Abstract
Alterations in T cell immunity occur with aging. Influenza causes significant morbidity and mortality in the elderly. We investigated the relationship of serum IgG responses with hemagglutinin inhibition (HI) antibody titers and the frequency of distinct T cell subsets in young and elderly people who received the inactivated influenza vaccine. Influenza vaccine-specific IgG responses correlated with the increase of HI antibody titers and the frequency of CD4+ T cells producing IFN-γ and IL-17 in young, but not elderly, people. Also, only in young people, such IgG responses correlated with the frequency of memory T cells, especially central memory cells, CD45RA− effector memory CD8+ T cells and IL-7 receptor alpha high effector memory CD8+ T cells with potent survival and proliferative capacity. These findings suggest that aging alters the association of influenza-vaccine specific IgG responses with HI antibody titers, cytokine-producing capacity and proportions of memory T cells in humans.
Keywords: influenza vaccine, T cells, humoral response, human, aging
1. Introduction
Age-associated alterations occur in the immune system that comprises innate and adaptive immunities [1–6]. These changes account in part for the development of pathologic conditions like infections, malignancies and inflammatory diseases that are associated with aging. T cells, a component of adaptive immunity, play a critical role in the immune system. T cells are involved in host defense against microorganisms and malignancy as well as in regulating other immune responses such as antibody production from B cells [2, 3]. Alterations in the number and function of T cells are found with aging [2, 3]. Aged mice have decreased generation of naïve T cells with atrophy of the thymus as well as expansion of memory T cells with reduced T cell receptor (TCR) repertoire diversity [2, 7–9]. In addition, changes in the effector function of T cells, including cytokine production, cell proliferation and cytotoxicity, have been found with aging [2, 3, 5, 10].
Memory T cells can be divided into several subsets. Central memory (CM) T cells with the expression of lymphoid tissue homing chemokine receptor 7 (CCR7) can migrate to secondary lymphoid tissues like the lymph nodes and spleen, while effector memory (EM) T cells can move to inflamed or infected peripheral tissues [11]. The cytokine IL-7 is essentially involved in the maintenance of memory T cells by promoting cell survival [12]. Indeed, two different subsets of EM CD8+ T cells with high and low levels of IL-7 receptor alpha chain expression (IL-7Rαhigh and low) exist in human peripheral blood. IL-7Rαhigh EM CD8+ T cells have potent cell proliferative and survival capacity compared to IL-7Rαlow EM CD8+ T cells [13]. Of interest, the proportions of naïve, CM and EM T cells as well as IL-7Rαhigh and low EM CD8+ T cells change with aging [13, 14].
CD4+ T helper (Th) cells can be divided into Th1, Th2 and Th17 cells based on the cytokines predominantly produced by individual Th cell subsets [15]. Th cells can promote humoral immune responses by producing cytokines as well as providing co-stimulation to B cells [16]. Recent studies reported increased antibody production and immunoglobulin isotype class switching by the cytokine IL-17 which is produced primarily by Th17 cells [17]. IFN-γ, the stereotypic cytokine produced by Th1 cells, could increase the generation of IgG2a while IL-4 derived from Th2 cells enhances the production of IgG1 in mice [18]. The findings from previous studies investigating the effect of aging on Th cell cytokines, in particular IFN-γ and IL-4, were largely inconsistent (reviewed in [2]). Of interest, we recently reported a decreased frequency of IL-17-producing memory CD4+ T cells in the peripheral blood of the elderly, compared to young people [10].
Influenza is common but can be a serious medical illness which causes significant morbidity and mortality in the elderly [19]. In fact, most deaths associated with influenza are seen in people age 65 or older [19]. Although the influenza vaccine can provide protection against an influenza virus infection, the vaccine response appears to decline with aging [4, 20–23]. The assay for hemagglutination inhibition (HI) antibody titers has been used to determine the response to influenza vaccine. In fact, the levels of the increase in serum HI antibody titers after influenza vaccination were lower in the elderly than in the young [20, 24]. Also, the elderly had decreased levels of influenza virus-specific CD4+ and CD8+ T cell responses compared to young adults after vaccination [22, 23, 25, 26]. Such impaired humoral and cellular immune responses to influenza vaccine could stem in part from alterations in the immune system with aging [21, 22]. The accumulation of memory CD8+ T cells expressing CD45RA or lacking CD28 expression, which may represent a functionally senescent cell population with reduced telomerase activity, was associated with a blunted increase in serum HI antibody titers in the elderly after influenza vaccination [27–29]. This finding suggests that the age-associated expansion of memory T cells could have a detrimental effect on the development of vaccine responses.
Here we studied the relationship of serum IgG responses with HI antibody titers as well as the frequency of T cell subsets with distinct cellular characteristics in young and elderly people who received influenza vaccine. Although serum IgG responses to influenza vaccine correlated with the increase of HI antibody titers in young people, a similar correlation was not found in elderly people. The frequency of CD4+ T cells producing IFN-γ and IL-17 correlated with IgG responses to influenza vaccine in the young but not in the elderly. In addition, such IgG responses correlated with the frequency of memory CD4+ and CD8+ T cells subsets, especially CM, IL-7Rαhigh EM and CD45RA− EM CD8+ T cells, only in the young. These findings suggest that aging affects the association of IgG and HI antibody responses specific for the influenza vaccine as well as the relationship of the cytokine-producing capacity and the proportions of memory T cells with the development of influenza vaccine-specific IgG responses in humans.
2. Materials and Methods
2.1. Human Subjects
Healthy elderly subjects 65 years of age (n = 26) or older and healthy young subjects 40 years of age or younger (n = 29) were recruited for this study. The mean age ± SD for young and elderly subjects was 24.9 years ± 2.2 and 75.3 years ± 6.8, respectively. The gender distribution was not different between the two groups (F:M, 16:13 and 15:11, respectively for young and elderly groups, P = 0.851 by Chi-square test). Twenty six of 29 young subjects and all 26 elderly subjects had received influenza vaccine in the previous year (P = 0.238 by Fisher’s exact test). Individuals who were taking immunosuppressive drugs or had a disease potentially affecting the immune system, including cancer and autoimmunity were excluded [10, 13, 24, 26, 30, 31]. All subjects were vaccinated in October 2011 with a commercially available inactivated subvirion trivalent 2011–2012 influenza vaccine containing the following strains: A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2) and B/Brisbane/60/2008. Peripheral blood was collected before vaccination and at a mean of 32 days (range, 29 – 36 days) after vaccination. Informed consent was obtained from all subjects. This work was approved by the institutional review committee of Yale University.
2.2. Flow cytometric analysis
Peripheral blood mononuclear cells (PBMCs) were prepared from blood on FicollPAQUE gradients. Cells were stained with antibodies to APC-Cy7-CD3, Pacific Blue-CD8, PE-Cy7-CCR7, PE-Cy5-CD45RA (all from BD Biosciences, San Jose, CA) and FITC-IL-7Rα (R&D Systems, Minneapolis, MN) or isotype antibodies. For intracellular cytokine staining, PBMCs were stimulated for 4 hours with a combination of phorbol myristate acetate (PMA, 50 ng/ml; Sigma-Aldrich, St. Louis, MO) and ionomycin (1 μg/ml; Sigma-Aldrich) or PBS (control) in the presence of Golgiplug (BD Biosciences). Stimulated cells were stained with antibodies to APC-Cy7-CD3, Alexa Fluor 700-CD4, PE-Cy5-CD8 (all from BD Biosciences). Cells were fixed, permeabilized and stained with antibodies to Alexa Fluor 488-IL-17A (eBioscience, San Diego, CA) and PE-Cy7-IFN-γ (BD Biosciences). Cells were analyzed using an LSRII® flow cytometer (BD Biosciences) and FlowJo software (Tree Star, Ashland, OR).
2.3. Determination of anti-influenza virus antibodies in serum
Collected serum samples were separated into aliquots and stored at a temperature of −80°C until assayed. Anti-influenza virus IgG antibodies in serum were measured by ELISA as previously described [26], with some modifications. Briefly, 96 well-microtiter plates were coated overnight at 4°C with lysates of individual strains of influenza virus (A/California, A/Perth and B/Brisbane, kindly provided from Sanofi-Pasteur US, Swiftwater, PA) in coating buffer at 5 ng/ml. After blocking with 1% BSA, plates were loaded with a 1:20,000 dilution of serum in 0.1% BSA in duplicates followed by incubation for two hours at room temperature. This dilution was selected based on the finding of a pilot study using two-fold serial dilutions of antigens and serum (data not shown). Plates were washed and incubated for one hour at room temperature with anti-human IgG antibodies conjugated with biotin (eBioscience). After washing, plates were incubated for 30 minutes with horseradish peroxidase (HRP) conjugated with avidin (eBioscience). Plates were then washed again and developed by adding 3,3′,5,5′-tetramethylbenzidine (TMB, eBioscience). The optical density (OD) was read at 405 nm. The OD values of individual samples were compared against the OD value of the same internal control serum through the experiments. HI assays on pre- and postvaccine serum samples were performed as described [24] to determine antibody titers against each of the strains of influenza virus included in the 2011–2012 influenza vaccine using antigen reagents specific to the vaccine. HI antibody seroconversion to a strain in the vaccine was defined as a 4-fold or greater increase in antibody titer between pre- and postvaccine serum samples [24].
2.4. Statistical Analysis
The unpaired or paired t- test, Pearson correlation and Chi-square test were used for statistical analyses as appropriate using SPSS 19.0 (SPSS Inc.). P values of less than 0.05 were considered statistically significant.
3. Results
3.1. Influenza virus-specific IgG responses correlate with the changes in HI antibody titers in the young but not in the elderly after influenza vaccination
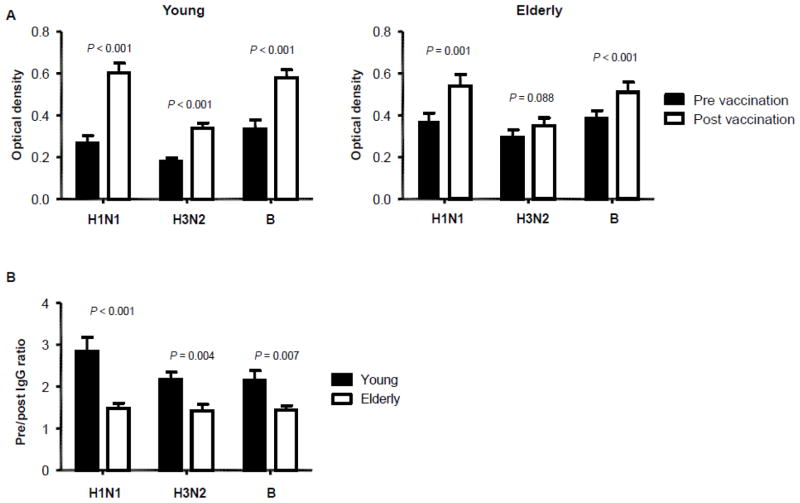
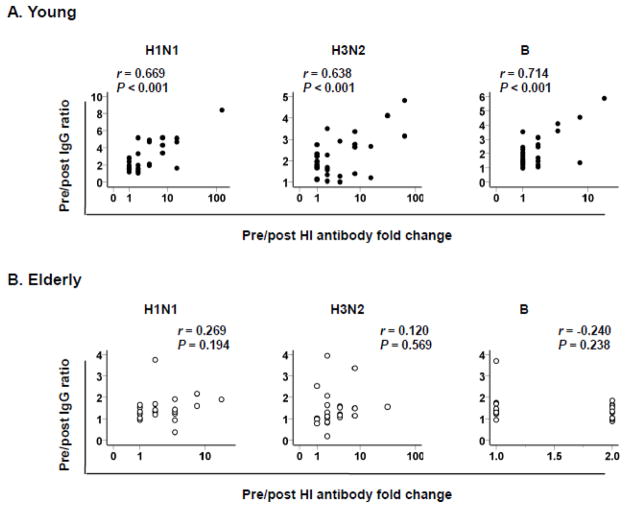
We measured serum levels of IgG specific for individual strains of influenza virus included in the vaccine in young and elderly adults by ELISA before and after influenza vaccination. These strains were A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2) and B/Brisbane/60/2008. Before vaccination, the elderly had higher levels of IgG specific for two A strains (H1N1 and H3N2) than the young although both groups had similar levels of IgG specific for strain B (Table I). The vaccination increased IgG levels for all three strains in the young whereas it increased IgG levels for only H1N1 and B strains in the elderly (Figure 1A–B). We obtained the pre-vaccination IgG to post vaccination IgG ratio (referred to as pre/post IgG ratio) for each strain. The young had higher pre/post IgG ratios for all three strains compared to the elderly (Figure 1C), indicating reduced IgG responses to influenza vaccine with aging. In addition to measuring total IgG responses to influenza vaccine, we determined HI antibody titers before and after influenza vaccination. The proportion of individuals with seroconversion for H1N1 and H3N2 strains was not different between young and elderly groups although the young group had a higher proportion of seroconverters for B strain than the elderly group (Supplementary Figure 1). We correlated pre/post IgG ratios with the results of the hemagglutination inhibition (HI) assay. In the young, pre/post IgG ratios were highly correlated with the changes in HI antibody titers before and after vaccination (Figure 2A). However, no such correlation was found in the elderly (Figure 2B).
Table I.
Pre- and post-vaccination levels of serum IgG specific for influenza virus strains in young and elderly people*
| Influenza vaccine strain | Serum IgG levels | P value | ||
|---|---|---|---|---|
| Young | Elderly | |||
| H1N1 | Pre | 0.27 ± 0.19 | 0.37 ± 0.19 | 0.046 |
| Post | 0.60 ± 0.25 | 0.54 ± 0.27 | 0.388 | |
| H3N2 | Pre | 0.18 ± 0.10 | 0.30 ± 0.18 | 0.006 |
| Post | 0.34 ± 0.13 | 0.35 ± 0.19 | 0.787 | |
| Brisbane | Pre | 0.34 ± 0.22 | 0.38 ± 0.20 | 0.404 |
| Post | 0.58 ± 0.20 | 0.51 ± 0.24 | 0.248 | |
data from 29 young and 26 elderly subjects; P values were obtained by two-tailed Student’s t-test
Figure 1. The increase in serum levels of influenza virus-specific IgG is lower in elderly people compared to young people after influenza vaccination.
(A–B) Serum levels of IgG specific for individual strains of influenza virus included in influenza vaccine 2011–2012 (A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2) and B/Brisbane/60/2008) were measured in young (n = 29) and elderly (n = 26) adults by ELISA before and ~32 days (range, 29–36 days) after influenza vaccination. (A) IgG levels in pre- and post-vaccination sera were compared in young and elderly people. (B) Ratios of pre- and post-vaccination IgG levels were compared between young and elderly people. P values were obtained by paired (A) or unpaired (B) t-test.
Figure 2. The ratios of pre/post vaccination IgG levels specific for influenza virus strains correlate with the changes in hemagglutinin inhibition (HI) antibody titers in young but not elderly people after influenza vaccination.
(A–B) Serum levels of IgG specific for individual strains of influenza virus included in influenza vaccine 2011–2012 (A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2) and B/Brisbane/60/2008) were measured in young (n = 29) and elderly (n = 26) adults by ELISA before and ~32 days (range, 29–36 days) after influenza vaccination. Serum HI antibody titers for the same strains of influenza virus were measured by HI assay. (AB) Ratios of Pre/post vaccination IgG levels specific for individual influenza viral strains were correlated with the changes in HI antibody titers in young and elderly people. P values were determined by Pearson correlation analysis.
3.2. Influenza vaccine-specific IgG responses correlate with the frequency of peripheral CD4+ T cells producing IFN-γ and IL-17 as well as with CD8+ T cells producing IFN-γ in the young but not in the elderly
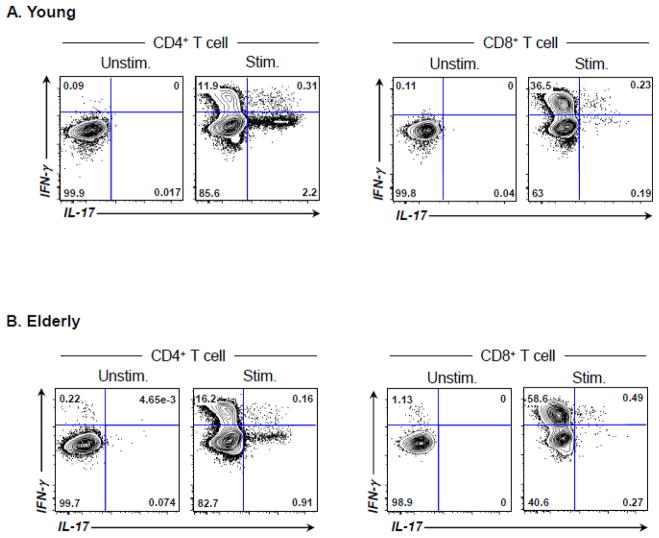
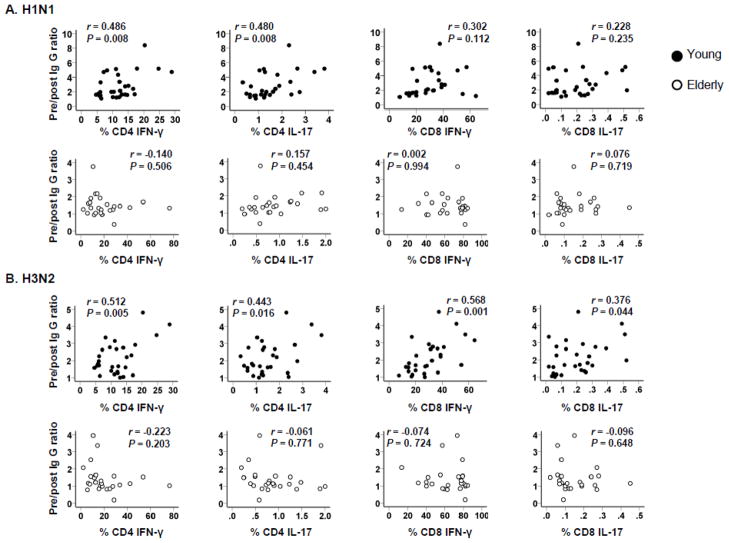
T cell immunity plays a role in the development of humoral immune responses. We measured the frequency of peripheral CD4+ and CD8+ T cells producing IFN-γ and IL-17 in response to PMA/ionomycin before vaccination (Figure 3, representative data) and correlated the results with IgG responses (pre-vaccination to post-vaccination IgG ratios). As previously reported [10], young and elderly people had an altered frequency of T cells producing these cytokines (Supplementary Table I). The frequency of CD4+ T cells producing IFN-γ and IL-17 correlated with pre to post-vaccination IgG ratios of H1N1 and H3N2 strains in the young but not in the elderly (Figure 4). The frequency of CD4+ T cells producing these cytokines did not correlate with pre to post-vaccination IgG ratios of the B strain in either group (data not shown). Although a large number of CD8+ T cells produced IFN-γ, CD8+ T cells producing IL-17 was rarely found by this assay (young vs. elderly, 0.2% ± 0.15 vs. 0.15% ± 0.1%, P = 0.145). In correlating with CD8+ T cells producing cytokines, the frequency of IFN-γ- and IL-17-producing cells correlated with pre/post IgG ratios of H3N2 strains (Figure 4). Although a similar trend was noticed between the frequency of IFN-γ-producing CD8+ T cells and pre to post-vaccination IgG ratios of H1N1 strain, it was not statistically significant. The frequency of CD8+ T cells producing IFN-γ and IL-17 did not correlate with pre to post-vaccination IgG ratios of B strain in young and elderly groups (data not shown). Overall, our data suggest that an age-associated alteration occurs in the relationship of IgG responses to influenza A virus in the influenza vaccine with cytokine production by T cells, especially CD4+ cells, in humans.
Figure 3. Flow cytometric data showing T cell subsets producing IFN-γ and IL-17 in human peripheral blood.
(A–B) PBMCs were obtained from the peripheral blood of healthy young (A) and elderly (B) individuals. (A) PBMCs were stimulated for 4 hours ex vivo with PMA and ionomycin or PBS (control) in the presence of Golgiplug. Cells were stained with antibodies to CD3, CD4 and CD8. Stained cells were then fixed, permeabilized and stained with antibodies to IL-17 and IFN-γ. The frequency of CD4+ T cells producing IL-17 and/or IFN-γ was measured using flow cytometry.
Figure 4. Correlation of CD4+ and CD8+ T cells producing IFN-γ and IL-17 with IgG responses to influenza vaccine in young and elderly people.
PBMCs were obtained from healthy young (n = 29) and elderly (n = 26) people before influenza vaccination. Cells were stimulated 4 hours with PMA/ionomycin or control (PBS) followed by flow cytometric analysis for CD4+ and CD8+ T cells producing IFN-γ and IL-17 as shown in Figure 3. Serum levels of IgG specific for influenza virus (A/California/7/2009 (H1N1) and A/Perth/16/2009 (H3N2)) in pre- and post-vaccination sera were determined by ELISA as described in Figure 1. Ratios of pre- and post-vaccination IgG levels were calculated and correlated with the frequency of CD4+ and CD8+ T cells producing cytokines. P values were determined by Pearson correlation analysis.
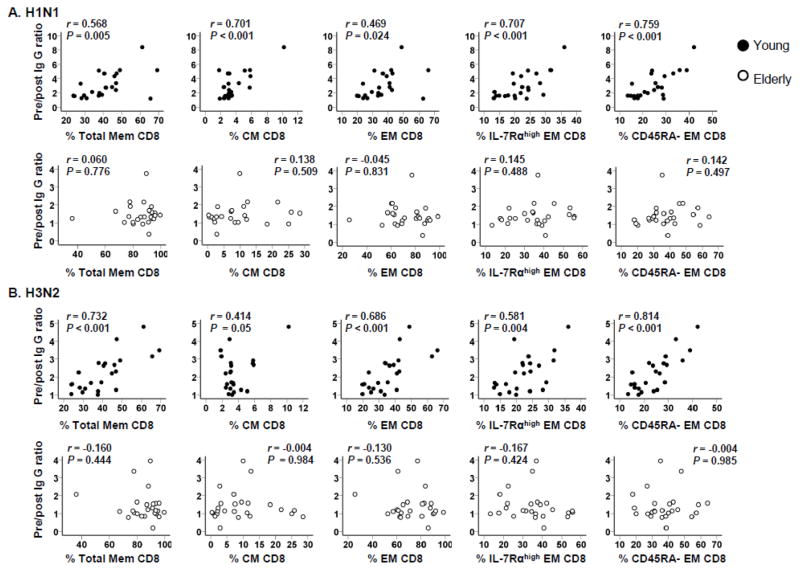
3.3. The frequency of memory T cell subsets correlates with influenza vaccine-specific IgG immune responses in the young but not in the elderly
Aging is associated with alterations in the frequency of T cell subsets [2, 7–9]. In concordance with findings from previous studies, we found that young and elderly people had an altered frequency of CD8+ T cell subsets in particular (Supplementary Table II) [13, 14]. Pre/post IgG ratios of H1N1 and H3N2 strains correlated with the frequency of memory CD8+ T cell subsets including central and effector memory cells as well as IL-7Rαhigh and CD45RA− effector memory CD8+ T cells in the young but not in the elderly (Figure 5). In contrast, the frequency of naïve CD8+ T cells inversely correlated with pre/post IgG ratios of H1N1 and H3N2 strains in the young but not in the elderly (data not shown). The correlative analysis of non-CD8+ T cells including CD4+ T cells showed similar results. The frequency of memory non-CD8+ T cells, particularly CM cells, correlated with pre/post IgG ratios of H1N1 and H3N2 strains in the young but not in the elderly (Supplementary Figure 2). There was no relationship noticed between the frequency of T cell subsets and pre/post IgG ratios of the B strain in both groups (data not show). These observations imply that aging affects the correlation of IgG responses to the frequency of T cell subsets in humans recognizing the influenza A vaccine strains.
Figure 5. Correlation of CD8+ T cell subsets with IgG responses to influenza vaccine in young and elderly people.
PBMCs from healthy young (n = 23) and elderly (n = 26) people were analyzed for naïve (CCR7+CD45RA+), central memory (CM, CCR7+CD45RA−), effector memory (EM, CCR7−CD45RA+/−), IL-7 receptor alpha high and low (IL-7Rαhigh and low) EM CD8+ T cells by flow cytometry before influenza vaccination. Serum levels of IgG specific for influenza virus (A/California/7/2009 (H1N1) and A/Perth/16/2009 (H3N2)) in pre- and post-vaccination sera were determined by ELISA as described in Figure 1. Ratios of pre- and post-vaccination IgG levels were calculated and correlated with the frequency of individual subsets of CD8+ T cells as indicated. Total memory CD8+ T cells include CM and EM CD8+ T cells. P values were determined by Pearson correlation analysis.
4. Discussion
In the current study, we investigated the relationship of serum IgG responses with HI antibody titers and the frequency of T cell subsets in young and elderly people who received influenza vaccine. Serum IgG responses to the influenza vaccine correlated with the increase of HI antibody titers in young people. However, a similar correlation was not found in elderly people. The frequency of CD4+ T cells producing IFN-γ and IL-17 correlated with IgG responses to influenza A viral strains included in the vaccine in young but not in elderly people. Also, only in the young, such IgG responses correlated with the frequency of memory T cells, especially CM, CD45RA− EM and IL-7Rαhigh EM CD8+ T cells with strong cell survival and proliferative capacity [13, 32]. These findings suggest that aging affects the association of IgG and HI antibody responses specific for influenza vaccine as well as the relationship of the cytokine-producing capacity and the proportions of memory T cells with influenza vaccine-specific IgG responses in humans.
Influenza vaccination induces both humoral and cellular immune responses [22]. Before influenza vaccination, elderly people in our study cohort had higher levels of IgG antibodies specific for H1N1 and H3N2 strains compared to young people. However, after vaccination, the increase of these antibodies was much higher in young people than in elderly people, suggesting reduced IgG responses to influenza vaccine with aging. Higher levels of IgG antibodies to H1N1 and H3N2 strains in elderly people could reflect previous infections and/or vaccination, which may affect the antibody response to subsequent vaccination. In our study, young and elderly people had similar rates of influenza vaccination in the year prior to the study participation (number of vaccinated subjects/total number of subjects, 26 of 29 vs. 26 of 26, respectively, P = 0.238 by Fisher’s exact test). Traditionally, humoral immune responses to influenza vaccine have been determined by the HI assay although the inactivated viruses in the vaccine contain additional antigens which can induce antibody production. In our study, we found a strong correlation between IgG responses and HI antibody titers for individual strains of influenza virus in the vaccine after vaccination in the young but not in the elderly. In accordance with our finding on young individuals, a recent study reported the correlation of HI antibody titers and the frequency of plasmablasts producing IgG specific for influenza vaccine [33]. An alteration in B cell repertoire also occurs with aging [34]. In addition, elderly people had a decreased frequency of influenza-vaccine specific plasmablasts compared to young people [35]. Such alterations could be responsible for the lack of the correlation between the total Ig responses and HI antibody titers in the elderly, leading to the generation of humoral responses to limited sets of antigens.
T cell immunity plays a critical role in host defense against microorganisms. This is achieved by providing help to other immune cells such as B cells as well as directly killing pathogen-infected cells [2]. T cell-derived cytokines including IFN-γ and IL-17 are known to promote humoral immunity. In fact, higher levels of IFN-γ and lower levels of IL-10 production from influenza virus-stimulated PBMCs better predicted protection from the influenza vaccine in the elderly compared to HI antibody titers [36]. A recent study showed that the influenza virus-infected IL-17 knockout mice had worse lung immunopathology and reduced survival with decreased B cell migration to the lung compared to infected wild-type mice [37]. Previous studies reported impaired CD4+ and CD8+ T cell responses to influenza vaccine in elderly people compared to young people [22, 23, 25, 26]. In our study, we found that the overall capacity of CD4+ T cells to produce IFN-γ and IL-17 in response to PMA and ionomycin correlated with the subsequent generation of IgG responses to influenza virus H1N1 and H3N2 in young, but not elderly people. This finding suggests that aging affects the relationship of such cytokine-producing capacity with IgG immune responses although the exact mechanism is unknown. A possible explanation could be related to any previous exposure to the same or similar influenza virus in elderly people, leading to the generation of long-lived plasma and memory B cells. Influenza vaccination could induce the development of secondary antibody response from these cells, which may not be as dependent on T cells as that of primary antibody response. In our study, the frequency of cytokine-producing CD4+ T cells and memory T cell subsets correlated with IgG responses specific for H1N1 and H3N2 strains but not for B strain in the young. It is unclear why such discrepancy occurred. A possible explanation for this finding could be the sample size of our study. Alternatively, our study population could have a recent influenza B infection affecting B cell repertoire.
Memory T cells that have previously experienced antigen stimulation can rapidly expand and produce cytokines upon encountering the same antigen. Aging is known to be associated with the expansion of memory T cells, especially memory CD8+ T cells, reflecting past microbial exposure [2, 3]. Although it is conceivable that such expansion of memory T cells is beneficial to host defense, it is unclear whether it could also help vaccine responses in young and elderly people. In the elderly, the accumulation of memory CD8+ T cells expressing CD45RA or lacking CD28 expression, which may represent a functionally senescent cell population, was associated with a diminished increase in serum HI antibody titers after influenza vaccination [27–29]. On the contrary, in influenza vaccinated-elderly people, the development of protective antibodies correlated with an increased frequency of memory CD8+ T cells that expressed CD62L, a selectin-type adhesion molecule acting as a lymphoid tissue homing receptor, after influenza vaccination [38]. T cells with the lymphoid tissue homing capacity can provide help to B cells in the lymphoid tissue like the spleen and lymph nodes where T and B cells interact to generate antibody producing cells [39]. These observations suggest that the expansion of different memory T cell subsets could have distinct effects in the development of humoral immune responses to influenza vaccine. Of interest, in our study, we found that the young but not the elderly had a positive correlation of the frequency of memory T cells, especially CCR7+ CM T cells with the capacity to migrate to lymph nodes and spleen, with IgG responses to H1N1 and H3N2 strains included in the vaccine. Similarly, the latter IgG responses correlated with the frequency of IL-7Rαhigh EM CD8+ T cells and CD45RA− EM CD8+ T cells. This observation is intriguing since IL-7Rαhigh EM CD8+ T cells and CD45RA− EM CD8+ T cells are known to have potent cell survival and proliferative capacity [13, 32]. The immune response induced by one strain of influenza A virus can provide a certain level of protection against infection with a different strain. This phenomenon of so-called heterosubtypic immunity involves antibodies and T cells which are cross-reactive to both strains of the virus [40]. It is possible that the correlation of IgG responses to influenza vaccine with the frequency of memory T cells in the young could be secondary in part to such heterosubtypic immunity. Despite the expansion of the memory T cell population with aging, this correlation was absent in the elderly. Reduced T cell repertoire diversity associated with aging could be a possible explanation for this lack of correlation [13, 22].
Taken together, our study showed that serum IgG responses to the influenza vaccine correlated with the increase of HI antibody titers in the young but not in the elderly. Similarly, the frequency of CD4+ T cells producing IFN-γ and IL-17 correlated with IgG responses to influenza A virus in the vaccine in the young but not in the elderly. In addition, only in the young, such IgG responses correlated with the frequency of memory T cells, especially CM, IL-7Rαhigh EM and CD45RA− EM CD8+ T cells with the potent cell survival and proliferative capacity [13, 32]. These findings suggest that aging alters the association of IgG and HI antibody responses specific for influenza vaccine as well as the relationship of cytokine-producing capacity and the proportions of memory T cells to the development of influenza vaccine-specific IgG responses in humans.
Supplementary Material
Highlights.
IgG responses to influenza vaccine decreased with aging
Association of IgG responses and HI titers in influenza vaccine altered with aging
Aging affected the link of T cell cytokines with IgG responses to influenza vaccine
Aging altered the link of T cell proportions with IgG responses to influenza vaccine
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (AG028069, AG030834 to IK; U19 AI089992 and contract 272201100019C-3-0-1 to R.R.M. and A.C.S; and K24 AG042489 to A.C.S). We thank Dr. Michael Decker at Sanofi-Pasteur US for providing inactivated influenza virus.
Abbreviations used in this paper
- TCR
T cell receptor
- HI
hemagglutinin inhibition
- CM
central memory (CM)
- IL-7Rαhigh
IL-7 receptor alpha high
- EM
effector memory
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller RA. Aging and Immune Function. In: Paul WE, editor. Fundamental Immunology. Lippincott-Raven; Philadelphia: 1999. pp. 947–966. [Google Scholar]
- 2.Lee N, Shin MS, Kang I. T-cell biology in aging, with a focus on lung disease. J Gerontol A Biol Sci Med Sci. 2012;67:254–263. doi: 10.1093/gerona/glr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorshkind K, Montecino-Rodriguez E, Signer RA. The ageing immune system: is it ever too old to become young again? Nat Rev Immunol. 2009;9:57–62. doi: 10.1038/nri2471. [DOI] [PubMed] [Google Scholar]
- 4.Kogut I, Scholz JL, Cancro MP, Cambier JC. B cell maintenance and function in aging. Semin Immunol. 2012 doi: 10.1016/j.smim.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 5.McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol. 2009;21:418–424. doi: 10.1016/j.coi.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol. 2005;17:468–475. doi: 10.1016/j.coi.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Akbar AN, Fletcher JM. Memory T cell homeostasis and senescence during aging. Curr Opin Immunol. 2005;17:480–485. doi: 10.1016/j.coi.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Effros RB, Cai Z, Linton PJ. CD8 T cells and aging. Crit Rev Immunol. 2003;23:45–64. doi: 10.1615/critrevimmunol.v23.i12.30. [DOI] [PubMed] [Google Scholar]
- 9.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JS, Lee WW, Kim SH, Kang Y, Lee N, Shin MS, Kang SW, Kang I. Age-associated alteration in naive and memory Th17 cell response in humans. Clin Immunol. 2011;140:84–91. doi: 10.1016/j.clim.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 12.Kim HR, Hwang KA, Park SH, Kang I. IL-7 and IL-15: biology and roles in T-Cell immunity in health and disease. Crit Rev Immunol. 2008;28:325–339. doi: 10.1615/critrevimmunol.v28.i4.40. [DOI] [PubMed] [Google Scholar]
- 13.Kim HR, Hong MS, Dan JM, Kang I. Altered IL-7R{alpha} expression with aging and the potential implications of IL-7 therapy on CD8+ T-cell immune responses. Blood. 2006;107:2855–2862. doi: 10.1182/blood-2005-09-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong MS, Dan JM, Choi JY, Kang I. Age-associated changes in the frequency of naive, memory and effector CD8+ T cells. Mech Ageing Dev. 2004;125:615–618. doi: 10.1016/j.mad.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010;11:674–680. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20:4–12. doi: 10.1038/cr.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, Fabien N, Cochat P, Pouteil-Noble C, Trolliet P, Durieu I, Tebib J, Kassai B, Ansieau S, Puisieux A, Eliaou JF, Bonnefoy-Berard N. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 18.Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, Vitetta ES. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 19.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. Jama. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 20.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 21.Chen WH, Kozlovsky BF, Effros RB, Grubeck-Loebenstein B, Edelman R, Sztein MB. Vaccination in the elderly: an immunological perspective. Trends Immunol. 2009;30:351–359. doi: 10.1016/j.it.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McElhaney JE. Influenza vaccine responses in older adults. Ageing Res Rev. 2011;10:379–388. doi: 10.1016/j.arr.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murasko DM, Bernstein ED, Gardner EM, Gross P, Munk G, Dran S, Abrutyn E. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol. 2002;37:427–439. doi: 10.1016/s0531-5565(01)00210-8. [DOI] [PubMed] [Google Scholar]
- 24.Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, Chen S, Towle V, Belshe RB, Fikrig E, Allore HG, Montgomery RR, Shaw AC. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010;184:2518–2527. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou X, McElhaney JE. Age-related changes in memory and effector T cells responding to influenza A/H3N2 and pandemic A/H1N1 strains in humans. Vaccine. 2011;29:2169–2177. doi: 10.1016/j.vaccine.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang I, Hong MS, Nolasco H, Park SH, Dan JM, Choi JY, Craft J. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J Immunol. 2004;173:673–681. doi: 10.4049/jimmunol.173.1.673. [DOI] [PubMed] [Google Scholar]
- 27.Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol. 2002;105:117–125. doi: 10.1006/clim.2002.5271. [DOI] [PubMed] [Google Scholar]
- 28.Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O’Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75:12182–12187. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Blasko I, Parson W, Bock G, Schonitzer D, Trannoy E, Grubeck-Loebenstein B. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 30.Hwang KA, Kim HR, Kang I. Aging and human CD4(+) regulatory T cells. Mech Ageing Dev. 2009;130:509–517. doi: 10.1016/j.mad.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee WW, Shin MS, Kang Y, Lee N, Jeon S, Kang I. The relationship of cytomegalovirus (CMV) infection with circulatory IFN-alpha levels and IL-7 receptor alpha expression on CD8(+) T cells in human aging. Cytokine. 2012 doi: 10.1016/j.cyto.2012.03.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260–4266. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 33.Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, Means AR, Kasturi SP, Khan N, Li GM, McCausland M, Kanchan V, Kokko KE, Li S, Elbein R, Mehta AK, Aderem A, Subbarao K, Ahmed R, Pulendran B. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol. 2011;12:786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson KL, Wu YC, Barnett Y, Duggan O, Vaughan R, Kondeatis E, Nilsson BO, Wikby A, Kipling D, Dunn-Walters DK. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009;8:18–25. doi: 10.1111/j.1474-9726.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasaki S, Sullivan M, Narvaez CF, Holmes TH, Furman D, Zheng NY, Nishtala M, Wrammert J, Smith K, James JA, Dekker CL, Davis MM, Wilson PC, Greenberg HB, He XS. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest. 2011;121:3109–3119. doi: 10.1172/JCI57834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, Ewen C, Kane KP, Bleackley RC. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176:6333–6339. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Chan CC, Yang M, Deng J, Poon VK, Leung VH, Ko KH, Zhou J, Yuen KY, Zheng BJ, Lu L. A critical role of IL-17 in modulating the B-cell response during H5N1 influenza virus infection. Cell Mol Immunol. 2011;8:462–468. doi: 10.1038/cmi.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwaiger S, Wolf AM, Robatscher P, Jenewein B, Grubeck-Loebenstein B. IL-4-producing CD8+ T cells with a CD62L++(bright) phenotype accumulate in a subgroup of older adults and are associated with the maintenance of intact humoral immunity in old age. J Immunol. 2003;170:613–619. doi: 10.4049/jimmunol.170.1.613. [DOI] [PubMed] [Google Scholar]
- 39.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 40.Kreijtz JH, Fouchier RA, Rimmelzwaan GF. Immune responses to influenza virus infection. Virus Res. 2011;162:19–30. doi: 10.1016/j.virusres.2011.09.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.