Abstract
Background
Currently, several studies assessed the role of Tai Chi (TC) in management of chronic obstructive pulmonary disease, but these studies have wide variation of sample and convey inconclusive results. We therefore undertook a meta-analysis to assess the effects of TC.
Methods
A computerized search through electronic databases was performed to obtain sample studies. The primary outcomes were 6-min walking distance (6MWD) and dyspnea. Secondary outcomes included health-related quality of life and pre-bronchodilator spirometry. Weighted mean differences (WMDs) and 95% confidence intervals (CIs) were calculated and heterogeneity was assessed with the I2 test. A random-effects meta-analysis model was applied.
Results
Eight randomized controlled trials involving 544 patients met the inclusion criteria. The pooled WMDs were 34.22 m (95% CI 21.25–47.20, P<0.00001) for 6 MWD, –0.86 units (95% CI –1.44––0.28, P = 0.004) for dyspnea, 70 ml (95% CI 0.02–0.13, P = 0.01) for FEV1, 120 ml (95% CI 0.00–0.23, P = 0.04) for FVC. TC significantly improved the Chronic Respiratory Disease Questionnaire total score, and the St George’s Respiratory Questionnaire score except impact score.
Conclusions
Findings suggest that TC may provide an effective alternative means to achieve results similar to those reported following participation in pulmonary rehabilitation programs. Further studies are needed to substantiate the preliminary findings and investigate the long-term effects of TC.
Introduction
Chronic obstructive pulmonary disease (COPD) is an important cause of morbidity and mortality worldwide [1]–[3]. Quadriceps weakness, decline of health-related quality of life (HRQoL) and exacerbations of symptoms such as dyspnea and cough with or without sputum production may contribute to the severity of COPD in individual patients [3]–[5]. At present, the primary goals in the management of COPD are to alleviate symptoms, slow down the deterioration of lung function, maintain or improve exercise capacity and HRQoL, which minimize the disabling effects and maximize the benefits for COPD patients [3]. The recent evidence-based clinical practice guidelines and statements showed that pulmonary rehabilitation programs (PRPs) are widely accepted as the most effective non-pharmacotherapy in the management of COPD [3], [6]–[10]. However, out-patient of PRPs are still not widely available and even those who participated in a PRP show poor adherence [11].
Tai Chi (TC), developed from ancient China as a defensive martial art, is a mind-body practice rooted in Chinese philosophies. TC provides mild to moderate aerobic activity, and lower-extremity, unsupported upper-extremity muscle strength training [12]. In recent decades, TC has gained popularity in Western countries as an alternative form of exercise. Studies have found positive effects of TC on balance control, cardiovascular fitness, pain, and fatigue, as well as effects on psychological well-being including enhanced mood and reduction of stress, anxiety and depression in both healthy participants and patients with chronic conditions [13]–[17]. In addition, TC can regulate breathing, strengthen the upper and lower limbs function, and especially can improve respiratory muscle and quadriceps muscle strength, which are essential aspects of COPD management [18]. Nowadays, there are published randomized controlled trials (RCTs) regarding the effect of TC in COPD patients [12], [19]–[21]. However, these studies have wide variation in sample size and different measures to evaluate outcomes, and convey inconclusive results. Therefore, we performed a meta-analysis to assess the effectiveness of TC as a method of PRP for COPD patients.
Study Characteristic, Quality and Bias Assessment
Methods
Data Sources and Searches
A computerized search was performed through PubMed, Embase, CNKI (China National Knowledge Infrastructure) databases and other websites (e.g., Cochrane Central Register of Controlled Trials, Google Scholar, and ClinicalTrials.gov) (up to Nov 2012), were searched for original research articles using the key terms “Tai Chi” and “COPD”. Results were limited to studies with human subjects and randomized controlled trials. No language restriction was imposed. Bibliographies of all potentially relevant retrieved studies, identified relevant articles (including unpublished and meta-analysis studies, a follow-up from reference lists of relevant articles and personal contact with experts in this field) and international guidelines were searched by hand.
The following inclusive selection criteria in PICOS order included: (i) population: patients with COPD; (ii) intervention: Tai Chi or Tai Chi Qigong with or without other treatments; (iii) comparison intervention: any type of control; and (iv) outcome measures: the primary outcome measures were 6-minute walking distance (6 MWD) and dyspnea, and secondary outcomes included HRQoL and pre-bronchodilator spirometry such as forced expiratory volume in one second (FEV1) and forced vital capacity (FVC); and (v) study design: RCT.
In our study, dyspnea was evaluated by Borg scale and higher Borg score indicates worse dyspnea. HRQoL was evaluated by Chronic Respiratory Disease Questionnaire (CRDQ) and St George’s Respiratory Questionnaire (SGRQ). The higher CRDQ score indicates more favorable while a lower SGRQ score indicates more favorable.
Data Extraction and Quality Assessment
For each study, we recorded the first author, year of publication, the sample size of the study population (intervention/control), type of TC protocol, study design, intervention group (TC with or without other treatments), control group (any type of other treatments), intervention duration and frequency, exercise time, and outcomes including intergroup differences. To assess eligibility, data and trial quality information from the papers selected for inclusion in the meta-analysis were extracted independently by two investigators (JHY and YZG). Any disagreements were resolved by discussion and consensus. A third investigator (HMY) was consulted in case of disagreement to improve accuracy. The analytical data missing from the primary reports were requested from their authors. When the same population was reported in several publications, we retained only the most informative article or complete study to avoid duplication of information.
Quality Assessment and Risk-of-bias Assessment
The methodological quality of each study was evaluated using the Jadad scale [22]. A score ≤2 indicates low quality and a score ≥3 indicates high quality [23]. The risk of bias was assessed using the Cochrane Handbook for Systematic Reviews of Interventions (Revman version 5.1.0, The Cochrane Collaboration 2011). Two authors (JHY and YZG) subjectively reviewed all studies and assigned a value of ‘high’, ‘low’, or ‘unclear’ to the following: (a) selection bias (Was there adequate generation of the randomization sequence? Was allocation concealment satisfactory?); (b) blinding (i.e., performance bias and detection bias) (Was there blinding of participants, personnel, and outcome assessment?); (c) attrition bias (Were incomplete outcome data sufficiently assessed and dealt with?); (d) reporting bias (Was there evidence of selective outcome reporting?); and (e) other biases (Was the study apparently free of other problems that could put it at a high risk of bias?).
Statistical Analysis
All data were combined using Revman 5.1.0. For continuous outcomes, a mean difference was calculated using weighted mean difference (WMD). All measures were estimated from each study with the associated 95% confidence intervals (CIs) and pooled across studies using a random-effects model [24]. Heterogeneity across studies was tested by using the I2 statistic, which was a quantitative measure of inconsistency across studies. Studies with an I2 statistic of 25% to 50% were considered to have low heterogeneity, those with an I2 statistic of 50% to 75% were considered to have moderate heterogeneity, and those with an I2 statistic of >75% were considered to have a high degree of heterogeneity [25]. If I2>50%, potential sources of heterogeneity were identified by sensitivity analyses conducted by omitting one study in each turn and investigating the influence of a single study on the overall pooled estimate. Potential publication bias for each analysis was assessed visually using a funnel plot. However, publication bias was not assessed because of the limited number (below 10) of studies included in each analysis. We undertook sensitivity analyses to explore the influence of the total effect for the primary outcomes according to methodological quality. A P value <0.05 was considered statistically significant. An intention-to-treat (ITT) analysis was applied and the overall treatment effect was compared with its minimum clinically important difference (MCID). Furthermore, this study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [26].
Results
Bibliographic Search Results
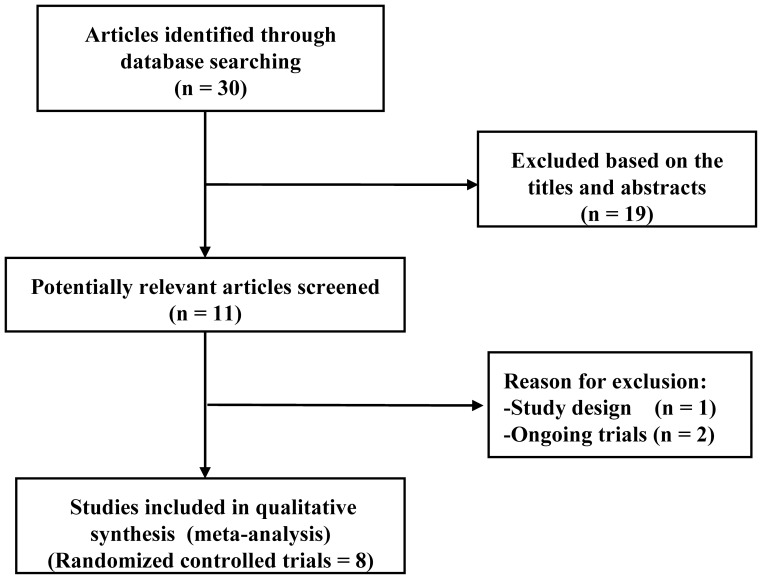
The initial search yielded 30 relevant publications, among which 22 were excluded for various reasons based on the inclusion criteria (PICOS). Reasons for exclusion are presented in Figure 1. Finally, eight RCTs [12], [19]–[21], [27]–[30] were selected for this meta-analysis and two of them from the same population or trial were pooled in our meta-analysis because some important outcomes were separately included in the two RCTs [19], [20]. Four RCTs were published in English and four in Chinese [27]–[30]. In addition, two ongoing RCTs (NCT01551953 and NCT01259245) were located from ClinicalTrials.gov.
Figure 1. Search strategy and flow chart of screened, excluded, and eventually analyzed articles.
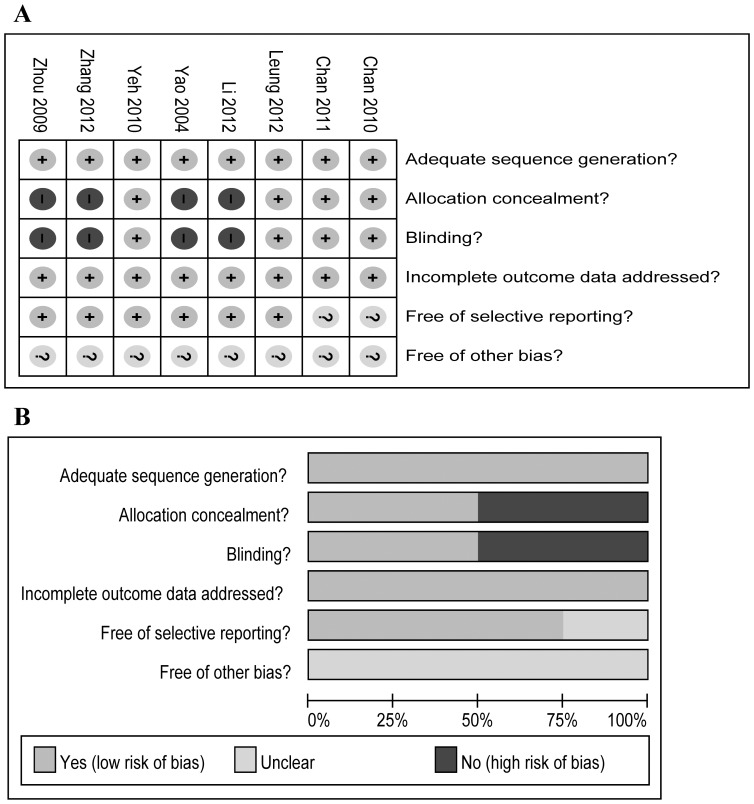
The principal characteristics of the selected studies are presented in Table 1. These studies were published between 2004 and 2012. The sample size ranged from 10 to 206 (total 544). Three RCTs reported 6 MWD [12], [19], [29] and dyspnea [19], [21], [28]. HRQoL was evaluated by CRDQ for two RCTs [12], [21] and by SGRQ for three RCTs [20], [27], [29]. Four RCTs [19], [27], [29], [30] reported FEV1 and three RCTs [27], [30] reported FVC. Follow-up ranged from 12 to 48 weeks and exercise time lasted 30–60 min. Two investigators (JHY and YZG) agreed on every item of the Jadad scores. The mean Jadad score of the studies included was 3 (SD = 1). Risk of bias analysis is presented in Figure 2. Four RCTs from China were not supplied with adequate allocation concealment and blinding [27]–[30].
Table 1. Characteristics of randomized controlled trials included in the meta-analysis.
| Study [ref] | Study design/Jadad score | Patients No. (M/F);Age, mean (I/C) | Grade or FEV1%pred | Study group (n) | TC form or style | Protocol | Adheren/Adverse effects | Outcomes |
| Chan [19], [20] | Single-blind RCT/4 | 206 (188/18); 71.7/73.6 | Class: I-III | TCQ (70); Exercise (69);Control (67) | 13-form TCQ | 12 wk × 2 times/wk;60 min/per time | 83%/None | FEV1, FVC, 6 MWD, Dyspnea, HRQoL |
| Yeh [12] | Partial single-blind RCT/4 | 10 (6/4); 65.0/66.0 | Mean class: 2.5 | TC (5); Control (5) | Yang-style short form | 12 wk × 2 times/wk;60 min/per time | 91%/None | 6 MWD, HRQoL |
| Leung [21] | Single-blind RCT/4 | 42 (27/15); Total agemean: 73.0 | Total mean FEV1%pred: 59% | TC (22); Control (20) | Short-form Sun-style TC | 12 wk × 2 times/wk;60 min/per time | 91%/None | 6 MWD, HRQoL, dyspnea |
| Li [27] | RCT/3 | 70 (55/15); 72.0/73.0 | Class: II-III | TC (35); Control (35) | 24-Short form TC | 24 wk × 1 time/day;60 min/per time | 86%/None | FEV1, FVC, HRQoL |
| Yao [28] | RCT/2 | 80 (45/35); 66.1/66.2 | Class: II-III | TC (40); Control (40) | Chen-style short form | 12 wk × 1 time/day;30 min/per time | 100%/None | Dyspnea |
| Zhang [29] | RCT/2 | 90 (51/39); 62.0/62.2 | Total mean FEV1% pred: 52% | TC (30); Exercise (30);Control (30) | 24-Short form TC | 48 wk × 1 time/day;30–60 min/per time | 100%/None | FEV1, 6 MWD, HRQoL |
| Zhou [30] | RCT/2 | 46 (28/18); 72/73 | Class: I-II | TC (23); Control (23) | 24-Short form TC | 16 wk × 5 times/day;40 min/per time | 100%/None | FEV1, FVC |
M/F, Male/Female; I/C, Intervention/Control; FEV1, forced expiratory volume in one second; RCT, randomized controlled trial; TCQ, Tai Chi Qigong; FVC, forced vital capacity; 6 MWD, 6-minute walking distance; HRQoL, Health-Related Quality of Life.
Figure 2. Risk-of-bias analysis.
(A) Risk-of-bias summary: the authors’ judgments about each risk-of-bias item for the each included studies. (B) Risk-of-bias graph: the authors’ judgments about each risk-of-bias item presented as percentages across all included studies.
The Primary Outcomes
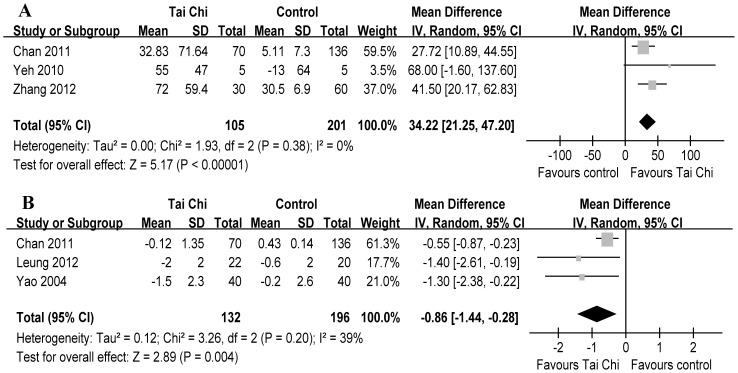
The aggregate results of these studies suggested that TC was associated with a statistical improving on 6 MWD (3 RCTs, WMD 34.22 m, 95% CI 21.25–47.20, P<0.00001, P for heterogeneity = 0.38, I2 = 0%) [12], [19], [29] (Fig. 3-A) and on dyspnea (3 RCTs, WMD –0.86 units, 95% CI –1.44––0.28, P = 0.004, P for heterogeneity = 0.20, I2 = 39%) [19], [21], [28] (Fig. 3-B). Subsequently, the mean changes of 6 MWD were greater than the MCID (≥26 m) [31]; however, the mean changes of dyspnea were lower than the MCID of Borg scale (≥1 unit) [32].
Figure 3. Meta-analysis of randomized controlled trials evaluating effects of Tai Chi on 6-min walking distance (A) and dyspnea (B) by the random-effects model.
Furthermore, we performed sensitivity analyses based on a random-effects model to explore the influence of the total effect according to methodological quality. Table 2 shows the results of sensitivity analyses excluding study with low quality for 6 MWD and dyspnea. However, these did not materially alter the overall combined WMD.
Table 2. Sensitivity analyses excluding trials with low quality for 6 MWD and dyspnea.
| Outcome | n (N) | WMD (95% CI) | P value | I2 (%) | Pheterogeneity |
| 6 MWD | |||||
| All included trials [12], [19], [29] | 306 (3) | 34.22 (21.25–47.20) | <0.00001 | 0 | 0.38 |
| High quality trials [12], [19] (Jadad score ≥3) | 216 (2) | 33.12 (6.22–60.03) | 0.02 | 18 | 0.27 |
| Low quality trial [29] (Jadad score <3) | 90 (1) | 41.50 (20.17–62.83) | 0.0001 | – | – |
| Dyspnea | |||||
| All included trials [19], [21], [28] | 318 (3) | –0.86 (–1.44––0.28) | 0.004 | 39 | 0.20 |
| High quality trials [19], [21] (Jadad score ≥3) | 248 (2) | –0.77 (–1.49––0.04) | 0.004 | 44 | 0.18 |
| Low quality trial [28] (Jadad score <3) | 80 (1) | –1.30 (–2.38––0.22) | 0.02 | – | – |
6 MWD, 6-minute walking distance; n, number of patients; N, number of trials.
The Secondary Outcomes
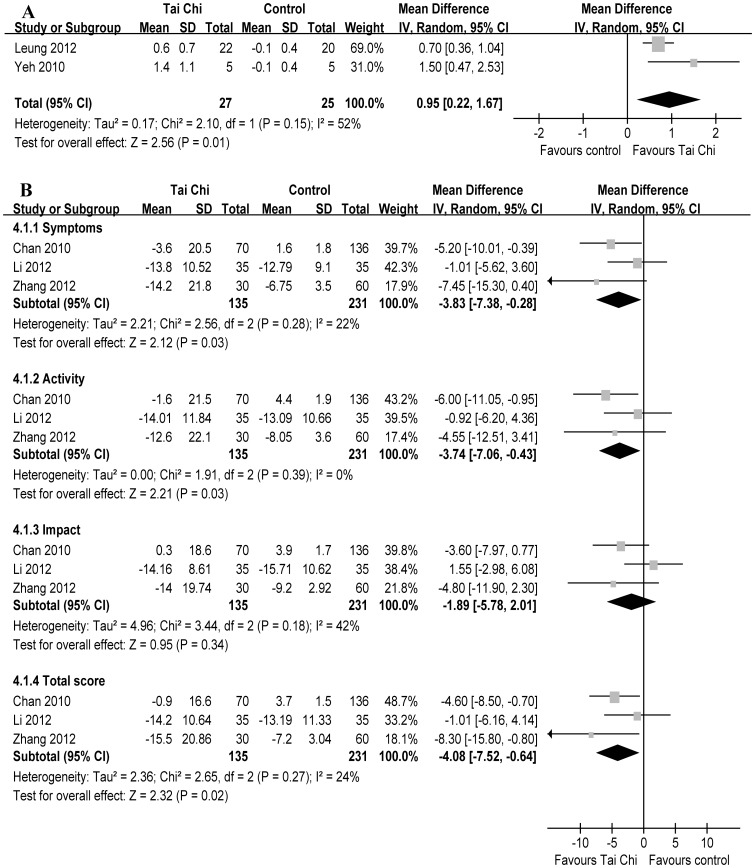
The aggregate results of HRQoL suggested TC was associated with a statistical improving on the CRDQ total score (2 RCTs, WMD 0.95 scores, 95% CI 0.22–1.67, P = 0.01, P for heterogeneity = 0.15, I2 = 52%) [12], [21] (Fig. 4-A). The pooled WMDs from3 RCTs [20], [27], [29] for SGRQ were –4.08 scores (95% CI –7.52––0.64, P = 0.02, P for heterogeneity = 0.27, I2 = 24%) for total score, –3.83 scores (95% CI –7.38––0.28, P = 0.03, P for heterogeneity = 0.28, I2 = 22%) for symptoms score, –3.74 scores (95% CI –7.06––0.43, P = 0.03, P for heterogeneity = 0.39, I2 = 0%) for activity score, –1.89 scores (95% CI –5.78–2.01, P = 0.34, P for heterogeneity = 0.18, I2 = 42%) for impact score (Fig. 4-B). Subsequently, the changes of the CRDQ total score were greater than the MCID (≥0.5 scores) [33]; however, the changes of SGRQ were similar to or greater than the MCID (≥4 units) except impact score [34].
Figure 4. Meta-analysis of randomized controlled trials evaluating effects of Tai Chi on health-related quality of life by the random-effects model.
Tai Chi was associated with a statistical improving on Chronic Respiratory Disease Questionnaire total score (A) and on St George’s Respiratory Questionnaire score except impact score (B).
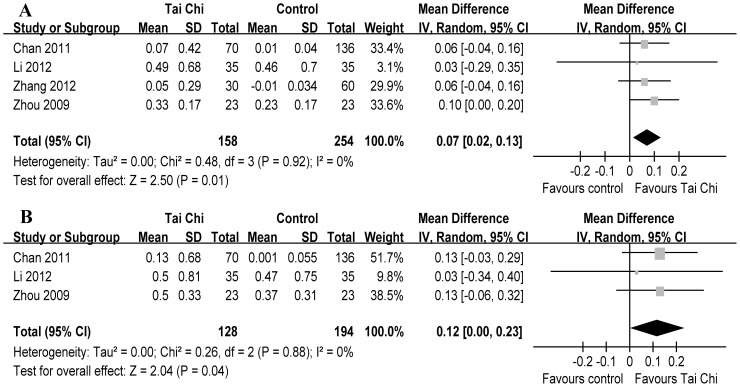
Regarding lung function, TC statistically increased FEV1 (4 RCTs, WMD 70 ml, 95% CI 0.02–0.13, P = 0.01, P for heterogeneity = 0.92, I2 = 0%) [19], [27], [29], [30] (Fig. 5-A) and FVC (3 RCTs, WMD 120 ml, 95% CI 0.00–0.23, P = 0.04, P for heterogeneity = 0.88, I2 = 0%) [19], [27], [30] (Fig. 5-B). Subsequently, the changes of FEV1 were lower than the MCID (≥100 ml) [35].
Figure 5. Meta-analysis of randomized controlled trials evaluating effects of Tai Chi on pre-bronchodilator spirometry by the random-effects model.
Tai Chi statistically increased forced expiratory volume in one second (A) and forced vital capacity (B).
Adherence and Adverse Effects
None of all RCTs reported any side effects or exercise-related problems. Chan et al. reported increasing dyspnea and joint pain which were not related to TC program [19]. The TC program attendance rate ranged from 83% to 100% (Table 1).
Discussion
The main purpose of the current meta-analysis is to evaluate the role of TC in COPD patients. The preliminary evidence suggested that TC may improve exercise capacity, dyspnea, quality of life, and lung function compared with general exercise control or routine care in COPD patients. However, there is currently a lack of adequate data and/or sufficient clinical evidence to support TC benefiting positive effects on these important clinical endpoints.
One of our results showed that TC was associated with statistical improvements regarding the outcomes included. However, it should be emphasized that the results were needed to compare with the MCID inasmuch as not all statistically significant differences are clinically relevant when interpreting clinical measures [36]. The changes of 34.22 m for 6 MWD were greater than the MCID (≥26 m) [31], which indicates that there should be a clinical effect for TC in COPD patients. However, there may be a moderate or even a slight effect with regard to 6 MWD for TC compared with other approaches such as upper limb and lower limb exercise training [9], [37]. Next, the changes of dyspnea were lower than the recommended MCID of Borg scale (≥1 unit) [32]. However, the changes of 0.86 units showed that there may be a better effect regarding to dyspnea for TC compared with upper extremity exercise [38]. Moreover, sensitivity analyses excluding low quality trial did not materially alter the pooled results, which adds robustness to our primary findings. In terms of HRQoL, the changes in CRDQ total score exceeded the MCID (≥0.5 scores) [33], which is consistent with the result from a research [21]. Unfortunately, we failed to further compare each domain of CRDQ with the MCID due to currently insufficient data. In addition, the changes in SGRQ were similar to or even greater than the MCID (≥4 units) except impact score [34]. Therefore, according to the findings, we believe that TC may improve HRQoL in patients with COPD. Of note, although imperfect with disease state, morbidity, mortality and functional status, FEV1 and FVC are the most commonly reported forced expiratory spirometry because of good reproducibility, ease of measurement, and correlation [35]. The changes of 70 ml for FEV1 were lower than the MCID (≥100 ml) [35]. Notwithstanding, taking into account the damaged lung function is difficult to reverse [6], the encouraging results showed that there may be a potential advantage for TC compared with other approaches of PRPs such as inspiratory muscle training, nutritional supplementation, education, etc [6].
Another concern with TC was adverse events and adherence. In view of the overall studies, no adverse events related to TC were reported. It is also worth noting TC adherence was very high, and that patient withdrawal in TC groups was very low, with most drop-out being due to hospitalization or communication barriers. These indicate TC appears to be generally safe and engenders good compliance among patients. Based on these findings, TC is well tolerated and enjoyed by the majority of the participants. Given that there are no special equipment requirements, lower costs and potential physical benefits, TC may represent an interesting alternative to conventional exercise training and incorporation into PRPs for COPD patients, and should be considered and worthy of further study.
Several limitations of the current meta-analysis should be taken into account. First, the number of studies is very limited and not more than 2–4 studies are available for the main outcomes, which limits the conclusion. Besides, these studies have wide variation in sample size. Overestimation of the treatment effect is more likely in smaller trials compared with larger trials. Second, different intervention forms represent probably one of the most important confounders. Particularly, for an intervention such as TC where heterogeneity is the standard, it becomes important to understand what was done. Style, emphasis, intensity, instructor characteristics all may make a substantial difference and greatly affect interpretation of results. Third, there was a certain risk of bias and heterogeneity among the included trials, especially among four RCTs from China with lack of adequate allocation concealment and blinding. The targeted population varied greatly (e.g., age, gender, ethnicity, GOLD class). Considering that mainly in patients that becuase of cultural or age related issues may be not so prone to be involved in TC training, these factors may contribute to the heterogeneity and have a potential influence on the results. Next, several caveats must be considered. Since there were limited data in analyzing each outcome and different measures to evaluate outcomes which may have a potentially negative impact, results should be interpreted with caution. Finally, some missing and unpublished data may lead to bias.
Nonetheless, the present study also provides additional interesting clues about a novel exercise to PRPs that may be useful for future research on this topic. Firstly, blinding prevents ascertainment bias and protects the sequence after allocation [39]. Although it may be difficult to blind to investigators, participants, and outcome assessors in TC study, we should try to apply the appropriate blinding such as blind outcome assessments. Secondly, it is crucial that PRPs tailor COPD patients; however, TC movements are far from “standard” and there is much variability. Exercise intensity will vary greatly depending on the fitness of the individual, the style, depth of stances, the vigour of performing the movements, etc. Further studies need to pay attention to the suitable?TC form for COPD patients. In addition, six to twelve weeks of PRPs benefit in several outcomes that decline gradually over 12 to 18 months in patients with COPD [6]. However, our study showed that the follow-up only ranged from 12 to 48 weeks; thus far the long-term effects of TC and the optimal exercise duration remain unclear. Next, it remains unknown which aspects of TC are responsible for the beneficial effects, and how TC differs from other forms of exercise (e.g., walking, upper limb and lower limb strength and endurance training, etc.). Finally, most studies lacked other objective outcome measures such as exacerbation rate, peripheral muscle strength, balance, survival and immune function, especially at the molecular level [40]. Therefore, further studies should focus on them to better understand the specific mechanism and obtain more reliable and convincing evidence for the efficacy of TC in patients with COPD.
In summary, the current encouraging evidence suggests that TC may improve exercise capacity, dyspnea, HRQoL, and lung function in COPD patients; thus, TC should be encouraged to be a potential approach to PRPs. However, considering the potential bias and heterogeneity of available data and the limited RCTs, further larger-scale RCTs are needed to substantiate the preliminary findings and investigate the long-term effects of TC as well as the tailoring of the rehabilitation intervention for COPD patients.
Funding Statement
The authors have no support or funding to report.
References
- 1. Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, et al. (2006) Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 27: 397–412. [DOI] [PubMed] [Google Scholar]
- 2. Dalal AA, Shah M, Lunacsek O, Hanania NA (2011) Clinical and economic burden of patients diagnosed with COPD with comorbid cardiovascular disease. Respir Med 105: 1516–1522. [DOI] [PubMed] [Google Scholar]
- 3. Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, et al. (2011) Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 155: 179–191. [DOI] [PubMed] [Google Scholar]
- 4. Beauchamp MK, Sibley KM, Lakhani B, Romano J, Mathur S, et al. (2012) Impairments in systems underlying control of balance in COPD. Chest 141: 1496–1503. [DOI] [PubMed] [Google Scholar]
- 5. Shrikrishna D, Patel M, Tanner RJ, Seymour JM, Connolly BA, et al. (2012) Quadriceps wasting and physical inactivity in patients with COPD. Eur Respir J 40: 1115–1122. [DOI] [PubMed] [Google Scholar]
- 6. Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, et al. (2007) Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest 131: 4S–42S. [DOI] [PubMed] [Google Scholar]
- 7. Altenburg WA, de Greef MH, ten Hacken NH, Wempe JB (2012) A better response in exercise capacity after pulmonary rehabilitation in more severe COPD patients. Respir Med 106: 694–700. [DOI] [PubMed] [Google Scholar]
- 8. Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, et al. (2006) American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med 173: 1390–1413. [DOI] [PubMed] [Google Scholar]
- 9. Lacasse Y, Wong E, Guyatt GH, King D, Cook DJ, et al. (1996) Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. Lancet 348: 1115–1119. [DOI] [PubMed] [Google Scholar]
- 10. Troosters T, Casaburi R, Gosselink R, Decramer M (2005) Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 172: 19–38. [DOI] [PubMed] [Google Scholar]
- 11. Garrod R, Marshall J, Barley E, Jones PW (2006) Predictors of success and failure in pulmonary rehabilitation. Eur Respir J 27: 788–794. [DOI] [PubMed] [Google Scholar]
- 12. Yeh GY, Roberts DH, Wayne PM, Davis RB, Quilty MT, et al. (2010) Tai chi exercise for patients with chronic obstructive pulmonary disease: a pilot study. Respir Care 55: 1475–1482. [PMC free article] [PubMed] [Google Scholar]
- 13. Li F, Harmer P, Fitzgerald K, Eckstrom E, Stock R, et al. (2012) Tai chi and postural stability in patients with Parkinson’s disease. N Engl J Med 366: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin CL, Lin CP, Lien SY (2013) [The effect of tai chi for blood pressure, blood sugar, blood lipid control for patients with chronic diseases: a systematic review]. Hu Li Za Zhi 60: 69–77. [DOI] [PubMed] [Google Scholar]
- 15. Peng PW (2012) Tai chi and chronic pain. Reg Anesth Pain Med 37: 372–382. [DOI] [PubMed] [Google Scholar]
- 16. Pan L, Yan J, Guo Y, Yan JH (2013) Effects of Tai Chi training on exercise capacity and quality of life in patients with chronic heart failure: a meta-analysis. Eur J Heart Fail 15: 316–323. [DOI] [PubMed] [Google Scholar]
- 17.Yeh GY, Wood MJ, Wayne PM, Quilty MT, Stevenson LW, et al.. (2012) Tai Chi in Patients With Heart Failure With Preserved Ejection Fraction. Congest Heart Fail. In press. [DOI] [PMC free article] [PubMed]
- 18. Liu B, Liu ZH, Zhu HE, Mo JC, Cheng DH (2011) Effects of tai chi on lower-limb myodynamia in the elderly people: a meta-analysis. J Tradit Chin Med 31: 141–146. [DOI] [PubMed] [Google Scholar]
- 19. Chan AW, Lee A, Suen LK, Tam WW (2011) Tai chi Qigong improves lung functions and activity tolerance in COPD clients: a single blind, randomized controlled trial. Complement Ther Med 19: 3–11. [DOI] [PubMed] [Google Scholar]
- 20. Chan AW, Lee A, Suen LK, Tam WW (2010) Effectiveness of a Tai chi Qigong program in promoting health-related quality of life and perceived social support in chronic obstructive pulmonary disease clients. Qual Life Res 19: 653–664. [DOI] [PubMed] [Google Scholar]
- 21.Leung RW, McKeough ZJ, Peters MJ, Alison JA (2012) Short-form Sun-style Tai Chi as an exercise training modality in people with COPD. Eur Respir J. In press. [DOI] [PubMed]
- 22. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, et al. (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 23. Kjaergard LL, Villumsen J, Gluud C (2001) Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 135: 982–989. [DOI] [PubMed] [Google Scholar]
- 24. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 25. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Q, Fang WH, Liu C (2012) [The effect of taijiquan combined with respiratory exercise training on rehabilitation of patients with stable chronic obstructive pulmonary disease]. Chin J Rehabil Med 27: 825–828. [Google Scholar]
- 28. Yao YP (2004) [Effect of tai chi chuan on chronic obstructive pulmonary disease]. Chin J Rehabil Theory Practice 10: 439–440. [Google Scholar]
- 29. Zhang LH, Wu JJ, Wang ZC (2012) [Effects of 24-form Tai Chi with Respiratory Rehabilitation Training on Pulmonary Function and Quality of Life of Patients with COPD]. Acta U Trad Med Sine Pharm Shanghai 26: 53–56. [Google Scholar]
- 30. Zhou Y, Wu L, Wang DX (2009) [The Rehabilitative Effects of Taijiquan Training with East Medical on Middle-elderly COPD Patients]. J Jilin I Phys Educ 25: 54–55. [Google Scholar]
- 31. Puhan MA, Chandra D, Mosenifar Z, Ries A, Make B, et al. (2011) The minimal important difference of exercise tests in severe COPD. Eur Respir J 37: 784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ries AL (2005) Minimally clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg Scale, and Visual Analog Scale. COPD 2: 105–110. [DOI] [PubMed] [Google Scholar]
- 33. Schunemann HJ, Puhan M, Goldstein R, Jaeschke R, Guyatt GH (2005) Measurement properties and interpretability of the Chronic respiratory disease questionnaire (CRQ). COPD 2: 81–89. [DOI] [PubMed] [Google Scholar]
- 34. Jones PW (2005) St. George’s Respiratory Questionnaire: MCID. COPD 2: 75–79. [DOI] [PubMed] [Google Scholar]
- 35. Donohue JF (2005) Minimal clinically important differences in COPD lung function. COPD 2: 111–124. [DOI] [PubMed] [Google Scholar]
- 36. Beaton DE, Bombardier C, Katz JN, Wright JG, Wells G, et al. (2001) Looking for important change/differences in studies of responsiveness. OMERACT MCID Working Group. Outcome Measures in Rheumatology. Minimal Clinically Important Difference. J Rheumatol 28: 400–405. [PubMed] [Google Scholar]
- 37. Costi S, Crisafulli E, Antoni FD, Beneventi C, Fabbri LM, et al. (2009) Effects of unsupported upper extremity exercise training in patients with COPD: a randomized clinical trial. Chest 136: 387–395. [DOI] [PubMed] [Google Scholar]
- 38. Pan L, Guo YZ, Yan JH, Zhang WX, Sun J, et al. (2012) Does upper extremity exercise improve dyspnea in patients with COPD? A meta-analysis. Respir Med 106: 1517–1525. [DOI] [PubMed] [Google Scholar]
- 39. Schulz KF, Grimes DA (2002) Blinding in randomised trials: hiding who got what. Lancet 359: 696–700. [DOI] [PubMed] [Google Scholar]
- 40. Saatcioglu F (2013) Regulation of gene expression by yoga, meditation and related practices: A review of recent studies. Asian J Psychiatr 6: 74–77. [DOI] [PubMed] [Google Scholar]