Abstract
Background
To monitor the impact of human papillomavirus types 16 and 18 vaccine on HPV infection dynamics in the Netherlands, we started an ongoing study in sexually transmitted infection (STI) clinics in 2009. Here, we analyze baseline type-specific HPV DNA and HPV-specific antibody positivity rates.
Methods
We enrolled 3569 men and women, 16–24 years of age, from 14 STI clinics, and estimated genital and anal HPV DNA and antibody positivity rates of 7 main carcinogenic HPV types. Generalized estimating equations regression analyses were applied to determine risk factors for, and associations between, type-specific HPV DNA and antibody positivity.
Results
Genital HPV DNA positivity rates were higher in women than in men; anal HPV DNA was especially high in men who have sex with men (MSM). HPV antibody seropositivity rates were also highest in women and MSM. High-risk sexual behavior was predictive of both HPV DNA and antibody positivity. Despite a strong correlation in serological profiles for multiple HPV types, seropositivity was independently associated with homologous HPV DNA detection.
Conclusions
HPV DNA and antibody positivity rates are higher in women and MSM than in heterosexual men, but their association is similar across gender. This suggests a site-specific natural course of infection.
Introduction
Human papillomavirus (HPV) is a common sexually transmitted virus known for its causal relation to cervical cancer. There are more than 100 HPV genotypes, with more than 15 carcinogenic types [1], [2]. In many countries, HPV vaccination has been introduced in sexually naïve girls to prevent infections with HPV-16/-18, which are most commonly found in cervical cancers. It is not yet known what the impact of HPV vaccination will be on HPV dynamics in partially vaccinated populations. Monitoring of type-specific HPV prevalence in both vaccinated and nonvaccinated people is, therefore, of great importance.
HPV infection does not always induce an immune response that results in HPV-specific antibodies (Ab) [3], [4], [5]. Even if women are diagnosed with precancerous cervical lesions that test positive for HPV DNA, they might still be negative for serum HPV Ab [6]. Whether HPV infection will lead to seroconversion depends on several factors, such as specific HPV types, persistence of infection, HPV DNA viral load, and site of infection [3], [4], [7], [8], [9], [10], [11]. In contrast to natural infection, HPV vaccination induces an immune response with high concentrations of HPV Ab, by far exceeding the HPV Ab concentrations found in nonvaccinated populations [12]. In addition, studies showed that vaccination against HPV-16/-18 can result in cross-protection against phylogenetically related genotypes [13], [14]. Therefore, it is possible that vaccination may not only result in a decline in HPV-16/-18 prevalence, but also in a decline in phylogenetically related genotypes such as HPV-31, -33, and -45. Conversely, unwanted effects like type replacement, i.e., the potential for nonvaccine HPV types to occupy the vacated ecologic niches, can occur as a result of the elimination of HPV-16/-18 [15]. This hypothesis has neither been confirmed nor rejected by epidemiological studies. As a result of reduced exposure to HPV-16/-18, an effect can also be expected among nonvaccinated men and women [16], [17], [18], [19].
The aim of our study was to describe HPV DNA and HPV-specific Ab detection rates of women, men who have sex with women only (MSW), and men who have sex with men (MSM), all of whom were without benefit of HPV vaccination. Furthermore, we explored associations between homologous and heterologous pairs of HPV DNA and HPV Ab types. This description will serve as a baseline measurement to which we can compare future monitoring rounds on HPV dynamics within the Netherlands.
Materials and Methods
Ethics Statement
The medical ethics committee of the University of Utrecht, the Netherlands, confirmed in writing that they waived the need for separate ethical approval and the need for written consent. This anonymous study used serum already collected for routine STI consultation, therefore no additional invasive procedures were needed. All eligible participants were informed about the purpose of the study prior to the regular STI consultation and full information was provided about the samples to be collected and the additional questionnaire to be administered. Only participants who consented verbally with all conditions were included in the study.
Study Population and Design
In 2009, the bivalent HPV vaccine was introduced in the Netherlands among 12- to 16-year-old girls. To monitor the effects of HPV-16/-18 vaccination on type-specific HPV dynamics in a young highly sexually active population, the PASSYON (PApillomavirus Surveillance among STI clinic YOungsters in the Netherlands) study was set up [20]. This biennial cross-sectional study includes 16- to 24-year-old male and female attendees of the sexually transmitted infection (STI) clinic. In 2009 and 2011, the first two rounds of this study took place in 14 STI clinics throughout the Netherlands; 10 STI clinics participated in both rounds.
A genital self-sample (vaginal or penile) of all participants was requested (sampling technique is described elsewhere [20]). In addition, MSM were requested for an anal sample as well. Procedures were similar in both rounds, however, in 2011, females and MSW also were invited to submit an anal sample. In addition, participation included: the use of serum already collected during routine consultation or collection of an extra blood sample if routine serum was not available; filling out a questionnaire; and permission to link the anonymous standardized data collected during the intake of the routine consultation with STI test results (e.g., Chlamydia trachomatis, Neisseria gonorrhoea, syphilis, HIV). The questionnaire covered sexual risk factors such as condom use, number of sex partners, and history of STI. Demographic data were also collected. Ethnicity was not recorded, but we asked the participants which population they belonged to, regardless of country of birth, and used their responses as proxies.
HPV DNA detection
All swabs were suspended in 1 ml universal transport medium buffer and stored at −20°C until processing. After thawing, swabs were vortexed and 200 µL of the sample was spiked with phocine herpes virus-1. DNA was subsequently extracted using the MagnaPure platform (Total Nucleic Acid Isolation Kit, Roche) and eluted in 100 µL elution buffer. HPV-DNA was amplified using the SPF10 primer set according to the manufacturer's instructions (DDL Diagnostic Laboratory, the Netherlands [21]). HPV-specific amplicons were detected using the DNA enzyme-linked immunoassay (HPV-DEIA, DDL Diagnostic Laboratory, the Netherlands). Amplicons of HPV-positive samples were subsequently analyzed in Line probe assay (HPV-LiPA, DDL Diagnostic Laboratory, the Netherlands). In this report, only the seven carcinogenic HPV types, which can also be detected in the Ab detection assay, are reported (i.e., HPV-16, -18, -31, -33, -45, -52, -58). Only persons positive for one of the seven HPV-types are reported as being HPV positive.
Serum antibody detection
Serum samples were stored at −80°C until analysis. HPV-specific serum antibodies against L1 virus-like particles (VLPs) for serotypes -16, -18, -31, -33, -45, -52 and -58 were assessed using a VLP-based multiplex immunoassay in which the VLPs were coupled to a set of seven distinct fluorescent microspheres. VLPs were kindly donated by GSK. Most samples were analysed with a 1/100 or 1/200 dilution, some in 1/2000 dilution. Serum samples were assumed to be antibody seropositive at cut-offs determined previously with this assay: 9, 13, 27, 11, 19, 14, 31 Luminex Units/millilitre (LU/ml) for HPV-16, -18, -31, -33, -45, -52, -58, respectively. The serological measurements and control steps have been described in more detail elsewhere [22].
Statistical Analyses
Since no significant differences were observed in HPV DNA or Ab positivity rates between the first two rounds (2009 and 2011), we chose to combine these rounds in this study to gain more power. We included only HIV-negative persons having a urogenital DNA sample as well as a serum sample. Women who reported HPV vaccination were excluded in these baseline analyses. We did not exclude men who reported to be HPV vaccinated (n = 17), since Ab concentrations of HPV-16/-18 were not significantly different from those of persons who reported not to be HPV vaccinated, and since men are not (yet) vaccinated in the Netherlands for HPV, it was unlikely that they actually had been vaccinated. Type-specific positivity rates for the seven detectable HPV DNA and HPV Ab types were estimated, stratified by women, MSW, and MSM. Positivity rates were estimated by dividing the number of type-specific HPV DNA positives by the total number tested, with 95% confidence intervals (CI) based on Wilson's score method. The Student's t-test was used to compare positivity rates. When the number testing positive was below five the Fisher's exact test was used.
Generalized estimating equations (GEE) analyses were performed to assess independent risk factors associated with the presence of HPV DNA, and separate analyses were performed to asses independent risk factors associated with HPV Ab seropositivity. GEE models are marginal and take into account multiple measurements, making them highly suitable for estimating population-averaged risks associated with repeated measures [23]. GEE models estimate conditional log odds ratios (βx) between an independent risk factor (x) and a multivariate outcome variable, while assuming a particular correlation structure between the repeated measures, in this case multiple HPV types [24]. In assessing independent risk factors for the presence of HPV DNA and for HPV Ab seropositivity, we used an ‘exchangeable correlation’ structure with a common odds ratio alpha, which is appropriate when the association between pairs of HPV types can be assumed to be constant. Analyses were stratified by gender (i.e., women, MSW, MSM), since univariable analyses showed that all three outcome variables (genital HPV DNA, anal HPV DNA, and HPV Ab) differed significantly between women, MSW, and MSM. Multivariable analyses were performed by including the following variables into a GEE model: age, ethnicity, years sexually active, number of lifetime sex partners, number of sex partners in the last six months, condom use in the last six months with a steady partner, condom use in the last six months with a casual partner, a chlamydia or gonorrhea infection in the past, a current urogenital chlamydia infection, a current anogenital chlamydia or gonorrhea infection, passive anal sex in the past six months, and the seven different HPV genotypes. Subsequently, all nonsignificant (p≥0.05) variables were removed by backward selection.
GEE models were also used to estimate possible type-specific associations between the presence of HPV DNA and HPV Ab. These analyses were also stratified by gender, but could not be performed for MSM because of the small group size. For these analyses, we used a saturated model for the independent risk factors and started with a fully parameterized model for the pair-wise log odds ratios between all 14 genital HPV DNA and HPV Ab outcome variables. The starting log odds ratio model was composed of 14×13/2 = 91 linear parameter combinations, one for each unique binary pair of type-specific DNA and/or Ab of the seven HPV types. All nonsignificant parameters were subsequently deleted from the model using Bonferroni-corrected p values based on Wald tests, and this procedure was repeated until all remaining parameters were significant. Final estimates were obtained after orthonormal transformation of the log odds ratio estimation matrix. In modelling the association between pairs of responses, we made use of the alternating logistic regression algorithm of the SAS GENMOD procedure [25].
Results
Characteristics study population
In 2009 and 2011, 1637 and 1932 participants, respectively, were tested for a genital HPV infection. Of these 3569 participants, 3439 (96%) were also tested for HPV Ab. After exclusion of 6 HIV-positive persons and 105 self-reported HPV-vaccinated women, a study population of 3328 participants remained, of whom two-thirds were females (n = 2233). A subgroup of 118 (5%) women, 56 (6%) MSW, and 124 (72%) MSM also had a test result for anal HPV DNA infection.
Characteristics stratified for women, MSW, and MSM are given in Table 1. The median age of the 3328 participants was 21 years (IQR: 20–23 years). Of all men, 16% (n = 173) reported having sex with men. Sexual behavioral factors showed a median age of first intercourse of 16 years (IQR: 15–17 years) and a median number of lifetime partners of 7 (IQR: 4–12) (6, 9, and 12 lifetime partners for women, MSW, and MSM, respectively). The percentage of persons with a current or past anogenital chlamydia or gonorrhoea infection is given in Table 2. Among women and MSW, 14% had a current urogenital chlamydia and 2% had a current gonorrhea infection. For MSM this was 5% and 4%, respectively. A current anal chlamydia and/or gonorrhea infection was reported for 1% of women and 14% of MSM. No anal infections were seen in MSW.
Table 1. Demographics and characteristics of the study population: women, MSW, and MSM.
| Female | MSW | MSM | Total | ||||||
| N = 2233 | N = 922 | N = 173 | N = 3328 | ||||||
| n | (%) | n | (%) | n | (%) | n | (%) | ||
| Year of participation | |||||||||
| 2009 | 1069 | (47.9) | 414 | (44.9) | 68 | (39.3) | 1551 | (46.6) | |
| 2011 | 1164 | (52.1) | 508 | (55.1) | 105 | (60.7) | 1777 | (53.4) | |
| Age | |||||||||
| 16–18 years | 249 | (11.2) | 61 | (6.6) | 17 | (9.8) | 327 | (9.8) | |
| 19–21 years | 974 | (43.6) | 351 | (38.1) | 71 | (41.0) | 1396 | (41.9) | |
| 22–24 years | 1010 | (45.2) | 510 | (55.3) | 85 | (49.1) | 1605 | (48.2) | |
| Ethnicity | |||||||||
| Dutch | 1925 | (86.2) | 741 | (80.4) | 137 | (79.2) | 2803 | (84.2) | |
| Non-Dutch | 308 | (13.8) | 181 | (19.6) | 36 | (20.8) | 525 | (15.8) | |
| Educational level * | |||||||||
| Low | 512 | (22.9) | 281 | (30.5) | 57 | (32.9) | 850 | (25.5) | |
| High | 1655 | (74.1) | 605 | (65.6) | 109 | (63.0) | 2369 | (71.2) | |
| Unknown | 66 | (3.0) | 36 | (3.9) | 7 | (4.0) | 109 | (3.3) | |
| Years sexual active | |||||||||
| 0–2 years | 411 | (18.4) | 97 | (10.5) | 32 | (18.5) | 540 | (16.2) | |
| 3–4 years | 576 | (25.8) | 224 | (24.3) | 47 | (27.2) | 847 | (25.5) | |
| ≥5 years | 1179 | (52.8) | 562 | (61.0) | 87 | (50.3) | 1828 | (54.9) | |
| Unknown | 67 | (3.0) | 39 | (4.2) | 7 | (4.0) | 113 | (3.4) | |
| Steady or casual sex partner | |||||||||
| No partner | 1009 | (45.2) | 348 | (37.7) | 71 | (41.0) | 1428 | (42.9) | |
| Steady partner | 878 | (39.3) | 409 | (44.4) | 61 | (35.3) | 1348 | (40.5) | |
| Casual partner | 237 | (10.6) | 99 | (10.7) | 21 | (12.1) | 357 | (10.7) | |
| Steady and casual partner | 52 | (2.3) | 28 | (3.0) | 14 | (8.1) | 94 | (2.8) | |
| Unknown | 57 | (2.6) | 38 | (4.1) | 6 | (3.5) | 101 | (3.0) | |
| Condom use steady partner, past 6 months | |||||||||
| Inconsistent condom use | 726 | (32.5) | 337 | (36.6) | 45 | (26.0) | 1108 | (33.3) | |
| Consistent condom use | 201 | (9.0) | 98 | (10.6) | 30 | (17.3) | 329 | (9.9) | |
| No steady partner | 1251 | (56.0) | 449 | (48.7) | 92 | (53.2) | 1792 | (53.8) | |
| Unknown | 55 | (2.5) | 38 | (4.1) | 6 | (3.5) | 99 | (3.0) | |
| Condom use casual partner, past 6 months | |||||||||
| Inconsistent condom use | 728 | (32.6) | 349 | (37.9) | 31 | (17.9) | 1108 | (33.3) | |
| Consistent condom use | 834 | (37.3) | 317 | (34.4) | 117 | (67.6) | 1268 | (38.1) | |
| No casual partner | 620 | (27.8) | 220 | (23.9) | 19 | (11.0) | 859 | (25.8) | |
| Unknown | 51 | (2.3) | 36 | (3.9) | 6 | (3.5) | 93 | (2.8) | |
| Number of sex partners, lifetime | |||||||||
| 1–4 partners | 762 | (34.1) | 187 | (20.3) | 29 | (16.8) | 978 | (29.4) | |
| 5–9 partners | 804 | (36.0) | 249 | (27.0) | 29 | (16.8) | 1082 | (32.5) | |
| ≥10 partners | 612 | (27.4) | 442 | (47.9) | 113 | (65.3) | 1167 | (35.1) | |
| Unknown | 55 | (2.5) | 44 | (4.8) | 2 | (1.2) | 101 | (3.0) | |
| Number of sex partners, past 6 months | |||||||||
| 0–1 partners | 806 | (36.1) | 232 | (25.2) | 28 | (16.2) | 1066 | (32.0) | |
| 2–3 partners | 1048 | (46.9) | 379 | (41.1) | 62 | (35.8) | 1489 | (44.7) | |
| ≥4 partners | 375 | (16.8) | 310 | (33.6) | 83 | (48.0) | 768 | (23.1) | |
| Unknown | 4 | (0.2) | 1 | (0.1) | 0 | (0.0) | 5 | (0.2) | |
| Passive anal intercourse, past 6 months | |||||||||
| No | 1938 | (86.8) | 917 | (99.5) | 44 | (25.4) | 2899 | (87.1) | |
| Yes | 253 | (11.3) | 5 | (0.5) | 129 | (74.6) | 387 | (11.6) | |
| Unknown | 42 | (1.9) | 0 | (0.0) | 0 | (0.0) | 42 | (1.3) | |
High educational level includes university of science and university of professional education, low educational level includes all other forms of education.
MSM = men who have sex with men; MSW = men who have sex with women only.
Table 2. Past and current (ano)genital chlamydia and gonorrhea infections: women, MSW, and MSM.
| Female | MSW | MSM | Total | ||||||
| N = 2233 | N = 922 | N = 173 | N = 3328 | ||||||
| n | (%) | n | (%) | n | (%) | n | (%) | ||
| Chlamydia or gonorrhea ever | |||||||||
| No | 1424 | (63.8) | 568 | (61.6) | 107 | (61.8) | 2099 | (63.1) | |
| Yes | 420 | (18.8) | 127 | (13.8) | 41 | (23.7) | 588 | (17.7) | |
| Never tested before | 326 | (14.6) | 190 | (20.6) | 16 | (9.2) | 532 | (16.0) | |
| Unknown | 63 | (2.8) | 37 | (4.0) | 9 | (5.2) | 109 | (3.3) | |
| Current urogenital chlamydia infection | |||||||||
| No | 1917 | (85.8) | 792 | (85.9) | 164 | (94.8) | 2873 | (86.3) | |
| Yes | 307 | (13.7) | 122 | (13.2) | 8 | (4.6) | 437 | (13.1) | |
| Unknown/not tested | 9 | (0.4) | 8 | (0.9) | 1 | (0.6) | 18 | (0.5) | |
| Current urogenital gonorrhea infection | |||||||||
| No | 2194 | (98.3) | 897 | (97.3) | 164 | (94.8) | 3255 | (97.8) | |
| Yes | 32 | (1.4) | 17 | (1.8) | 7 | (4.0) | 56 | (1.7) | |
| Unknown/not tested | 7 | (0.3) | 8 | (0.9) | 2 | (1.2) | 17 | (0.5) | |
| Current anal chlamydia infection | |||||||||
| No | 96 | (4.3) | 5 | (0.5) | 121 | (69.9) | 222 | (6.7) | |
| Yes | 24 | (1.1) | 0 | (0.0) | 16 | (9.2) | 40 | (1.2) | |
| Unknown/not tested | 2113 | (94.6) | 917 | (99.5) | 36 | (20.8) | 3066 | (92.1) | |
| Current anal gonorrhea infection | |||||||||
| No | 340 | (15.2) | 8 | (0.9) | 141 | (81.5) | 489 | (14.7) | |
| Yes | 7 | (0.3) | 0 | (0.0) | 13 | (7.5) | 20 | (0.6) | |
| Unknown/not tested | 1886 | (84.5) | 914 | (99.1) | 19 | (11.0) | 2819 | (84.7) | |
MSM = men who have sex with men; MSW = men who have sex with women only.
HPV DNA positivity and HPV-specific antibody seropositivity
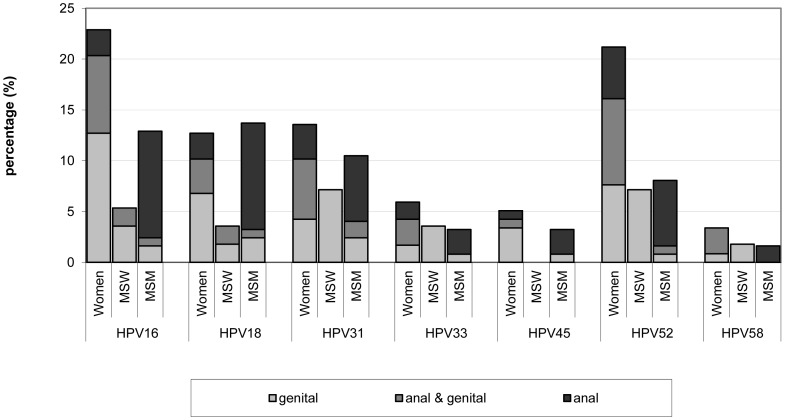
Women had the highest genital HPV DNA positivity rate (45%) with the highest type-specific rate for HPV-16 (17% of all women). Among men, MSM had lower positivity rates for genital (penile) DNA than MSW, 16% versus 26%, respectively. Although low genital HPV DNA positivity rates were seen in MSM, anal HPV DNA positivity rates were high. Within the subgroups that also provided anal samples (124 MSM, 118 women, and 56 MSW), similar anal HPV DNA positivity rates were seen in MSM (33%) and women (32%), whereas anal HPV DNA positivity was rare in MSW (4%) (Figure 1). HPV seropositivity was highest in women (49%), followed by MSM (34%) and MSW (19%).
Figure 1. Positivity rate for genital and anal DNA detection in women, MSW and MSM.
Black gives the positivity rate for persons with a HPV DNA detection only at the genitals, dark grey gives the positivity rate for persons with an HPV DNA infection genital as well as anal, and light grey are the positivity rates when persons are only detected with HPV DNA anally. The figures includes women (N = 118), MSW (N = 56) and MSM (N = 124) of whom a genital and an anal sample were available. MSW = men who have sex with women only; MSM = men who have sex with men.
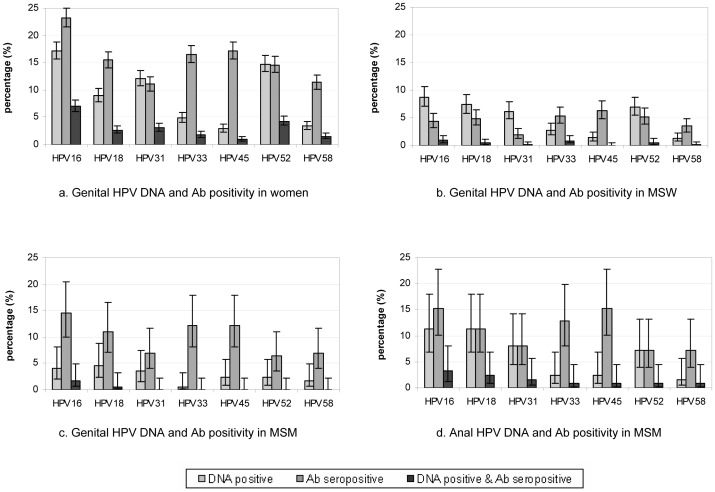
Comparing HPV DNA positivity and HPV Ab seropositivity rates by gender, the main differences were the lower positivity rates for HPV Ab -16, -18, -31, and -52 compared to homologous HPV DNA positivity rates in MSW (Figure 2a–c). Women and MSM, however, had generally higher positivity rates for HPV Ab in comparison to homologous HPV DNA positivity rates. When comparing anal and genital infections in MSM, the anal HPV DNA positivity rates were higher than the genital HPV DNA positivity rates, although not significant (p = 0.25) (Figure 2c–d). Subsequently, the percentage of simultaneous detection of HPV DNA and homologous HPV Ab increased. The difference between anal HPV DNA positivity and HPV Ab seropositivity in MSM was similar to the genital rates observed in women. When observing women and MSM with both a genital as well as an anal sample, differences were seen for HPV Ab seropositivity among those infected with HPV DNA at one anatomical site only. Although numbers were small, women tended to be more often HPV Ab seropositive when only a genital HPV DNA infection was present compared to MSM (45% versus 18%, respectively (p = 0.16)). Whereas, HPV Ab seropositivity rates when only an anal HPV DNA infection was present were quite similar between women and MSM (29% versus 21%, respectively (p = 0.71)).
Figure 2. Genital (and anal) HPV DNA and HPV-specific antibody seropositivity in women, MSW, and MSM.
Figure 2a–c gives the genital HPV DNA (light grey), HPV-specific antibody seropositivity (dark grey), as well as the percentage of persons detected with genital HPV DNA as well as HPV antibodies (black) with 95% confidence intervals in women (N = 2233), MSW (N = 922), and MSM (N = 173) per HPV genotype. Figure 2d gives the anal HPV DNA (light grey), HPV-specific antibody seropositivity (dark grey) as well as the percentage of persons detected with anal HPV DNA as well as HPV antibodies (black) with 95% confidence intervals in MSM (N = 124). Ab = antibody; MSM = men who have sex with men; MSW = men who have sex with women only.
Simultaneous detection of type-specific HPV DNA and homologous HPV Ab genotype was most frequent for genotype 16 among all genders and sites. From Figure 2a–b, the odds ratio for HPV-16 Ab seropositivity together with HPV-16 DNA positivity is directly estimated at 2.9 for women and at 3.3 for MSW. However, given the presence of HPV-16 DNA, the probability of testing positive for HPV-16 Ab is much higher for women than for MSW, 41% versus 11%, respectively (p<0.01).
Determinants of HPV DNA positivity and HPV-specific antibody seropositivity
Multivariable analyses resulted in different risk factors for genital HPV DNA positivity, anal HPV DNA positivity, and HPV Ab seropositivity. Most noticeable was the significant association of ethnicity with HPV Ab seropositivity for women as well as MSM, whereas ethnicity was not significantly associated with HPV DNA positivity. Persons who reported they were non-Dutch were more likely to be HPV Ab seropositive. Furthermore, in women the site of the current chlamydia infection corresponded with the site of the associated HPV DNA positivity: a genital chlamydia infection was associated with genital HPV DNA infection and an anal chlamydia infection with an anal HPV DNA infection. In MSM, reporting passive anal sex in the past six months was associated with HPV Ab seropositivity. All significant risk factors resulting from the multivariable GEE analysis are shown in Table 3, 4 and 5.
Table 3. Adjusted odds ratio and 95% confidence interval for the detection of genital and anal HPV DNA and HPV antibodies using multilevel analyses (generalized estimating equations [GEE)]) in women.
| Genital HPV DNA | Anal HPV DNA | HPV antibodies | |||||
| AOR | (95% CI) | AOR | (95% CI) | AOR | (95% CI) | ||
| Ethnicity | |||||||
| Dutch | NS | NS | Reference | ||||
| Non-Dutch | 1.46 | (1.19–1.78) | |||||
| Years sexual active | |||||||
| 0–2 years | NS | NS | Reference | ||||
| 3–4 years | 0.95 | (0.73–1.22) | |||||
| ≥5 years | 1.20 | (0.95–1.51) | |||||
| Number of sex partners, lifetime | |||||||
| 1–4 partners | Reference | Reference | Reference | ||||
| 5–9 partners | 1.37 | (1.15–1.62) | 0.31 | (0.10–0.93) | 1.40 | (1.15–1.70) | |
| ≥10 partners | 2.02 | (1.70–2.40) | 0.94 | (0.32–2.76) | 2.23 | (1.82–2.74) | |
| Condom use steady partner, past 6 months | |||||||
| Inconsistent condom use | NS | NS | Reference | ||||
| Consistent condom use | 0.79 | (0.59–1.04) | |||||
| No steady partner | 0.82 | (0.70–0.96) | |||||
| Condom use casual partner, past 6 months | |||||||
| Inconsistent condom use | Reference | NS | NS | ||||
| Consistent condom use | 0.94 | (0.82–1.08) | |||||
| No casual partner | 0.66 | (0.56–0.79) | |||||
| History of chlamydia or gonorrhea | |||||||
| No | Reference | Reference | Reference | ||||
| Yes | 1.29 | (1.10–1.51) | 4.52 | (1.46–14.02) | 1.83 | (1.53–2.19) | |
| Never tested before | 1.08 | (0.90–1.30) | 1.32 | (0.52–3.39) | 0.80 | (0.63–1.03) | |
| Current urogenital chlamydia infection | |||||||
| No | Reference | NS | NS | ||||
| Yes | 1.26 | (1.06–1.50) | |||||
| Current anal chlamydia/gonorrhea infection | |||||||
| No | Reference | Reference | Reference | ||||
| Yes | 0.50 | (0.28–0.91) | 3.45 | (1.01–11.73) | 1.33 | (0.76–2.36) | |
| Not tested | 0.77 | (0.66–0.91) | 0.52 | (0.25–1.10) | 0.65 | (0.54–0.79) | |
| HPV genotype | |||||||
| HPV16 | Reference | Reference | Reference | ||||
| HPV18 | 0.49 | (0.41–0.59) | 0.54 | (0.21–1.42) | 0.59 | (0.51–0.68) | |
| HPV31 | 0.65 | (0.55–0.77) | 0.81 | (0.37–1.80) | 0.38 | (0.32–0.45) | |
| HPV33 | 0.24 | (0.19–0.30) | 0.21 | (0.06–0.77) | 0.63 | (0.55–0.72) | |
| HPV45 | 0.15 | (0.11–0.19) | 0.14 | (0.03–0.60) | 0.66 | (0.58–0.76) | |
| HPV52 | 0.82 | (0.70–0.97) | 1.44 | (0.69–3.03) | 0.54 | (0.47–0.62) | |
| HPV58 | 0.17 | (0.13–0.22) | 0.22 | (0.05–0.86) | 0.40 | (0.34–0.46) | |
| Odds of having one HPV genotype when having another HPV genotype within an individual (Alpha) | |||||||
| 1.54 | (1.35–1.76) | 1.81 | (1.01–3.23) | 4.34 | (3.79–4.98) | ||
The following variables were included into the crude GEE model: age, ethnicity, years sexually active, number of lifetime sex partners, number of sex partners in the last six months, condom use in the last six months with a steady partner, condom use in the last six months with a casual partner, a chlamydia or gonorrhea in the past, a current urogenital chlamydia infection, a current anal chlamydia or gonorrhea infection, passive anal intercourse in the past six months, and the seven different HPV genotypes.
AOR = adjusted odds ratio; CI = confidence interval; NI = not included in the multivariable model; NS = not significant in multivariable model.
Table 4. Adjusted odds ratio and 95% confidence interval for the detection of genital and anal HPV DNA and HPV antibodies using multilevel analyses (generalized estimating equations [GEE)]) in men who have sex with women only (MSW).
| Genital HPV DNA | Anal HPV DNA* | HPV antibodies | |||||
| AOR | (95% CI) | AOR | (95% CI) | AOR | (95% CI) | ||
| Age (y) | |||||||
| 16–18 | Reference | Reference | |||||
| 19–21 | 1.42 | (0.67–2.99) | 1.99 | (0.93–4.27) | |||
| 22–24 | 2.02 | (0.98–4.19) | 2.19 | (1.08–4.41) | |||
| Number of sex partners, lifetime | |||||||
| 1–4 partners | Reference | NS | |||||
| 5–9 partners | 1.39 | (0.85–2.29) | |||||
| ≥10 partners | 2.23 | (1.45–3.43) | |||||
| Condom use steady partner, past 6 months | |||||||
| Inconsistent condom use | Reference | NS | |||||
| Consistent condom use | 0.33 | (0.18–0.60) | |||||
| No steady partner | 0.74 | (0.56–0.98) | |||||
| History of chlamydia or gonorrhea | |||||||
| No | Reference | NS | |||||
| Yes | 1.49 | (1.05–2.12) | |||||
| Never tested before | 0.86 | (0.58–1.27) | |||||
| HPV genotype | |||||||
| HPV16 | Reference | Reference | |||||
| HPV18 | 0.86 | (0.62–1.21) | 1.10 | (0.73–1.66) | |||
| HPV31 | 0.68 | (0.47–0.97) | 0.41 | (0.24–0.71) | |||
| HPV33 | 0.29 | (0.19–0.47) | 1.27 | (0.86–1.86) | |||
| HPV45 | 0.16 | (0.09–0.28) | 1.44 | (0.95–2.18) | |||
| HPV52 | 0.78 | (0.55–1.10) | 1.14 | (0.78–1.66) | |||
| HPV58 | 0.14 | (0.08–0.26) | 0.77 | (0.50–1.17) | |||
| Odds of having one HPV genotype when having another HPV genotype within an individual (Alpha) | |||||||
| 2.18 | (1.46–3.24) | 8.73 | (5.94–12.83) | ||||
The following variables were included into the crude GEE model: age, ethnicity, years sexually active, number of lifetime sex partners, number of sex partners in the last six months, condom use in the last six months with a steady partner, condom use in the last six months with a casual partner, a chlamydia or gonorrhea in the past, a current urogenital chlamydia infection, passive anal intercourse in the past six months, and the seven different HPV genotypes.
The model for anal HPV DNA could not be run for MSW due to small numbers.
AOR = adjusted odds ratio; CI = confidence interval; NI = not included in the multivariable model; NS = not significant in multivariable model.
Table 5. Adjusted odds ratio and 95% confidence interval for the detection of genital and anal HPV DNA and HPV antibodies using multilevel analyses (generalized estimating equations [GEE)]) in men who have sex with men (MSM).
| Genital HPV DNA* | Anal HPV DNA | HPV antibodies | |||||
| AOR | (95% CI) | AOR | (95% CI) | AOR | (95% CI) | ||
| Ethnicity | |||||||
| Dutch | NS | Reference | |||||
| Non-Dutch | 2.65 | (1.29–5.46) | |||||
| Years sexual active | |||||||
| 0–2 years | Reference | NS | |||||
| 3–4 years | 6.23 | (1.51–25.65) | |||||
| ≥5 years | 7.74 | (1.88–31.92) | |||||
| Number of sex partners, lifetime | |||||||
| 1–4 partners | NS | Reference | |||||
| 5–9 partners | 0.95 | (0.24–3.78) | |||||
| ≥10 partners | 2.96 | (1.02–8.57) | |||||
| Condom use casual partner, past 6 months | |||||||
| Inconsistent condom use | NS | Reference | |||||
| Consistent condom use | 1.10 | (0.39–3.10) | |||||
| No casual partner | 5.38 | (1.45–20.02) | |||||
| Passive anal intercourse, past 6 months | |||||||
| No | NS | Reference | |||||
| Yes | 2.67 | (1.25–5.72) | |||||
| HPV genotype | |||||||
| HPV16 | Reference | Reference | |||||
| HPV18 | 1.00 | (0.45–2.20) | 0.65 | (0.38–1.12) | |||
| HPV31 | 0.68 | (0.28–1.64) | 0.39 | (0.20–0.76) | |||
| HPV33 | 0.19 | (0.06–0.65) | 0.81 | (0.46–1.43) | |||
| HPV45 | 0.19 | (0.05–0.71) | 0.75 | (0.44–1.29) | |||
| HPV52 | 0.53 | (0.20–1.40) | 0.38 | (0.20–0.72) | |||
| HPV58 | 0.13 | (0.03–0.58) | 0.34 | (0.17–0.69) | |||
| Odds of having one HPV genotype when having another HPV genotype within an individual (Alpha) | |||||||
| 1.79 | (0.94–3.43) | 8.12 | (4.43–14.91) | ||||
The following variables were included into the crude GEE model: age, ethnicity, years sexually active, number of lifetime sex partners, number of sex partners in the last six months, condom use in the last six months with a steady partner, condom use in the last six months with a casual partner, a chlamydia or gonorrhea in the past, a current urogenital chlamydia infection, a current anal chlamydia or gonorrhea infection, passive anal intercourse in the past six months, and the seven different HPV genotypes.
The model for genital HPV DNA could not be run for MSM due to small numbers.
AOR = adjusted odds ratio; CI = confidence interval; NI = not included in the multivariable model; NS = not significant in multivariable model.
Association between genital HPV DNA and HPV-specific antibody genotypes
The log odds ratio model for genital HPV DNA positivity and HPV Ab seropositivity could be reduced from a total of 91 parameters to as few as 7 independent parameters in women. Table 6 shows the resulting estimates of the common odds ratios for any pair of the following: (1) homologous HPV DNA and HPV Ab genotypes; (2) HPV DNA genotypes; (3) HPV Ab genotypes; and (4) heterologous DNA and Ab genotypes. In addition to these associations, we found significantly increased odds ratios for DNA and Ab of HPV-58; DNA of HPV-31 and HPV-33; and Ab detection for a subset of genotypes (i.e., HPV-33, -45, -52, -58). For example, seropositivity for HPV-33 Ab in combination with HPV-45 Ab is a frequently occurring test result, as well as seropositivity for HPV-33 Ab with HPV-52 Ab. For MSW, the number of independent parameters that could be estimated was smaller than for women, which is in line with the smaller sample size and lower positivity rate in MSW as compared to women. Associations were similar between the sexes (Table 6), except that a much stronger association was observed between pairs of HPV Ab genotypes in MSW compared with women.
Table 6. Association (odds ratio and 95% confidence interval) between genital HPV DNA and HPV-specific antibody genotypes, for women and MSW.
| Women | MSW | |||
| OR | (95% CI) | OR | (95% CI) | |
| Any pair of homologous HPV DNA and HPV Ab genotypes | 2.55 | (2.19–2.96) | 2.02 | (1.28–3.20) |
| Any pair of HPV DNA genotypes | 1.54 | (1.35–1.76) | 2.07 | (1.36–3.15) |
| Any pair of HPV Ab genotypes | 4.14 | (3.61–4.76) | 9.07 | (6.01–13.69) |
| Any pair of heterologous DNA and Ab genotypes | 1.21 | (1.09–1.33) | 1.01 | (0.66–1.53) |
Ab = antibody; OR = odds ratio; CI = confidence interval; MSW = men who have sex with women only.
The results of the log odds ratio model provided a justification for the assumption of an ‘exchangeable correlation’ structure in assessing independent risk factors for the presence of HPV DNA and for HPV Ab seropositivity. The associations between HPV DNA genotypes or between HPV Ab genotypes estimated by the log odds ratio model corresponded well with the alphas estimated in the multivariable GEE analyses (Table 3, 4 and 5). Note that the common odds ratios estimated by assuming an exchangeable correlation structure are slightly higher (with exception of the odds ratio for HPV Ab in MSW), because these incorporate the small elevations related to certain HPV types in the log odds ratio model. Having verified the assumptions underlying an exchangeable correlation structure, we then compared the associations between HPV DNA genotypes or HPV Ab genotypes in MSM and those in women and in MSW. We found that MSM have similar associations between anal HPV DNA genotypes as women (Table 3 and 5). Associations between HPV Ab genotypes of MSM overlap with those of women and MSW when taking the 95% confidence intervals into account.
Discussion
Positivity rates were high in this young and sexually active population, without the benefit of HPV vaccination. HPV DNA positivity and HPV Ab seropositivity were higher in women than in MSW, with MSM in between, but the association between detection of type-specific DNA and serum Ab was similar across gender. The PASSYON study sheds new light on differences in the natural history of HPV infection between men and women, indirectly providing evidence for a site-specific natural course of infection.
In women, HPV DNA positivity rates were lower for each HPV genotype than HPV Ab seropositivity rates, whereas in MSW, type-specific HPV DNA positivity rates were higher than HPV Ab seropositivity rates with the exceptions of HPV Ab-33, -45, and -58. These specific Ab types had high positivity rates in all genders, which was confirmed by the results of the GEE analyses. Significantly higher association between Ab-33 and -45 and between Ab-33 and -58 was found, meaning that when an individual is positive for one of these Ab types, he or she is also frequently found positive for the other type. The gender differences we observed in HPV DNA positivity and HPV Ab seropositivity may be related to the type of tissue being infected. The dry keratinized tissue of the penis is much harder for the virus to infect than the soft mucosal tissue of the vagina or the anus, therefore men are less likely than women to develop a humoral response [8], [9], [10], [11]. Results in MSM support the hypothesis that tissue could play an important role in HPV Ab seropositivity, since high HPV Ab seropositivity rates in combination with high anal HPV DNA positivity rates were found in MSM, whereas genital HPV DNA positivity was low. The ratios between HPV Ab and anal HPV DNA positivity rates in MSM were similar to the ratios of HPV Ab and vaginal/anal HPV DNA positivity rates in women. To examine whether gender-specific factors other than anatomical site could influence seroconversion, we also compared the HPV Ab seropositivity rates within women and MSM who provided both genital and anal samples. Women with only a genital HPV DNA infection were more often seropositive for the homologous HPV Ab serotype as compared to MSM with only a penile HPV DNA infection, whereas HPV Ab seropositivity rates were comparable for women and MSM with only an anal HPV DNA infection. These findings support the hypothesis that penile HPV infections will result less often in seroconversion than vaginal or anal HPV infections. However, our study was not adequately powered to detect differences in seropositivity rate between women and MSM who were infected at one anatomical site only, and we cannot rule out the possibility that additional factors (e.g. genetic, behavioural) might contribute to gender-specific differences in seroconversion. We can only conjecture that anatomical site is a determinant in the natural course of HPV infection. Obviously, a cross-sectional design is inherently limited in drawing conclusions about dynamic associations and it would be worthwhile to further investigate the relation between site-specific HPV infection and the humoral immune response in a longitudinal study.
Multivariable GEE analyses helped clarify risk factors for HPV positivity. One risk factor for a HPV DNA infection in women was a current chlamydia infection. The site-specific relation between a chlamydia infection and an HPV infection was noticeable: a current genital chlamydia infection was associated with genital HPV DNA positivity and a current anal chlamydia infection with anal HPV DNA positivity. The underlying mechanism could be less efficient clearance because of this concurrent infection, resulting in a persistent infection, or easier invasion into the body. There were also behavioral factors, such as reported number of lifetime sex partners, associated with HPV DNA positivity and HPV Ab seropositivity. These results are in line with previous studies [11], [26], [27], [28], [29], [30].
The added value of GEE analyses is the possibility to include all type-specific HPV DNA and HPV Ab outcomes in one model, thereby making optimal use of the available data. In addition, including both HPV DNA and HPV Ab test results in one model gave us the option to estimate associations between homologous and heterologous pairs of HPV DNA and HPV Ab genotypes. Despite the difference in HPV DNA positivity and HPV Ab seropositivity between women and MSW, no significant gender difference was seen in the association between a homologous or heterologous pair of HPV DNA and HPV Ab types. Equal odds ratios for the associations between HPV DNA positivity and HPV Ab seropositivity in women and MSW do not imply that the natural course of the infection would be similar between the sexes. As shown, women have a higher probability to be seropositive when HPV DNA positive then MSW. A higher degree of persistence could be an explanation for the higher seropositivity in women. Furthermore, a higher association between any two HPV Ab genotypes in MSW was observed as compared to women. This higher association in MSW could be due to the less frequent occurrence of seroconversion. It is conceivable that characteristics related to seroconversion are restricted to specific persons such that, on a population level, it will lead to a stronger clustering of seropositivity in MSW than in women, where seroconversion is more common.
We also found type-specific HPV DNA and HPV Ab associations and found some associations between homologous HPV DNA and HPV Ab types, and between two heterologous HPV DNA types.
These associations could have occurred because of the high level of multiple HPV infections. However, the HPV types tested in the VLP-MIA are phylogenetically related to each other, and belong to the alpha-7 species (HPV-18, -45) and alpha-9 species (HPV-16, -31, -33, -52, -58) [31]. Since a high percentage of homology is found between HPV types of the same species, cross-reactive HPV-specific antibodies could account for the type-specific associations.
To summarize, the higher HPV DNA and Ab positivity rates in women and MSM as compared to heterosexual men, and the similar HPV DNA and Ab associations across gender suggest a site-specific natural course of infection. These findings in combination with the determinants for HPV DNA positivity and HPV Ab seropositivity can help us interpret the results of future rounds with more accuracy and will enable us to detect changes in HPV infection dynamics, both of vaccine and nonvaccine types.
Acknowledgments
The authors thank the following for their valuable contributions to the design or execution of the study: Mariet Feltkamp, Titia Heijman, Elske van Logchem, Adam Meijer, Chris Meijer, Hester de Melker, Martijn van Rooijen, Rutger Schepp, Peter Snijders, Jan Sonsma, and Hans van Vliet. Furthermore, the STI clinics, including all nurses and physicians, within the municipal health centers and the hospitals are acknowledged for their permission to collect data from their patients and their effort. The authors acknowledge the medical microbiologic laboratories and the analysts for storage and testing of the samples.
Medical Microbiological Laboratories: D.S. Luijt (Laboratory for Infectious Diseases Groningen), J.W.A. Rossen (St. Elisabeth Hospital Tilburg), M. Schutten (Erasmus Medical Center Rotterdam), R. Schuurman (University Medical Center Utrecht), A.G.C.L. Speksnijder (Public Health Laboratory Amsterdam), P. Wolffs (University Hospital Maastricht);
Municipal Health Services: G. Aalfs (MHS Drenthe), H. van Buel-Bruins (MHS IJsselland), P.J. Cornelissen (MHS Rivierenland), T.E. Doorn (University Medical Center Utrecht), H.M. Götz (MHS Rotterdam-Rijnmond), F. de Groot (MHS Groningen), C.J.P.A. Hoebe (MHS Zuid Limburg), P. Hut-van Vliet (MHS Fryslân), C.J.G. Kampman (MHS Twente), H. van Kruchten (MHS Hart voor Brabant), A.P. van Leeuwen (MHS Amsterdam), M. Pelgrim (MHS Gelderland-Midden).
Funding Statement
This work was supported by the Ministry of Health, Welfare and Sport, the Netherlands. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, et al. (2009) A review of human carcinogens–Part B: biological agents. Lancet Oncol 10: 321–322. [DOI] [PubMed] [Google Scholar]
- 2. Munoz N, Castellsague X, de Gonzalez AB, Gissmann L (2006) Chapter 1: HPV in the etiology of human cancer. Vaccine 24 Suppl 3S3/1–10. [DOI] [PubMed] [Google Scholar]
- 3. Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, et al. (2000) Comparison of Human Papillomavirus Types 16, 18, and 6 Capsid Antibody Responses Following Incident Infection. The Journal of Infectious Diseases 181: 1911–1919. [DOI] [PubMed] [Google Scholar]
- 4. Ho GY, Studentsov YY, Bierman R, Burk RD (2004) Natural history of human papillomavirus type 16 virus-like particle antibodies in young women. Cancer Epidemiol Biomarkers Prev 13: 110–116. [DOI] [PubMed] [Google Scholar]
- 5. Steele J, Collins S, Wen K, Ryan G, Constandinou-Williams C, et al. (2008) Measurement of the humoral immune response following an incident human papillomavirus type 16 or 18 infection in young women by a pseudovirion-based neutralizing antibody assay. Clin Vaccine Immunol 15: 1387–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vonka V, Hamsikova E, Kanka J, Ludvikova V, Sapp M, et al. (1999) Prospective study on cervical neoplasia IV. Presence of HPV antibodies. Int J Cancer 80: 365–368. [DOI] [PubMed] [Google Scholar]
- 7. Carter JJ, Madeleine MM, Shera K, Schwartz SM, Cushing-Haugen KL, et al. (2001) Human Papillomavirus 16 and 18 L1 Serology Compared across Anogenital Cancer Sites. Cancer Res 61: 1934–1940. [PubMed] [Google Scholar]
- 8. Edelstein ZR, Carter JJ, Garg R, Winer RL, Feng Q, et al. (2011) Serum Antibody Response Following Genital α9 Human Papillomavirus Infection in Young Men. Journal of Infectious Diseases 204: 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu B, Viscidi RP, Lee JH, Wu Y, Villa LL, et al. (2011) Human papillomavirus (HPV) 6, 11, 16, and 18 seroprevalence is associated with sexual practice and age: results from the multinational HPV Infection in Men Study (HIM Study). Cancer Epidemiol Biomarkers Prev 20: 990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Newall AT, Brotherton JML, Quinn HE, McIntyre PB, Backhouse J, et al. (2008) Population Seroprevalence of Human Papillomavirus Types 6, 11, 16, and 18 in Men, Women, and Children in Australia. Clinical Infectious Diseases 46: 1647–1655. [DOI] [PubMed] [Google Scholar]
- 11. Stone KM, Karem KL, Sternberg MR, McQuillan GM, Poon AD, et al. (2002) Seroprevalence of Human Papillomavirus Type 16 Infection in the United States. Journal of Infectious Diseases 186: 1396–1402. [DOI] [PubMed] [Google Scholar]
- 12. De Carvalho N, Teixeira J, Roteli-Martins CM, Naud P, De Borba P, et al. (2010) Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine 28: 6247–6255. [DOI] [PubMed] [Google Scholar]
- 13. Brown DR, Kjaer SK, Sigurdsson K, Iversen O-E, Hernandez-Avila M, et al. (2009) The Impact of Quadrivalent Human Papillomavirus (HPV; Types 6, 11, 16, and 18) L1 Virus-Like Particle Vaccine on Infection and Disease Due to Oncogenic Nonvaccine HPV Types in Generally HPV-Naive Women Aged 16–26 Years. The Journal of Infectious Diseases 199: 926–935. [DOI] [PubMed] [Google Scholar]
- 14. Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow SN, et al. (2009) Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. The Lancet 374: 301–314. [DOI] [PubMed] [Google Scholar]
- 15. Durham DP, Poolman EM, Ibuka Y, Townsend JP, Galvani AP (2012) Reevaluation of epidemiological data demonstrates that it is consistent with cross-immunity among human papillomavirus types. J Infect Dis 206: 1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bogaards JA, Coupe VM, Xiridou M, Meijer CJ, Wallinga J, et al. (2011) Long-term impact of human papillomavirus vaccination on infection rates, cervical abnormalities, and cancer incidence. Epidemiology 22: 505–515. [DOI] [PubMed] [Google Scholar]
- 17. Brisson M, Van de Velde N, Boily MC (2011) Different population-level vaccination effectiveness for HPV types 16, 18, 6 and 11. Sex Transm Infect 87: 41–43. [DOI] [PubMed] [Google Scholar]
- 18. Donovan B, Grulich AE (2011) The quadrivalent HPV vaccine is effective prophylaxis against HPV-related external genital lesions in young men. Evid Based Med 16: 157–158. [DOI] [PubMed] [Google Scholar]
- 19. Smith MA, Lew JB, Walker RJ, Brotherton JM, Nickson C, et al. (2011) The predicted impact of HPV vaccination on male infections and male HPV-related cancers in Australia. Vaccine 29: 9112–9122. [DOI] [PubMed] [Google Scholar]
- 20. Vriend HJ, Boot HJ, van der Sande MA (2012) Type-Specific Human Papillomavirus Infections Among Young Heterosexual Male and Female STI Clinic Attendees. Sex Transm Dis 39: 72–78. [DOI] [PubMed] [Google Scholar]
- 21. Molijn A, Kleter B, Quint W, van Doorn LJ (2005) Molecular diagnosis of human papillomavirus (HPV) infections. J Clin Virol 32 Suppl 1S43–51. [DOI] [PubMed] [Google Scholar]
- 22.Scherpenisse M, Mollers M, Schepp RM, Boot HJ, de Melker HE, et al.. (2012) Seroprevalence of seven high-risk HPV types in The Netherlands. Vaccine jn press. [DOI] [PubMed]
- 23.Fitzmaurice GM, Laird NM, Ware JH (2004) Applied Longitudinal Analysis. New York: Wiley-Interscience.
- 24. Xue X, Gange SJ, Zhong Y, Burk RD, Minkoff H, et al. (2010) Marginal and mixed-effects models in the analysis of human papillomavirus natural history data. Cancer Epidemiol Biomarkers Prev 19: 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carey V, Zeger SL, Diggle PJ (1993) Modelling multivariate binary data with alternating logistic regressions. Biometrika 80: 517–526. [Google Scholar]
- 26. Markowitz LE, Sternberg M, Dunne EF, McQuillan G, Unger ER (2009) Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health and Nutrition Examination Survey 2003–2004. J Infect Dis 200: 1059–1067. [DOI] [PubMed] [Google Scholar]
- 27. Nielsen A, Kjaer SK, Munk C, Iftner T (2008) Type-specific HPV infection and multiple HPV types: prevalence and risk factor profile in nearly 12,000 younger and older Danish women. Sex Transm Dis 35: 276–282. [DOI] [PubMed] [Google Scholar]
- 28. Nielson CM, Harris RB, Dunne EF, Abrahamsen M, Papenfuss MR, et al. (2007) Risk factors for anogenital human papillomavirus infection in men. J Infect Dis 196: 1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nyitray AG, da Silva RJ, Baggio ML, Lu B, Smith D, et al. (2011) The prevalence of genital HPV and factors associated with oncogenic HPV among men having sex with men and men having sex with women and men: the HIM study. Sex Transm Dis 38: 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shikary T, Bernstein DI, Jin Y, Zimet GD, Rosenthal SL, et al. (2009) Epidemiology and risk factors for human papillomavirus infection in a diverse sample of low-income young women. J Clin Virol 46: 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H (2004) Classification of papillomaviruses. Virology 324: 17–27. [DOI] [PubMed] [Google Scholar]