 3.98 | 12.31 |
| HS7 | 4.29 | 3.53 | 12.94 |
| HS8 | 2.98 | 4.66 | 18.56 |
| Mean | 4.11 | 11.03 |
Table 2.
Percentage dilution recovery for the PRO-C3 assay using human-, rat-, and mouse samples. Human serum (HS), Human plasma (HP), Rat serum (RS), Mouse serum (MS), Mouse plasma (MP)
| PRO-C3 ng/ml | HS (n=2) | HP (n=3) | RS (n=10) | MS (n=2) | MP (n=2) |
|---|---|---|---|---|---|
| Undiluted | 100% | - | 100% | 100% | - |
| Dilution 1:2 | 98 | 100% | 116 | 96 | 100% |
| Dilution 1:4 | 103 | 91 | 110 | 118 | 114 |
| Dilution 1:8 | 114 | 87 | - | - | - |
| Dilution 1:16 | - | 92 | - | - | - |
| Mean | 105 | 90 | 113 | 107 | 114 |
Table 3.
Spiking recovery of calibrator peptide in human serum or plasma, and human AF in human serum or plasma. The recovery was calculated as percent recovery of calculated peptide/AF in serum/plasma compared to pure serum/plasma. Concentration of calibrator peptide were 38.16 ng/ml (StdB), 19.08 ng/ml (StdC), 9.54 ng/ml (StdD), 4.77 ng/ml (StdE), 2.39 ng/ml (StdF) and 1.19 ng/ml (StdG). AF was added in 2-fold dilution starting from 1:2
| Added Std | Serum (n=3) sRE% | Plasma (n=3) sRE% | Added AF | Serum (n=3) sRE% | Plasma (n=3) sRE% |
|---|---|---|---|---|---|
| StdB | 16 | 15 | 2x | 101 | 103 |
| StdC | 29 | 25 | 4x | 103 | 108 |
| StdD | 42 | 38 | 8x | 106 | 113 |
| StdE | 58 | 54 | 16x | 103 | 112 |
| StdF | 70 | 69 | 32x | 104 | 115 |
| StdG | 82 | 83 | 64x | 103 | 110 |
| Buffer | 92 | 100 | Buffer | 99 | 104 |
| Mean sRE% | 56 | 100 | Mean sRE% | 55 | 111 |
Table 4.
Interference of hemoglobin, lipemia and biotin in human serum added in various concentrations. All data are shown as percent recovery compared to pure serum
| Hemoglobin | Lipemia | Biotin | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| mmol/L | RE% | mmol/L | RE% | mmol/L | RE% | |
| 0.5 | 68 | 0.56 | 101 | 160,000 | 134 | |
| 0.25 | 74 | 0.28 | 103 | 80,000 | 113 | |
| 0.13 | 81 | 0.14 | 99 | 40,000 | 96 | |
| 0.063 | 81 | 0.07 | 104 | 20,000 | 97 | |
| 0.031 | 82 | 0.04 | 101 | 10,000 | 94 | |
| 0.016 | 86 | 0.00 | 100 | 5,000 | 87 | |
| 0.005 | 95 | 2,500 | 100 | |||
| 0.000 | 100 | 0 | 100 | |||
| Mean | 83 | 101 | 103 | |||
Table 5.
Analyte stability in three human serum and plasma samples in four freeze/thaw cycles. All data are shown as mean percent recovery compared to 1 freeze/thaw cycle
| Freeze/thaw cycle | Serum Mean recovery % | EDTA plasma Mean recovery % | Heparin plasma Mean recovery % | Citrate plasma Mean recovery % |
|---|---|---|---|---|
| 1 | 100% | 100% | 100% | 100% |
| 2 | 103 | 102 | 103 | 109 |
| 3 | 99 | 99 | 98 | 103 |
| 4 | 102 | 100 | 98 | 100 |
Comparison between PRO-C3 and UniQ PIIINP
Serum levels of PRO-C3 did not correlate significantly to serum levels of UniQ PIIINP in healthy humans (R2=0.12, p=ns) (Figure 4).
Figure 4.

Comparison between PRO-C3 and UniQ PIIINP serum levels in 20 randomly selected serum samples from healthy human individuals; ns=non-significant difference.
PRO-C3 levels in CCl4 treated rat
Serum levels of PRO-C3 were statistically elevated in CCl4 treated rats compared to vehicle rats at week 8 (+30.17% increase, p<0.001), week 12 (+26.58% increase, p<0.05), and week 16 (+44.15% increase, p<0.05), however not in week 20 (+6.24% increase, p=ns) (Figure 5A). When rats were stratified into quartiles according to total amount of collagen in the liver assessed by Sirius Red staining, PRO-C3 was statistically elevated in all four quartiles compared to vehicle animals (quartile 1 (Q1) +21% increase, p<0.01; Q2 +21% increase, p<0.05; Q3 +33% increase, p<0.001; Q4 +56% increase, p<0.001) (Figure 5B). Furthermore, serum levels of PRO-C3 were correlated to the total collagen in the liver. PRO-C3 correlated significant to collagen in CCl4 rats (r=0.46, p<0.01), however not in vehicle rats (r=0.07, p=ns) (Figure 5C and D).
Figure 5.
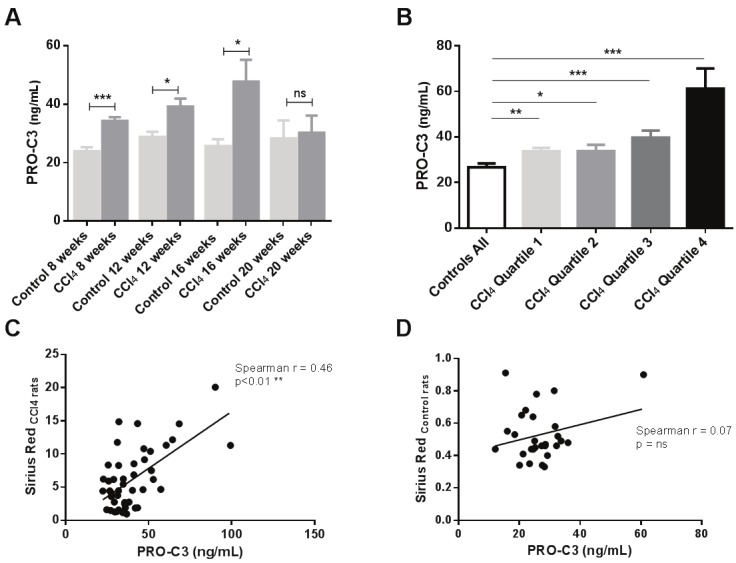
PRO-C3 levels measured in the CCl4 inhalation rat model: A. Serum levels of PRO-C3 was assessed in vehicle rats at termination (controls) as well as in CCl4 treated rats at termination (CCl4) at week 8, 12, 16, and 20. Results shown are mean ± standard error of the mean (SEM); B. Serum levels of PRO-C3 in vehicle rats and CCl4 rats stratified in quartiles according to total collagen in the liver; C, D) Correlation between PRO-C3 and Sirius red in CCl4 rats and in vehicle rats. Asterisks indicate statistical significance as indicated by bars. (*=p<0.05; **=p<0.01; ***=p<0.001,ns= non-significant difference).
PRO-C3 levels related to muscle mass
Although levels of PRO-C3 did not differ significantly between intervention time points when correlated against muscle mass at baseline, PRO-C3 showed a significant positive correlation (R2=0.4416, P=0.0361) (Figure 6), meaning that higher PRO-C3 levels strongly predict higher muscle mass in healthy individuals not exposed to any intervention.
Figure 6.
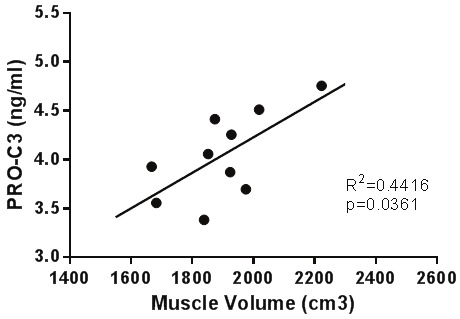
Serum PRO-C3 titers at baseline correlate significantly with quad muscle mass measured by thigh MRI using Pearson correlation. Data have not been transformed.
Discussion
We developed and evaluated a novel competitive ELISA using a monoclonal antibody to detect the N-protease mediated cleavage of the N-terminal propeptide of type III collagen (i.e. PRO-C3). The main findings were: 1) A high reactivity towards the PRO-C3 peptide sequence and native reactivity in human and rodent serum, plasma and AF was observed, while being specific towards the N-protease cleavage site; 2) A technically stable assay was developed for detecting PRO-C3 in human and rodent material. The assay had acceptable intra- and inter assay variation, dilution recovery and low limit of detection; 3) Serum PRO-C3 levels were significantly elevated in CCl4 treated rats at weeks 8, 12, and 16 compared to vehicle rats and correlated significantly to total hepatic collagen in the diseased rats; 4) Human serum PRO-C3 levels correlated significantly to muscle mass at baseline. The data combined suggest that the PRO-C3 marker can be related to different pathologies involving type III collagen formation.
The presently described PRO-C3 ELISA is different and may be superior to other commercial available assays since other commercial PIIINP assays available utilize either a monoclonal- or polyclonal antibody, from which the precise epitope is not known. Figure 1 shows the sequence alignment of human (▪) and rat (~) α1-chain of N-terminal propeptide of type III collagen. The sequence lacking underline corresponds to the initial N-terminal telopeptide. The monoclonal antibody NB61N-62 is specific towards the C-terminal sequence CPTGPQNYSP which is part of the N-terminal propeptide of type III collagen (red box). N-protease cleaves the propeptide at a Pro-Gln bond [31,32] corresponding to amino acid 153 and 154 in the human and rat PIIINP, respectively, generating a cleavage specific neo-epitope to which a specific antibody can be directed against. Fragments with one or more additional amino acids from the telopeptide are therefore not recognized by the antibody which was shown in the PRO-C3 ELISA using the elongated peptide described in the present work. In 1993, Brocks et al [5] described a radioimmunoassay based on antibody raised against a synthetic peptide which represents the 14 C-terminal amino acids of rat and bovine N-terminal propeptide of type III collagen. The antibody recognition site was described as the same site which our NB61N-62 monoclonal antibody recognizes, whereas Brocks et al described an assay using polyclonal antibodies. The assay would not be specific for the cleaved propeptide as the N-terminal propeptide contains multiple Pro-Gln bonds at which the N-protease might cleave. Therefore the assay described by Brocks et al potentially also reflects degradation of type III collagen when the propeptide is attached to the telopeptide. However, the ELISA described here with the specific NB61N-62 antibody assesses true formation of type III collagen and not degradation. The fact that our ELISA measures different from the commercial UniQ PIIINP is further verified by correlation of serum samples measured in both assays indicated by R2=0.12.
Characterization and technical validation of PRO-C3
Serum, plasma and AF from human, rat and mouse was selected for characterization of the ELISAs. Native reactivity was very high towards human AF, healthy human serum and plasma, and for healthy rat serum, plasma and AF, while high reactivity was observed for mouse serum and plasma, indicating that the antibody recognizes PRO-C3 in these matrices, and is specific towards the calibrator peptide sequence. Furthermore, no reactivity was observed towards the elongated and non-sense peptides, indicating that the antibody is neo-epitope specific towards the cleavage site on the propeptide.
The size of the PRO-C3 fragment recognized by the NB61N-62 antibody was characterized by Western Blot in human and rat AF. Two bands around 52-60 kDa was observed for rat, while only one band was observed for human. These results are in agreement with previous findings characterizing the human propeptide with a size of 42 kDa [3]. The small size difference between our findings and Niemelas characterization could be explained by different characterization methods. Niemela characterize purified PIIINP from human ascites fluid, while we characterize the size of the propeptide in amniotic fluid. Furthermore, changes in technical conditions might also explain the differences.
The technical evaluation of the ELISA revealed that dilution recovery of rodent material was improved by using a different buffer system compared to human samples. The two PRO-C3 competitive ELISAs employing the same monoclonal antibody allows good translational science. Both human and rodent PRO-C3 ELISAs were technically stable with acceptable intra- and inter-assay variations. Furthermore, dilution recovery was acceptable within 100±20%.
PRO-C3 is related to liver fibrosis
During liver fibrosis the amount of ECM components are known to be highly increased up to 6 fold [33] including type III collagen, and it is well known that the N-terminal propeptide of type III collagen is a marker for describing liver fibrosis [34-37]. In the present study we evaluated the novel PRO-C3 marker in a rat model of liver fibrosis, and found that serum PRO-C3 was significantly elevated at termination after 8, 12 and 16 weeks and when stratified into quartiles of the total amount of collagen compared to controls. At the 20 week termination point serum PRO-C3 had regressed back to control levels. These data indicate that this marker reflects fibrogenesis rather than degradation since the serum PRO-C3 were high initially in this model. According to literature, PIIINP can both be a marker of formation and degradation as the removal of the propeptide is sometimes incomplete leaving the propeptide attached to the molecule during fibrillar assembly [3]. However, we speculate that this novel PRO-C3 reflects pure formation rather than degradation since this ELISA is specific towards the cleavage neo-epitope of the propeptide.
PRO-C3 for the evaluation of muscle volume
Our results indicate that the serum PRO-C3 is a novel biomarker of muscle mass. Several studies have indicated that anabolic treatment results in increased levels of serum PIIINP [17,19], in a manner where higher PIIINP indicated better anabolic response to treatment. However, these results were obtained using the Orion Diagnostica PIIINP radioimmuno assay, the antibody specificity of which is undisclosed, whereas our antibody is directed at the C-terminal end of the N-terminal propetide, i.e. where the propeptide is cleaved from the intact type III collagen ensuring specificity towards the only the type III collagen propeptide and not other cleaved fragments containing the same sequence.
The most obvious interpretation of this would be that the continual turnover of type III collagen in muscle results in a steady output of PIIINP, leading to a steady-state plasma concentration of which a big enough fraction is muscle-derived to result in a correlation between muscle mass and plasma PIIINP.
It is well established that type I and III collagens in the intramuscular connective tissue sheaths surrounding the entire muscle, fascicles of muscle fibers and individual fibers are highly abundant. Muscle fibers project contractile forces through the sides of the fibers and into the ECM, which “carries” the forces to the muscle aponeuroses. Hence, the amount of muscle collagens must be strongly related to total muscle mass. Obviously, this relationship requires further validation in larger studies.
Also if a biomarker of a given quality, displays sufficient validity and reliability then it will by definition also be a marker of change in that quality. As the change in total muscle mass in the present study is quite small and the sample size too small for this purpose, it remains to be shown if the presently described PRO-C3 is also biomarker of change in muscle mass.
Limitations
The study carries some limitations. Firstly, the assessment of serum PRO-C3 as a biochemical marker for liver fibrosis was carried out in inbred laboratory rats, which were synchronous exposed to CCl4 to induce liver disease, thus this animal model does not fully represent the highly complicated presentation of clinical liver fibrosis. Secondly, the muscle volume study was only carried out in eleven individuals. Therefore future investigations of how PRO-C3 is related to muscle volume should be tested in larger clinical cohorts.
Conclusion
In conclusion we have shown that this non-radioimmune and neo-epitope specific ELISA for PRO-C3 may reflect true formation since the antibody employed in the PRO-C3 assay only recognizes the N-terminal type III procollagen when released from intact type III collagen. Furthermore, we have shown that the PRO-C3 ELISA is technically robust and is a related to pathologies such as liver fibrosis and to muscle mass.
Acknowledgement
We acknowledge the funding from the Danish science foundation (“Den Danske Forskningsfond”) and the Danish Agency for Science Technology and Innovation (“Forsknings- og Innovationsstyrelsen”).
References
- 1.Bao X, Zeng Y, Wei S, Wang G, Liu C, Sun Y, Chen Q, Li H. Developmental changes of Col3a1 mRNA expression in muscle and their association with intramuscular collagen in pigs. J Genet Genomics. 2007;34:223–228. doi: 10.1016/S1673-8527(07)60023-X. [DOI] [PubMed] [Google Scholar]
- 2.Jensen LT, Host NB. Collagen: scaffold for repair or execution. Cardiovasc Res. 1997;33:535–539. doi: 10.1016/s0008-6363(96)00247-7. [DOI] [PubMed] [Google Scholar]
- 3.Niemela O, Risteli L, Parkkinen J, Risteli J. Purification and characterization of the N-terminal propeptide of human type III procollagen. Biochem J. 1985;232:145–150. doi: 10.1042/bj2320145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang WM, Ge G, Lim NH, Nagase H, Greenspan DS. TIMP-3 inhibits the procollagen N-proteinase ADAMTS-2. Biochem J. 2006;398:515–519. doi: 10.1042/BJ20060630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brocks DG, Steinert C, Gerl M, Knolle J, Neubauer HP, Gunzler V. A radioimmunoassay for the N-terminal propeptide of rat procollagen type III. Application to the study of the uptake of the N-terminal propeptide of procollagen type III in isolated perfused rat liver. Matrix. 1993;13:381–387. doi: 10.1016/s0934-8832(11)80043-0. [DOI] [PubMed] [Google Scholar]
- 6.Rohde H, Vargas L, Hahn E, Kalbfleisch H, Bruguera M, Timpl R. Radioimmunoassay for type III procollagen peptide and its application to human liver disease. Eur J Clin Invest. 1979;9:451–459. doi: 10.1111/j.1365-2362.1979.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 7.Van den Steen PE, Opdenakker G, Wormald MR, Dwek RA, Rudd PM. Matrix remodelling enzymes, the protease cascade and glycosylation. Biochim Biophys Acta. 2001;1528:61–73. doi: 10.1016/s0304-4165(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 8.Cuzner ML, Opdenakker G. Plasminogen activators and matrix metalloproteases, mediators of extracellular proteolysis in inflammatory demyelination of the central nervous system. J Neuroimmunol. 1999;94:1–14. doi: 10.1016/s0165-5728(98)00241-0. [DOI] [PubMed] [Google Scholar]
- 9.Meduri GU, Tolley EA, Chinn A, Stentz F, Postlethwaite A. Procollagen types I and III aminoterminal propeptide levels during acute respiratory distress syndrome and in response to methylprednisolone treatment. Am J Respir Crit Care Med. 1998;158:1432–1441. doi: 10.1164/ajrccm.158.5.9801107. [DOI] [PubMed] [Google Scholar]
- 10.Teare JP, Sherman D, Greenfield SM, Simpson J, Bray G, Catterall AP, Murray-Lyon IM, Peters TJ, Williams R, Thompson RP. Comparison of serum procollagen III peptide concentrations and PGA index for assessment of hepatic fibrosis. Lancet. 1993;342:895–898. doi: 10.1016/0140-6736(93)91946-j. [DOI] [PubMed] [Google Scholar]
- 11.Scheja A, Akesson A, Horslev-Petersen K. Serum levels of aminoterminal type III procollagen peptide and hyaluronan predict mortality in systemic sclerosis. Scand J Rheumatol. 1992;21:5–9. doi: 10.3109/03009749209095054. [DOI] [PubMed] [Google Scholar]
- 12.Lin YH, Ho YL, Wang TD, Liu CP, Kao HL, Chao CL, Chien KL, Hung CS, Wu VC, Tsai IJ, Yen RF, Shiau YC, Chen WJ. The relation of amino-terminal propeptide of type III procollagen and severity of coronary artery disease in patients without myocardial infarction or hibernation. Clin Biochem. 2006;39:861–866. doi: 10.1016/j.clinbiochem.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Teppo AM, Tornroth T, Honkanen E, Gronhagen-Riska C. Urinary amino-terminal propeptide of type III procollagen (PIIINP) as a marker of interstitial fibrosis in renal transplant recipients. Transplantation. 2003;75:2113–2119. doi: 10.1097/01.TP.0000066809.60389.48. [DOI] [PubMed] [Google Scholar]
- 14.Han XY, Wang W, Komulainen J, Koskinen SO, Kovanen V, Vihko V, Trackman PC, Takala TE. Increased mRNAs for procollagens and key regulating enzymes in rat skeletal muscle following downhill running. Pflugers Arch. 1999;437:857–864. doi: 10.1007/s004240050855. [DOI] [PubMed] [Google Scholar]
- 15.Koskinen SO, Ahtikoski AM, Komulainen J, Hesselink MK, Drost MR, Takala TE. Short-term effects of forced eccentric contractions on collagen synthesis and degradation in rat skeletal muscle. Pflugers Arch. 2002;444:59–72. doi: 10.1007/s00424-002-0792-2. [DOI] [PubMed] [Google Scholar]
- 16.Crameri RM, Langberg H, Teisner B, Magnusson P, Schroder HD, Olesen JL, Jensen CH, Koskinen S, Suetta C, Kjaer M. Enhanced procollagen processing in skeletal muscle after a single bout of eccentric loading in humans. Matrix Biol. 2004;23:259–264. doi: 10.1016/j.matbio.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Chen F, Lam R, Shaywitz D, Hendrickson RC, Opiteck GJ, Wishengrad D, Liaw A, Song Q, Stewart AJ, Cummings CE, Beals C, Yarasheski KE, Reicin A, Ruddy M, Hu X, Yates NA, Menetski J, Herman GA. Evaluation of early biomarkers of muscle anabolic response to testosterone. J Cachexia Sarcopenia Muscle. 2011;2:45–56. doi: 10.1007/s13539-011-0021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longobardi S, Keay N, Ehrnborg C, Cittadini A, Rosen T, Dall R, Boroujerdi MA, Bassett EE, Healy ML, Pentecost C, Wallace JD, Powrie J, Jorgensen JO, Sacca L. Growth hormone (GH) effects on bone and collagen turnover in healthy adults and its potential as a marker of GH abuse in sports: a double blind, placebo-controlled study. The GH-2000 Study Group. J Clin Endocrinol Metab. 2000;85:1505–1512. doi: 10.1210/jcem.85.4.6551. [DOI] [PubMed] [Google Scholar]
- 19.Bhasin S, He EJ, Kawakubo M, Schroeder ET, Yarasheski K, Opiteck GJ, Reicin A, Chen F, Lam R, Tsou JA, Castaneda-Sceppa C, Binder EF, Azen SP, Sattler FR. N-terminal propeptide of type III procollagen as a biomarker of anabolic response to recombinant human GH and testosterone. J Clin Endocrinol Metab. 2009;94:4224–4233. doi: 10.1210/jc.2009-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson AE, Meinhardt U, Hansen JL, Walker IH, Stone G, Howe CJ, Leung KC, Seibel MJ, Baxter RC, Handelsman DJ, Kazlauskas R, Ho KK. Pharmacodynamics of growth hormone abuse biomarkers and the influence of gender and testosterone: a randomized double-blind placebo-controlled study in young recreational athletes. J Clin Endocrinol Metab. 2008;93:2213–2222. doi: 10.1210/jc.2008-0402. [DOI] [PubMed] [Google Scholar]
- 21.Zachariae H, Heickendorff L, Sogaard H. The value of amino-terminal propeptide of type III procollagen in routine screening for methotrexate-induced liver fibrosis: a 10-year follow-up. Br J Dermatol. 2001;144:100–103. doi: 10.1046/j.1365-2133.2001.03959.x. [DOI] [PubMed] [Google Scholar]
- 22.Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarcuska P, Janicko M, Veseliny E, Jarcuska P, Skladany L. Circulating markers of liver fibrosis progression. Clin Chim Acta. 2010;411:1009–1017. doi: 10.1016/j.cca.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Frei A, Zimmermann A, Weigand K. The N-terminal propeptide of collagen type III in serum reflects activity and degree of fibrosis in patients with chronic liver disease. Hepatology. 1984;4:830–834. doi: 10.1002/hep.1840040505. [DOI] [PubMed] [Google Scholar]
- 25.Fabris P, Marranconi F, Bozzola L, Biasin MR, De Lazzari F, Plebani M, Benedetti P, Tositti G, Pellizzer G, Stecca C, de LF. Fibrogenesis serum markers in patients with chronic hepatitis C treated with alpha-IFN. J Gastroenterol. 1999;34:345–350. doi: 10.1007/s005350050272. [DOI] [PubMed] [Google Scholar]
- 26.Gefter ML, Margulies DH, Scharff MD. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977;3:231–236. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- 27.Warming L, Hassager C, Christiansen C. Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporos Int. 2002;13:105–112. doi: 10.1007/s001980200001. [DOI] [PubMed] [Google Scholar]
- 28.Segovia-Silvestre T, Reichenbach V, Fernandez-Varo G, Vassiliadis E, Barascuk N, Morales-Ruiz M, Karsdal MA, Jimenez W. Circulating CO3-610, a degradation product of collagen III, closely reflects liver collagen and portal pressure in rats with fibrosis. Fibrogenesis Tissue Repair. 2011 Aug 3;4:19. doi: 10.1186/1755-1536-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clária J, Jiménez W. Experimental Models of Cirrhosis and Ascites. Second edition 2005. [Google Scholar]
- 30.Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol. 2009;107:1172–1180. doi: 10.1152/japplphysiol.00290.2009. [DOI] [PubMed] [Google Scholar]
- 31.Nusgens BV, Goebels Y, Shinkai H, Lapiere CM. Procollagen type III N-terminal endopeptidase in fibroblast culture. Biochem J. 1980;191:699–706. doi: 10.1042/bj1910699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le GC, Somerville RP, Kesteloot F, Powell K, Birk DE, Colige AC, Apte SS. Regulation of procollagen amino-propeptide processing during mouse embryogenesis by specialization of homologous ADAMTS proteases: insights on collagen biosynthesis and dermatosparaxis. Development. 2006;133:1587–1596. doi: 10.1242/dev.02308. [DOI] [PubMed] [Google Scholar]
- 33.Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001;21:351–372. doi: 10.1055/s-2001-17556. [DOI] [PubMed] [Google Scholar]
- 34.Parkes J, Guha IN, Roderick P, Harris S, Cross R, Manos MM, Irving W, Zaitoun A, Wheatley M, Ryder S, Rosenberg W. Enhanced Liver Fibrosis (ELF) test accurately identifies liver fibrosis in patients with chronic hepatitis C. J Viral Hepat. 2011;18:23–31. doi: 10.1111/j.1365-2893.2009.01263.x. [DOI] [PubMed] [Google Scholar]
- 35.Lieber CS, Weiss DG, Paronetto F. Value of fibrosis markers for staging liver fibrosis in patients with precirrhotic alcoholic liver disease. Alcohol Clin Exp Res. 2008;32:1031–1039. doi: 10.1111/j.1530-0277.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee MH, Cheong JY, Um SH, Seo YS, Kim DJ, Hwang SG, Yang JM, Han KH, Cho SW. Comparison of surrogate serum markers and transient elastography (Fibroscan) for assessing cirrhosis in patients with chronic viral hepatitis. Dig Dis Sci. 2010;55:3552–3560. doi: 10.1007/s10620-010-1219-0. [DOI] [PubMed] [Google Scholar]
- 37.Nobili V, Parkes J, Bottazzo G, Marcellini M, Cross R, Newman D, Vizzutti F, Pinzani M, Rosenberg WM. Performance of ELF serum markers in predicting fibrosis stage in pediatric non-alcoholic fatty liver disease. Gastroenterology. 2009;136:160–167. doi: 10.1053/j.gastro.2008.09.013. [DOI] [PubMed] [Google Scholar]


