Abstract
Background: Emerging evidence has suggested that Notch signaling pathway may be involved in the development, progression and metastasis of prostate cancer (PCa). In the present study, we investigated the expression levels of Jagged-1 and Notch-1 in human prostate tumors and their associations with PCa progression and metastasis. Methods: Immunohistochemistry (IHC) for Jagged-1 and Notch-1 was performed on tissue microarray (TMA) slides containing 286 formalin-fixed and paraffin-embedded (FFPE) tissue specimens with various prostatic pathologies, including benign changes, high grade prostatic intraepithelial neoplasia (HGPIN), low- and high-grade PCas as well as metastatic PCa. Results: Cytoplasmic and membranous IHC scores for Jagged-1 in both metastatic PCa and high grade PCa were significantly higher than those in low grade PCa and in benign prostatic tissues. Similarly, cytoplasmic IHC scores of Notch-1 in both metastatic PCa and high grade PCa were significantly elevated compared with those observed in low grade PCa and in benign prostatic tissues. A statistically significant correlation was identified between the expression of Jagged-1 and Notch-1 in human prostatic tissues. Furthermore, significantly more highly expressed Jagged-1 in membrane was observed in Caucasian patients with high-grade or metastatic PCa (vs. African Americans) and in PCa patients with positive surgical margins (vs. negative surgical margins). Conclusion: Our results provide strong evidence that up-regulation of Jagged1-Notch1 signaling plays a role in PCa progression and metastasis and suggest that Jagged-1 and Notch-1 may be useful markers in distinguishing indolent and aggressive PCas.
Keywords: Prostate cancer (PCa), cancer metastasis, Jagged-1, Notch-1, tissue microarray (TMA), immunohistochemistry (IHC)
Introduction
Although prostate cancer (PCa) mortality has decreased in recent years, PCa remains the most frequent non-cutaneous male malignancy and the second leading cause of cancer-related deaths in men in the United States [1]. Significant efforts have been made to understand the biological and molecular mechanisms driving PCa development, progression and metastasis. Localized disease is rarely fatal, while the vast majority of PCa-related mortality is due to metastatic disease. It is essential to reveal the molecular determinants involved in the processes of PCa progression and metastasis, to develop novel prognostic biomarkers as well as mechanism-based targeted prevention and/or treatment strategies against metastatic PCa.
Notch signaling pathway plays a crucial role in the development and homeostasis of tissues by regulating cell-fate decision, proliferation, differentiation, and apoptosis. Four Notch receptors (Notch1-4) and five ligands (Jagged-1, 2, Delta-1, 3, 4) have been described in mammals [2,3]. Generally, the modular structure of Notch receptors consists of an extracellular ligand binding domain (NEC) and a transmembrane domain (NTM), which is cleaved upon ligand-mediated receptor activation to generate a Notch intracellular domain (NIC). Upon ligand binding, NEC dissociates from NTM, and NTM undergoes a series of programmed proteolytic events, first at the extracellular surface by α-secretase (ADAM10, or in some cases, ADAM17), and then within the phospholipid bilayer. This intramembranous cleavage is mediated by γ-secretase. NIC is then released from the inner surface of cell membranes and translocated into nucleus where it activates the transcription of the target genes [4]. Additionally, cytoplasmic signaling by NIC has also been described in some cell types [5,6]. Because Notch signaling plays a key role in cell fate decisions including cell proliferation and apoptosis, dysregulated activity of Notch signaling is often associated with tumorigenesis. Up-regulated expression of Notch receptors and their ligands has been reported in numerous solid and some hematopoietic malignancies [7-27]. Moreover, a number of studies have suggested an involvement of Notch signaling in cancer angiogenesis and metastasis [28-31].
Several studies have demonstrated that Notch signaling is required for embryonic and postnatal prostatic growth and development, for proper cell lineage specification within the prostate, as well as for adult prostate maintenance and regeneration following castration and hormone replacement [32,33]. Emerging evidence has also suggested that Notch signaling may play an important role in PCa development, progression and metastasis [34]. It was reported that Notch signaling was overexpressed in various PCa cell lines, including highly metastatic PCa cell lines, as compared with prostate cell lines derived from normal tissue [35-37]. Elevated expression of Notch-1 was also observed in malignant prostatic epithelial cells of primary and metastatic tumors of transgenic mouse models of PCa [35,38]. Furthermore, it was shown that Notch ligand Jagged-1 was overexpressed in metastatic human PCa when compared with localized PCa or benign prostatic tissue [39] and down-regulation of Notch-1 and Jagged-1 inhibited PCa cell growth, migration, and invasion [40-42]. More recently, Sethi et al reported that Notch-1 expression was significantly elevated in human PCa bone metastasis [43]. Finally, Notch and Hedgehog signaling have been recently found to be necessary for the maintenance of PCa tumor-initiating cells or “cancer stem-like cells” [44]. Taken together, these studies imply that up-regulation of Notch signaling may have a crucial role in the development, progression and metastasis of PCa.
However, the literature is not unanimous on the role of Notch in PCa. For example, Shou et al. reported that the expression of Notch ligands was low or undetectable in PCa cells, and that overexpression of a constitutively active form of Notch-1 inhibited the proliferation of various PCa cells, suggesting that Notch acts as a tumor suppressor [35]. Wang et al reported that expression of Notch-1 and its effector Hey-1 gene in human prostate adenocarcinoma were found significantly down-regulated compared with normal control tissues [33]. Whelan et al reported that Notch-1 signaling is lost in prostate adenocarcinoma and Notch-1 may play a protective role in human prostate tumor formation by promoting PTEN gene expression [45]. These seemingly contradictory findings may be a reflection of the heterogeneity of prostate cancers, with Notch playing different roles within different tumor cell types, and/or during different stages of tumor progression. Therefore, more in-depth investigations are necessary to address this controversy. In the present study, we describe IHC analysis of Jagged-1 and Notch-1 proteins in human PCa and demonstrate elevated expression of Jagged-1 and Notch-1 in high grade and metastatic PCas, but not in low-grade PCas or HGPIN lesions. Our findings support a model in which Notch-1 signaling is involved in high-grade and metastatic PCa, but not in low-grade lesions. It is possible that, like TGF-β in breast cancer, Notch signaling may have dual and opposite roles in early versus late stages of tumor progression. Additionally, our findings suggest that expression of Notch-1 and Jagged-1 may be a candidate biomarker for aggressive, metastasis-prone disease.
Materials and methods
Patient population, tissue collection, and tissue microarray (TMA) construction
The protocol was approved by the University of Mississippi Medical Center Institutional Review Board. Clinical information and pathological reports were retrieved from the University database. This study included 166 patients who had undergone prostatectomy, transurethral resection of the prostate, or resection of tissue with metastatic prostate cancer at the University of Mississippi Medical Center from 1992 to 2010. Ninety-four out of 166 patients were African American and seventy-two were Caucasian. From formalin-fixed and paraffin-embedded (FFPE) surgical specimens of 166 patients, 286 sites including benign prostatic changes, high grade prostatic intraepithelial neoplasia (HGPIN), localized PCa and metastatic PCa were selected. The localized prostate cancers were further divided into two groups according to the Gleason grade (not Gleason Sum): The localized prostate cancers with Gleason grade III and below were defined as a low grade group, while those with Gleason grade IV and above were defined as high grade group. After histological features in hematoxylin and eosin stained slides were re-confirmed by two researchers including one pathologist, they were topographically correlated with the corresponding paraffin blocks. A 2 mm cylindrical core from each selected site of the primary FFPE block was transferred to the composite paraffin blocks to construct TMA blocks by using a Beecher MTA1 Manual Tissue Arrayer. The resulting TMA blocks were then sectioned at 5μm in thickness for IHC study.
Immunohistochemical staining
TMA sections were placed in a 58-60°C oven overnight for tissue to adhere. The sections were deparaffinized in xylene, rehydrated through graded ethanol and washed with PBS before being treated with 1X Reveal in a Decloaking Chamber (Biocare Medical, CA) for antigen retrieval following the manufacturer’s protocol. After rinsed in PBS for 15 min, the sections were soaked in 3% H2O2 in PBS for 20 min. to quench endogenous peroxidase activity. Sections were incubated for 60 min in 3% normal rabbit serum (Vector Laboratories, CA) in PBS at room temperature to block non-specific binding sites and then probed with primary antibodies (anti-Jagged 1 (C-20) and anti-Notch 1 (C-20), Santa Cruz Biotechnologies, CA). Sections were incubated with diluted primary antibodies (1μg/ml for both anti-Jagged 1 and anti-Notch 1 antibodies) prepared in PBS containing 1.5% normal rabbit serum for one hour (Jagged-1) or two hours (Notch-1) in a hydrated chamber at room temperature. TMA sections incubated with 1μg/ml normal goat IgG (Santa Cruz Biotechnologies, CA), run in parallel with each stain, were used as negative controls. Following extensive washing, antigen-antibody complexes were detected using the Vectastain Elite ABC kit (Vector Laboratories, CA) according to the manufacturer’s protocol. Staining was performed with ImmPactTM DAB peroxidase substrate kit (Vector Laboratories, CA). Sections were then counterstained in Gill’s hematoxylin and dehydrated in ascending grades of ethanol before clearing in xylene and mounting under a coverslip using Cytoseal XYL. The levels of Jagged-1 and Notch-1 expression in each specimen were scored according to the extent (percent of stained cells) and intensity of staining. The score for the extent of the IHC stained area was scaled as 0 for no IHC signal at all, 1 for < 10%, 2 for 10–50%, and 3 for > 50% of tumor cells stained. The score for IHC intensity was also scaled as 0 for no IHC signal, 1 for weak, 2 for moderate, and 3 for strong IHC signals. The final score used in the analysis was calculated by multiplying the extent score and intensity score, with a maximum score equal to 9.
Statistical analysis
Statistical analysis was performed using SPSS for windows (SPSS 19.0, Chicago, IL). One-way ANOVA was used in the analysis of intergroup difference of Jagged-1 and Notch-1 IHC mean scores. Two sample T-test was performed to analyze the difference of IHC mean scores between any two groups. The Satterthwaite T test was used whenever the IHC scores in two groups had unequal variances. A comparison was considered significant if the two-sided P value was less than 0.05. Bivariate correlation analysis was employed to examine the association of clinical and pathological parameters with the expression levels of Jagged-1 and Notch-1.
Results
Of the 243 prostatic specimens available with the staining of Jagged-1 antibody in our cohort, 238 (98%) showed expression of Jagged-1 determined by IHC: 238 (100%) showed cytoplasmic expression, 108 (45%) showed membranous expression and 108 (45%) showed both cytoplasmic and membranous expression. Of the 251 prostatic specimens available with Notch-1 antibody staining, cytoplasmic expression of Notch-1 was revealed in 92 specimens (37%). The rates of detectable Notch-1 expression in cytoplasm were 27% (14 of 52) in benign prostatic tissue group, 39% (11 of 28) in HGPIN group, 23% (13 of 57) in low grade PCa group, 41% (34 of 82) in high grade PCa group and 63% (20 of 32) in metastatic PCa group, respectively. Because membranous expression of Notch-1 was only observed in 6 cases (one in benign prostatic tissue group, one in metastatic PCa group and four in high grade PCa group), we excluded it from our data analysis.
Representative examples of Jagged-1 and Notch-1 staining in different groups (prostatic tissue with benign changes, HGPIN, low grade PCa, high grade PCa, and metastatic PCa) are shown in Figure 1. ANOVA analysis revealed a significant difference in Jagged-1 cytoplasmic IHC mean scores among the groups (F = 15.150, p = 4.75E-11). Further analysis of the differences between any two groups indicated that Jagged-1 cytoplasmic staining scores in metastatic PCa (5.92 ± 2.12, Mean ± SD) and in high grade PCa (5.74 ± 2.45) were significantly higher than those in low grade PCa (3.55 ± 2.02, p = 9.02E-07 and 4.65E-08, respectively), in HGPIN (4.50 ± 2.34, p = 0.023 and 0.030, respectively), and in prostatic tissues with benign changes (3.39 ± 1.85, p = 7.96E-07 and 3.12E-08, respectively, Table 1). For Jagged-1 membranous staining, a statistically significant difference was also uncovered by ANOVA analysis among the groups (F = 8.252, p = 3.00E-06) and the IHC mean scores in metastatic PCa (3.05 ± 3.52) and in high grade PCa (2.41 ± 2.90) were also significantly higher than those in low grade PCa (0.71 ± 1.42, p = 0.001 and 8.07E-06, respectively) and in prostatic tissue with benign changes (0.82 ± 1.28, p = 0.002 and 4.08E-05, respectively) (Table 2). Similarly, ANOVA analysis indicated a significant difference in Notch-1 cytoplasmic IHC mean scores among the groups (F = 9.063, p = 7.62E-07). Notch-1 cytoplasmic IHC scores in metastatic PCa (2.22 ± 2.11) and in high grade PCa (1.10 ± 1.68) were significantly elevated as compared with the IHC mean scores observed in low grade PCa (0.51 ± 1.15, p = 1.24E-04 and 0.015, respectively) and in prostate tissue with benign changes (0.48 ± 1.01, p = 8.86E-05 and 0.008, respectively) (Table 3). Therefore, simultaneously elevated expression of Jagged-1 and Notch-1 in high grade and metastatic PCa groups observed in our study indicate that the up-regulation of Jagged 1-Notch1 signaling may play a role in the progression and metastasis of PCa. The distribution of staining showed marked differences with tumor state. The prostatic stroma and smooth muscle cells showed strong Notch-1 staining in benign prostate tissues, HGPIN, low grade tumors and to some extent high grade tumors, whereas epithelial cells show absent or weak signal. Conversely, metastatic lesions show uniform Notch-1 staining in neoplastic epithelial cells (Figure 1). Jagged-1 expression, on the other hand, appeared to be largely limited to epithelial cells, but increased in intensity from barely detectable in normal prostatic epithelium to intense staining in metastatic carcinomas.
Figure 1.
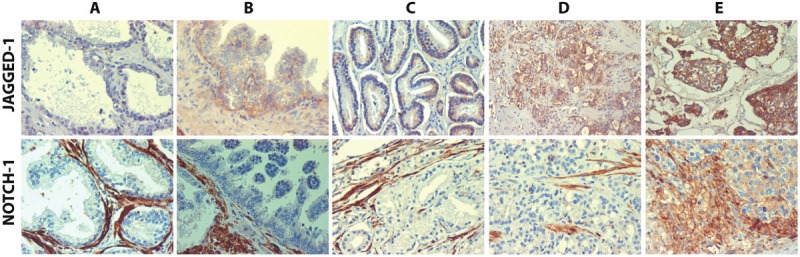
Representative examples of Jagged-1 (upper panel) and Notch-1 (lower panel) expression levels observed in prostatic tissue with benign changes (A); HGPIN (B); low grade PCa (C); high grade PCa (D) and metastatic PCa (E).
Table 1.
Comparison of Jagged-1 cytoplasmic IHC scores among different pathological categories(2-tailed p value)
| Cytoplasmic Staining Score (n/Mean ± SD) | |||||
|---|---|---|---|---|---|
|
|
|||||
| Category | Benign prostate | HGPIN | Low grade PCa | High grade PCa | Metastatic PCa |
|
| |||||
| (42/3.39 ± 1.85) | (24/4.50 ± 2.34) | (63/3.55 ± 2.02) | (83/5.74 ± 2.45) | (31/5.92 ± 2.12) | |
| Benign | ― | 0.038 | 0.692 | 3.12E-08 | 7.96E-07 |
| HGPIN | ― | 0.063 | 0.030 | 0.023 | |
| Low grade PCa | ― | 4.65E-08 | 9.02E-07 | ||
| High grade PCa | ― | 0.721 | |||
| Metastatic PCa | ― | ||||
Table 2.
Comparison of Jagged-1 membranous IHC scores among different pathological categories (2-tailed p value)
| Cytoplasmic Staining Score (n/Mean ± SD) | |||||
|---|---|---|---|---|---|
|
|
|||||
| Category | Benign prostate | HGPIN | Low grade PCa | High grade PCa | Metastatic PCa |
|
| |||||
| (42/0.82 ± 1.28) | (24/1.71 ± 2.41) | (63/0.71 ± 1.42) | (83/2.41 ± 2.90) | (31/3.05 ± 3.52) | |
| Benign | ― | 0.105 | 0.695 | 4.08E-05 | 0.002 |
| HGPIN | ― | 0.068 | 0.278 | 0.100 | |
| Low grade PCa | ― | 8.07E-06 | 0.001 | ||
| High grade PCa | ― | 0.331 | |||
| Metastatic PCa | ― | ||||
Table 3.
Comparison of Notch-1 cytoplasmic IHC scores among different pathological categories(2-tailed p value)
| Cytoplasmic Staining Score (n/Mean ± SD) | |||||
|---|---|---|---|---|---|
|
|
|||||
| Category | Benign prostate | HGPIN | Low grade PCa | High grade PCa | Metastatic PCa |
|
| |||||
| (52/0.48 ± 1.01) | (28/0.68 ± 1.14) | (57/0.51 ± 1.15) | (82/1.10 ± 1.68) | (32/2.22 ± 2.11) | |
| Benign | ― | 0.428 | 0.873 | 0.008 | 8.86E-05 |
| HGPIN | ― | 0.535 | 0.139 | 0.001 | |
| Low grade PCa | ― | 0.015 | 1.24E-04 | ||
| High grade PCa | ― | 0.010 | |||
| Metastatic PCa | ― | ||||
To determine whether there is an association between the expression of Jagged-1 and Notch-1 in human prostatic tissues, especially in high grade and metastatic PCa tissues in which a simultaneously elevated expression of Jagged-1 and Notch-1 was observed, correlation analysis was performed. First, with all the human prostatic specimens effectively stained with Jagged-1 and/or Nothch-1 in our study (n = 216), it was revealed that Notch-1 cytoplasmic expression was significantly correlated with both Jagged-1 cytoplasmic expression (r = 0.389, p = 3.36E-09) and membranous expression (r = 0.181, p = 0.008); Second, with all the PCa specimens (including localized and metastatic PCas, n = 152), a statistically significant correlation was found between Notch-1 and Jagged-1 cytoplasmic expression (r = 0.347, p = 1.17E-05) and a correlation with an approaching statistical significance (r = 0.154, p = 0.057) was found between Notch-1 cytoplasmic expression and Jagged-1 membranous expression; Last, with the specimens pooled just from high grade and metastatic PCa groups (n = 101), a statistically significant correlation was still observed between Notch-1 and Jagged-1 cytoplasmic expression (r = 0.256, p = 0.010). However, no significant correlation between Notch-1 cytoplasmic expression and Jagged-1 membranous expression was observed (r = 0.09, p = 0.373). These data suggest that Jagged-1 may be functionally involved in Notch-1 receptor activation in the processes of cancer development, progression and metastasis of PCa.
It is well known that African American race, advanced age, high PSA level at the time of diagnosis, and positive surgical margins as well as capsular invasion in radical prostatectomy are associated with unfavorable prognosis in PCa. In order to determine the relationship between these existing indicators and the expression levels of Jagged-1 and Notch-1, Jagged-1 and Notch-1 IHC scores were compared between different groups of patients according to race (African American and Caucasian), age (≤ 60 years old or > 60 years old), positivity of surgical resection margins (positive or negative), capsular invasion (positive or negative) and PSA level at the time of diagnosis of PCa. It was found that IHC mean scores of Jagged-1 membranous staining in Caucasian patients were significantly higher than those found in African American patients in high grade PCa group (3.32 ± 3.09 vs. 1.64 ± 2.51, p = 0.009) and in metastatic PCa group (6.44 ± 2.74 vs. 1.87 ± 2.98, p = 0.001) (Table 4). However, there were no statistical differences in the mean scores of Jagged-1 and Notch-1 cytoplasmic staining between African American and Caucasian patients (Tables 4 and 5). Also, there was no statistical difference in Jagged-1 and Notch-1 IHC scores between two age groups (Table not shown). In addition, no correlation between the expression levels of Jagged-1/Notch-1 and PSA level at the time of diagnosis was observed (Table not shown). When we analyzed the subset of prostate cancer patients (n = 85) on whom radical prostatectomy was performed, the IHC mean score of Jagged-1 membranous staining was found to be approaching statistical significance in the positive surgical margin group as compared with the negative surgical margin group (p = 0.051) (Figure 2). Similarly, a trend towards of increased IHC score of Jagged-1 membranous staining was also observed in the positive capsular invasion group as compared with the negative capsular invasion group (p = 0.054) (Figure 3). These results obtained from clinical data analysis indicate that high Jagged-1 membranous expression may be race-related and associated with the prognosis of PCa, therefore may be more precise for predicting the prognosis of PCa compared to cytoplasmic expression of Jagged-1.
Table 4.
Comparison of Jagged-1 IHC scores between African Americans and Caucasians
| Histology | No. (black/white) | Cytoplasmic staining (Mean ± SD) | Membranous staining (Mean ± SD) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Black | White | P | Black | White | P | ||
| Benign | 17/25 | 2.92 ± 1.29 | 3.72 ± 2.12 | > 0.05 | 0.76 ±1.19 | 0.86 ± 1.36 | > 0.05 |
| HGPIN | 20/4 | 4.43 ± 2.21 | 4.86 ± 3.33 | > 0.05 | 1.75 ± 2.37 | 1.50 ± 3.00 | > 0.05 |
| Low grade PCa | 38/25 | 3.16 ±1.95 | 4.14 ± 2.00 | > 0.05 | 0.70 ± 1.34 | 0.74 ± 1.58 | > 0.05 |
| High grade PCa | 45/38 | 5.53 ± 2.29 | 5.99 ± 2.64 | > 0.05 | 1.64 ± 2.51 | 3.32 ± 3.09 | 0.009 |
| Metastatic PCa | 23/8 | 5.74 ± 2.00 | 6.44 ± 2.50 | > 0.05 | 1.87 ± 2.98 | 6.44 ± 2.74 | 0.001 |
Table 5.
Comparison of Notch-1 IHC scores between African Americans and Caucasians
| Histology | No. (black/white) | Cytoplasmic staining ( Mean ± SD) | |||
|---|---|---|---|---|---|
|
| |||||
| Black | White | P | |||
| Benign | 24/28 | 0.75 ± 1.29 | 0.25 ± 0.65 | > 0.05 | |
| HGPIN | 20/8 | 0.68 ± 1.18 | 0.69 ± 1.10 | > 0.05 | |
| Low grade PCa | 34/23 | 0.56 ±1.32 | 0.46 ± 0.85 | > 0.05 | |
| High grade PCa | 45/37 | 1.27 ± 1.94 | 0.91 ± 1.29 | > 0.05 | |
| Metastatic PCa | 23/7 | 2.36 ± 2.11 | 1.71 ± 2.20 | > 0.05 | |
Figure 2.
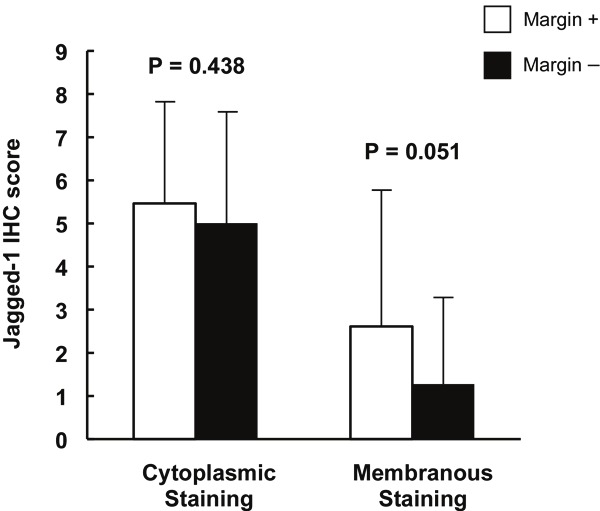
Comparison of Jagged-1 IHC scores between positive and negative surgical margin groups. Of 85 prostate cancer patients who were performed with radical prostatectomy, 27 had positive surgical margin and 58 had negative surgical margin. Columns, mean of IHC scores; bars, ± SD..
Figure 3.
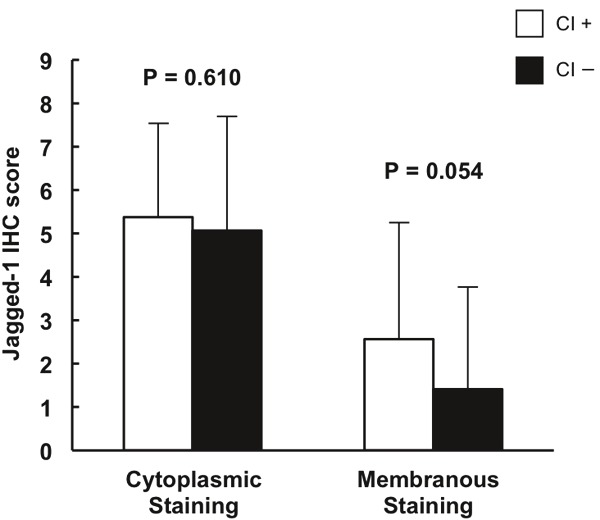
Comparison of Jagged-1 IHC scores between positive and negative capsular invasion (CI) groups. Of 85 prostate cancer patients who were performed with radical prostatectomy, 24 had positive capsular invasion and 61 had negative capsular invasion. Columns, mean of IHC scores; bars, ± SD.
Discussion
In this study, we examined the expression levels and subcellular location of Jagged-1 and Notch-1 IHC staining in human prostatic tissues with various prostatic pathologies. The determination of subcellular location of a Notch receptor/ligand may provide useful clues regarding the activity status of the Notch signaling pathway that may be more informative than simple staining intensity and/or fraction of positive cells. Notch receptors are matured (by glycosylation and furin-mediated cleavage of precursor proteins) in the endoplasmic reticulum (perinuclear); they interact with their ligands (Jagged and Delta) at the membrane, and are then cleaved and translocated to the nucleus. Once in the nucleus, active Notch (NotchIC) modulates transcription of numerous genes via its interaction with CSL and possibly also through other indirect mechanisms [46,47]. Notch IC seldom accumulates in the nucleus due to rapid degradation; therefore it is very difficult to detect it in the nucleus by routine IHC on FFPE sections. For Notch ligand Jagged-1, membrane localization may indicate that the ligand is most likely functional, but ligand endocytosis is required for Notch activation by ligands [47,48]. Thus, cytoplasmic localization of Jagged-1 may indicate active ligand endocytosis and hence functional activity. In the current study, we found that Notch-1 receptor staining was more predominantly marked in the cytoplasm (37%) than in membrane (2%) and no detectable nuclear staining of Notch-1 was observed. This does not imply that Notch-1 is non-functional. Rapid degradation of nuclear NotchIC may prevent its detection, and cytoplasmic, non-canonical signaling [5,6] may be functionally important in this case. We have observed similar staining patterns in breast cancer specimens and breast cancer models [49]. Also, Jagged-1 staining was found most marked in the cytoplasm (98%) compared with membranous staining (44%). Furthermore, both cytoplasmic and membranous expression of Jagged-1 was found significantly correlated with Notch-1 cytoplasmic expression (p = 3.36E-09 and p = 0.008, respectively). Therefore, these results suggest that Jagged-1 may be functionally involved in Notch-1 receptor activation in human prostatic tissues with various prostatic pathologies.
It should be noted that overall expression of Notch-1 determined by IHC in human prostatic tissues is relatively low, based on the IHC scores observed. From our preliminary titration experiment for anti-Notch1 antibody, we noticed that at 1μg/ml, the staining of prostatic stromal cells (which served as an internal control) in benign prostatic tissues was already fully saturated, reaching to the highest IHC score available. Therefore, we chose this concentration as an endpoint. When Notch-1 antibody was used for IHC at this concentration, benign secretory cells and adenocarcinoma foci in most cases of benign control and low grade PCa groups were found to exhibit very weak or undetectable Notch-1 expression. However, there was a clear tendency showing an increased rate of detectable Notch-1 expression from benign prostatic tissue group (27%) and low grade PCa group (23%) to high grade PCa group (41%) and metastatic PCa group (63%). Mean IHC scores of cytoplasmic Notch-1 in metastatic PCa and in high grade PCa were significantly elevated as compared with the IHC scores observed in low grade PCa and in benign prostate tissues. Moreover, Notch-1 cytoplasmic expression was significantly correlated with Jagged-1 cytoplasmic expression in high grade and metastatic PCa groups (r = 0.256, p = 0.010). Together, these data demonstrate that Notch-1 expression is more closely associated with high-grade and metastatic PCa, suggesting that Jagged-1 mediated Notch-1 activation may participate in the progression and metastasis of PCa.
Interestingly, our data indicated that among patients with high grade and metastatic PCa, mean IHC scores of Jagged-1 membranous staining in Caucasians were significantly higher than those found in African Americans. Many studies have indicated that the incidence rate and progression of PCa are race-related: PCa more commonly affects black men than white or Hispanic men and African-Americans have twice the risk of non-Hispanic whites for presenting with advanced-stage PCa [50,51]. In the current study, no statistical differences in the mean IHC scores of Jagged-1 and Notch-1 cytoplasmic staining were observed between African American and Caucasian patients. The functional significance of this difference in membranous Jagged-1 staining remains unclear. It may relate to slower Notch activation kinetics (and therefore less Jagged-1 endocytosis), or to more functionally active ligand being present at the membrane. However, our observation that the elevation of Jagged-1 membranous expression was solely found in Caucasian patients with high grade or metastatic PCa suggests that the evaluation of Jagged-1 membranous expression may be a potential prognostic biomarker in Caucasian PCa patients. Additionally, based on the analyses with a subset of PCa patients on whom radical prostatectomy was performed, a marginally significant elevation of Jagged-1 membranous staining scores was identified in the positive surgical margin group as compared with the negative margin group (P = 0.051). Similarly, the elevation of Jagged-1 membranous expression in PCa patients with positive capsular invasion was also found to be approaching statistical significance compared with patients with negative capsular invasion. Both surgical resection margins in radical prostatectomy and capsular invasion are well known indicators for the prognosis of PCa [52-55]. These findings associate high Jagged-1 membranous expression with an unfavorable prognosis of PCa. Although further evaluations of Jagged-1 membranous expression in larger populations are still needed to validate our findings, our results suggest that Jagged-1 may be a promising candidate as a molecular prognostic biomarker for PCa.
We are not the first to associate the up-regulation of Notch signaling with PCa metastasis. A number of previous reports have proposed a link between the over-expression of Jagged-1 or Notch-1 and PCa metastasis. For example, it was reported that Jagged-1 was highly expressed in metastatic PCa compared with localized PCa or benign prostatic tissue and high Jagged-1 expression in a subset of clinically localized tumors was significantly associated with recurrence [39]. Bone metastases from PCa patients expressed Notch-1 protein in osteoblastic PCa metastatic cells, in both an extranuclear and nuclear pattern [56]. Notch-1 was significantly over-expressed in PCa bone metastasis compared with primary PCa [43]. Our results are consistent with, and expand these previous findings and support the notion that up-regulation of a Jagged1-Notch1 signaling axis may contribute to the progression and metastasis of human PCa. Recently, Domingo-Domenech et al. reported that the Notch and Hedgehog signaling pathways were overexpressed in a subpopulation of PCa cells that survive Docetaxel exposure and exhibit potent tumor-initiating capacity. Their data also demonstrated that targeting Notch and Hedgehog signaling depleted this population of PCa cells through inhibition of survival molecules AKT and Bcl-2 [44]. Taken together, our results corroborate a growing body of evidence that Notch signaling pathway may contribute to the progression, metastasis, and chemotherapy resistance of PCa. Whether Notch plays a different role in pre-neoplastic lesions is an interesting question that deserved further investigation.
Our findings are novel and significant in some aspects. First, we divided localized PCa into two categories (low grade PCa and high grade PCa) according to Gleason grade and demonstrated that the significant elevation of Jagged-1 and Notch-1 expression compared with benign prostatic tissues took place solely in high grade PCa, but not in low grade PCa. In fact, the mean IHC scores for both Jagged-1 and Notch-1 in low grade PCa were the same as those observed in benign prostatic tissues and were significantly lower than those in high grade and metastatic PCa groups. Secondly, we performed IHC analysis for both Jagged-1 and Notch-1 in human PCa specimens from 166 men and revealed for the first time a simultaneously significant elevation of Jagged-1 and Notch-1 co-expression in high grade and metastatic PCas as compared with either low grade PCa or benign prostatic tissues. Lastly, our results indicated that the elevation of Jagged-1 membranous expression may be race-related and associated with an unfavorable prognosis. Our findings are consistent with the hypothesis that elevated expression of Jagged-1 and Notch-1 in neoplastic epithelial cells is associated with a biologically aggressive, metastatic phenotype in PCa cells.
Our findings suggest that Jagged-1 and Notch-1 may serve as useful markers in distinguishing indolent and aggressive PCas and Jagged1-Notch1 signaling could be a potential therapeutic target for the treatment of PCa.
Acknowledgement
The authors thank Dr. Antonio Pannuti (Cancer Institute, UMMC) for his help with figure preparation and Dr. Xu Zhang (Cancer Institute and Center of Biostatistics and Bioinformatics, UMMC) for her expert assistance in statistical analyses.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Miele L. Notching signaling. Clin Cancer Res. 2006;12:1074–1079. doi: 10.1158/1078-0432.CCR-05-2570. [DOI] [PubMed] [Google Scholar]
- 3.Miele L, Miao H, Nickoloff BJ. NOTCH signaling as a novel cancer therapeutic target. Curr Cancer Drug Targets. 2006;6:313–323. doi: 10.2174/156800906777441771. [DOI] [PubMed] [Google Scholar]
- 4.Shih IeM, Wang TL. Notch signaling, gamma-secretase inhibitors, and cancer therapy. Cancer Res. 2007;5:1879–1882. doi: 10.1158/0008-5472.CAN-06-3958. [DOI] [PubMed] [Google Scholar]
- 5.Sade H, Krishna S, Sarin A. he anti-apoptotic effect of Notch-1 requires p56lck-dependent, Akt/PKB-mediated signaling in T cells. J Biol Chem. 2004;279:2937–2944. doi: 10.1074/jbc.M309924200. [DOI] [PubMed] [Google Scholar]
- 6.Perumalsamy LR, Marcel N, Kulkarni S, Radtke F, Sarin A. Distinct spatial and molecular features of notch pathway assembly in regulatory T cells. Sci Signal. 2012;5:ra53. doi: 10.1126/scisignal.2002859. [DOI] [PubMed] [Google Scholar]
- 7.Maliekal TT, Bajaj J, Giri V, Subramanyam D, Krishna S. The role of Notch signaling in human cervical cancer: implications for solid tumors. Oncogene. 2008;27:5110–5114. doi: 10.1038/onc.2008.224. [DOI] [PubMed] [Google Scholar]
- 8.Yeasmin S, Nakayama K, Rahman MT, Rahman M, Ishikawa M, Iida K, Otsuki Y, Kobayashi H, Nakayama S, Miyazaki K. Expression of nuclear Notch3 in cervical squamous cell carcinomas and its association with adverse clinical outcomes. Gynecol Oncol. 2010;117:409–416. doi: 10.1016/j.ygyno.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Reedijk M, Odorcic S, Chang L, Zhang H, Miller N, McCready DR, Lockwood G, Egan SE. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 2005;65:8530–8537. doi: 10.1158/0008-5472.CAN-05-1069. [DOI] [PubMed] [Google Scholar]
- 10.Dickson BC, Mulligan AM, Zhang H, Lockwood G, O’Malley FP, Egan SE, Reedijk M. High-level JAG1 mRNA and protein predict poor outcome in breast cancer. Mod Pathol. 2007;20:685–693. doi: 10.1038/modpathol.3800785. [DOI] [PubMed] [Google Scholar]
- 11.Guo S, Liu M, Gonzalez-Perez RR. Role of Notch and its oncogenic signaling crosstalk in breast cancer. Biochim Biophys Acta. 2011;1815:197–213. doi: 10.1016/j.bbcan.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dang TP, Gazdar AF, Virmani AK, Sepetavec T, Hande KR, Minna JD, Roberts JR, Carbone DP. Chromosome 19 translocation, overexpression of Notch3, and human lung cancer. J Natl Cancer Inst. 2000;92:1355–1357. doi: 10.1093/jnci/92.16.1355. [DOI] [PubMed] [Google Scholar]
- 13.Collins BJ, Kleeberger W, Ball DW. Notch in lung development and lung cancer. Semin Cancer Biol. 2004;14:357–364. doi: 10.1016/j.semcancer.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Konishi J, kawaguchi KS, Vo H, Haruki N, Gonzalez A, Carbone DP, Dang TP. Gamma-secretase inhibitor prevents Notch3 activation and reduces proliferation in human lung cancers. Cancer Res. 2007;67:8051–8057. doi: 10.1158/0008-5472.CAN-07-1022. [DOI] [PubMed] [Google Scholar]
- 15.Reedijk M, Odorcic S, Zhang H, Chetty R, Tennert C, Dickson BC, Lockwood G, Gallinger S, Egan SE. Activation of Notch signaling in human colon adenocarcinoma. Int J Oncol. 2008;33:1223–1229. doi: 10.3892/ijo_00000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Li B, Ji ZZ, Zheng PS. Notch1 regulates the growth of human colon cancers. Cancer. 2010;116:5207–5218. doi: 10.1002/cncr.25449. [DOI] [PubMed] [Google Scholar]
- 17.Lin JT, Chen MK, Yeh KT, Chang CS, Chang TH, Lin CY, Wu YC, Su BW, Lee KD, Chang PJ. Association of high levels of Jagged-1 and Notch-1 expression with poor prognosis in head and neck cancer. Ann Surg Oncol. 2010;17:2976–2983. doi: 10.1245/s10434-010-1118-9. [DOI] [PubMed] [Google Scholar]
- 18.Patard JJ, Leray E, Rioux-Leclercq N, Cindolo L, Ficarra V, Zisman A, De La Taille A, Tostain J, Artibani W, Abbou CC, Lobel B, Guillé F, Chopin DK, Mulders PF, Wood CG, Swanson DA, Figlin RA, Belldegrun AS, Pantuck AJ. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J. Clin. Oncol. 2005;23:2763–2771. doi: 10.1200/JCO.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 19.Sun S, Du R, Gao J, Ning X, Xie H, Lin X, Liu J, Fan D. Expression and clinical significance of Notch receptors in human renal cell carcinoma. Pathology. 2009;41:335–341. doi: 10.1080/00313020902885003. [DOI] [PubMed] [Google Scholar]
- 20.Aparicio LM, Villaamil VM, Gallego GA, Caínzos IS, Campelo RG, Rubira LV, Estévez SV, Mateos LL, Perez JL, Vázquez MR, Calvo OF, Bolós MV. Expression of Notch1 to -4 and their ligands in renal cell carcinoma: a tissue microarray study. Cancer Genomics Proteomics. 2011;8:93–101. [PubMed] [Google Scholar]
- 21.Wang M, Li JT, Zeng YX, Hou JH, Lin QQ. Expression and Significance of Notch1, P21WAF1 and involucrin in nasopharyngeal carcinoma. Ai Zheng. 2005;24:1230–1234. [PubMed] [Google Scholar]
- 22.Zhang Y, Peng J, Zhang H, Zhu Y, Wan L, Chen J, Chen X, Lin R, Li H, Mao X, Jin K. Notch1 signaling is activated in cells expressing embryonic stem cell proteins in human primary nasopharyngeal carcinoma. J Otolaryngol Head Neck Surg. 2010;39:157–166. [PMC free article] [PubMed] [Google Scholar]
- 23.Xu X, Zhao Y, Xu M, Dai Q, Meng W, Yang J, Qin R. Activation of Notch signal pathway is associated with a poorer prognosis in acute myeloid leukemia. Med Oncol. 2011;28:S483–489. doi: 10.1007/s12032-010-9667-0. [DOI] [PubMed] [Google Scholar]
- 24.Jiang L, Wu J, Chen Q, Hu X, Li W, Hu G. Notch1 expression is upregulated in glioma and is associated with tumor progression. J Clin Neurosci. 2011;18:387–390. doi: 10.1016/j.jocn.2010.07.131. [DOI] [PubMed] [Google Scholar]
- 25.Mazur PK, Einwächter H, Lee M, Sipos B, Nakhai H, Rad R, Zimber-Strobl U, Strobl LJ, Radtke F, Klöppel G, Schmid RM, Siveke JT. Notch2 is required for progression of pancreatic intraepithelial neoplasia and development of pancreatic ductal adenocarcinoma. Proc Natl Acad Sci USA. 2010;107:13438–13443. doi: 10.1073/pnas.1002423107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang M, Jiang B, Xu B, Lu W, Guo Q, Xie Q, Zhang B, Dong X, Chen D, Wu Y. Delta like ligand 4 induces impaired chemo-drug delivery and enhanced chemoresistance in pancreatic cancer. Cancer Lett. 2013;330:11–21. doi: 10.1016/j.canlet.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Jundt F, Anagnostopoulos I, Förster R, Mathas S, Stein H, Dörken B. Activated Notch1 signaling promotes tumor cell proliferation and survival in Hodgkin and anaplastic large cell lymphoma. Blood. 2002;99:3398–3403. doi: 10.1182/blood.v99.9.3398. [DOI] [PubMed] [Google Scholar]
- 28.Zeng Q, Li S, Chepeha DB, Giordano TJ, Li J, Zhang H, Polverini PJ, Nor J, Kitajewski J, Wang CY. Crosstalk between tumor and endothelial cells promotes tumor angiogenesis by MAPK activation of Notch signaling. Cancer Cell. 2005;1:13–23. doi: 10.1016/j.ccr.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Hellström M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalén M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 30.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 31.Hu YY, Zheng MH, Zhang R, Liang YM, Han H. Notch signaling pathway and cancer metastasis. Adv Exp Med Biol. 2012;727:186–198. doi: 10.1007/978-1-4614-0899-4_14. [DOI] [PubMed] [Google Scholar]
- 32.Wang XD, Shou J, Wong P, French DM, Gao WQ. Notch1-expressing cells are indispensable for prostatic branching morphogenesis during development and re-growth following castration and androgen replacement. J Biol Chem. 2004;279:24733–24744. doi: 10.1074/jbc.M401602200. [DOI] [PubMed] [Google Scholar]
- 33.Wang XD, Leow CC, Zha J, Tang Z, Modrusan Z, Radtke F, Aguet M, de Sauvage FJ, Gao WQ. Notch signaling is required for normal prostatic epithelial cell proliferation and differentiation. Dev Biol. 2006;290:66–80. doi: 10.1016/j.ydbio.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Leong KG, Gao WQ. The Notch pathway in prostate development and cancer. Differentiation. 2008;76:699–716. doi: 10.1111/j.1432-0436.2008.00288.x. [DOI] [PubMed] [Google Scholar]
- 35.Shou J, Ross S, Koeppen H, de Sauvage FJ, Gao WQ. Dynamics of Notch expression during murine prostate development. Cancer Res. 2001;61:7291–7297. [PubMed] [Google Scholar]
- 36.Scorey N, Fraser SP, Patel P, Pridgeon C, Dallman MJ, Djamgoz MB. Notch signaling and votage-gated Na(+) channel activity in human prostate cancer cells: Independent modulation of in vitro motility. Prostate Cancer Prostatic Dis. 2006;9:399–406. doi: 10.1038/sj.pcan.4500894. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Li Y, Banerjee S, Kong D, Ahmad A, Nogueira V, Hay N, Sarkar FH. Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cell growth, migration and invasion, and induces apoptosis via inactivation of Akt, mTOR, and NF-kappaB signaling pathways. J Cell Biochem. 2010;109:726–736. doi: 10.1002/jcb.22451. [DOI] [PubMed] [Google Scholar]
- 38.Gipp J, Gu G, Crylen C, Kasper S, Bushman W. Hedgehog pathway activity in the LADY prostate tumor model. Mol Cancer. 2007;6:19. doi: 10.1186/1476-4598-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santagata S, Demichelis F, Riva A, Varambally S, Hofer MD, Kutok JL, Kim R, Tang J, Montie JE, Chinnaiyan AM, Rubin MA, Aster JC. JAGGED1 expression is associated with prostate cancer metastasis and recurrence. Cancer Res. 2004;64:6854–6857. doi: 10.1158/0008-5472.CAN-04-2500. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Wang Z, Ahmed F, Banerjee S, Li Y, Sarkar FH. Down-regulation of Jagged1 induces cell growth inhibition and S phase arrest in prostate cancer cells. Int J Cancer. 2006;119:2071–2077. doi: 10.1002/ijc.22077. [DOI] [PubMed] [Google Scholar]
- 41.Hafeez BB, Adhami VM, Asim M, Siddiqui IA, Bhat KM, Zhong W, Saleem M, Din M, Setaluri V, Mukhtar H. Targeted knockdown of Notch1 inhibits invasion of human prostate cancer cells concomitant with inhibition of matrix metalloproteinase-9 and urokinase plasminogen activator. Clin Cancer Res. 2009;15:452–459. doi: 10.1158/1078-0432.CCR-08-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z, Li Y, Ahmad A, Banerjee S, Azmi AS, Kong D, Wojewoda C, Miele L, Sarkar FH. Down-regulation of Notch-1 is associated with Akt and FoxM1 in inducing cell growth inhibition and apoptosis in prostate cancer cells. J Cell Biochem. 2011;12:78–88. doi: 10.1002/jcb.22770. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Sethi S, Macoska J, Chen W, Sarkar FH. Molecular signature of epithelial-mesenchymal transition (EMT) in human prostate cancer bone metastasis. Am J Transl Res. 2011;3:90–99. [PMC free article] [PubMed] [Google Scholar]
- 44.Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco R, Bonal DM, Charytonowicz E, Gladoun N, de la Iglesia-Vicente J, Petrylak DP, Benson MC, Silva JM, Cordon-Cardo C. Suppression of Acquired Docetaxel Resistance in Prostate Cancer through Depletion of Notch- and Hedgehog-Dependent Tumor-Initiating Cells. Cancer Cell. 2012;22:373–388. doi: 10.1016/j.ccr.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whelan JT, Kellogg A, Shewchuk BM, Hewan-Lowe K, Bertrand FE. Notch-1 signaling is lost in prostate adenocarcinoma and promotes PTEN gene expression. J Cell Biochem. 2009;107:992–1001. doi: 10.1002/jcb.22199. [DOI] [PubMed] [Google Scholar]
- 46.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 47.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Borgne R. Regulation of Notch signalling by endocytosis and endosomal sorting. Curr Opin Cell Biol. 2006;18:213–222. doi: 10.1016/j.ceb.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 49.Rizzo P, Miao H, D’Souza G, Osipo C, Song LL, Yun J, Zhao H, Mascarenhas J, Wyatt D, Antico G, Hao L, Yao K, Rajan P, Hicks C, Siziopikou K, Selvaggi S, Bashir A, Bhandari D, Marchese A, Lendahl U, Qin JZ, Tonetti DA, Albain K, Nickoloff BJ, Miele L. Cross-talk between notch and the estrogen receptor in breast cancer suggests novel therapeutic approaches. Cancer Res. 2008;68:5226–5235. doi: 10.1158/0008-5472.CAN-07-5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gallagher RP, Fleshner N. Prostate cancer: 3. Individual risk factors. CMAJ. 1998;159:807–813. [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffman RM, Gilliland FD, Eley JW, Harlan LC, Stephenson RA, Stanford JL, Albertson PC, Hamilton AS, Hunt WC, Potosky AL. Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2001;93:388–395. doi: 10.1093/jnci/93.5.388. [DOI] [PubMed] [Google Scholar]
- 52.Karakiewicz PI, Eastham JA, Graefen M, Cagiannos I, Stricker PD, Klein E, Cangiano T, Schröder FH, Scardino PT, Kattan MW. Prognostic impact of positive surgical margins in surgically treated prostate cancer: multi-institutional assessment of 5831 patients. Urology. 2005;66:1245–1250. doi: 10.1016/j.urology.2005.06.108. [DOI] [PubMed] [Google Scholar]
- 53.Pfitzenmaier J, Pahernik S, Tremmel T, Haferkamp A, Buse S, Hohenfellner M. Positive surgical margins after radical prostatectomy: do they have an impact on biochemical or clinical progression? BJU Int. 2008;102:1413–1418. doi: 10.1111/j.1464-410X.2008.07791.x. [DOI] [PubMed] [Google Scholar]
- 54.Wright JL, Dalkin BL, True LD, Ellis WJ, Stanford JL, Lange PH, Lin DW. Positive surgical margins at radical prostatectomy predict prostate cancer specific mortality. J Urol. 2010;183:2213–2218. doi: 10.1016/j.juro.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wheeler TM, Dillioglugil O, Kattan MW, Arakawa A, Soh S, Suyama K, Ohori M, Scardino PT. Clinical and pathological significance of the level and extent of capsular invasion in clinical stage T1-2 prostate cancer. Hum Pathol. 1998;29:856–862. doi: 10.1016/s0046-8177(98)90457-9. [DOI] [PubMed] [Google Scholar]
- 56.Zayzafoon M, Abdulkadir SA, McDonald JM. Notch signaling and ERK activation are important for the osteomimetic properties of prostate cancer bone metastatic cell lines. J Biol Chem. 2004;279:3662–3670. doi: 10.1074/jbc.M308158200. [DOI] [PubMed] [Google Scholar]


