Abstract
Insulin-like growth factor binding proteins (IGFBPs) are important components of insulin growth factor (IGF) signaling pathways. One of the binding proteins, IGFBP-5, enhances the actions of IGF-1, which include the enhanced proliferation of smooth muscle cells. In the present study, we examined the expression and the biological effects of IGFBP-5 in vascular smooth muscle cells (VSMCs) from spontaneously hypertensive rats (SHR) and Wistar Kyoto rats (WKY). The levels of IGFBP-5 mRNA and protein were found to be higher in the VSMC from SHR than in those from WKY. Treatment with recombinant IGFBP-5-stimulated VSMC proliferation in WKY to the levels observed in SHR. In the VSMCs of WKY, incubation with angiotensin (Ang) II or IGF-1 dose dependently increased IGFBP-5 protein levels. Transfection with IGFBP-5 siRNA reduced VSMC proliferation in SHR to the levels exhibited in WKY. In addition, recombinant IGFBP-5 significantly up-regulated ERK1/2 phosphorylation in the VSMCs of WKY as much as those of SHR. Concurrent treatment with the MEK1/2 inhibitors, PD98059 or U0126 completely inhibited recombinant IGFBP-5-induced VSMC proliferation in WKY, while concurrent treatment with the phosphatidylinositol-3 kinase inhibitor, LY294002, had no effect. Furthermore, knockdown with IGFBP-5 siRNA inhibited ERK1/2 phosphorylation in VSMC of SHR. These results suggest that IGFBP-5 plays a role in the regulation of VSMC proliferation via ERK1/2 MAPK signaling in hypertensive rats.
Keywords: ERK1/2 MAPK, IGFBP-5, Insulin growth factor, Proliferation, Spontaneously hypertensive rats
INTRODUCTION
Insulin-like growth factor-1 (IGF-1) is a potent mitogen and antiapoptotic factor for VSMCs, thus, potentially reducing the effects of IGF-1 might be beneficial for certain pathologic conditions, such as hypertension and the early stages of atherosclerotic plaque formation characterized by the hyperplasia of VSMCs [1]. The bio-availability of IGF-1 is regulated by six IGF binding proteins (IGFBPs), which have higher IGF binding affinity than IGF receptors [2]. IGFBPs also play important roles in determining whether VSMCs proliferate or migrate in response to IGF-1stimulation and IGFBP-5, in particular, plays an important role in these processes [3,4]. IGFBP-5 not only modulates IGF-1 actions, but it also stimulates cell migration by interacting with cell-surface heparin sulfate proteoglycans [4]. Zheng et al., reported that IGFBP-5 is cleaved by physiologic concentrations of thrombin, which increases free IGF-1 levels, and contributes to the mitogenic effects of thrombin [5]. In addition, previous studies have shown that porcine aortic smooth muscle cells secrete IGFBP-5, and IGFBP-5 enhances IGF-I-stimulated DNA synthesis [6]. IGFBP-5 stimulates growth and IGF-1 secretion in human intestinal smooth muscle cells by activating p38 MAP kinase-dependent and ERK1/2-dependent pathways that are independent of IGF-1 [7].
VSMC proliferation contributes to the arterial remodeling associated with hypertension [8]. Indeed, the VSMCs from SHR proliferate faster than those from normotensive WKY [9,10]. Furthermore, in SHR, insulin-like growth factor-1 (IGF-1) induced vasorelaxant effects were found to be impaired before the onset of hypertension, indicating that this effect could influence the development of hypertension [11]. In addition, it was also found that IGF-1, IGF-1R, and IGFBP-1 through IGFBP-5 were undetectable in the SMCs of normal coronary arteries, but were markedly increased in atherectomy specimens [12]. However, the intracellular mechanism of IGFBP-5 in atherosclerotic lesion remains unidentified. Furthermore, no previous study has addressed the effect of IGFBP-5 on hypertension. Therefore, the goals of this study were to determine whether IGFBP-5 exerts a direct effect on VSMC proliferation in SHR. and to identify the intracellular signaling pathways involved.
METHODS
Materials
Dulbecco's modified eagle medium (DMEM) and fetal bovine serum (FBS) were purchased from Hyclone (South Logan, UT, USA). Pro-prep protein extract solution was purchased from Intron Biotechnology (Sungnam, Korea). The ECL western blot detection kit was purchased from Neuronex. The PCR primer oligonucleotides for IGF-1 (forward, cagacaagcccacaggctat; reverse, ctgggtcttgggcatgtc), IGF-2 (forward, ggagaattcgtctgattgtccag; reverse, tttctctccgtgctgttctctc), IGFBP-1 (forward, ccagggactcagctgccgtgcg; reverse, ggcgttccacaggatgggctg), IGFBP-2 (forward, tctattagaagcaggaacggag; reverse, gcagtaaaccacagccagtc), IGFBP-3 (forward, gacacccagaacttctcctcc; reverse, catacttgtccac acaccagc), IGFBP-4 (forward, tgtctctgcttgtgctggga; reverse, tcagtccctgtttcctggga), IGFBP-5 (forward, caggagttcaaagccagcccac; reverse, cgaaggcgtggcactgaaagtc) and for β-actin (forward, taggcaggcctcttttctca and reverse, agaggggacctg ggtttaga) were obtained from Bioneer (Daejeon, Korea). Recombinant IGFBP-5, recombinant IGF-1, and IGFBP-5 antibody were purchased from R&D systems (Minneapolis, MN, USA). Phospho-ERK antibody was purchased from Cell Signaling Technology (Beverly, MA, USA). 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) and β-actin antibody were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ang II, PD98059, U0126, and LY294002 were purchased from Calbiochem (San Diego, CA, USA), and IGFBP-5 siRNA (Eugene, OR, USA) and Lipofectamine 2000 (Carlsbad, CA, USA) were purchased from Invitrogen.
Rats
Male SHR and WKY weighing 200~250 g were used in this study. All experimental animals received autoclaved food and bedding such as to minimize exposure to viral or microbial pathogens. The rats were cared for in accordance with the Guide for the Care and Use of Experimental Animals of Yeungnam Medical Center.
Cell culture
Aortic VSMCs were isolated from 24-week-old male SHRs and age-matched WKY (200 to 250 g). VSMCs were grown in 10% FBS-DMEM containing 1% antibiotics and incubated in a CO2 incubator (95% CO2, 37℃). VSMCs from passages 4 to 9 at 70~90% confluence were used and cell growth was arrested by incubating cells in serum-free DMEM for 16 to 24 hrs prior to use.
Western blot analysis
Whole cell extracts were prepared by lysing cells in pro-prep protein extract solution. Protein concentrations were quantified with protein assay reagent (Bio-Rad, Hercules, CA, USA). Briefly, equal amounts of protein were mixed with sodium dodecyl sulfate (SDS) sample buffer and incubated for 5 min at 100℃ before loading. Total protein samples (30 µg) were subjected to 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) for 1 hr and 30 min at 100 V. The separated proteins were then transferred onto PVDF membranes for 1 hr at 30 mA using a SD Semi-dry Transfer Cell. The membranes were blocked with 5% non-fat milk in phosphate buffered saline (PBS) containing 0.05% Tween 20 (PBS-T) for 2 hrs at room temperature and then incubated with the primary antibodies at a dilution of 1:1,000 in 5% skim milk in PBS overnight at 4℃. The membranes were then washed with PBS-T and incubated with the secondary antibodies in 5% skim milk in PBS for 1 hr at room temperature. Finally, after rinses with the wash buffer, the membranes were exposed to ECL western blot analysis detection reagents.
Cell proliferation assay
For the cell counting assays, the VSMCs from WKY and SHR were seeded onto 24-well plates at a density of 1×104 cells per well in DMEM. After stimulation with 10% FBS, the cells were counted every day for four days using a hemocytometer. For the MTT assays, the VSMCs were seeded onto 24-well plates at a density of 1×104 cells per well in DMEM supplemented with 10% FBS. After the different treatments, 50 µl of an 1 mg/ml MTT solution was added to each well (0.1 mg/well) and incubated for 4 hrs. The supernatants were then aspirated, and the formazan crystals were solubilized in 200 µl of dimethyl sulfoxide (DMSO). The supernatants (100 µl) of these solutions were placed in the wells of 96-well plates. The cell proliferations were determined using a microplate reader measuring absorbance at 570 nm.
Reverse transcription-polymerase chain reaction
Total RNA was extracted from the VSMCs using a single-step guanidine thiocyanate/phenol chloroform extraction procedure, using Trizol® reagent (Molecular Research Center, Cincinnati, OH, USA), according to the manufacturer's instructions. Total RNA was reverse transcribed into single-stranded cDNA after 50 min incubation at 50℃ and a 5 min incubation at 85℃ in a final volume of 20 µl, using a SuperScript III RT-PCR kit (Invitrogen, Carlsbad, CA, USA). PCR amplification was performed on cDNA made from 100 ng of mRNA, using specific oligonucleotide primers for IGF-1, IGF-2, IGFBP-1 to 5, and β-actin. cDNAs were heated for 1 min at 94℃, then amplified using 29 cycles for each primer (94℃ for 30 sec, 60℃ for 30 sec, 72℃ for 45 sec) and using 28 cycles for β-actin (94℃ for 45 sec, 55℃ for 1 min, 72℃ for 1 min), followed by a 10 min extension at 72℃. The PCR products were resolved on a 1.2% agarose gel and visualized by ethidium bromide staining.
Transfection of IGFBP-5 siRNA
The VSMCs were transfected with siRNA using Lipofectamine 2000 reagent, according to the manufacturer's instructions. Aliquots of 1×104 cells were plated onto 60-mm dishes one day before transfection and grown to approximately 70% confluency. The cells were then transfected with 2.5 µl IGFBP-5 siRNA and 5 µl of Lipofectamine for 6 hrs in Opti-MEM®I reduced serum media (Invitrogen, Carlsbad, CA, USA), and incubated for 48 hrs. Protein levels were determined by western blot, and cell proliferation was determined using an MTT assay.
RESULTS
IGFBP-5 expressions higher on the VSMCs of SHR than those of WKY
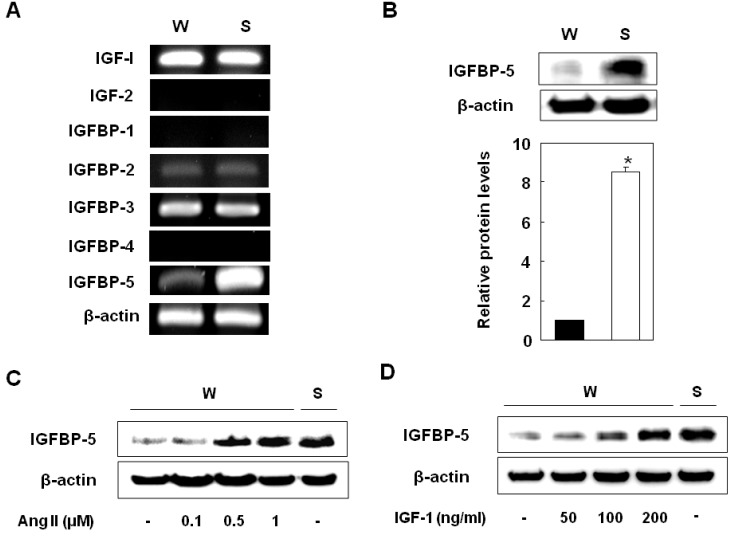
We first examined the mRNA levels of IGF-1, -2, IGFBP-1 to 4, and IGFBP-5 on the VSMCs of SHR and WKY. The expression of IGF-1, IGFBP-2, and IGFBP-3 were similar on both, and IGF-2, IGFBP-1, and IGFBP-4 were not expressed in the VSMCs of either SHR or WKY. Interestingly, IGFBP-5 expression was significantly higher on the VSMCs of SHR than on the VSMCs of WKY (Fig. 1A and 1B). To confirm whether hypertension induced IGFBP-5, we treated the VSMCs of WKY with angiotensin II and found that IGFBP-5 protein levels significantly increased to levels that were comparable to the levels in SHR (Fig. 1C). In the VSMCs of WKY, treatment with IGF-1 strongly and dose-dependently induced IGFBP-5 protein expression and treatment with IGF-1 (200 ng/ml) increased IGFBP-5 protein expression to levels that were observed in SHR (Fig. 1D). These results indicated that conditions of faster growing VSMCs such as hyperplasia or hypertension induced IGFBP-5 expression.
Fig. 1.
IGFBP-5 was more highly expressed on the VSMCs of SHR than on the VSMCs of WKY. Gene expressions of IGF-1, 2 and IGFBPs in the VSMCs of WKY and SHR (A). Expression levels of IGFBP-5 protein in the VSMCs of WKY and SHR (B). Cells were treated with Ang II (0.1, 0.5, and 1 µM) for 24 hrs (C) and then treated with recombinant IGF-1 (50, 100, and 200 ng/ml) for 24 hrs (D). After treatment, mRNAs were detected by RT-PCR and proteins by Western blot. These data are representative of three experiments. Results are represented as the mean±S.E.M. (n=3). *p value<0.001 compared with VSMCs of WKY.
The effect of IGFBP-5 knock down on the proliferation of VSMCs from SHR
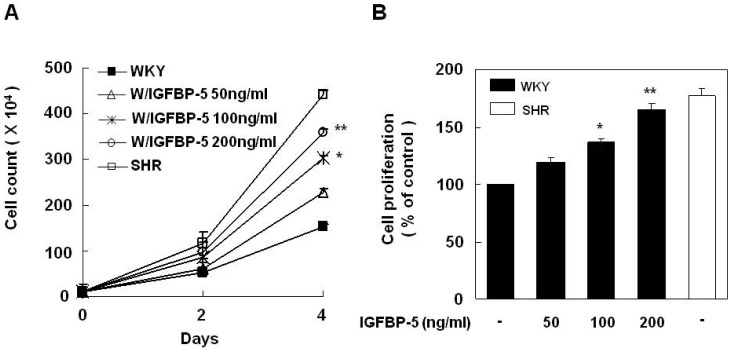
We next sought to determine the effect of IGFBP-5 on the VSMCs of SHR and WKY. We first analyzed VSMC proliferation in response to IGFBP-5 recombinant protein in WKY. Cell counts and MTT assays revealed that recombinant IGFBP-5 dose-dependently up-regulated VSMC proliferation in WKY to levels that were observed in SHR (Fig. 2). To study the role of IGFBP-5, endogenous IGFBP-5 was knocked down using adenovirus-delivered IGFBP-5 siRNA. Transfection with IGFBP-5 siRNA efficiently reduced the endogenous level of IGFBP-5 in the VSMCs of SHR (Fig. 3A). Depletion of IGFBP-5 almost completely inhibited VSMC proliferation in SHR to the levels of those in WKY (Fig. 3B and 3C). These results indicated that IGFBP-5 plays a critical role in the regulation of VSMC proliferation in SHR.
Fig. 2.
Recombinant IGFBP-5 increased the proliferation of the VSMCs from WKY. Cells were treated with IGFBP-5 (50, 100, and 200 ng/ml) for 24 hrs. Cell proliferation was determined by counting cells (A) and performing an MTT assay (B). Results are represented as the mean±S.E.M. (n=4). *p value<0.05 compared with VSMCs of WKY. **p value<0.001 compared with VSMCs of WKY.
Fig. 3.
Knockdown of IGFBP-5-inhibited VSMC proliferation in SHR. Cells were transfected with control siRNA or IGFBP-5 siRNA and then IGFBP-5 protein levels were determined by Western blot (A). Cell proliferation was determined using an MTT assay (B) and cell counting (C). Results are represented as the mean±S.E.M. (n=4). *p value<0.001 compared with VSMCs of WKY, **p value <0.001 compared with VSMC of SHR.
IGFBP-5 induced VSMC proliferation via ERK phosphorylation
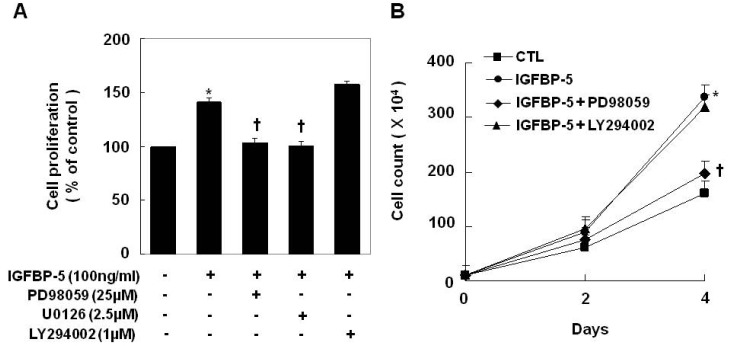
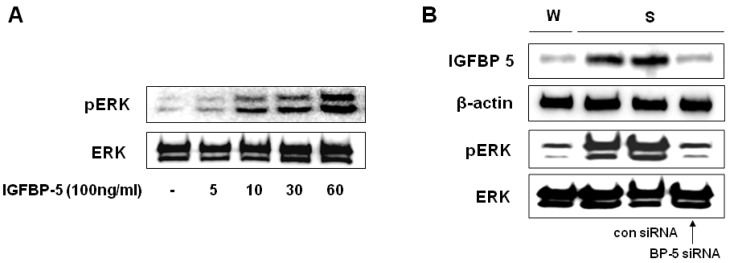
Thus far, we have established that IGFBP-5-induced VSMC proliferation is required for the activation of insulin signaling pathways, including the PI3 kinase and ERK1/2 pathways. Treatment with PD98059 or U0126, specific inhibitors of MEK1/2 significantly decreased VSMC proliferation in SHR. However, treatment with LY294002, a PI-3 kinase inhibitor had no inhibitory effect on VSMC proliferation in SHR (Fig. 4). To confirm the effects of IGFBP-5 on the ERK1/2 phosphorylation, transduction with IGFBP-5 recombinant protein efficiently stimulated ERK1/2 phosphorylation in the VSMCs of WKY (Fig. 5A). Furthermore, transfection with IGFBP-5 siRNA completely blocked ERK1/2 phosphorylation in the VSMCs of SHR (Fig. 5B). These results indicated that IGFBP-5 up-regulation in the VSMC of SHR is closely linked with ERK1/2 phosphorylation.
Fig. 4.
MEK1/2 inhibitors inhibited IGFBP-5-induced VSMC proliferation. Cells were pretreated with PD98059 (25 µM), U0126 (2.5 µM), or LY294002 (1 µM) for 1 hr and then stimulated with IGFBP-5 (100 ng/ml) for 24 hrs. Cell proliferation was determined by an MTT assay (A) and by cell counting (B). Results are represented as the mean±S.E.M. (n=4). *p value<0.001 compared with VSMCs of WKY, †p value<0.001 compared with IGFBP-5-treated VSMCs of WKY.
Fig. 5.
IGFBP-5 regulated ERK phosphorylation in VSMC of SHR. Cells were treated with IGFBP-5 (100 ng/ml) for 5, 10, 30, or 60 min (A) Cells were transfected with control siRNA or IGFBP-5 siRNA (B), and then ERK 1/2 phosphorylation was determined by Western blot. Results are represented as the mean±S.E.M. (n=4).
DISCUSSION
In the present study, we examined whether IGFBP-5 is involved in the proliferation of VSMCs from hypertensive rats. IGFBP-5 was expressed in the VSMCs from porcine arteries by IGF-1, but the IGFBP-5 was undetectable in rat arteries [1]. Our results showed the expression pattern of the five IGFBPs in the VSMCs from hypertensive and normotensive rat arteries (Fig. 1A). In particular, IGFBP-5 was the only IGFBP that was expressed at a higher level in the VSMCs from hypertensive compared to those from normotensive rats (Fig. 1A). IGFBP-5 mRNA levels and protein expression levels were higher in the VSMCs from hypertensive compared to those from normotensive rats. There are some controversies surrounding the association of the IGF/IGFBP system with cardiovascular diseases. Calao et al. reported that circulating IGF1 and IGFBP-3 levels were negatively correlated with common cardiovascular risk factors in healthy subjects [13]. However, Kawachi et al. showed that circulating IGF1 and IGFBP-3 were associated with early carotid atherosclerosis [14]. IGFBP-3 was reported to be associated with presence and extent of coronary arteriosclerosis [15] and with the development of carotid atherosclerosis in hypertensive patients [16]. In a recent data, they showed that IGFBP-5 immunoreactivity was increased in old human arteries compared with young human arteries and was also strong in atherosclerotic plaques [17]. To our knowledge, this is the first study to report the increased expression of IGFBP-5 in the VSMCs from hypertensive rat arteries.
Our results provide the first evidence of the involvement of IGFBP-5 in VSMC of hypertensive rats through the ERK1/2 signaling pathway. We demonstrated that IGFBP-5 plays an important role in hypertension through these findings: 1) transduction with normotensive VSMC with recombinant of IGFBP-5 stimulated excessive cell proliferation; 2) knockdown of IGFBP-5 in hypertensive VSMC reversed the cell proliferation. Although a variety of evidence suggests that insulin/IGF signaling pathways are important in hypertension of many organisms [18], and IGFBPs are important components of these signaling pathways, the role of IGFBPs in hypertension was not well understood. Because IGF-1 and IGF-2 are essential for growth and development and bioavailable IGFs are tightly regulated by IGFBPs, regulation of IGFs by IGFBPs was known to affect cell proliferation, differentiation, and survival. Especially, IGFBP-5 expression is regulated by various signaling molecules in vitro, including IGF-1, IGF-2, insulin and dexamethasone [19], and IGF-1 is the most important regulator of in vitro IGFBP-5 expression in many cell types from different species. Furthermore, IGF-1 induced expression of IGFBP-5 can occur by directly stimulating IGFBP-5 gene transcription [20]. Consistent with previous reports, IGF-I dose-dependently induced IGFBP-5 protein expression in normotensive VSMCs (Fig. 1D). The role of IGFBP-5 in cell growth is complicated, and it has been reported that IGFBP-5 can either stimulate [21] or inhibit cell proliferation [22,23]. These conflicting effects may be cell- and context-specific, and it has also been suggested that IGF-dependent and -independent mechanisms are involved [24]. IGFBP-5 may regulate normotensive VSMC proliferation, and in the present study, recombinant IGFBP-5 was found to stimulate the proliferation of the VSMCs from normotensive rats (Fig. 2). In addition, knock down of IGFBP-5 siRNA completely inhibited VSMC proliferation in hypertensive rats (Fig. 3A and 3B). It has been suggested that IGFBP-5-inducible cellular senescence in endothelial cells contributes to vascular aging and the development of age-associated cardiovascular disease [17]. Therefore, our results combined with the report suggest that IGFBP-5 contributes to the proliferation of VSMCs in hypertension.
The involvement of the MAP kinase/ERK pathway in regulating osteoblast cell proliferation has been well established [25]. It is therefore tempting to speculate that the IGFBP-5-induced increase in ERK phosphorylation coud be involved in mediating the IGFBP-5 effects on cell proliferation. We confirmed that IGFBP-5 directly affects VSMC proliferation via ERK1/2 activation. In the present study, concurrent treatment with the MEK1/2 inhibitors, PD98059 or U0126 completely inhibited recombinant IGFBP-5-induced VSMC proliferation in WKY, while concurrent treatment with the phosphatidylinositol-3 kinase inhibitor, LY294002 had no effect (Fig. 4). In a recent paper, IGFBP-1 alone was found to stimulate VSMC proliferation and ERK1/2 activation dose-dependently in atherosclerotic lesions [26]. In addition, recombinant IGFBP-5 increased ERK1/2 phosphorylation in the VSMCs of normotensive arteries to levels observed in the hypertensive arteries (Fig. 5A). Furthermore, knock down of IGFBP-5 siRNA completely inhibited ERK1/2 phosphorylation on the VSMCs of SHR to the level observed in WKY rats (Fig. 5B). Further studies are needed to establish the cause and effects association between the IGFBP-5 interaction with ERK1/2 phosphorylation and cell proliferation to provide experimental data that the IGFBP-5 leads to an increase in ERK phosphorylation, which mediates the IGFBP-5 effects on cell proliferation.
In summary, our results demonstrate that IGFBP-5 is endogenously up-regulated in the VSMCs of hypertensive rats, and suggest that IGFBP-5 stimulates VSMC proliferation in normotensive rat arteries by activating the ERK1/2 MAPK signaling pathway. Accordingly, we believe that IGFBP-5 induction in VSMCs potentially represents an important potential mechanism to regulate VSMC proliferation in arteries and contributes to the development of hypertension.
ACKNOWLEDGEMENTS
This work was supported by a grant from Yeungnam University and from the National Research Foundation of Korea (NRF) funded by the Korean government (MEST) (2012-0000288) (2012).
ABBREVIATIONS
- IGFBP-5
insulin-like growth factor binding protein-5
- VSMC
vascular smooth muscle cell
- SHR
spontaneously hypertensive rats
- WKY
Wistar Kyoto rats
- ERK1/2
extracellular signal regulated kinase 1/2
- MAPK
mitogen activated protein kinase
- FBS
fetal bovine serum
- ECL
electrochemiluminescence
- MTT
3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolidium bromide
- DMEM
dulbecco's modified eagle's medium
- SDS
sodium dodecyl sulfate
- PBS
phosphate buffered saline
- DMSO
dimethyl sulfoxide
- PVDF
polyvinylidene difluoride
References
- 1.Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol. 2004;24:435–444. doi: 10.1161/01.ATV.0000105902.89459.09. [DOI] [PubMed] [Google Scholar]
- 2.Clemmons DR. Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nat Rev Drug Discov. 2007;6:821–833. doi: 10.1038/nrd2359. [DOI] [PubMed] [Google Scholar]
- 3.Duan C. Specifying the cellular responses to IGF signals: roles of IGF-binding proteins. J Endocrinol. 2002;175:41–54. doi: 10.1677/joe.0.1750041. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh T, Gordon RE, Clemmons DR, Busby WH, Jr, Duan C. Regulation of vascular smooth muscle cell responses to insulin-like growth factor (IGF)-I by local IGF-binding proteins. J Biol Chem. 2003;278:42886–42892. doi: 10.1074/jbc.M303835200. [DOI] [PubMed] [Google Scholar]
- 5.Zheng B, Clarke JB, Busby WH, Duan C, Clemmons DR. Insulin-like growth factor-binding protein-5 is cleaved by physiological concentrations of thrombin. Endocrinology. 1998;139:1708–1714. doi: 10.1210/endo.139.4.5945. [DOI] [PubMed] [Google Scholar]
- 6.Duan C, Hawes SB, Prevette T, Clemmons DR. Insulin-like growth factor-I (IGF-I) regulates IGF-binding protein-5 synthesis through transcriptional activation of the gene in aortic smooth muscle cells. J Biol Chem. 1996;271:4280–4288. doi: 10.1074/jbc.271.8.4280. [DOI] [PubMed] [Google Scholar]
- 7.Kuemmerle JF, Zhou H. Insulin-like growth factor-binding protein-5 (IGFBP-5) stimulates growth and IGF-I secretion in human intestinal smooth muscle by Ras-dependent activation of p38 MAP kinase and Erk1/2 pathways. J Biol Chem. 2002;277:20563–20571. doi: 10.1074/jbc.M200885200. [DOI] [PubMed] [Google Scholar]
- 8.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 9.Resink TJ, Scott-Burden T, Baur U, Bühler FR. Increased proliferation fate and phosphoinositide turnover in cultured smooth muscle cells from spontaneously hypertensive rats. J Hypertens Suppl. 1987;5:S145–S148. [PubMed] [Google Scholar]
- 10.Choi HC, Lee KY, Lee DH, Kang YJ. Heme oxygenase-1 induced by aprotinin inhibits vascular smooth muscle cell proliferation through cell cycle arrest in hypertensive rats. Korean J Physiol Pharmacol. 2009;13:309–313. doi: 10.4196/kjpp.2009.13.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vecchione C, Colella S, Fratta L, Gentile MT, Selvetella G, Frati G, Trimarco B, Lembo G. Impaired insulin-like growth factor I vasorelaxant effects in hypertension. Hypertension. 2001;37:1480–1485. doi: 10.1161/01.hyp.37.6.1480. [DOI] [PubMed] [Google Scholar]
- 12.Grant MB, Wargovich TJ, Ellis EA, Tarnuzzer R, Caballero S, Estes K, Rossing M, Spoerri PE, Pepine C. Expression of IGF-I, IGF-I receptor and IGF binding proteins-1, -2, -3, -4 and -5 in human atherectomy specimens. Regul Pept. 1996;67:137–144. doi: 10.1016/s0167-0115(96)00124-3. [DOI] [PubMed] [Google Scholar]
- 13.Colao A, Spiezia S, Di Somma C, Pivonello R, Marzullo P, Rota F, Musella T, Auriemma RS, De Martino MC, Lombardi G. Circulating insulin-like growth factor-I levels are correlated with the atherosclerotic profile in healthy subjects independently of age. J Endocrinol Invest. 2005;28:440–448. doi: 10.1007/BF03347225. [DOI] [PubMed] [Google Scholar]
- 14.Kawachi S, Takeda N, Sasaki A, Kokubo Y, Takami K, Sarui H, Hayashi M, Yamakita N, Yasuda K. Circulating insulin-like growth factor-1 and insulin-like growth factor binding protein-3 are associated with early carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:617–621. doi: 10.1161/01.ATV.0000154486.03017.35. [DOI] [PubMed] [Google Scholar]
- 15.Schuler-Luttmann S, Monnig G, Enbergs A, Schulte H, Breithardt G, Assmann G, Kerber S, von Eckardstein A. Insulin-like growth factor-binding protein-3 is associated with the presence and extent of coronary arteriosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:E10–E15. [PubMed] [Google Scholar]
- 16.Watanabe T, Itokawa M, Nakagawa Y, Iguchi T, Katagiri T. Increased levels of insulin-like growth factor binding protein-3 in hypertensive patients with carotid atherosclerosis. Am J Hypertens. 2003;16:754–760. doi: 10.1016/s0895-7061(03)00985-3. [DOI] [PubMed] [Google Scholar]
- 17.Kim KS, Seu YB, Baek SH, Kim MJ, Kim KJ, Kim JH, Kim JR. Induction of cellular senescence by insulin-like growth factor binding protein-5 through a p53-dependent mechanism. Mol Biol Cell. 2007;18:4543–4552. doi: 10.1091/mbc.E07-03-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bach LA. The insulin-like growth factor system in kidney disease and hypertension. Curr Opin Nephrol Hypertens. 2012;21:86–91. doi: 10.1097/MNH.0b013e32834dc1a2. [DOI] [PubMed] [Google Scholar]
- 19.Schneider MR, Wolf E, Hoeflich A, Lahm H. IGF-binding protein-5: flexible player in the IGF system and effector on its own. J Endocrinol. 2002;172:423–440. doi: 10.1677/joe.0.1720423. [DOI] [PubMed] [Google Scholar]
- 20.Duan C, Liimatta MB, Bottum OL. Insulin-like growth factor (IGF)-I regulates IGF-binding protein-5 gene expression through the phosphatidylinositol 3-kinase, protein kinase B/Akt, and p70 S6 kinase signaling pathway. J Biol Chem. 1999;274:37147–37153. doi: 10.1074/jbc.274.52.37147. [DOI] [PubMed] [Google Scholar]
- 21.Cobb LJ, Salih DA, Gonzalez I, Tripathi G, Carter EJ, Lovett F, Holding C, Pell JM. Partitioning of IGFBP-5 actions in myogenesis: IGF-independent anti-apoptotic function. J Cell Sci. 2004;117:1737–1746. doi: 10.1242/jcs.01028. [DOI] [PubMed] [Google Scholar]
- 22.Butt AJ, Dickson KA, McDougall F, Baxter RC. Insulin-like growth factor-binding protein-5 inhibits the growth of human breast cancer cells in vitro and in vivo. J Biol Chem. 2003;278:29676–29685. doi: 10.1074/jbc.M301965200. [DOI] [PubMed] [Google Scholar]
- 23.Salih DA, Tripathi G, Holding C, Szestak TA, Gonzalez MI, Carter EJ, Cobb LJ, Eisemann JE, Pell JM. Insulin-like growth factor-binding protein 5 (Igfbp5) compromises survival, growth, muscle development, and fertility in mice. Proc Natl Acad Sci USA. 2004;101:4314–4319. doi: 10.1073/pnas.0400230101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hampel B, Fortschegger K, Ressler S, Chang MW, Unterluggauer H, Breitwieser A, Sommergruber W, Fitzky B, Lepperdinger G, Jansen-Dürr P, Voglauer R, Grillari J. Increased expression of extracellular proteins as a hallmark of human endothelial cell in vitro senescence. Exp Gerontol. 2006;41:474–481. doi: 10.1016/j.exger.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Lau KH, Baylink DJ. Molecular mechanism of action of fluoride on bone cells. J Bone Miner Res. 1998;13:1660–1667. doi: 10.1359/jbmr.1998.13.11.1660. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Razuvaev A, Folkersen L, Hedin E, Roy J, Brismar K, Hedin U. The expression of IGFs and IGF binding proteins in human carotid atherosclerosis, and the possible role of IGF binding protein-1 in the regulation of smooth muscle cell proliferation. Atherosclerosis. 2012;220:102–109. doi: 10.1016/j.atherosclerosis.2011.10.032. [DOI] [PubMed] [Google Scholar]