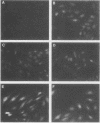
Abstract
The E1a gene of adenovirus encodes two proteins, 289 and 243 amino acids long, which have positive (transactivator) and negative (enhancer repressor) RNA polymerase II transcriptional regulatory properties and cell transformation activities including cooperation with an activated ras gene. The E1a transforming functions more closely correlate with the repressor property than with transactivation in that both E1a proteins express the repressor and transformation functions while only the 289-amino-acid protein is an efficient transactivator. To understand whether the transcriptional regulatory activities of E1a are related to its ras cooperation activity, we generated a series of mutant E1a expression vectors by linker insertion mutagenesis of the 289-amino-acid protein. Here we describe a new class of mutants which although defective for enhancer repression still can cooperate with the ras oncogene in cell transformation. The mutants are also defective in transcription transactivation. Our data suggest that enhancer repression and transformation via ras cooperation are separate E1a functions and that cooperation with ras does not rely on either of the RNA polymerase II transcription regulatory functions of E1a. We also show that mutations which inactivate enhancer repression are not confirmed to a single critical domain necessary for repression. We therefore propose that the integrity of the overall configuration of the E1a proteins is important for the repression activity.
Full text
PDF

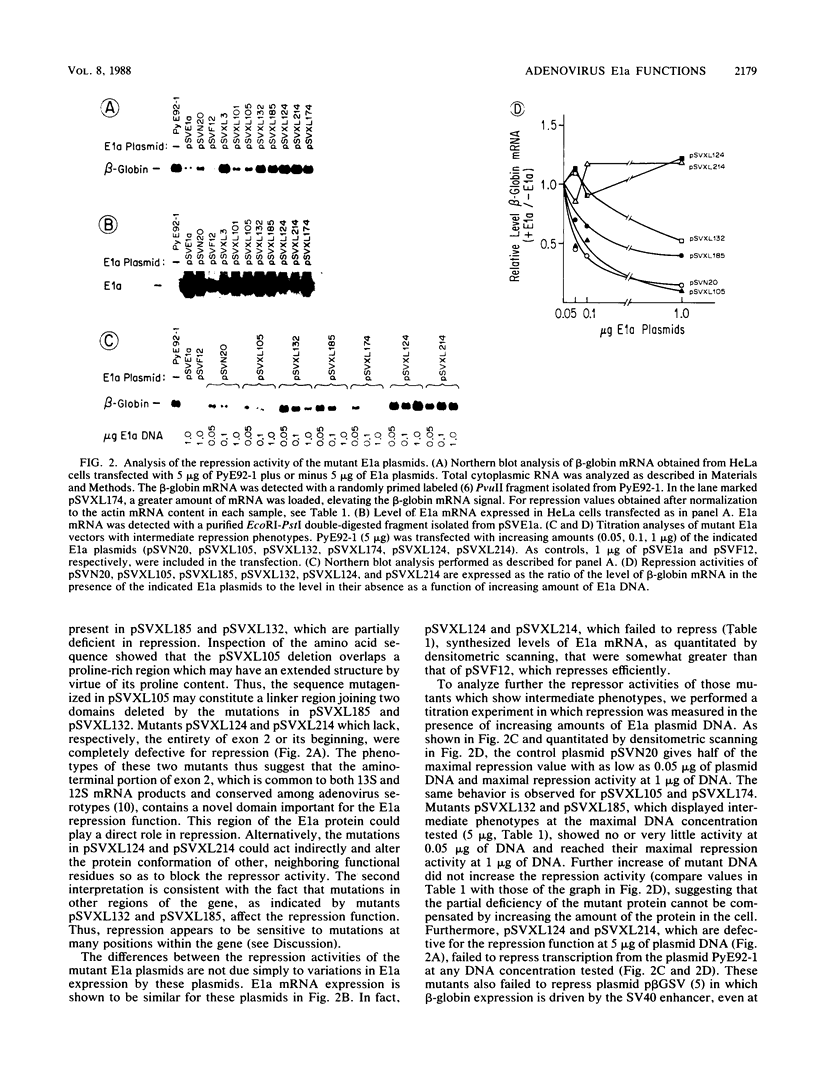
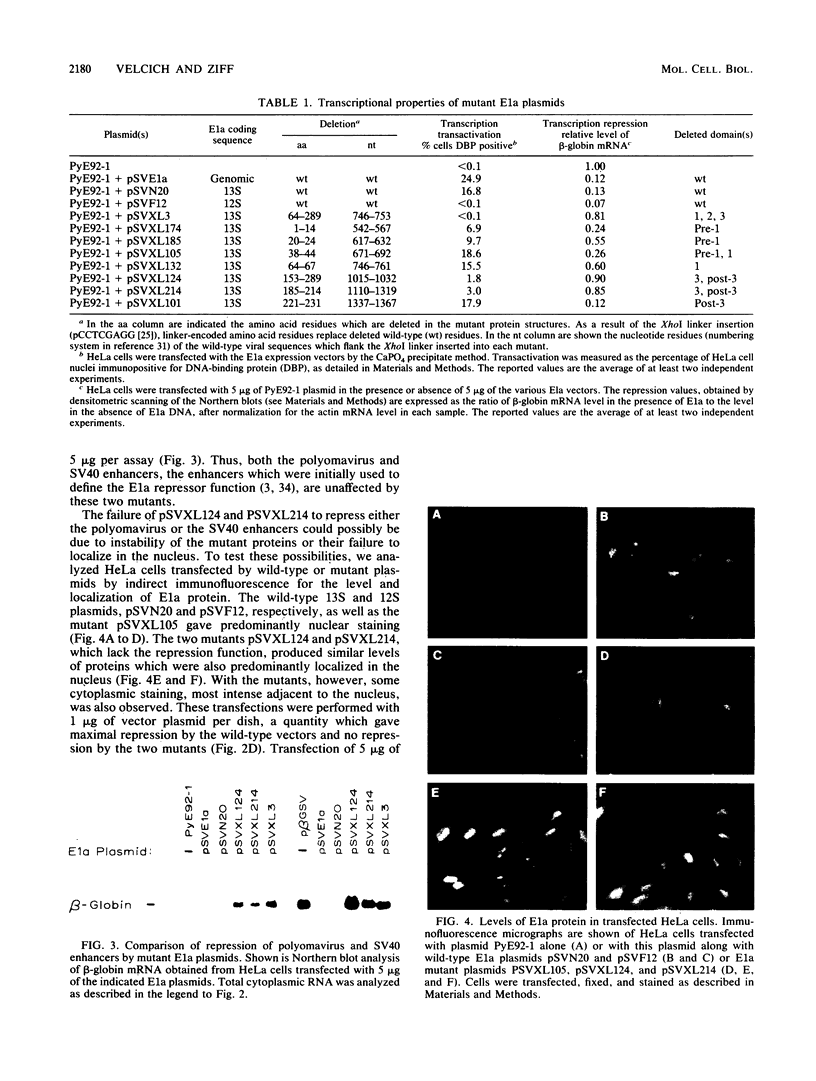



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Berk A. J. Functions of adenovirus E1A. Cancer Surv. 1986;5(2):367–387. [PubMed] [Google Scholar]
- Borrelli E., Hen R., Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984 Dec 13;312(5995):608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- Branton P. E., Bayley S. T., Graham F. L. Transformation by human adenoviruses. Biochim Biophys Acta. 1985;780(1):67–94. doi: 10.1016/0304-419x(84)90007-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Franza B. R., Jr, Maruyama K., Garrels J. I., Ruley H. E. In vitro establishment is not a sufficient prerequisite for transformation by activated ras oncogenes. Cell. 1986 Feb 14;44(3):409–418. doi: 10.1016/0092-8674(86)90462-9. [DOI] [PubMed] [Google Scholar]
- Glenn G. M., Ricciardi R. P. Adenovirus 5 early region 1A host range mutants hr3, hr4, and hr5 contain point mutations which generate single amino acid substitutions. J Virol. 1985 Oct;56(1):66–74. doi: 10.1128/jvi.56.1.66-74.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hen R., Borrelli E., Chambon P. Repression of the immunoglobulin heavy chain enhancer by the adenovirus-2 E1A products. Science. 1985 Dec 20;230(4732):1391–1394. doi: 10.1126/science.2999984. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Miller J. S., Porter D., Roberts B. E. E1a regions of the human adenoviruses and of the highly oncogenic simian adenovirus 7 are closely related. J Virol. 1985 Feb;53(2):399–409. doi: 10.1128/jvi.53.2.399-409.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston R. E., Baldwin A. S., Sharp P. A. Transcription control by oncogenes. Cell. 1985 May;41(1):3–5. doi: 10.1016/0092-8674(85)90049-2. [DOI] [PubMed] [Google Scholar]
- Kuppuswamy M. N., Chinnadurai G. Relationship between the transforming and transcriptional regulatory functions of adenovirus 2 E1a oncogene. Virology. 1987 Jul;159(1):31–38. doi: 10.1016/0042-6822(87)90344-8. [DOI] [PubMed] [Google Scholar]
- Lillie J. W., Green M., Green M. R. An adenovirus E1a protein region required for transformation and transcriptional repression. Cell. 1986 Sep 26;46(7):1043–1051. doi: 10.1016/0092-8674(86)90704-x. [DOI] [PubMed] [Google Scholar]
- Lillie J. W., Loewenstein P. M., Green M. R., Green M. Functional domains of adenovirus type 5 E1a proteins. Cell. 1987 Sep 25;50(7):1091–1100. doi: 10.1016/0092-8674(87)90175-9. [DOI] [PubMed] [Google Scholar]
- Montell C., Courtois G., Eng C., Berk A. Complete transformation by adenovirus 2 requires both E1A proteins. Cell. 1984 Apr;36(4):951–961. doi: 10.1016/0092-8674(84)90045-x. [DOI] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Resolving the functions of overlapping viral genes by site-specific mutagenesis at a mRNA splice site. Nature. 1982 Feb 4;295(5848):380–384. doi: 10.1038/295380a0. [DOI] [PubMed] [Google Scholar]
- Moran E., Grodzicker T., Roberts R. J., Mathews M. B., Zerler B. Lytic and transforming functions of individual products of the adenovirus E1A gene. J Virol. 1986 Mar;57(3):765–775. doi: 10.1128/jvi.57.3.765-775.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran E., Mathews M. B. Multiple functional domains in the adenovirus E1A gene. Cell. 1987 Jan 30;48(2):177–178. doi: 10.1016/0092-8674(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Moran E., Zerler B., Harrison T. M., Mathews M. B. Identification of separate domains in the adenovirus E1A gene for immortalization activity and the activation of virus early genes. Mol Cell Biol. 1986 Oct;6(10):3470–3480. doi: 10.1128/mcb.6.10.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne T. F., Gaynor R. B., Berk A. J. The TATA homology and the mRNA 5' untranslated sequence are not required for expression of essential adenovirus E1A functions. Cell. 1982 May;29(1):139–148. doi: 10.1016/0092-8674(82)90098-8. [DOI] [PubMed] [Google Scholar]
- Perricaudet M., Akusjärvi G., Virtanen A., Pettersson U. Structure of two spliced mRNAs from the transforming region of human subgroup C adenoviruses. Nature. 1979 Oct 25;281(5733):694–696. doi: 10.1038/281694a0. [DOI] [PubMed] [Google Scholar]
- Ruben M., Bacchetti S., Graham F. L. Integration and expression of viral DNA in cells transformed by host range mutants of adenovirus type 5. J Virol. 1982 Feb;41(2):674–685. doi: 10.1128/jvi.41.2.674-685.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Schneider J. F., Fisher F., Goding C. R., Jones N. C. Mutational analysis of the adenovirus E1a gene: the role of transcriptional regulation in transformation. EMBO J. 1987 Jul;6(7):2053–2060. doi: 10.1002/j.1460-2075.1987.tb02470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. H., Kegler D. M., Ziff E. B. Vector expression of adenovirus type 5 E1a proteins: evidence for E1a autoregulation. Mol Cell Biol. 1985 Oct;5(10):2684–2696. doi: 10.1128/mcb.5.10.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler K. R., Eng C. Y., Berk A. J. An adenovirus early region 1A protein is required for maximal viral DNA replication in growth-arrested human cells. J Virol. 1985 Mar;53(3):742–750. doi: 10.1128/jvi.53.3.742-750.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabel S., Argos P., Philipson L. The release of growth arrest by microinjection of adenovirus E1A DNA. EMBO J. 1985 Sep;4(9):2329–2336. doi: 10.1002/j.1460-2075.1985.tb03934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. W., Ziff E. B. Repression of insulin gene expression by adenovirus type 5 E1a proteins. Mol Cell Biol. 1987 Mar;7(3):1164–1170. doi: 10.1128/mcb.7.3.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens C., Harlow E. Differential splicing yields novel adenovirus 5 E1A mRNAs that encode 30 kd and 35 kd proteins. EMBO J. 1987 Jul;6(7):2027–2035. doi: 10.1002/j.1460-2075.1987.tb02467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson C., Akusjärvi G. Adenovirus 2 early region 1A stimulates expression of both viral and cellular genes. EMBO J. 1984 Apr;3(4):789–794. doi: 10.1002/j.1460-2075.1984.tb01886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velcich A., Kern F. G., Basilico C., Ziff E. B. Adenovirus E1a proteins repress expression from polyomavirus early and late promoters. Mol Cell Biol. 1986 Nov;6(11):4019–4025. doi: 10.1128/mcb.6.11.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velcich A., Ziff E. Adenovirus E1a proteins repress transcription from the SV40 early promoter. Cell. 1985 Mar;40(3):705–716. doi: 10.1016/0092-8674(85)90219-3. [DOI] [PubMed] [Google Scholar]
- Velcich A., Ziff E. Gene regulation: repression of activators. Nature. 1984 Dec 13;312(5995):594–595. doi: 10.1038/312594b0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Zerler B., Moran B., Maruyama K., Moomaw J., Grodzicker T., Ruley H. E. Adenovirus E1A coding sequences that enable ras and pmt oncogenes to transform cultured primary cells. Mol Cell Biol. 1986 Mar;6(3):887–899. doi: 10.1128/mcb.6.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers J., Olson L., Tyndall C., Schaffner W. Transcriptional 'enhancers' from SV40 and polyoma virus show a cell type preference. Nucleic Acids Res. 1982 Dec 20;10(24):7965–7976. doi: 10.1093/nar/10.24.7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ormondt H., Galibert F. Nucleotide sequences of adenovirus DNAs. Curr Top Microbiol Immunol. 1984;110:73–142. doi: 10.1007/978-3-642-46494-2_4. [DOI] [PubMed] [Google Scholar]