Abstract
Prolyl 4 hydroxylases (P4H) are iron- and 2-oxoglutamate-dependent dioxygenase enzymes and hypoxia-inducible transcription factor (HIF)-P4Hs play a critical role in the regulating oxygen homeostasis in the local tissues as well in the systemic circulation. Over a period of time, a number of prolyl hydroxylase inhibitors and activators have been developed. By employing the pharmacological tools and transgenic knock out animals, the critical role of these enzymes has been established in the pathophysiology of number of diseases including myocardial infarction, congestive heart failure, stroke, neurodegeneration, inflammatory disease, respiratory diseases, retinopathy and others. The present review discusses the different aspects of these enzymes including their pathophysiological role in disease development.
Keywords: Hypoxia inducible factor, Inflammation, Ischemia, Prolyl hydroxylase
INTRODUCTION
Prolyl hydroxylases belong to the family of iron- and 2-oxoglutamate-dependent dioxygenase enzyme and its several distinct isoforms have been identified. The hypoxia-inducible factor (HIF) prolyl hydroxylase enzymes, termed as prolyl hydroxylase domain (PHD), play an important role in oxygen regulation in the physiological network. Its three isoforms including PHD1, PHD2 and PHD3 have been identified in different tissues and organs [1].
Cells recognize and respond to hypoxia by accumulating the transcription factor hypoxia-inducible factor 1 (HIF-1), composed of an oxygen-sensitive inducible HIF-1α and a constitutive HIF-1β subunits. PHD enzyme are involved in the degradation of HIF-1α sub-unit by regulating its hydroxylation of 402/504 proline residues. Under hypoxic conditions, the lack of oxygen leads to stabilization of HIF-1α to form HIF heterodimer which is subsequently translocated to the nucleus. The binding of the HIF-heterodimer to specific DNA sequences within the nucleus, named hypoxia-responsive elements, triggers the trans-activation of target genes. The nature of target gene and type of expressed proteins may vary depending upon the type of tissues and disease conditions. The present review describes the different aspects of these PHD enzymes including the therapeutic implications of its modulators in different disease states.
TYPES OF PROLYL 4-HYDROXYLASES
Collagen Prolyl 4-Hydroxylases
Collagen prolyl 4-hydroxylases (C-P4Hs) are located within the lumen of the endoplasmic reticulum and catalyze the hydroxylation of prolines in -X-Pro-Gly- sequences in collagens and more than 15 other proteins that have collagen-like domains [2]. These C-P4Hs have a central role in the biosynthesis of collagens as 4-hydroxyproline residues are essential for the formation of the collagen triple helix.
1. Nematode collagen P4Hs
The nematode C. elegans has a large gene family of more than 150 members that encodes cuticle collagens [3]. The PHY-1/PHY-2/PDI2 mixed tetramer is the main P4H form in wild-type C. elegans, along with a small amount of PHY-1/PDI dimers, while PHY-2/PDI dimers have not been detected. The PHY-1, PHY-2, and PDI2 subunits of nematodes C-P4H are complementary to α(I), α(II), and β2 subunits of vertebrates C- P4H [4]. P4H α subunit from D. melanogaster has been cloned and characterized and consists of 516 amino acid residues and shows 34~35% and 31% sequence identities to the vertebrate α subunits and the C. elegans PHY-1, respectively [5]. C-P4Hs have also been cloned and characterized from the parasite filarial nematodes Onchocerca volvulus and Brugia malayi [6,7].
2. Plant and viral P4Hs
Although plants have no collagens, yet 4-hydroxyproline is found in many plant glycoproteins. Unlike the animal P4Hs, partially purified P4Hs from unicellular and multicellular green algae have been shown to be monomeric in nature [8]. In addition, higher plant P4Hs has also been noted to exist as monomers [9]. Partly purified plant P4Hs have been shown to effectively hydroxylate poly (L-proline) [10]. However, plant P4Hs have been unable to hydroxylate free prolines suggesting that a poly (L-proline) type II helix conformation is required in the substrate for hydroxylation [10]. None of the animal P4Hs use poly (L-proline) as a substrate, but it has been noted to be an effective competitive inhibitor of the former [11,12]. Arabidopsis thaliana genome has been noted to contain six genes encoding α-subunit like short polypeptides of 280~332 residues that show 21~27% amino acid sequence identity to the catalytic C-terminal regions of the human P4H α subunits [13].
4-Hydroxyproline has been reported to be absent in viral and bacterial proteins, but viral and bacterial genomes are also known to encode polypeptides with proline-rich repeats and even short collagen-like sequences [14-16]. A viral P4H has been cloned from an algal virus, Paramecium bursaria Chlorella virus-1 (PBCV-1). PBCV-1 P4H was found to hydroxylate prolines in both positions in the -Pro-Ala-Pro-Lysrepeats but those preceding the alanines are hydroxylated more efficiently [15].
3. Vertebrate collagen P4Hs
Collagen P4Hs, from all vertebrate sources so far has been studied, are composed of α2β2 tetramers in which the β subunit is identical to protein disulfide isomerase (PDI) [11,12]. C-P4H had long been assumed to be of one type only, with no isoenzymes, however, now several isoforms of catalytic α subunit have been identified in humans, mice, Caenorhabditis elegans and Drosophila melanogaster [5,17,18]. Both the α(I) and α(II) subunits associate with β subunit to form [α(I)]2 β2 or [α(II)]2 β2 tetramers, called the type I and type II enzymes, respectively [17,19]. Insect cell co-expression data strongly argue against the existence of mixed vertebrate α(I) α(II) β2 tetramers [17].
The human α(I) subunit consists of 517 amino acids and a signal peptide of 17 additional residues, whereas the α(II) subunit consists of 514 amino acids and a signal peptide of 21 residues. The overall amino acid sequence identity between the human α(I) and α(II) subunits is 64%, and highest degree of identity (80%), is observed within the catalytic C-terminal regions [17,20]. Type I C-P4H is the main form in the most cell types and tissues, while the type II enzyme has been shown to represent approximately 30% of the total P4H activity in cultured human WI-38 and HT-1080 cells and approximately 5~15% in various chick embryo tissues [17]. However, type II P4H represents at least 70% and 80% of the total P4H activity in cultured mouse chondrocytes and cartilage, respectively [21], and is thus likely to have a major role in the development of cartilage, cartilagenous bone and capillaries in vertebrates.
HYPOXIA INDUCIBLE FACTOR-PROLYL 4-HYDROXYLASE (HIF-P4H) SYSTEM
Discovery, types and distribution of HIF-1
Semenza and Wang discovered the HIF-1, a protein with DNA binding activity, by identifying the presence of hypoxia response element (HRE; 5'-RCGTG-3') in the erythropoietin gene [22]. The two isoforms or subunits of HIF-1 viz., HIF-1α (inducible) and HIF-1β (constitutive) have been identified that form a heterodimeric complex to regulate target gene in response to hypoxia [23]. HIF-1 β, also known as the aryl hydrocarbon nuclear translocator (ARNT), was originally identified as a binding partner of the aryl hydrocarbon receptor [24] and was known much earlier as compared to its binding partner HIF-1α [25]. The subsequent studies revealed the ubiquitous presence of HIF-1α in the human and the mouse tissues and described its general role in multiple physiological responses to hypoxia [26]. HIF-1α and HIF-1β proteins belong to the basic helix-loop-helix-Per-ARNT-Sim (bHLH-PAS) protein family [23] and bHLH and PAS motifs are essential for dimerization of these subunits and subsequent DNA binding [27]. HIF-2α (also termed as HIF-like factor and HIF-related factor) was identified and cloned in the lung, endothelium, and carotid body [28-30]. HIF-3α, mainly expressed in the Purkinje cells of the cerebellum and corneal epithelium, was subsequently discovered. A splice variant of HIF-3α does not possess endogenous transactivation activity and mainly functions as inhibitory PAS (IPAS) to prevent the binding of HIF-1α to its target DNA binding site [31].
Structurally, HIF-1α protein possesses N-terminal (NTAD) and C-terminal (C-TAD) as two transactivation domains in its C-terminal half part that are involved in activating the transcriptional process [32]. The C-TAD particularly interacts with CBP/p300 (acting as co-activators) to activate gene transcription [33]. The hydroxylation of an asparagine residue in the C-TAD inhibits the association of HIF-1α with co-activators CBP/p300 and thus inhibits its transcriptional activity [34,35]. HIF-1 α also possess an oxygen-dependent degradation domain (ODDD) that mediates oxygen-regulated stability [35].
HIF-P4H enzymes
HIF-P4Hs, a novel and distinct family of cytoplasmic prolyl 4-hydroxylases, plays a critical role in the regulation of the hypoxia-inducible transcription factor (HIF-1α) [36,37]. No overall amino acid sequence homology has been detected between the collagen-P4Hs and HIF-P4Hs, with the exception of critical residues in catalytic domain. It has been reported that human type I and type II collagen-P4Hs do not hydroxylate 19-residue synthetic peptide corresponding to the sequence around Pro564 in HIF-1α [38]. HIF-P4Hs have been identified in humans, C. elegans and D. melanogaster. Three human cytoplasmic HIF- P4H isoenzymes that hydroxylate HIF-1α have been identified. The three different human HIF-P4Hs shows a 42~59% sequence identity to one another but no distinct sequence similarity to the collagen P4Hs [37,39,40]. Like collagen-P4Hs these novel enzymes require Fe2+, 2-oxoglutarate, O2, and ascorbate as co-factor for catalytic activity. Although all three enzymes are widely expressed in many tissues, they exhibit tissue-specific overexpression. P4H2 are abundant in adipose tissue [41], P4H3 in the heart and placenta [41,42], and P4H1 in the testis [42]. The differences of the enzyme activity of P4Hs, sub-cellular localization and tissue distribution enables a graded or tissue-specific response to hypoxia.
Three isoforms of HIF-P4Hs have nearly identical Km value for O2, indicating that changes in the O2 are likely to have similar effects on the catalytic activities of all three isoenzymes. These Km values have been found to be slightly higher than the concentration of dissolved O2 in air and much higher than the Km for O2 of the type I collagen-P4H. The difference in Km values may correspond to different functions of the two classes of P4H. HIF-P4Hs have been to act as effective oxygen sensors, their Km values for O2 are close to atmospheric oxygen concentrations and even small decreases in O2 have been noted to influence their activities. However, type I collagen-P4H has been known to act in situations with very low O2 concentrations, which is in wounds and tissues of low vascularity. Therefore, the Km values for O2 of type I collagen-P4H is much lower.
Gene regulatory function and mechanism of HIF-1
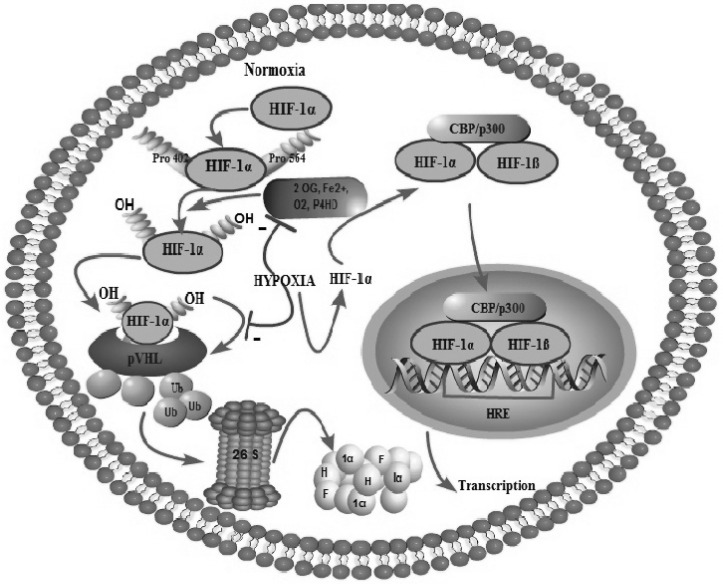
The hypoxia-inducible transcription factors (HIFs) has been noted to play a central role in the regulation of cellular and systemic O2 homeostasis [22]. The functional role of HIF-1α in regulating target gene expression in response to hypoxia is mainly under the control of oxygen sensing HIF-1α dioxygenases also termed as prolyl-4-hydroxylasedomain (P4HD) containing enzymes [23]. These enzymes are also termed as HIF-prolyl hydroxylase (HPH) and Egg-laying Nine (EGLN) and its three isoforms have been identified that include PHD1/HPH3/EGLN2, PHD2/HPH2/EGLN1, and PHD3/HPH1/EGLN3 [37]. These belong to the superfamily of non-heme iron (Fe2+)-containing 2-oxoglutarate (2-OG)-dependent oxygenases and these enzymes sense the cellular oxygen and use it as a co-substrate in hydroxylation reaction [43]. During normoxia (normal oxygen concentration), P4HD enzymes rapidly hydroxylate the proline residues i.e., proline 402 (Pro402) and 564 (Pro564) located within ODDD on the de novo synthesized cytoplasmic HIF-1α [44-46]. Once the two proline residues are converted to hydroxyproline, the hydroxylated HIF-1α fits accurately on the surface pocket of von Hippel-Lindau (pVHL) in a highly specific manner [47,48]. Before binding to HIF-1α, the pVHL associates with other proteins elongin C, elongin B, cullin-2, and Rbx1 to form the VCB-Cul2 E3 ligase complex [49]. The subsequent binding of HIF-1α to this multiprotein E3 complex causes polyubiquitination of HIF-1α, ultimately leading to its degradation by 26S proteasome [25].
However, hydroxylation of proline residues of HIF-1α does not occur during hypoxia due to inhibition of P4HD enzymes which in turn prevents its degradation. The persisting HIF-1α forms a stable hetero-dimer with HIF-β using bHLH and PAS motifs [38,49]. HIF-1α-HIF-β hetero-dimer translocates into the nucleus and binds to the HIF-responsive elements in a number of hypoxia-inducible genes, such as those for erythropoietin, vascular endothelial growth factor and glycolytic enzymes etc (Fig. 1) [38,49]. Interestingly, the gene for the α(I) subunit of human type I collagen P4H has been shown to be one of the hypoxia-inducible target genes of HIF-1α [50]. HIF-1α has been termed as 'master regulator of gene expression' during hypoxia and the consequences of HIF-1α activation are manifold [51]. HIF-1α has been known for its ability to stimulate glycolysis, angiogenesis and erythropoiesis at cellular level. HIF-1α has been reported to modulate the expression of many genes involved in cell survival including insulin-like growth factor-2 (IGF-2), transforming growth factor-α (TGF-α) and nitric oxide synthase-2 (NOS-2) [52-54]. HIF-1α also has the property to increase the glucose uptake and anaerobic metabolism through activation of glucose transporters GLUT-1 and GLUT-3, and glycolytic enzymes such as phosphoglycerate kinase-1 (PGK-1) and aldolase A (ALDA) [55]. These changes increase the tolerance of cells to hypoxic conditions. HIF-1α has also been shown to modulate activities at the tissue level by up-regulating genes involved in angiogenesis and blood flow, including genes for vascular endothelial growth factor (VEGF) and heme oxygenase-1 (HO-1) [56]. Finally, HIF-1α has also been noted to exert effects at the level of the whole organism, by inducing the erythropoietin (EPO) gene responsible for the generation of hemoglobin and consequently the oxygen-carrying capacity of blood.
Fig. 1.
The effects of O2 changes (hypoxia and normoxia) on the fate of HIF-1α and mechanism of HIF-induced transcriptional changes.
Stabilization of HIF-1α due to decreased functioning of P4H enzymes
The stability regulation and subsequent trans-activational functions of HIF-1α are mainly controlled by its post-translational modifications, such as hydroxylation, ubiquitination, acetylation, and phosphorylation [57]. It has been reported that mutation of both proline residues disrupts the interaction of HIF-1α with pVHL and increases its stability in the presence of normal oxygen levels, whereas mutation of either proline alone only partially stabilizes HIF-1α [45]. The half-life of HIF-1α may also be increased by inactivating the P4HDs by 2-OG analogs [38,40]. Fe2+ at the active site of the P4HDs is loosely bound by two histidine residues and one aspartic acid to form a 2-histidine-1-carboxylate coordination motif. The diminished availability of Fe2+ for the enzyme or substituting Fe2+ from the Fe2+ binding site inhibits the P4HD activity and stabilizes HIF-1α [46]. The genetic knockdown of P4HD2, not PHD1 or PHD3, by its specific small interfering RNA also stabilizes HIF-1α levels under normoxia [58].
PHYSIOLOGICAL ROLE OF HIF-P4H IN EMBRYONIC DEVELOPMENT
The large number of evidences has indicated that early placental environment is hypoxic [59]) and that HIF-1α is present in the placenta before 10 weeks [60]. HIF-1α has been shown to play a critical role during the development as evidenced by early embryonic lethality in HIF-1α knockout animals [61,62]. Murine embryos lacking HIF-1α display defects in cephalic vascularisation, cardiovascular functioning and neural tube formation. Tissue-specific knockouts have demonstrated HIF-1α is crucial for the survival of hypoxic chondrocytes and that modulation of chondrocyte proliferation, differentiation and growth arrest [63]. Hypoxia has been known to influence adipogenesis, via HIF-1α dependent pathway [64]. Finally, HIF-1α has been shown to play a role in brain development through the use of neural-specific HIF-1α knockout mice and in these mutated mice had reduced numbers of neural cells and suffered from hydrocephalus [65].
PROLYL 4-HYDROXYLASE MODULATORS
Prolyl 4-Hydroxylase inhibitors
Prolyl 4-hydroxylases inhibitors are divided into two following categories:
1. Peptide inhibitors
Poly (L-proline), with molecular weight 15,0000, is a highly effective competitive inhibitor of the vertebrate type I prolyl 4-hydroxylase with a Ki of 0.02µM. Peptides in which the proline residue is to be hydroxylated has been replaced by 5-oxaproline, a proline analog containing oxygen as a part of the five-membered ring, become suicide inactivators of prolyl 4-hydroxylases [66]. The most effective such peptide studied to date has the structure benzyloxycarbonyl-Phe-oxaproline-Gly-benzylester and inactivates type I prolyl 4-hydroxylase by 50% in 1 h at 0.8µM concentration.
2. Non-peptide inhibitors
Inhibitors of prolyl 4-hydroxylase have been characterized with respect to all its co- substrates. An interest has been focused especially on competitive inhibitors with respect to 2-oxoglutarate [67]. Pyridine 2,4-dicarboxylate and pyridine 2,5-dicarboxylate have functional groups that can interact with all the sub-sites of the 2-oxoglutarate binding site of P4H and inhibit the enzyme with Ki values of 2µM and 0.8µM, respectively [68,69]. These two compounds are only very weak inhibitors of 2-oxoglutarate dehydrogenase, an enzyme which differs from P4H in that its reaction mechanism does not involve any metal ion. N-oxalylglycine is another compound that interacts with 2-oxoglutarate binding site and inhibits the enzyme with Ki of 0.5~8µM [67,70].
3,4-Dihydroxybenzoate (EDHB) also has functional groups needed for binding with all the sub-sites of the 2-oxoglutarate binding site and inhibits the enzyme with a Ki of 5µM [71]. It differs from the pyridine derivatives in being competitive with respect to both 2-oxoglutarate and ascorbate, whereas the latter are uncompetitive with respect to ascorbate. L-Mimosine inhibits P4H by 50% at a 120µM concentration [72]. Three groups of compounds have also been identified that act as irreversible inactivators of P4H by a suicide mechanism. The first group consists of peptides containing 5-oxaproline, as discussed above. The second group includes coumalic acid, which acts as a 2-oxoglutarate analog but is only a weak inactivator, as it lacks a functional group needed to bind to the Fe2+ at a catalytic site [73]. The third group consists of the anthracyclines doxorubicin and daunorubicin, which inactivate P4H by 50% in 1 hr at a 60µM concentration [74]. Hydralazine, dealanylalahopcin and its analogues as human HIF-P4H inhibitors have also been reported [75,76].
Prolyl 4-Hydroxylases activators
α-Ketoglutarate (α-KG) is an activator of humans P4Hs and has been used employed as an important pharmacological tool. It is a key intermediate in the Krebs cycle, and is a rate-limiting cofactor of P4H. It also has a potent effect on increasing the proline pool during collagen production and it is involved in collagen metabolism through a variety of mechanisms including glutamine precursor [77].
THERAPEUTIC IMPLICATIONS OF HYPOXIA INDUCIBLE FACTOR-PROLYL 4-HYDROXYLASES (HIF-P4H) MODULATORS
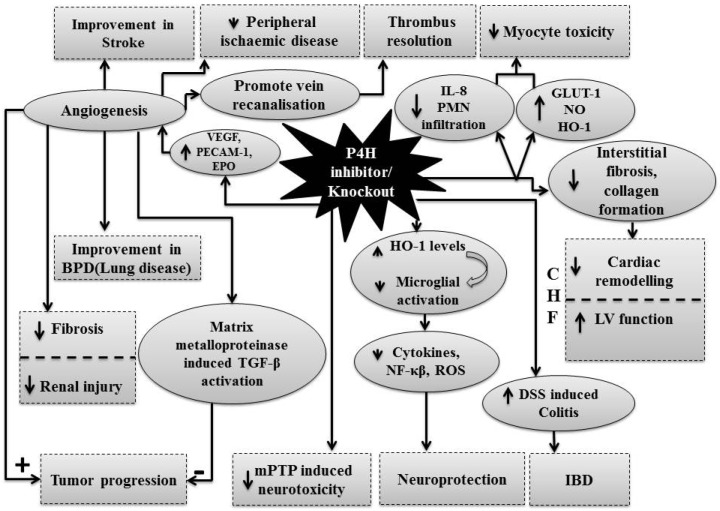
The pharmacological modulation of P4H enzymes have revealed the important role of HIF-P4H system in the pathophysiology of number of diseases and accordingly, these modulators have potential to attenuate these disease state (Fig. 2).
Fig. 2.
Therapeutic implications of HIF-P4H inhibitors in different diseases.
Myocardial infarction (MI)
Wright et al. [78] examined the consequences of activation of prolyl hydroxylase domain containing enzymes (PHD) in cultured neonatal myocytes using EDHB and dimethyloxalylglycine (DMOG) as P4H enzyme inhibitors. The treatment with these inhibitors and short duration hypoxia were found to induce the expression of different proteins including glucose transporter 1 (GLUT1), nitric oxide synthase (NOS) and heme oxygenase-1 (HO-1). In conjunction with these changes in gene expression and HIF-P4H inhibition, increased myocyte viability was observed in the face of metabolic inhibition with cyanide and 2-deoxyglucose. These results point to a key role for the HIF-P4H pathway in the phenotypic changes observed in a hypoxic myocyte and suggest a strategy to pharmacologically induce protection in heart. In another study, Ockaili et al. [79] reported that systemic administration of the potent HIF- P4H inhibitor, DMOG, prior to ischemia and reperfusion is associated with attenuated serum IL-8 levels, myocardial polymorphonuclear cells (PMN) infiltration and significantly reduced myocardial infarct size. It has also been demonstrated that silencing of HIF-P4H2 genes with small interfering RNAs (siRNAs) leads to cardioprotection against myocardial ischaemia.
Recently, it has been reported that the orally absorbed PHD inhibitor enzyme, GSK360A, modulates HIF-1α signaling to protect the failing heart following myocardial infarction [1]. The cardiomyocyte-specific knock out of PHD2 has been associated with protection from acute myocardial ischemic injury [80]. The earlier studies also demonstrated that the hearts of HIF-P4H-2 hypomorphic mice are more resistant to acute ischemia-reperfusion injury [81]. The conjugated linoleic acid-induced blockade of PHD1 and induction of HIF-2α in myocardium is associated with upregulation of pyruvate dehydrogenase lipoamide kinase isozyme 4 (PDK4) by activation of PPARα. It is described to reprogram the basal metabolism and protect against oxidative damage in myocardium in mice [82].
Congestive heart failure (CHF)
Left ventricular (LV) remodeling after myocardial infarction (MI) involves disruption of supporting structures, myocyte hypertrophy, and collagen deposition both at the site of infarction and at areas remote from the infarct. This process is adaptive initially but may progress to LV dilatation and dysfunction [83]. The final common pathway for collagen formation involves the activation of HIF-P4H, and accordingly, the inhibition of this enzyme after MI has been proposed to prevent interstitial fibrosis. Nwogu et al. [84] substantiated this proposal by showing the decreased fibrosis and collagen deposition in the presence of FG041, an orally available P4H inhibitor. In another study, P4H inhibitor was shown to improve the LV dysfunction and reduce the imbalance of matrix turnover and hypertrophy associated gene expression [85]. The conditional inactivation of PHD2 in mice sufficient to activate a subset of HIF target genes has been associated with premature mortality characterized by marked venous congestion and dilated cardiomyopathy [86].
Cancer
The activation of the HIF-1α system has been observed in numerous cancers due to induction of a hypoxic environment in the wake of rapidly dividing cancerous cells [87]. The different molecular mechanisms have been proposed for the up-regulation of the HIF-1α system in tumorogenesis [88] and it is apparent that its down-regulation may be an attractive target for cancer therapy. Bordoli and co-workers demonstrated that down-regulation of PHD2 leads to increased tumor growth in a hormone-dependent mammary carcinoma and the clinical samples of human breast cancer showed significantly shorter survival times of patients with low-level PHD2 over a period of 10 years. In the absence of PHD2, an increase in amphiregulin was reported and its levels were normalized after PHD2 reconstitution. Amphiregulin is an angiogenesis-related antibody and is regulated on the transcriptional level specifically by HIF-2, but not HIF-1. Accordingly, it has been proposed that PHD2/HIF-2/amphiregulin signaling is critical in regulating breast tumor progression and PHD2 is a potential tumor suppressor in breast cancer [89]. The studies have also indicated that PHDs function as tumor suppressors in human colorectal cancer (CRC). A recent clinical study has described that the low expression of PHD2 in CRC predicts poor survival (in early stage tumors) independent of HIF-1α [90]. Heindryckx and co-workers described the increased hepato-carcinogenesis and development of cholangiocarcinoma in PHD2 deficient mice in response to diethylnitrosamine, a carcinogenic agent. The growth of these tumors is limited as they rapidly outgrow their vascular supply and become hypoxic. Therefore, it is proposed that deficiency of PHD2-induced stabilization of HIF-1α promotes angiogenesis to accelerate the growth of these tumors in mutant mice suggesting PHD2 as good target for potential therapeutic intervention [91]. On the contrary, Klotzsche-von Ameln and co-workers demonstrated the anti-tumor effects due to inhibition of oxygen sensor PHD2 in tumor cells through matrix metalloproteinase- induced TGF-β activation pathway [92].
Respiratory diseases
Bronchopulmonary dysplasia (BPD), a disorder of preterm newborns, is characterized by impairment in lung microvasculature development and distal airway formation. It has been reported that pharmacological inhibition of HIF-P4H with FG-4045 is associated with an increased angiogenesis in lung due to augmented growth factors like vascular endothelial growth factor (VEGF) and platelet-endothelial cell adhesion molecule 1 (PECAM-1) [93]. The augmentation of lung angiogenesis with elevated HIF-1α, in primate model of BPD, suggests the potential usefulness of HIF-P4H inhibitors in the management of BPD in preterm newborns. The involvement of prolyl hydroxylases in the process of hypoxic pulmonary vascular remodeling in chronic obstructive pulmonary disease (COPD) via regulation of HIF-1α gene expression has been demonstrated. The levels of HIF-lα mRNA and protein levels in COPD group are shown to significantly higher as compared to normal subjects [94].
Peripheral vascular diseases
Peripheral vascular disease is one of ischemic disease and its treatment is still unsatisfactory. Using the rat sponge model for angiogenesis, Warnecke et al. [95] provided the evidences of increased vascularization with local injection of HIF prolyl hydroxylase inhibitor and projected it as novel target for the treatment of peripheral ischemic diseases. Within hours after a single application of HIF prolyl hydroxylase inhibitors such as L-Mim and S956711, the induction of cytoprotective genes including HO-1 was demonstrated suggesting their capability to mediate acute protection against hypoxic damage. Accordingly, it has been proposed that the topical application of HIF-P4H inhibitors could be clinically useful to augment vascularization in peripheral artery disease or to preserve organ transplant [95]. A recent study has shown that although the HIF1α levels in vein wall are not affected during thrombosis, yet its up-regulation in local vein wall promotes angiogenesis to recanalize the veins and resolve the thrombus [96].
Stroke
Stroke is an ischemic disease of the brain and its treatment like other ischemic diseases is still unsatisfactory. There have been studies suggesting that the HIF-1α prolyl hyroxylases are inhibited during ischemic preconditioning and pharmacological inhibitors of these enzymes may be viable targets for stroke therapy (Bergeron et al., 2000). Siddiq et al. [97] demonstrated the up-regulation of HIF dependent target genes like enolase, VEGF, P21waf1/cip1 and erythropoietin in the embryonic cortical neurons in vitro and even in adult rat brain in vivo as a consequence of HIF-P4H inhibition. The expression of these genes due to HIF-P4H inhibition has been shown to prevent oxidative stress-induced death in vitro and ischemic injury in vivo. Recently, the inhibition of prolyl hydroxylase and subsequent stabilization of HIF activity with oral administration of TM6008 has been shown to protect the neurons in the forebrain from focal ischemia by inhibiting apoptosis [98].
Neurodegenerative diseases
The HIF prolyl hydroxylase inhibitors are shown to prevent mitochondrial toxins- induced neuronal death, thus, implicating their therapeutic potential for Huntington's disease and Alzheimer's disease [99]. The pharmacological inhibition of prolyl hydroxylases by 3,4-dihydroxybenzoate (DHB) administration has been shown to produce protection against MPTP-induced neurotoxicity, animal model of Parkinson's disease [100]. The in vitro studies have demonstrated that DHB attenuates LPS-mediated induction of nitric oxide synthase and pro-inflammatory cytokines in murine BV2 microglial cells. Furthermore, it was also shown to reduce ROS production and activation of NFκB and MAPK pathways possibly due to up-regulation of HO-1 levels. The in vivo treatment with DHB also suppressed MPTP-induced microglial activation suggesting that its beneficial neuroprotective properties may be due to inhibition of microglial activation via HO-1 induction [101]. The earlier studies have shown HIF prolyl hydroxylase inhibition increases cell viability and potentiates dopamine release in dopaminergic cells and hence, prolyl hydroxylases may represent novel targets for therapeutic intervention in disorders characterized by dopamine homeostasis dysregulation like Parkinson's disease [102].
Kidney diseases
The intrinsic HIF activation is sub-maximal in acute kidney injury and the augmentation of HIF has been shown to ameliorate the acute disease manifestations of the kidney [103]. The activation of HIF has been shown to protect the kidney from acute ischemic cell death, while it promotes fibrosis in experimental models of chronic kidney diseases. Kapitsinou and co-workers demonstrated that the pharmacologic inhibition of HIF prolyl hydroxylation before acute kidney injury ameliorates fibrosis and prevents the development of anemia. Accordingly, the pre-ischemic targeting of the PHD/HIF pathway has been suggested as an effective therapeutic strategy for the prevention of chronic kidney disease resulting from acute injury [104].
Inflammation and related diseases
Using a pharmacologic approach leading to HIF-1α stabilization, and genetic manipulation of HIF-1α homologs in zebrafish, Elks and co-workers demonstrated the key role of HIF-1α in neutrophilic inflammation. Both approaches suggested that the activated HIF-1α delays resolution of inflammation and its activation leads to reduced neutrophil apoptosis and increased retention of neutrophils at the site of tissue injury, thereby delaying the resolution phase [105]. Using neutrophils from mice deficient in PHD3, the unique role for PHD3 in prolonging neutrophil survival during hypoxia (distinct from other hypoxia-associated changes in neutrophil function and metabolic activity) has been demonstrated. The reduced neutrophil survival due to PHD3 deficiency was associated with up-regulation of the proapoptotic mediator Siva1 and loss of its binding target Bcl-xL. An increased neutrophil apoptosis and clearance in PHD3-deficient mice has also been reported in in vivo models of inflammation (acute lung injury model and acute mouse model of colitis) [106].
The studies have shown that HIF prolyl hydroxylase inhibitors are protective in mouse models of inflammatory bowel disease (IBD). The PHD1(-/-), but not PHD2(+/-) or PHD3(-/-), mice are shown to less susceptible to the development of dextran sulphate sodium-induced colitis in terms of reduction in weight loss, disease activity, colon histology, neutrophil infiltration, and cytokine expression. Furthermore, the reduced susceptibility of PHD1(-/-) mice to colitis was associated with increased density of colonic epithelial cells due to decreased apoptosis and enhanced epithelial barrier function [107].
Oxygen-induced retinopathy
The major side effect of oxygen therapy for preterm infants is retinopathy which in turn leads to blindness in children. Duan and co-workers demonstrated that the exposure of 75% oxygen leads to degradation of retinal HIF-α proteins in the neonatal Smice expressing normal amounts of PHD2 and it was accompanied by massive loss of the retinal microvessels. PHD2 deficiency significantly stabilized HIF-1α, (HIF-2α to some extent), in neonatal retinal tissues, to protect retinal microvessels from oxygen-induced obliteration. Accordingly, it has been proposed that there is close association between PHD2-dependent HIF-α degradation and oxygen-induced retinal microvascular obliteration, and PHD2 may serve as promising therapeutic target to prevent oxygen-induced retinopathy [108].
Preconditioning
Three HIF-P4Hs (HIF-P4H1, HIF-P4H2, and HIF-P4H3) affect the proteosome-mediated degradation of HIF by catalyzing the hydroxylation of key proline residues in the HIF-1α subunit under normoxic conditions. When oxygen tension is reduced, HIF-P4H-mediated hydroxylation do not occur and HIF-1α accumulates in the nucleus leading to enhanced HIF-mediated gene transcription [109]. The involvement of HIF-P4Hs in preconditioning in the various tissues has been recently shown by many workers. Bernhardt et al. [110] has reported that hypoxic preconditioning or HIF-P4Hs inhibitors confer renal protection in ischemic acute renal failure [110]. Ran et al. [111] reported that hypoxic preconditioning shows neuroprotection against sustained ischemic insult and HIF-P4Hs inhibitors mimic this hypoxic preconditioning reflecting the involvement of HIF-P4Hs in hypoxic preconditioning. Prolyl 4-hydroxylase inhibition-induced preconditioning is also involved in myocardial protection. From our own laboratory, it was demonstrated that pharmacological preconditioning with EDHB (HIF-P4Hs inhibitor) mimicked the cardioprotective effects of remote renal preconditioning. Administration of α-KG (HIF-P4Hs activator) and diethyldithiocarbamic acid (NFκB inhibitor) were shown to abolish the cardioprotective effects of remote renal preconditioning and EDHB. Therefore, it was proposed that inhibition of HIF-P4H has a key role in remote renal preconditioning-induced cardioprotection and HIF- P4H inhibition triggers a transduction pathway involving NFκB activation [112].
In the model of cultured hippocampal slices, the application of anoxia preconditioning before oxygen-glucose deprivation is shown to prevent the neuronal damage and suppression of HIF-1α and HIF-3α mRNA expression. Furthermore, the effects of HIF prolyl-hydroxylase inhibition with 2,4-pyridinedicarboxylic acid diethyl ester pre-treatment were similar to anoxia preconditioning. It suggests that anoxia preconditioning increases anti-ischemic neuronal resistance which correlates with the changes of HIF-1α and HIF-3α expression [113]. It has been described that ischemic preconditioning produces reinforcing effect on HIF-1 accumulation during the subsequent hypoxic injury and HIF-1 induction during hypoxic preconditioning produces reinforcing effect to accumulate HIF-1 and develops a tolerance against a subsequent hypoxic neuronal injury [114].
Others
The loss of the oxygen sensor PHD1 is suggested to make the skeletal muscles and liver more resistant to ischemia reperfusion injury, thereby projecting it as critical target in ischemia reperfusion-induced injury in these organs [115]. Muz and co-workers described that PHD-2 is the major isoform that regulates HIF-α levels in RA fibroblast-like synoviocytes (RA FLS) suggesting the major importance of this enzyme in hypoxia- and angiogenesis- dependent inflammatory diseases such as RA [116].
CONCLUSION
The preclinical studies have projected prolyl 4 hydroxylase as critical target in the pathophysiology of number of diseases. Therefore, its pharmacological modulators may also be clinically effective in management of diseases.
ACKNOWLEDGEMENTS
The authors are grateful to Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala, India for supporting this study and providing technical facilities for the work.
ABBREVIATIONS
- HIF
hypoxia-inducible factor
- PHD
prolyl hydroxylase domain
- C-P4Hs
collagen prolyl 4-hydroxylases
- PDI
protein disulfide isomerase
- HRE
hypoxia response element
- ARNT
aryl hydrocarbon nuclear translocator
- ODDD
oxygen-dependent degradation domain
- 2-OG
2-oxoglutarate
- pVHL
von Hippel-Lindau
- IGF-2
insulin-like growth factor-2
- TGF-α
transforming growth factor-α
- NOS-2
nitric oxide synthase-2
- PGK-1
phosphoglycerate kinase-1
- ALDA
aldolase A
- VEGF
vascular endothelial growth factor
- HO-1
heme oxygenase-1
- EPO
erythropoietin
- EDHB
dihydroxybenzoate
- DMOG
dimethyloxalylglycine
- PMN
polymorphonuclear cells
- LV
left ventricular
- MI
myocardial infarction
- BPD
bronchopulmonary dysplasia
- PECAM-1
platelet-endothelial cell adhesion molecule 1
- COPD
chronic obstructive pulmonary disease
- IBD
inflammatory bowel disease
- FLS
fibroblast-like synoviocytes
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
References
- 1.Bao W, Qin P, Needle S, Erickson-Miller CL, Duffy KJ, Ariazi JL, Zhao S, Olzinski AR, Behm DJ, Pipes GC, Jucker BM, Hu E, Lepore JJ, Willette RN. Chronic inhibition of hypoxia-inducible factor prolyl 4-hydroxylase improves ventricular performance, remodeling, and vascularity after myocardial infarction in the rat. J Cardiovasc Pharmacol. 2010;56:147–155. doi: 10.1097/FJC.0b013e3181e2bfef. [DOI] [PubMed] [Google Scholar]
- 2.Myllyharju J, Kivirikko KI. Collagens and collagen-related diseases. Ann Med. 2001;33:7–21. doi: 10.3109/07853890109002055. [DOI] [PubMed] [Google Scholar]
- 3.Johnstone IL. Cuticle collagen genes. Expression in Caenorhabditis elegans. Trends Genet. 2000;16:21–27. doi: 10.1016/s0168-9525(99)01857-0. [DOI] [PubMed] [Google Scholar]
- 4.Myllyharju J, Kukkola L, Winter AD, Page AP. The exoskeleton collagens in Caenorhabditis elegans are modified by prolyl 4-hydroxylases with unique combinations of subunits. J Biol Chem. 2002;277:29187–29196. doi: 10.1074/jbc.M203824200. [DOI] [PubMed] [Google Scholar]
- 5.Annunen P, Koivunen P, Kivirikko KI. Cloning of the alpha subunit of prolyl 4-hydroxylase from Drosophila and expression and characterization of the corresponding enzyme tetramer with some unique properties. J Biol Chem. 1999;274:6790–6796. doi: 10.1074/jbc.274.10.6790. [DOI] [PubMed] [Google Scholar]
- 6.Merriweather A, Guenzler V, Brenner M, Unnasch TR. Characterization and expression of enzymatically active recombinant filarial prolyl 4-hydroxylase. Mol Biochem Parasitol. 2001;116:185–197. doi: 10.1016/s0166-6851(01)00317-6. [DOI] [PubMed] [Google Scholar]
- 7.Winter AD, Myllyharju J, Page AP. A hypodermally expressed prolyl 4-hydroxylase from the filarial nematode Brugia malayi is soluble and active in the absence of protein disulfide isomerase. J Biol Chem. 2003;278:2554–2562. doi: 10.1074/jbc.M210381200. [DOI] [PubMed] [Google Scholar]
- 8.Kaska DD, Myllylä R, Günzler V, Gibor A, Kivirikko KI. Prolyl 4-hydroxylase from Volvox carteri. A low-Mr enzyme antigenically related to the alpha subunit of the vertebrate enzyme. Biochem J. 1988;256:257–263. doi: 10.1042/bj2560257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wojtaszek P, Smith CG, Bolwell GP. Ultrastructural localisation and further biochemical characterisation of prolyl 4-hydroxylase from Phaseolus vulgaris: comparative analysis. Int J Biochem Cell Biol. 1999;31:463–477. doi: 10.1016/s1357-2725(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 10.Kivirikko KI, Myllyla R, Pihlajaniemi T. Harding JJ, Crabbe JC. Post-Translational Modifications of Proteins. Boca Raton, FL: CRC Press; 1992. Hydroxylation of proline and lysine residues in collagens and other animal and plant proteins; pp. 1–51. [Google Scholar]
- 11.Kivirikko KI, Myllyharju J. Prolyl 4-hydroxylases and their protein disulfide isomerase subunit. Matrix Biol. 1998;16:357–368. doi: 10.1016/s0945-053x(98)90009-9. [DOI] [PubMed] [Google Scholar]
- 12.Kivirikko KI, Pihlajaniemi T. Collagen hydroxylases and the protein disulfide isomerase subunit of prolyl 4-hydroxylases. Adv Enzymol Relat Areas Mol Biol. 1998;72:325–398. doi: 10.1002/9780470123188.ch9. [DOI] [PubMed] [Google Scholar]
- 13.Hieta R, Myllyharju J. Cloning and characterization of a low molecular weight prolyl 4-hydroxylase from Arabidopsis thaliana. Effective hydroxylation of proline-rich, collagen-like, and hypoxia-inducible transcription factor alpha-like peptides. J Biol Chem. 2002;277:23965–23971. doi: 10.1074/jbc.M201865200. [DOI] [PubMed] [Google Scholar]
- 14.Smith MC, Burns N, Sayers JR, Sorrell JA, Casjens SR, Hendrix RW. Bacteriophage collagen. Science. 1998;279:1834. doi: 10.1126/science.279.5358.1831g. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson M, Myllyharju J, Tu H, Hellman M, Kivirikko KI. Evidence for 4-hydroxyproline in viral proteins. Characterization of a viral prolyl 4-hydroxylase and its peptide substrates. J Biol Chem. 1999;274:22131–22134. doi: 10.1074/jbc.274.32.22131. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Keene DR, Bujnicki JM, Höök M, Lukomski S. Streptococcal Scl1 and Scl2 proteins form collagen-like triple helices. J Biol Chem. 2002;277:27312–27318. doi: 10.1074/jbc.M201163200. [DOI] [PubMed] [Google Scholar]
- 17.Annunen P, Helaakoski T, Myllyharju J, Veijola J, Pihlajaniemi T, Kivirikko KI. Cloning of the human prolyl 4-hydroxylase alpha subunit isoform alpha(II) and characterization of the type II enzyme tetramer. The alpha(I) and alpha(II) subunits do not form a mixed alpha(I)alpha(II)beta2 tetramer. J Biol Chem. 1997;272:17342–17348. doi: 10.1074/jbc.272.28.17342. [DOI] [PubMed] [Google Scholar]
- 18.Friedman L, Higgin JJ, Moulder G, Barstead R, Raines RT, Kimble J. Prolyl 4-hydroxylase is required for viability and morphogenesis in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2000;97:4736–4741. doi: 10.1073/pnas.97.9.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helaakoski T, Annunen P, Vuori K, MacNeil IA, Pihlajaniemi T, Kivirikko KI. Cloning, baculovirus expression, and characterization of a second mouse prolyl 4-hydroxylase alpha-subunit isoform: formation of an α2β2 tetramer with the protein disulfide-isomerase/β subunit. Proc Natl Acad Sci U S A. 1995;92:4427–4431. doi: 10.1073/pnas.92.10.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helaakoski T, Vuori K, Myllylä R, Kivirikko KI, Pihlajaniemi T. Molecular cloning of the alpha-subunit of human prolyl 4-hydroxylase: the complete cDNA-derived amino acid sequence and evidence for alternative splicing of RNA transcripts. Proc Natl Acad Sci U S A. 1989;86:4392–4396. doi: 10.1073/pnas.86.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Annunen P, Autio-Harmainen H, Kivirikko KI. The novel type II prolyl 4-hydroxylase is the main enzyme form in chondrocytes and capillary endothelial cells, whereas the type I enzyme predominates in most cells. J Biol Chem. 1998;273:5989–5992. doi: 10.1074/jbc.273.11.5989. [DOI] [PubMed] [Google Scholar]
- 22.Semenza GL, Koury ST, Nejfelt MK, Gearhart JD, Antonarakis SE. Cell-type-specific and hypoxia-inducible expression of the human erythropoietin gene in transgenic mice. Proc Natl Acad Sci U S A. 1991;88:8725–8729. doi: 10.1073/pnas.88.19.8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reyes H, Reisz-Porszasz S, Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992;256:1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- 25.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 26.Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- 27.Crews ST. Control of cell lineage-specific development and transcription by bHLH-PAS proteins. Genes Dev. 1998;12:607–620. doi: 10.1101/gad.12.5.607. [DOI] [PubMed] [Google Scholar]
- 28.Ema M, Hirota K, Mimura J, Abe H, Yodoi J, Sogawa K, Poellinger L, Fujii-Kuriyama Y. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 1999;18:1905–1914. doi: 10.1093/emboj/18.7.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 30.Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 1998;12:3320–3324. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makino Y, Cao R, Svensson K, Bertilsson G, Asman M, Tanaka H, Cao Y, Berkenstam A, Poellinger L. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature. 2001;414:550–554. doi: 10.1038/35107085. [DOI] [PubMed] [Google Scholar]
- 32.Ruas JL, Poellinger L, Pereira T. Functional analysis of hypoxia-inducible factor-1 alpha-mediated transactivation. Identification of amino acid residues critical for transcriptional activation and/or interaction with CREB-binding protein. J Biol Chem. 2002;277:38723–38730. doi: 10.1074/jbc.M205051200. [DOI] [PubMed] [Google Scholar]
- 33.Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 34.Kamura T, Sato S, Iwai K, Czyzyk-Krzeska M, Conaway RC, Conaway JW. Activation of HIF1alpha ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc Natl Acad Sci U S A. 2000;97:10430–10435. doi: 10.1073/pnas.190332597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pugh CW, O'Rourke JF, Nagao M, Gleadle JM, Ratcliffe PJ. Activation of hypoxia-inducible factor-1; definition of regulatory domains within the alpha subunit. J Biol Chem. 1997;272:11205–11214. doi: 10.1074/jbc.272.17.11205. [DOI] [PubMed] [Google Scholar]
- 36.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 37.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 38.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 39.Bruick RK, McKnight SL. A conserved family of prolyl 4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 40.Ivan M, Haberberger T, Gervasi DC, Michelson KS, Günzler V, Kondo K, Yang H, Sorokina I, Conaway RC, Conaway JW, Kaelin WG., Jr Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci U S A. 2002;99:13459–13464. doi: 10.1073/pnas.192342099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oehme F, Ellinghaus P, Kolkhof P, Smith TJ, Ramakrishnan S, Hütter J, Schramm M, Flamme I. Overexpression of PH-4, a novel putative proline 4-hydroxylase, modulates activity of hypoxia-inducible transcription factors. Biochem Biophys Res Commun. 2002;296:343–349. doi: 10.1016/s0006-291x(02)00862-8. [DOI] [PubMed] [Google Scholar]
- 42.Lieb ME, Menzies K, Moschella MC, Ni R, Taubman MB. Mammalian EGLN genes have distinct patterns of mRNA expression and regulation. Biochem Cell Biol. 2002;80:421–426. doi: 10.1139/o02-115. [DOI] [PubMed] [Google Scholar]
- 43.Fraisl P, Aragonés J, Carmeliet P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat Rev Drug Discov. 2009;8:139–152. doi: 10.1038/nrd2761. [DOI] [PubMed] [Google Scholar]
- 44.Srinivas V, Zhang LP, Zhu XH, Caro J. Characterization of an oxygen/redox-dependent degradation domain of hypoxia-inducible factor alpha (HIF-alpha) proteins. Biochem Biophys Res Commun. 1999;260:557–561. doi: 10.1006/bbrc.1999.0878. [DOI] [PubMed] [Google Scholar]
- 45.Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Masson N, Ratcliffe PJ. HIF prolyl and asparaginyl hydroxylases in the biological response to intracellular O(2) levels. J Cell Sci. 2003;116:3041–3049. doi: 10.1242/jcs.00655. [DOI] [PubMed] [Google Scholar]
- 47.Hon WC, Wilson MI, Harlos K, Claridge TD, Schofield CJ, Pugh CW, Maxwell PH, Ratcliffe PJ, Stuart DI, Jones EY. Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL. Nature. 2002;417:975–978. doi: 10.1038/nature00767. [DOI] [PubMed] [Google Scholar]
- 48.Min JH, Yang H, Ivan M, Gertler F, Kaelin WG, Jr, Pavletich NP. Structure of an HIF-1alpha-pVHL complex: hydroxyproline recognition in signaling. Science. 2002;296:1886–1889. doi: 10.1126/science.1073440. [DOI] [PubMed] [Google Scholar]
- 49.Ivan M, Kaelin WG., Jr The von Hippel-Lindau tumor suppressor protein. Curr Opin Genet Dev. 2001;11:27–34. doi: 10.1016/s0959-437x(00)00152-0. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi Y, Takahashi S, Shiga Y, Yoshimi T, Miura T. Hypoxic induction of prolyl 4-hydroxylase alpha (I) in cultured cells. J Biol Chem. 2000;275:14139–14146. doi: 10.1074/jbc.275.19.14139. [DOI] [PubMed] [Google Scholar]
- 51.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 52.Melillo G, Musso T, Sica A, Taylor LS, Cox GW, Varesio L. A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J Exp Med. 1995;182:1683–1693. doi: 10.1084/jem.182.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999;59:3915–3918. [PubMed] [Google Scholar]
- 54.Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi P, Semenza GL. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003;63:1138–1143. [PubMed] [Google Scholar]
- 55.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 56.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brahimi-Horn C, Mazure N, Pouysségur J. Signalling via the hypoxia-inducible factor-1alpha requires multiple posttranslational modifications. Cell Signal. 2005;17:1–9. doi: 10.1016/j.cellsig.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 58.Berra E, Benizri E, Ginouvès A, Volmat V, Roux D, Pouysségur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003;22:4082–4090. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jaffe R, Jauniaux E, Hustin J. Maternal circulation in the first-trimester human placenta--myth or reality? Am J Obstet Gynecol. 1997;176:695–705. doi: 10.1016/s0002-9378(97)70572-6. [DOI] [PubMed] [Google Scholar]
- 60.Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3) J Clin Invest. 2000;105:577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HIF-1alpha-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Dev Biol. 1999;209:254–267. doi: 10.1006/dbio.1999.9253. [DOI] [PubMed] [Google Scholar]
- 63.Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, Johnson RS. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15:2865–2876. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2:331–341. doi: 10.1016/s1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 65.Tomita S, Ueno M, Sakamoto M, Kitahama Y, Ueki M, Maekawa N, Sakamoto H, Gassmann M, Kageyama R, Ueda N, Gonzalez FJ, Takahama Y. Defective brain development in mice lacking the Hif-1alpha gene in neural cells. Mol Cell Biol. 2003;23:6739–6749. doi: 10.1128/MCB.23.19.6739-6749.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Günzler V, Brocks D, Henke S, Myllylä R, Geiger R, Kivirikko KI. Syncatalytic inactivation of prolyl 4-hydroxylase by synthetic peptides containing the unphysiologic amino acid 5-oxaproline. J Biol Chem. 1988;263:19498–19504. [PubMed] [Google Scholar]
- 67.Baader E, Tschank G, Baringhaus KH, Burghard H, Günzler V. Inhibition of prolyl 4-hydroxylase by oxalyl amino acid derivatives in vitro, in isolated microsomes and in embryonic chicken tissues. Biochem J. 1994;300:525–530. doi: 10.1042/bj3000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hanauske-Abel HM, Günzler V. A stereochemical concept for the catalytic mechanism of prolylhydroxylase: applicability to classification and design of inhibitors. J Theor Biol. 1982;94:421–455. doi: 10.1016/0022-5193(82)90320-4. [DOI] [PubMed] [Google Scholar]
- 69.Majamaa K, Hanauske-Abel HM, Günzler V, Kivirikko KI. The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for 2-oxoglutarate decarboxylation in a ligand reaction at the enzyme-bound ferrous ion. Eur J Biochem. 1984;138:239–245. doi: 10.1111/j.1432-1033.1984.tb07907.x. [DOI] [PubMed] [Google Scholar]
- 70.Cunliffe CJ, Franklin TJ, Hales NJ, Hill GB. Novel inhibitors of prolyl 4-hydroxylase 3 Inhibition by the substrate analogue N-oxaloglycine and its derivatives. J Med Chem. 1992;35:2652–2658. doi: 10.1021/jm00092a016. [DOI] [PubMed] [Google Scholar]
- 71.Majamaa K, Günzler V, Hanauske-Abel HM, Myllylä R, Kivirikko KI. Partial identity of the 2-oxoglutarate and ascorbate binding sites of prolyl 4-hydroxylase. J Biol Chem. 1986;261:7819–7823. [PubMed] [Google Scholar]
- 72.McCaffrey TA, Pomerantz KB, Sanborn TA, Spokojny AM, Du B, Park MH, Folk JE, Lamberg A, Kivirikko KI, Falcone DJ, et al. Specific inhibition of eIF-5A and collagen hydroxylation by a single agent. Antiproliferative and fibrosuppressive effects on smooth muscle cells from human coronary arteries. J Clin Invest. 1995;95:446–455. doi: 10.1172/JCI117684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Günzler V, Hanauske-Abel HM, Myllylä R, Mohr J, Kivirikko KI. Time-dependent inactivation of chick-embryo prolyl 4-hydroxylase by coumalic acid. Evidence for a syncatalytic mechanism. Biochem J. 1987;242:163–169. doi: 10.1042/bj2420163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Günzler V, Hanauske-Abel HM, Myllylä R, Kaska DD, Hanauske A, Kivirikko KI. Syncatalytic inactivation of prolyl 4-hydroxylase by anthracyclines. Biochem J. 1988;251:365–372. doi: 10.1042/bj2510365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schlemminger I, Mole DR, McNeill LA, Dhanda A, Hewitson KS, Tian YM, Ratcliffe PJ, Pugh CW, Schofield CJ. Analogues of dealanylalahopcin are inhibitors of human HIF prolyl hydroxylases. Bioorg Med Chem Lett. 2003;13:1451–1454. doi: 10.1016/s0960-894x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 76.Knowles HJ, Tian YM, Mole DR, Harris AL. Novel mechanism of action for hydralazine: induction of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by inhibition of prolyl hydroxylases. Circ Res. 2004;95:162–169. doi: 10.1161/01.RES.0000134924.89412.70. [DOI] [PubMed] [Google Scholar]
- 77.Son ED, Choi GH, Kim H, Lee B, Chang IS, Hwang JS. Alpha-ketoglutarate stimulates procollagen production in cultured human dermal fibroblasts, and decreases UVB-induced wrinkle formation following topical application on the dorsal skin of hairless mice. Biol Pharm Bull. 2007;30:1395–1399. doi: 10.1248/bpb.30.1395. [DOI] [PubMed] [Google Scholar]
- 78.Wright G, Higgin JJ, Raines RT, Steenbergen C, Murphy E. Activation of the prolyl hydroxylase oxygen-sensor results in induction of GLUT1, heme oxygenase-1, and nitric-oxide synthase proteins and confers protection from metabolic inhibition to cardiomyocytes. J Biol Chem. 2003;278:20235–20239. doi: 10.1074/jbc.M301391200. [DOI] [PubMed] [Google Scholar]
- 79.Ockaili R, Natarajan R, Salloum F, Fisher BJ, Jones D, Fowler AA, 3rd, Kukreja RC. HIF-1 activation attenuates postischemic myocardial injury: role for heme oxygenase-1 in modulating microvascular chemokine generation. Am J Physiol Heart Circ Physiol. 2005;289:H542–H548. doi: 10.1152/ajpheart.00089.2005. [DOI] [PubMed] [Google Scholar]
- 80.Hölscher M, Silter M, Krull S, von Ahlen M, Hesse A, Schwartz P, Wielockx B, Breier G, Katschinski DM, Zieseniss A. Cardiomyocyte-specific prolyl-4-hydroxylase domain 2 knock out protects from acute myocardial ischemic injury. J Biol Chem. 2011;286:11185–11194. doi: 10.1074/jbc.M110.186809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hyvärinen J, Hassinen IE, Sormunen R, Mäki JM, Kivirikko KI, Koivunen P, Myllyharju J. Hearts of hypoxia-inducible factor prolyl 4-hydroxylase-2 hypomorphic mice show protection against acute ischemia-reperfusion injury. J Biol Chem. 2010;285:13646–13657. doi: 10.1074/jbc.M109.084855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang J, Li D. Effect of conjugated linoleic acid on inhibition of prolyl hydroxylase 1 in hearts of mice. Lipids Health Dis. 2012;11:22. doi: 10.1186/1476-511X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Francis GS, McDonald KM, Cohn JN. Neurohumoral activation in preclinical heart failure. Remodeling and the potential for intervention. Circulation. 1993;87(5 Suppl):IV90–IV96. [PubMed] [Google Scholar]
- 84.Nwogu JI, Geenen D, Bean M, Brenner MC, Huang X, Buttrick PM. Inhibition of collagen synthesis with prolyl 4-hydroxylase inhibitor improves left ventricular function and alters the pattern of left ventricular dilatation after myocardial infarction. Circulation. 2001;104:2216–2221. doi: 10.1161/hc4301.097193. [DOI] [PubMed] [Google Scholar]
- 85.Fielitz J, Philipp S, Herda LR, Schuch E, Pilz B, Schubert C, Günzler V, Willenbrock R, Regitz-Zagrosek V. Inhibition of prolyl 4-hydroxylase prevents left ventricular remodelling in rats with thoracic aortic banding. Eur J Heart Fail. 2007;9:336–342. doi: 10.1016/j.ejheart.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 86.Minamishima YA, Moslehi J, Bardeesy N, Cullen D, Bronson RT, Kaelin WG., Jr Somatic inactivation of the PHD2 prolyl hydroxylase causes polycythemia and congestive heart failure. Blood. 2008;111:3236–3244. doi: 10.1182/blood-2007-10-117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hewitson KS, Schofield CJ. The HIF pathway as a therapeutic target. Drug Discov Today. 2004;9:704–711. doi: 10.1016/S1359-6446(04)03202-7. [DOI] [PubMed] [Google Scholar]
- 88.Huang LE, Bunn HF. Hypoxia-inducible factor and its biomedical relevance. J Biol Chem. 2003;278:19575–19578. doi: 10.1074/jbc.R200030200. [DOI] [PubMed] [Google Scholar]
- 89.Bordoli MR, Stiehl DP, Borsig L, Kristiansen G, Hausladen S, Schraml P, Wenger RH, Camenisch G. Prolyl-4-hydroxylase PHD2- and hypoxia-inducible factor 2-dependent regulation of amphiregulin contributes to breast tumorigenesis. Oncogene. 2011;30:548–560. doi: 10.1038/onc.2010.433. [DOI] [PubMed] [Google Scholar]
- 90.Xie G, Zheng L, Ou J, Huang H, He J, Li J, Pan F, Liang H. Low expression of prolyl hydroxylase 2 is associated with tumor grade and poor prognosis in patients with colorectal cancer. Exp Biol Med (Maywood) 2012;237:860–866. doi: 10.1258/ebm.2012.011331. [DOI] [PubMed] [Google Scholar]
- 91.Heindryckx F, Kuchnio A, Casteleyn C, Coulon S, Olievier K, Colle I, Geerts A, Libbrecht L, Carmeliet P, Van Vlierberghe H. Effect of prolyl hydroxylase domain-2 haplodeficiency on the hepatocarcinogenesis in mice. J Hepatol. 2012;57:61–68. doi: 10.1016/j.jhep.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 92.Klotzsche-von Ameln A, Muschter A, Heimesaat MM, Breier G, Wielockx B. HIF prolyl hydroxylase-2 inhibition diminishes tumor growth through matrix metalloproteinase-induced TGFβ activation. Cancer Biol Ther. 2012;13:216–223. doi: 10.4161/cbt.13.4.18830. [DOI] [PubMed] [Google Scholar]
- 93.Asikainen TM, Waleh NS, Schneider BK, Clyman RI, White CW. Enhancement of angiogenic effectors through hypoxia-inducible factor in preterm primate lung in vivo. Am J Physiol Lung Cell Mol Physiol. 2006;291:L588–L595. doi: 10.1152/ajplung.00098.2006. [DOI] [PubMed] [Google Scholar]
- 94.Chen YR, Dai AG, Hu RC, Kong CC. The expression of hypoxia-inducible factor-1alpha and its hydroxylases in pulmonary arteries of patient with chronic obstructive pulmonary disease. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2012;28:234–238. [PubMed] [Google Scholar]
- 95.Warnecke C, Griethe W, Weidemann A, Jürgensen JS, Willam C, Bachmann S, Ivashchenko Y, Wagner I, Frei U, Wiesener M, Eckardt KU. Activation of the hypoxia-inducible factor-pathway and stimulation of angiogenesis by application of prolyl hydroxylase inhibitors. FASEB J. 2003;17:1186–1188. doi: 10.1096/fj.02-1062fje. [DOI] [PubMed] [Google Scholar]
- 96.Evans CE, Humphries J, Waltham M, Saha P, Mattock K, Patel A, Ahmad A, Wadoodi A, Modarai B, Burnand K, Smith A. Upregulation of hypoxia-inducible factor 1 alpha in local vein wall is associated with enhanced venous thrombus resolution. Thromb Res. 2011;128:346–351. doi: 10.1016/j.thromres.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Siddiq A, Ayoub IA, Chavez JC, Aminova L, Shah S, LaManna JC, Patton SM, Connor JR, Cherny RA, Volitakis I, Bush AI, Langsetmo I, Seeley T, Gunzler V, Ratan RR. Hypoxia-inducible factor prolyl 4-hydroxylase inhibition. A target for neuroprotection in the central nervous system. J Biol Chem. 2005;280:41732–41743. doi: 10.1074/jbc.M504963200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takizawa S, Nagata E, Luo HR. Novel neuroprotective agents: a HIF activator and an Akt activator. Rinsho Shinkeigaku. 2012;52:911–912. doi: 10.5692/clinicalneurol.52.911. [DOI] [PubMed] [Google Scholar]
- 99.Niatsetskaya Z, Basso M, Speer RE, McConoughey SJ, Coppola G, Ma TC, Ratan RR. HIF prolyl hydroxylase inhibitors prevent neuronal death induced by mitochondrial toxins: therapeutic implications for Huntington's disease and Alzheimer's disease. Antioxid Redox Signal. 2010;12:435–443. doi: 10.1089/ars.2009.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee DW, Rajagopalan S, Siddiq A, Gwiazda R, Yang L, Beal MF, Ratan RR, Andersen JK. Inhibition of prolyl hydroxylase protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity: model for the potential involvement of the hypoxia-inducible factor pathway in Parkinson disease. J Biol Chem. 2009;284:29065–29076. doi: 10.1074/jbc.M109.000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chinta SJ, Rajagopalan S, Ganesan A, Andersen JK. A possible novel anti-inflammatory mechanism for the pharmacological prolyl hydroxylase inhibitor 3,4-dihydroxybenzoate: implications for use as a therapeutic for Parkinson's disease. Parkinsons Dis. 2012;2012:364684. doi: 10.1155/2012/364684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Johansen JL, Sager TN, Lotharius J, Witten L, Mørk A, Egebjerg J, Thirstrup K. HIF prolyl hydroxylase inhibition increases cell viability and potentiates dopamine release in dopaminergic cells. J Neurochem. 2010;115:209–219. doi: 10.1111/j.1471-4159.2010.06917.x. [DOI] [PubMed] [Google Scholar]
- 103.Nangaku M, Rosenberger C, Heyman SN, Eckardt KU. HIF regulation in kidney disease. Clin Exp Pharmacol Physiol. 2012 [Epub ahead of print] [Google Scholar]
- 104.Kapitsinou PP, Jaffe J, Michael M, Swan CE, Duffy KJ, Erickson-Miller CL, Haase VH. Preischemic targeting of HIF prolyl hydroxylation inhibits fibrosis associated with acute kidney injury. Am J Physiol Renal Physiol. 2012;302:F1172–F1179. doi: 10.1152/ajprenal.00667.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Elks PM, van Eeden FJ, Dixon G, Wang X, Reyes-Aldasoro CC, Ingham PW, Whyte MK, Walmsley SR, Renshaw SA. Activation of hypoxia-inducible factor-1α (Hif-1α) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood. 2011;118:712–722. doi: 10.1182/blood-2010-12-324186. [DOI] [PubMed] [Google Scholar]
- 106.Walmsley SR, Chilvers ER, Thompson AA, Vaughan K, Marriott HM, Parker LC, Shaw G, Parmar S, Schneider M, Sabroe I, Dockrell DH, Milo M, Taylor CT, Johnson RS, Pugh CW, Ratcliffe PJ, Maxwell PH, Carmeliet P, Whyte MK. Prolyl hydroxylase 3 (PHD3) is essential for hypoxic regulation of neutrophilic inflammation in humans and mice. J Clin Invest. 2011;121:1053–1063. doi: 10.1172/JCI43273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, Scholz CC, Fraisl P, Lasitschka F, Mollenhauer M, Saunders SP, Maxwell PH, Carmeliet P, Fallon PG, Schneider M, Taylor CT. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology. 2010;139:2093–2101. doi: 10.1053/j.gastro.2010.06.068. [DOI] [PubMed] [Google Scholar]
- 108.Duan LJ, Takeda K, Fong GH. Prolyl hydroxylase domain protein 2 (PHD2) mediates oxygen-induced retinopathy in neonatal mice. Am J Pathol. 2011;178:1881–1890. doi: 10.1016/j.ajpath.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cioffi CL, Liu XQ, Kosinski PA, Garay M, Bowen BR. Differential regulation of HIF-1 alpha prolyl-4-hydroxylase genes by hypoxia in human cardiovascular cells. Biochem Biophys Res Commun. 2003;303:947–953. doi: 10.1016/s0006-291x(03)00453-4. [DOI] [PubMed] [Google Scholar]
- 110.Bernhardt WM, Câmpean V, Kany S, Jürgensen JS, Weidemann A, Warnecke C, Arend M, Klaus S, Günzler V, Amann K, Willam C, Wiesener MS, Eckardt KU. Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol. 2006;17:1970–1978. doi: 10.1681/ASN.2005121302. [DOI] [PubMed] [Google Scholar]
- 111.Ran R, Xu H, Lu A, Bernaudin M, Sharp FR. Hypoxia preconditioning in the brain. Dev Neurosci. 2005;27:87–92. doi: 10.1159/000085979. [DOI] [PubMed] [Google Scholar]
- 112.Kant R, Diwan V, Jaggi AS, Singh N, Singh D. Remote renal preconditioning-induced cardioprotection: a key role of hypoxia inducible factor-prolyl 4-hydroxylases. Mol Cell Biochem. 2008;312:25–31. doi: 10.1007/s11010-008-9717-5. [DOI] [PubMed] [Google Scholar]
- 113.Lushnikova I, Orlovsky M, Dosenko V, Maistrenko A, Skibo G. Brief anoxia preconditioning and HIF prolyl-hydroxylase inhibition enhances neuronal resistance in organotypic hippocampal slices on model of ischemic damage. Brain Res. 2011;1386:175–183. doi: 10.1016/j.brainres.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 114.Giusti S, Fiszer de Plazas S. Neuroprotection by hypoxic preconditioning involves upregulation of hypoxia-inducible factor-1 in a prenatal model of acute hypoxia. J Neurosci Res. 2012;90:468–478. doi: 10.1002/jnr.22766. [DOI] [PubMed] [Google Scholar]
- 115.Schneider M, Van Geyte K, Fraisl P, Kiss J, Aragonés J, Mazzone M, Mairbäurl H, De Bock K, Jeoung NH, Mollenhauer M, Georgiadou M, Bishop T, Roncal C, Sutherland A, Jordan B, Gallez B, Weitz J, Harris RA, Maxwell P, Baes M, Ratcliffe P, Carmeliet P. Loss or silencing of the PHD1 prolyl hydroxylase protects livers of mice against ischemia/reperfusion injury. Gastroenterology. 2010;138:1143–1154. doi: 10.1053/j.gastro.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 116.Muz B, Larsen H, Madden L, Kiriakidis S, Paleolog EM. Prolyl hydroxylase domain enzyme 2 is the major player in regulating hypoxic responses in rheumatoid arthritis. Arthritis Rheum. 2012;64:2856–2867. doi: 10.1002/art.34479. [DOI] [PubMed] [Google Scholar]