Abstract
MicroRNAs (miRNAs) are small, endogenous, non-protein–coding RNAs that are an important means of posttranscriptional gene regulation. Deletion of Dicer, a key miRNA processing enzyme, is embryonic lethal in mice, and tissue-specific Dicer deletion results in developmental defects. Using a conditional knockout model, we generated mice lacking Dicer in the adrenal cortex. These Dicer-knockout (KO) mice exhibited perinatal mortality and failure of the adrenal cortex during late gestation between embryonic day 16.5 (E16.5) and E18.5. Further study of Dicer-KO adrenals demonstrated a significant loss of steroidogenic factor 1-expressing cortical cells that was histologically evident as early as E16.5 coincident with an increase in p21 and cleaved-caspase 3 staining in the cortex. However, peripheral cortical proliferation persisted in KO adrenals as assessed by staining of proliferating cell nuclear antigen. To further characterize the embryonic adrenals from Dicer-KO mice, we performed microarray analyses for both gene and miRNA expression on purified RNA isolated from control and KO adrenals of E15.5 and E16.5 embryos. Consistent with the absence of Dicer and the associated loss of miRNA-mediated mRNA degradation, we observed an up-regulation of a small subset of adrenal transcripts in Dicer-KO mice, most notably the transcripts coded by the genes Nr6a1 and Acvr1c. Indeed, several miRNAs, including let-7, miR-34c, and miR-21, that are predicted to target these genes for degradation, were also markedly down-regulated in Dicer-KO adrenals. Together these data suggest a role for miRNA-mediated regulation of a subset of genes that are essential for normal adrenal growth and homeostasis.
The adrenal glands are bilateral structures located superior to the kidneys that have essential functions in maintaining electrolyte and metabolic homeostasis as well as in regulating the stress response. The adrenal is comprised of two embryologically and functionally distinct cell types: the adrenal cortex, which is derived from the coelomic epithelia and intermediate mesoderm known as the urogenital ridge, and the adrenal medulla, which is comprised of neuroendocrine cells derived from the neural crest (1, 2).
The adrenal cortex initially forms as a coalescence of cells known as the adrenogonadal primordium (AGP) at approximately embryonic day 9 (E9.0) (3). It is at this time that steroidogenic factor 1 (Sf1), a key regulator of steroidogenic enzymes in the adrenal cortex and steroid-secreting cells of the gonads, begins to be expressed in the AGP (4). By E12.0, a distinct adrenal primordium consisting of fetal adrenocortical cells separates from the AGP. Medullary precursor cells from the neural crest begin migrating into and populating the fetal adrenal cortex (1). Shortly thereafter, mesenchymal cells from the surrounding stroma coalesce to form the adrenal capsule, which is where a population of adrenocortical precursor cells resides (5). As development progresses, the fetal adrenal cortex is replaced by the adult or definitive cortex, which contains peripheral stem/progenitor cells that continuously replenish the dying cells of the inner gland throughout the life of the organism (6).
MicroRNAs (miRNAs) are short, endogenous, noncoding RNA transcripts first described in Caenorhabditis elegans (7). The canonical function of miRNAs is the posttranscriptional regulation of gene expression, a process mediated by the binding of a given miRNA to partially complementary sequences in the 3′-untranslated region (UTR) of the target gene mRNA. In conjunction with a protein complex, known as the miRNA-induced silencing complex (miRISC), miRNAs bind to target mRNA transcripts to inhibit translation either by destabilizing the target transcript and facilitating degradation or by inhibiting the translational machinery (8, 9). Both mechanisms have the effect of subsequently inhibiting the protein expression of specific genes within a cell, providing an additional layer of regulatory control over gene expression.
Dicer is the ribonuclease III enzyme required for maturation of pre-miRNAs into double-stranded miRNAs. Mice deficient in Dicer do not survive beyond E8.5, indicating that miRNAs are crucial for normal development (10). Recent studies involving tissue-specific Dicer-knockout (KO) mice reveal that Dicer is required for normal organogenesis and maintenance in a variety of tissues including heart, lung, skin, muscle, and the adrenal gland (11–18). Dicer has been shown to be required for the maintenance of both embryonic and tissue stem cells (19, 20), suggesting a role for Dicer and miRNA expression in regulating organ formation and/or homeostasis.
In this study, we used a genetic approach to ablate Dicer in the steroidogenic cells of the adrenal cortex. The resulting adrenocortical Dicer-KO mice displayed normal adrenal development through E14.5. However, by E18.5, the adrenal cortex had completely failed, resulting in the absence of cortical tissue, consistent with the similar results of a previous report (18). miRNA and mRNA array analyses showed that adrenals from Dicer-KO mice had distinct expression profiles relative to wild-type (WT) controls, including the up-regulation of Nr6a1 and Acvr1c and concurrent down-regulation of several miRNAs, most notably let-7, miR-101b, miR-10a, and miR-21. Importantly, these down-regulated miRNAs are predicted to target the mRNA transcripts that are up-regulated in Dicer-KO adrenals.
Materials and Methods
Mice
All experiments involving mice were performed in accordance with an institutionally approved protocol under the auspice of the University Committee on Use and Care of Animals at the University of Michigan. Veterinary care was provided by the Unit for Laboratory Animal Medicine staff at the University of Michigan based on standards in the Guide for Care and Use of Laboratory Animals, the Animal Welfare Act Regulations, and the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Sf1-Cre mice were obtained and described previously (21, 22). Mice carrying the floxed Dicer allele (Dicer1tm1Bdh/J) were purchased from The Jackson Laboratory (Bar Harbor, Maine). To obtain Sf1-Cre/Dicerlox/lox mice, Sf1-Cre/Dicer+/lox, and Dicerlox/lox mice were mated together. Females from each mating pair were monitored for seminal plugs, and the morning of detection was designated as E0.5. Pregnant females were killed at the designated time points, and harvested embryos were staged using Theiler staging criteria (www.emouseatlas.org). Genotyping for the Sf1-Cre and Dicerlox allele was performed as previously described (21, 23).
Adrenal histology, immunohistochemistry, and immunofluorescence
Tissues were fixed and paraffin embedded as previously described (22), and 7-μm sections were cut and placed on microscope slides for further manipulation. Antigen retrieval for immunohistochemistry was performed as described previously (22). Antibody staining was performed with VECTASTAIN ABC kits (Vector Laboratories, Burlingame, California) according to the manufacturer's protocol. Tissue sections were blocked in antibody diluent solution for 1 hour at room temperature and then incubated overnight at 4°C with anti-p21 (1:100; BD Pharmingen, San Diego, California), anti–cleaved-caspase 3 (1:100; Cell Signaling Technology, Danvers, Massachusetts) or anti–phospho-H2A.X (1:50; Cell Signaling). The following day, sections were washed and incubated with biotinylated secondary antibodies for 1 hour at room temperature and subsequent staining via 3,3′-diaminobenzidine (DAB) (Sigma, St Louis, Misouri) was performed according to the manufacturer's instructions. DAB-stained tissue sections were then counterstained with either diluted (1:10 deionized water) eosin or hematoxylin. Coverslips were mounted using Permount (ThermoFisher, Waltham, Massachusetts) and sections imaged using light microscopy.
Immunofluorescence was carried out in a similar manner except that tissue sections were blocked with PBS/2% nonfat dry milk/2% normal goat serum. Sections were then incubated overnight at 4°C with anti-Sf1 (1:1000, custom antibody), anti-tyrosine hydroxylase (1:300; Millipore, Billerica, Massachusetts), anti-proliferating cell nuclear antigen (PCNA) (1:500; Santa Cruz Biotechnology, Santa Cruz, California), anti-CD3 (1:250; Abcam, Cambridge, Massachusetts), anti-CD68 (1:200; Abcam), or anti-CD20 (1:100; Santa Cruz). The following morning, slides were washed and incubated with Dylight 488-conjugated goat antirabbit or Dylight 549-conjugated goat antimouse (1:1000; Jackson ImmunoResearch Laboratories, West Grove, Pennsylvania) in the dark at room temperature for 1 hour. All antibodies were diluted in PBS containing 0.2% nonfat dry milk and 0.2% normal goat serum. The fluorescently labeled tissue sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (1:1000; Sigma-Aldrich, St Louis, Missouri) and coverslips mounted using Tris-buffered Fluorogel (Electron Microscopy Sciences, Hatfield, Pennsylvania). Sections were visualized by fluorescent microscopy.
Hematoxylin and eosin staining was performed by standard procedures.
Microarrays
Timed matings were established to generate Sf1-Cre/Dicerlox/lox KO embryos. At E15.5 and E16.5, embryos were harvested from pregnant females, and adrenals from each embryo were microdissected and stored separately in RNAlater solution (Life Technologies, Carlsbad, California) until genotyping confirmed Dicer KO status. The adrenals from control and Dicer-KO littermates were pooled separately from 4 individual litters, resulting in a total of 4 control (cre−) and 4 Dicer-KO biological replicates at both E15.5 and E16.5. Total RNA was isolated using the RNAqueous Micro kit (Life Technologies, Carlsbad, California) in accordance with the manufacturer's protocol to preserve small RNA recovery. Isolated RNA was quantified on a Nanodrop 2000c spectrophotometer (Thermo Scientific, Wilmington, Delaware) and submitted to the University of Michigan Microarray Core Facility where samples were quality checked and finally analyzed with both Affymetrix Mouse 430 version 2.0 gene expression arrays and ABI miRNA OpenArrays. RNA preparation for the ABI miRNA OpenArray platform was performed according to the manufacturer's protocols (Applied Biosystems, Carlsbad, California). Complete data from both arrays can be found in the Gene Expression Omnibus (GEO) series at accession number GSE45812.
Quantitative real-time PCR
Up to 1 μg of total RNA isolated from E15.5 and E16.5 embryonic adrenals was reverse transcribed using the iScript system (Bio-Rad Laboratories, Hercules, California) to generate cDNA. The resulting cDNA was amplified with appropriate primers using Power SYBR Green PCR Master Mix and analyzed on an ABI 7300 real-time PCR system. Data analysis was performed using the 2−ΔΔCT method (24). Gene expression was normalized to mouse β-actin. Primers for each amplified gene are as follows: β-actin (Actb), forward 5′-CTAAGGCCAACCGTGAAAAG-3′ and reverse 5′-ACCAGAGGCATACAGGGACA-3′; Nr6a1 isoform 1 (Nr6a1_1), forward 5′-GCAACGGTTTCTGTCAGGAT-3′ and reverse 5′-GCCAAGTGTTAAACTGTCAAGTCTCT-3′; Nr6a1 isoform 2 (Nr6a1_2), forward 5′-GCTTGCCAGAGATCCGATAC-3′ and reverse 5′-AGTGCAGCACCACCTTAAAGA-3′; and Nr6a1 isoform 3 (Nr6a1_3), forward 5′-GAGAGCAACCAGCCCTCA-3′ and reverse 5′-ATCCCTGAATGCCATGAATC-3′.
Statistical analysis
Microarray data were normalized using the robust multiarray average (RMA) algorithm (25). miRNA data were normalized to the U6 rRNA value on the corresponding subpanel of the OpenArray. For both types of arrays, differential gene expression between conditions was determined using the limma package by applying linear modeling followed by the empirical Bayes method to compute significance (26). The resulting P values were adjusted for multiple testing by the Benjamini-Hochberg method (27). Genes with an absolute log2-fold change greater than or equal to 1.5 with an adjusted P value < .05 were considered differentially expressed and statistically significant. For DAVID analysis, each collection of differentially expressed genes was evaluated for gene-enrichment by submitting the Entrez Gene identifiers to DAVID (david.abcc.ncifcrf.gov) and running the default analysis. Functional classifications with a false discovery rate (FDR) ≤5% were considered significant (28). A modified Fisher's exact test was used to assess over representation of predicted binding sites for each of the differentially expressed miRNAs in the 3′-UTRs of the up-regulated gene list.
Results
Sf1-Cre/Dicerlox/lox (Dicer-KO) mice die shortly after birth
The Cre-mediated excision of Dicer in the developing adrenal cortex resulted in a marked adrenal defect that proved to be lethal. Based on our breeding strategy, embryonic and postweaning offspring were expected to demonstrate Mendelian genotypic ratios in which 25% of progeny should have been positive for the Sf1-Cre/Dicerlox/lox genotype. However, of 52 mice genotyped at weaning, none were Dicer-KO animals. The expected Mendelian ratios for the Sf1-Cre/Dicerlox/lox genotype were seen only at the embryonic stages. Of 96 embryos tested, 25 were Sf1-Cre/Dicerlox/lox. Mortality among Sf1-Cre/Dicerlox/lox animals occurred 1 to 2 days after parturition, with no Sf1-Cre/Dicerlox/lox offspring surviving beyond this time point. We conclude that Sf1-Cre/Dicerlox/lox offspring invariably died shortly after birth, presumably due to adrenal failure. This perinatal lethality in Dicer-KO animals is supported by a previous report (18).
Dicer-KO mice exhibit adrenal failure late in embryonic development
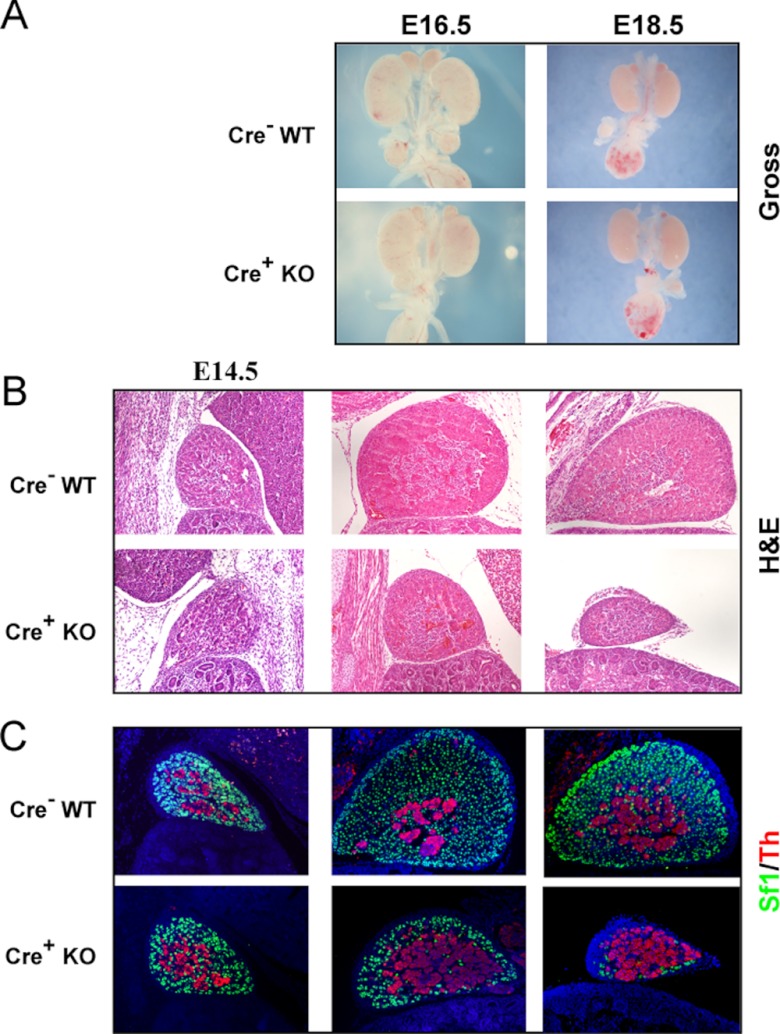
To determine the developmental stage at which Dicer is essential for adrenal organogenesis, we performed detailed analyses of adrenals from embryos at 3 embryonic time points: E14.5, E16.5, and E18.5. Grossly, there was no appreciable difference between control and Dicer-KO adrenals at E14.5 or E16.5. However, the size of the adrenals in E18.5 Dicer-KO animals was markedly smaller than control counterparts (Figure 1A), consistent with either growth failure or destruction of the developing adrenal cortex. We then analyzed the histology of Dicer-KO adrenals. We were unable to detect significant histological changes at E14.5 (Figure 1B) between control and Dicer-KO adrenals. This implied that the fetal adrenal cortex in Dicer-KO animals underwent normal specification and formation and that Dicer loss in Sf1-positive fetal adrenal cells was not detrimental to early adrenal development. However, as shown in Figure 1B, the adrenal cortex from E16.5 Dicer-KO animals appeared smaller in cross-sections but maintained distinct cortical and medullary demarcations. The most significant phenotype in Dicer-KO animals occurred at E18.5. Adrenals from Dicer-KO animals at this time point demonstrated a marked loss of Sf1-expressing cortical cells, albeit with some E18.5 Dicer-KO adrenals exhibiting more residual Sf1-expressing cortical cells than others (compare Figures 1C and 2B), consistent with the stochastic nature of cre-mediated excision that, together with other genetic and environmental factors, routinely results in phenotypic variability. Regardless, there was a consistent, dramatic loss of Sf1-expressing cortical cells in all E18.5 Dicer-KO adrenals compared with control (cre− littermates) adrenals. Interestingly, the adrenal medulla, which is derived from a separate cell lineage than the cortex, persisted in the Dicer-KO adrenals.
Figure 1.
Analysis of adrenals from Sf1-Cre/Dicerlox/lox mice. A, Gross photos of adrenals and kidneys from E16.5 and E18.5 Dicer-KO (cre+ KO) embryos and cre− littermate controls (WT). Photos of the E16.5 time point were taken at a 2× higher magnification than the E18.5 photos. B and C, Histological comparison of adrenal sections from Dicer-KO (cre+ KO) embryos and cre− littermate controls (WT) at E14.5, E16.5, and E18.5 by hematoxylin and eosin (H&E) (B) and immunofluorescent straining (C) for Sf1 and tyrosine hydroxylase (Th). In C, sections were counterstained with DAPI (blue) before visualization and images were merged to show colocalization. Images in B and C are taken at ×100 magnification.
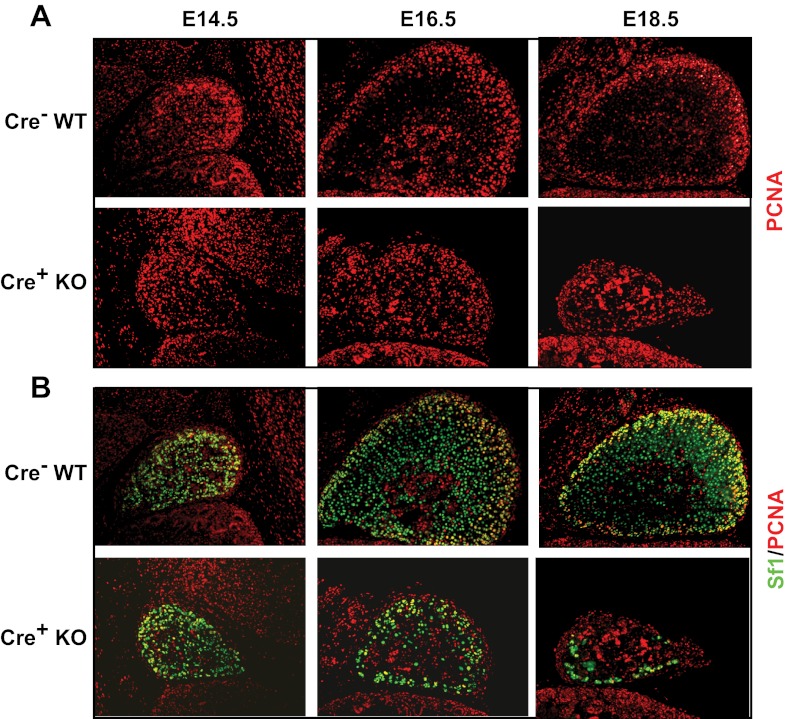
Figure 2.
Assessment of proliferation in adrenals from Sf1-Cre/Dicerlox/lox mice. Immunofluorescent staining of adrenals from Dicer-KO (cre+ KO) embryos and cre− littermate controls (WT) at E14.5, E16.5, and E18.5. A, PCNA alone. B, Sf1 (green) and PCNA (red). Images are taken at ×100 magnification.
Immunofluorescent staining was also performed on control and Dicer-KO adrenal sections. At E14.5, both the adrenal cortex and medulla appeared to be intact, as evidenced by anti-Sf1 (cortex-specific) and anti-tyrosine hydroxylase (medulla-specific) staining (Figure 1C). However, at E16.5, the Sf1-positive cortex is dramatically thinner in the KO mice compared with control mice. By E18.5, Dicer-KO animals had very few residual Sf1-expressing adrenocortical cells. Tyrosine hydroxylase-expressing medullary cells persisted at E18.5 in Dicer-KO adrenals, despite the severe cortical failure (Figure 1C).
In summary, Dicer-KO adrenals initiate organogenesis normally, as evidenced by the formation of the fetal adrenal cortex, followed by the normal infiltration of neural crest-derived medullary precursor cells and encapsulation by mesenchymal cells of the surrounding intermediate mesoderm. However, beginning at E16.5, Dicer-KO adrenals exhibited a gradual loss of Sf1-positive cortical cells that resulted in the complete absence of the cortex by E18.5. This marked phenotype was incompatible with life, as evidenced by the perinatal lethality observed in Dicer-KO animals.
Embryonic Dicer-KO adrenals exhibit normal proliferation but evidence of increased cell death
We next examined cortical cell proliferation and apoptosis. In the adrenal, proliferating cortical cells are most abundant in the outer peripheral region of the newly forming definitive cortex (29). Adrenal sections from control and Dicer-KO embryos were co-stained with anti-Sf1 and anti-PCNA to localize proliferating cells. Staining with PCNA alone is shown in Figure 2A and the colocalization in Figure 2B. Our results demonstrated that proliferating cells localized to the peripheral cortex remained detectable in Dicer-KO adrenals, suggesting that loss of Dicer in definitive cortical cells did not preclude the proliferation of peripheral cells. However, although overall proliferation in the adrenal cortex (reflected in PCNA immunoreactivity) did not differ between control and KO adrenals, the number of PCNA-stained Sf1-positive cells did appear to be somewhat diminished in Dicer-KO adrenals, particularly in the older E18.5 mice. However, the decrease in number of Sf1-expressing cells in E18.5 Dicer-KO adrenals may be confounding the interpretation of whether loss of proliferation resulted in decreased Sf1 cells or vice versa.
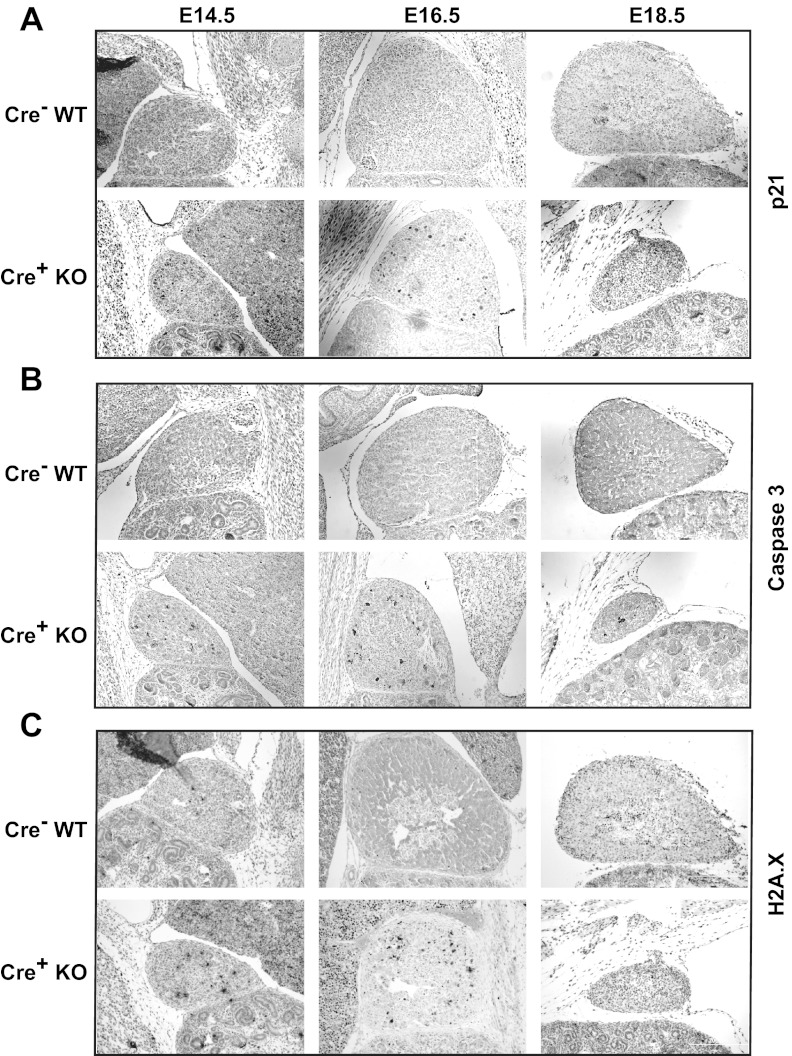
Therefore, we then determined whether Dicer loss in the adrenal cortex induced cell cycle arrest and apoptosis. In adrenals from E14.5 embryos, staining for the apoptosis markers, Cdkn1a (p21) and cleaved-caspase 3, was limited primarily to the cortex and was clearly increased in Dicer-KO adrenals compared with their WT littermates (Figure 3, A and B). This was surprising because we were unable to otherwise appreciate an apparent phenotype in Dicer-KO adrenals at this time point. A similar increase in apoptotic markers was seen at E16.5. In addition, phospho-γ-H2A.X staining was present in Dicer-KO adrenals at E14.5 and E16.5, consistent with double-stranded DNA damage (Figure 3C). In summary, Dicer-KO adrenals showed evidence of increased cell cycle arrest and apoptotic activity in the cortex, consistent with the loss of Sf1-positive cortical cells observed at E18.5.
Figure 3.
Assessment of cell cycle arrest/apoptosis and DNA damage in adrenals from Sf1-Cre/Dicerlox/lox mice. DAB immunohistochemistry of adrenals Dicer-KO (cre+ KO) embryos and cre− littermate controls (WT) at E14.5, E16.5, and E18.5. A, p21 (Cdkn1a), a cell cycle inhibitor. B, Cleaved-caspase 3, an apoptotic marker. C, Phospho-γ-H2A.X, an indicator of DNA damage repair. Tissues were counterstained with hematoxylin or eosin. Images are taken at ×100 magnification.
Dicer-KO adrenals demonstrate a unique mRNA expression profile
Dicer-KO mice demonstrated marked hypoplasia of the adrenal cortex that began with increased cellular death at E14.5 and continued until E18.5, at which time the adrenals demonstrated a marked decrease in Sf1-expressing cortical cells. Whether this adrenal failure reflected dysregulation of miRNA biogenesis or a cellular toxicity effect resulting from the accumulation of unprocessed pre-miRNAs is unclear. Further analysis assessed gene transcript expression in both control and Dicer-KO adrenals at E15.5 and E16.5.
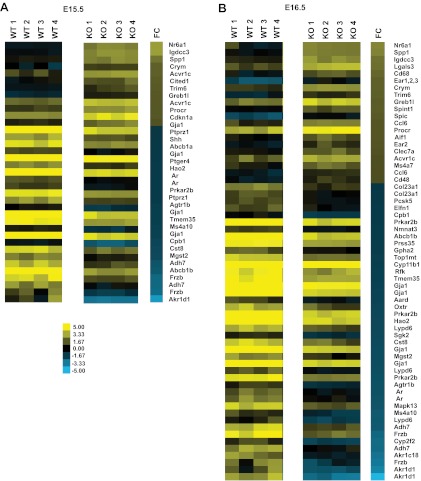
Figure 4 shows heatmaps from Affymetrix arrays illustrating the differentially expressed gene transcripts in Dicer-KO adrenals compared with control adrenals at E15.5 (Figure 4A) and E16.5 (Figure 4B). Shown are only those genes that have greater than 1.5-fold change and P ≤ .05. In E15.5 Dicer-KO adrenals, 10 differentially up-regulated and 19 differentially down-regulated genes were observed relative to control adrenals. Similarly, there were fewer differentially up-regulated genes in E16.5 Dicer-KO adrenals than differentially down-regulated genes (19 up-regulated vs 31 down-regulated). Most of these differentially expressed genes were observed in both E15.5 and E16.5 in Dicer-KO adrenals (detailed in Table 1).
Figure 4.
Gene expression differences between adrenals from control and Sf1-Cre/Dicerlox/lox embryos at E15.5 and E16.5. Heatmaps generated from Affymetrix gene expression arrays illustrate differentially expressed genes in adrenals from Dicer-KO and control (WT, cre− littermates) embryos at E15.5 (A) and E16.5 (B). Data were filtered by excluding probe sets with an FDR of ≥.05 and a log2-fold change of ≤1.5 or ≥−1.5. Yellow bars indicate an increase over the mean chip intensity, and blue indicates a decrease over mean intensity. FC represents log2-fold change between the 4 Dicer-KO litters/replicates compared with the 4 WT litters/replicates.
Table 1.
Differentially Expressed mRNAs and miRNAs in Adrenals From Dicer-Deficient Mice Compared With WT Littermates
| Genes Differentially Expressed in Adrenals from E15.5 and E16.5 Dicer-KO Embryos |
miRNAs Decreased in Both E15.5 and E16.5 Dicer-KO Embryos | |
|---|---|---|
| Increased in Dicer-KO | Decreased in Dicer-KO | |
| Acvr1c | Abcb1b | miR-674* |
| Crym | Adh7 | miR-10a |
| Greb1l | Agtr1b | miR-21 |
| Igdcc3 | Akr1d1 | miR-501* |
| Nr6a1 | Ar | let-7d |
| Procr | Cpb1 | miR-107 |
| Spp1 | Cst8 | miR-672 |
| Trim6 | Frzb | miR-34c |
| Gja1 | miR-34b-3p | |
| Hao2 | miR-292-3p | |
| Mgst2 | miR-193* | |
| Ms4a10 | miR-202 | |
| Prkar2b | miR-293 | |
| miR-365 | ||
| miR-34c* | ||
| miR-674 | ||
Differentially down-regulated gene transcripts in Dicer-KO adrenals included those related to steroidogenic pathways, such as Cyp11b1, and 2 of the most down-regulated transcripts common to both E15.5 and E16.5 time points, Akr1d1 and Adh7. Additionally, Frzb, a secreted Wnt antagonist, was also highly down-regulated in E15.5 and E16.5 Dicer-KO adrenals. This result was intriguing, as data from our lab and others demonstrate a role for the Wnt/β-catenin signaling pathway in adrenal development and maintenance and in the pathology of adrenocortical neoplasia (22, 30, 31).
When we compared all of the differentially up-regulated transcripts in Dicer-KO adrenals relative to control adrenals at E15.5 and E16.5, genes related to inflammatory and immune processes appeared to be overrepresented in the data. We performed a DAVID (Database for Annotation, Visualization, and Integrated Discovery) analysis that identified enriched gene ontology (GO) terms and functionally related gene groups. Comparison across time points and genetic background (cre− control vs Dicer-KO) demonstrated that E16.5 Dicer-KO adrenals were particularly enriched for GO terms related to immune and inflammatory response pathway genes (Table 2). These data are suggestive of an inflammatory process or a cell-mediated immune response occurring in Dicer-KO adrenals that could either contribute to or be a consequence of the observed cortical cell death.
Table 2.
DAVID Enrichment Analysis Illustrating Differentially Expressed Genes Enriched for Specific GO Terms in Adrenals From E16.5 Dicer-KO Compared With Control (Top) and in Adrenals From E16.5 Compared With E15.5 Dicer-KO (Bottom)a
| Gene Ontology Gene List | Fold Enrichment | P Value |
|---|---|---|
| Enrichment in GO terms in differentially expressed genes in adrenals from E16.5 embryos | ||
| Innate immune response | 5.22 | 9.72 × 10−7 |
| Positive regulation of immune response | 5.20 | 2.27 × 10−8 |
| Activation of immune response | 5.19 | 1.78 × 10−5 |
| Immune effector process | 4.73 | 1.36 × 10−6 |
| Positive regulation of immune system process | 4.34 | 7.13 × 10−9 |
| Regulation of cytokine production | 4.29 | 4.71 × 10−6 |
| Positive regulation of response to stimulus | 4.00 | 6.00 × 10−7 |
| Regulation of cell activation | 3.82 | 1.92 × 10−5 |
| Cell activation | 3.78 | 4.72 × 10−8 |
| Leukocyte activation | 3.40 | 6.92 × 10−6 |
| Immune response | 3.32 | 2.48 × 10−11 |
| Response to wounding | 3.11 | 2.07 × 10−7 |
| Cell surface | 2.83 | 8.55 × 10−6 |
| Enrichment in GO terms in differentially expressed genes in adrenals from E16.5 compared with E15.5 embryos | ||
| Adaptive immune response | 12.58 | 1.80 × 10−5 |
| Inflammatory response | 8.05 | 2.36 × 10−7 |
| Response to wounding | 6.53 | 5.22 × 10−8 |
| External side of plasma membrane | 6.19 | 3.07 × 10−5 |
| Cell surface | 5.86 | 6.22 × 10−7 |
| Immune response | 5.45 | 5.78 × 10−8 |
| Carbohydrate binding | 5.36 | 3.33 × 10−5 |
| Defense response | 5.06 | 1.17 × 10−6 |
Data are filtered for FDR ≤0.05 and fold changes ≥1.5.
The most up-regulated gene in Dicer-KO adrenals at both E15.5 and E16.5 time points was Nr6a1, or germ cell nuclear factor (Gcnf), followed by a consistent increase in expression of a number of other genes, most notably Acvr1c (Alk7). The interpretation of Nr6a1 expression levels, however, is confounded by the experimental design. The presence of approximately 10 kb of the 3′ end of Nr6a1 in the bacterial artificial chromosome (BAC) used to generate the Sf1-Cre transgene potentially contributed to the observed increased Nr6a1 expression in Sf1-Cre/Dicerlox/lox mice. Therefore, using adrenals from E15.5 and E16.5 control and Dicer-KO embryos, we performed quantitative real-time PCR of the 3 Nr6a1 isoforms expressed from the Nr6a1genomic locus. Importantly, only primers for isoform 2 would be expected to amplify the portion of the Nr6a1 sequence present in the BAC. Therefore, to specifically determine the extent of isoform 2 expression from the BAC, we first determined Nr6a1 isoform-specific transcript levels in the adrenals of adult mice carrying the Sf1-Cre transgene without the floxed Dicer allele compared with the adrenals of WT animals. Although we observed no increase in expression of Nr6a1 isoforms 1 and 3, a minor increase in expression of Nr6a1 isoform 2 indicated only modest 3′ transcriptional leakage from the transgene (Figure 5A). In contrast, when we examined isoform expression in the adrenals of the Dicer-KO mice (Sf1-Cre/Dicerlox/lox) compared with the adrenals of WT animals (4 samples at E15.5 and 4 at E16.5), we observed a significantly higher levels of isoform 2 (up to 20-fold) as well as a 2- to 10-fold up-regulation of isoforms 1 and 3, confirming a bona fide up-regulation of Nr6a1 in Dicer-KO adrenals (Figure 5, B and C). Taken together, these data confirm that Nr6a1 was up-regulated resulting from loss of Dicer in the adrenal cortex.
Figure 5.
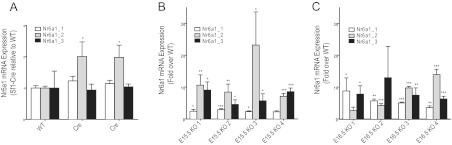
Quantitative real-time PCR to confirm Nr6a1 expression in adrenals from Sf1-Cre/Dicerlox/lox and Sf1-Cre–only tissues. Total RNA was isolated from adrenals and reverse transcribed and quantitative PCR performed as described in Materials and Methods. A, Comparison of Nr6a1 expression in adrenals from adult mice expressing only the Sf1-Cre transgene (Cre), relative to WT animals. B and C, Expression of the three Nr6a1 transcript isoforms in 4 individual samples of E15.5 and E16.5 Sf1-Cre/Dicerlox/lox adrenals relative to their corresponding WT control samples. Statistical significance was determined using unpaired t tests. *, P < .05; **, P ≤ .01; ***, P ≤ .001.
Down-regulated miRNAs in Dicer-KO adrenals are predicted to target up-regulated gene transcripts
To determine which specific miRNAs were affected in Dicer-KO adrenals, we analyzed RNA isolated from E15.5 and E16.5 WT and Dicer-KO adrenals on OpenArray miRNA arrays. As expected, differentially expressed miRNAs in Dicer-KO adrenals were down-regulated relative to controls; miRNAs with greater than 1.5-fold difference and P ≤ .05 are displayed on the heatmap shown in Figure 6. Of these differentially expressed miRNAs, 16 were common between E15.5 and E16.5 (detailed in Table 1). Of these down-regulated miRNAs, miR-34c, miR-21, miR-10a, and let-7d were among the most interesting candidates for future studies due to the large body of literature available regarding their function.
Figure 6.
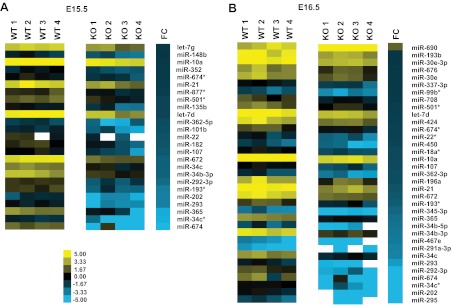
Differentially expressed miRNAs in adrenals from Sf1-Cre/Dicerlox/lox embryos. Heatmaps were generated from OpenArray rodent miRNA analysis as described in Materials and Methods. A, Differentially expressed miRNAs at both E15.5 and E16.5 in Dicer-KO adrenals relative to control (WT, cre− littermates) adrenals were filtered to select only those whose expression was changed significantly with a P value ≤ .05. Only miRNAs with a log2-fold change of ≥1.5 or ≤−1.5 were included. FC represents log2-fold change between the 4 Dicer-KO litters/replicates/samples compared with the 4 WT samples.
To identify predicted targets of miRNAs, we used both TargetScan (targetscan.org) and miRanda (microRNA.org). We compared the predicted targets of differentially expressed miRNAs with genes that were differentially expressed in both E15.5 and E16.5 Dicer-KO adrenals. The intersection of these, along with the number of specific miRNA sites in each gene, is shown in Table 3. Interestingly, 4 genes that were elevated both in E15.5 and in E16.5 Dicer-KO adrenals, Nr6a1, Igdcc3, Acvr1c, and Greb1l, were consistently identified as targets for a small subset of miRNAs that were commonly down-regulated in E15.5 and E16.5 Dicer-KO adrenals (Table 3). This subset includes let-7, miR-10a, and miR-21. Using a modified Fisher's exact test, we found that there was a significant overrepresentation of targets for let-7d (P < .01) and miR-101b (P < .01) in the differentially expressed gene list at E15.5. Finally, we compared the predicted binding sites (as suggested by the TargetScan algorithm) for let-7 in the 3′-UTRs of Nr6a1 and Acvr1c. As illustrated in Table 4, the seed sequences recognized by let-7 are strongly conserved among vertebrate organisms. Shown are human, mouse, dog, and horse, but other species as evolutionarily distant as elephant exhibit similar conservation. This phylogenetic conservation of predicted let-7 binding sites among various species supports the notion that these binding sites may indeed be functional. Similar analyses for predicted miR-10 and miR-101 interactions with Nr6a1 are shown in Table 4. In summary, results from the arrays performed on E15.5 and E16.5 control versus Dicer-KO mouse adrenals revealed a unique gene expression profile. The set of elevated transcripts was enriched for genes implicated in the immune/inflammatory response, suggesting a potential contributory component to the apoptosis and ultimate aplastic phenotype observed in Dicer-KO adrenal glands. A number of other transcripts, most notably coded by Nr6a1 and Acvr1c, were also highly up-regulated in Dicer-KO adrenal glands. Concurrent miRNA profiling suggested a strong correlation between these two differentially expressed genes and down-regulated miRNAs that may target them.
Table 3.
Intersection of mRNAs and Predicted Targets of miRNAs That Are Differentially Expressed in Adrenals From Dicer-KO Embryosa
| E15.5 Differentially Expressed miRNAs |
E16.5 Differentially Expressed miRNAs |
||||
|---|---|---|---|---|---|
| miRNA | Predicted Targets | Number of Predicted Sites | miRNA | Predicted Targets | Number of Predicted Sites |
| let-7g | Nr6a1 | 2 | let-7d | Nr6a1 | 2 |
| let-7-d | Igdcc3 | 2 | Igdcc3 | 2 | |
| Acvr1c | 1 | Acvr1c | 1 | ||
| Greb1l | 1 | Greb1l | 1 | ||
| miR-101b | Nr6a1 | 1 | |||
| Acvr1c | 1 | ||||
| Greb1l | 1 | ||||
| miR-10a | Nr6a1 | 1 | miR-10a | Nr6a1 | 1 |
| miR-21 | Acvr1c | 1 | miR-21 | Acvr1c | 1 |
| miR-182 | Acvr1c | 1 | |||
| miR-362 | Greb1l | 1 | |||
Shown are only those predicted by both TargetScan and miRanda.
Table 4.
Conservation of Selected miRNA Target Sequences in Nr6a1 Transcripts in Mouse (Mmu), Human (Has), Dog (Cfa), and Horse (Eca)a
| Nr6a1_Let-7(370) | Nr6a1_Let-7(1228) | Nr6a1_miR-10(2290) | Nr6a1_miR-101(4416) | |
|---|---|---|---|---|
| CUACCUCA | CUACCUCA | ACAGGGUA | GUACUGUA | |
| Mmu | UGACAAAGACAACUACCUCAAUGGAAACAGGU | GGG--CAUUAAACUACCUCAUGUUUCUAAGGG | GGCUUCUUUUAAACAGGGUAAAGUAAAUGGGC | AGCAAGUAUUAGGUACUGUAUUUGAACCAAUA |
| Hsa | UGACAAAGAUGACUACCUCAAUGGAAAUGGGG | GGGG-CUUUAAAUACCUCAGGUUCCUAAUGG | GGCUUUUUUUAAACAGGGUAAAGUGAAUGUGU | AGCAAGUGUUAGGUACUGUAUUUGAACCAAUA |
| Cfa | UGACAAAGACGACUACCUCAGUGGAAAGGGAG | GGGGCCUUGAAACUACCUCAUAUUCCUAAGGG | GGCUUUUUUUAAACAGGGUAAAGUGAAUGUGU | AGCAAAUGUUAGGUACUGUAUUUGAACCAAUA |
| Eca | UGACAAAGACGACUACCUCAGUGGAAAGGGGG | GGGG-CUUUAAACUACCUCAUGUUCCUAAGGG | GGCUUUUUU-AAACAGGGUAAAGUGAAUGUGU | AGCAAAUGUUAGGUACUGUAUUUGAACCAAUA |
Consensus miRNA seed/binding sequences are underlined, and the nucleotide position in the transcript is shown in parentheses.
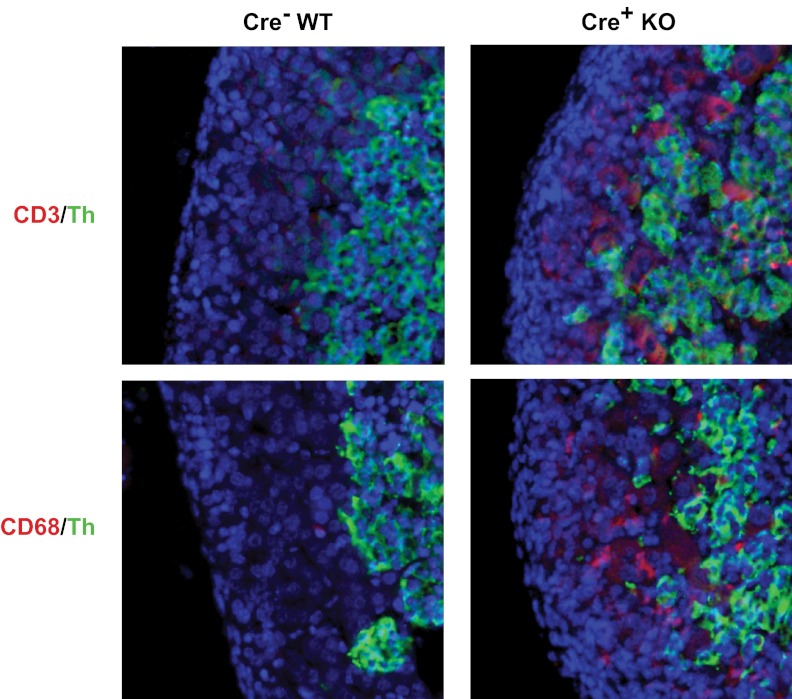
Because the mRNA array data indicated that immune response genes were elevated in adrenals from Dicer-KO embryos, we examined expression of CD3 and CD20, representative of T cells and B cells, respectively, and of CD68, which is expressed on cells of the macrophage lineage and elevated in the mRNA array. Little or no staining was observed in E14.5 or E16.5 control of KO adrenals. However, adrenals from E18.5 Dicer-KO embryos displayed immunoreactivity with both anti-CD68 and anti-CD3, whereas their WT littermates did not (Figure 7). The B cell marker, CD20, was not detected in either controls or Dicer-KO adrenals (data not shown).
Figure 7.
Expression of CD3 and CD68 in adrenals from E18.5 Sf1-Cre/Dicerlox/lox embryos. Immunohistochemistry of adrenals from E18.5 Dicer-KO (cre+ KO) embryos and cre− littermate controls (WT). Top panel shows immunofluorescence staining for CD3 (red) and Th (green). Bottom panel shows immunofluorescence staining form CD68 (red) and Th (green). Sections were counterstained with DAPI (blue) before visualization, and images were merged to show colocalization.
Discussion
Dicer inactivation has been associated with cellular senescence, apoptosis, and reduced proliferation in a number of biological models (32–35). We hypothesized that Dicer inactivation in the developing adrenal cortex would result in organ failure. Despite normal fetal adrenal formation at early stages, Dicer-KO animals exhibited severe adrenal aplasia at E18.5. Because Sf1 is expressed early in adrenal development, between E9 and E10, we expected Cre-mediated excision of Dicer to occur in this timeframe with concomitant early adrenal failure. However, the onset of phenotypic changes in Dicer-KO mice was not evident until E14.5 to E16.5. This delay may be due either to varying half-lives of Dicer protein and/or of mature miRNAs in the developing adrenal or to differing temporal sensitivities of the adrenal to miRNA-mediated gene regulation. A significant delay has been reported between Cre-mediated Dicer excision and depletion of specific miRNAs in the developing mouse inner ear and in Purkinje cells (36, 37). We observed increased apoptosis at E14.5 in Dicer-KO adrenals, which coincides with the time at which the fetal cortex begins to transition to the adult cortex (38). Our lab has recently defined a subset of fetal adrenocortical cells that undergo a change in transcriptional programming, populate the adrenal capsule, and become adrenal stem/progenitor cells responsible for maintaining the adult adrenal cortex (Wood, M. A., and G. D. Hammer, manuscript submitted for publication) (5). Such a transition would be predicted to require significant changes in gene regulation and could make the adrenal cortex more vulnerable to Dicer inactivation.
The phenotype observed in the adrenal cortex of Dicer-KO animals could not be clearly associated with a change in proliferation but with increased cell cycle arrest and apoptosis. As cited earlier, many of the previous tissue-specific Dicer loss-of-function studies reported an increase in apoptosis. Additionally, it is known that Dicer inactivation in primary cell cultures results in the induction of a DNA damage checkpoint, and subsequent p19Arf-p53 signaling, leading to increased cellular senescence (32). The increased phospho-γ-H2A.X staining observed in Dicer-KO adrenals was consistent with DNA damage. Adrenals from Dicer-KO embryos also demonstrated a significant up-regulation of the apoptosis marker, cleaved-caspase 3, and of p21, a cell cycle inhibitor that in part mediates cellular senescence.
Because Dicer is required for proper processing of miRNAs, Dicer loss would be expected to result in the accumulation of precursor miRNAs, which could have toxic effects. Mice injected with short hairpin RNA vectors into the liver exhibit a decrease in the expression of several liver-specific miRNAs, which appears to be a result of oversaturating the endogenous miRNA processing machinery, and evidence of toxicity (39, 40). It is unknown what effects the analogous overabundance of immature miRNA species may have on cellular homeostasis.
Dicer is also reported to exhibit miRNA-independent cell survival functions, which could be contributing factors in Dicer loss-of-function phenotypes. A recent report by Kaneko et al (41) demonstrates the requirement for Dicer in clearing Alu and Alu-like B1/B2 RNAs in the retinal pigmented epithelium of humans and mice, respectively. Loss of Dicer in these cells resulted in degeneration of the retinal pigmented epithelium and was not dependent on dysfunctional miRNA biogenesis. Similarly, Dicer has been implicated in the silencing of centromeric chromatin and regulation of differentiation in mouse embryonic stem cells through a non–miRNA-dependent mechanism (19). These reports support the possibility that Dicer loss-of-function phenotypes, although due primarily to impaired miRNA biogenesis, may also be the result of defects in other Dicer-dependent pathways such as RNA interference.
To address the molecular basis of the Dicer-KO adrenal phenotype, we profiled mRNA and miRNA expression in Dicer-KO adrenals at E15.5 and E16.5. It should be noted that cell death is evident as early as E14.5 (Figure 3) and could indicate an early decrease in cortical cell number, potentially confounding interpretation of these results. Thus, the initial or primary effect of loss of Dicer remains unclear. However, to additionally control for potential changes in gene expression secondary to changes in cell mass, we calculated the expression levels of the up- and down-regulated genes in comparison with β-actin in the expression arrays. By this calculation, all genes listed as regulated in Table 1 remain respectively up- or down-regulated in Dicer-KO compared with WT adrenals, consistent with an overall regulation of gene expression per unit cell mass.
The mRNA microarray revealed a significant down-regulation of numerous potentially interesting genes such as Frzb, Adh7, and Akr1d1. Frzb, a secreted antagonist of Wnt signaling (42, 43), is of particular interest based on the significant role of Wnt/β-catenin signaling in adrenal development. Our lab and others have shown that canonical Wnt/β-catenin signaling is required for normal adrenal development and maintenance because targeted disruption of β-catenin in the mouse adrenal results in marked adrenal hypoplasia and stabilization of β-catenin is associated with hyperplasia and ultimate tumor formation (22, 31, 44). Because Frzb is specifically expressed in the rodent adrenal (45), a significant down-regulation of a Wnt inhibitor such as Frzb may reflect a compensatory increase in Wnt/β-catenin signaling in Dicer-KO adrenals. Akr1d1 codes for a 5β-reductase that is responsible for the 5β reduction of corticosterone and cortisol as well as androstenedione, progesterone, and 17-OH-progesterone (46). Its potential function in the metabolism of steroid hormones and its down-regulation in the setting of Dicer loss-of-function suggest a possible role for Akr1d1 in the developing mouse adrenal cortex. Likewise, perturbation of Adh7 expression is interesting in that it is expressed in both the embryonic and adult mouse adrenal cortex (47). Adh7 is an alcohol dehydrogenase that serves as a dehydrogenase in the oxidation of retinol, a required step in the synthesis of retinoic acid from vitamin A. Although its exact function in the adrenal gland is not fully understood, it has been hypothesized that Adh7 expression in the developing adrenal may facilitate the synthesis of retinoic acid, which could be distributed to the embryo in an endocrine manner (48). Furthermore, Adh7 expression in the adult adrenal cortex has been described as a radial, spoke-like distribution. Given that cortical maintenance and differentiation is believed to originate from a population of adrenal progenitor cells residing in the outermost subcapsular region of the adrenal (5, 6), it is also possible that Adh7 may have an important function in regulating this process.
More importantly, however, the array data revealed down-regulation of miRNAs predicted to target Nr6a1 and Acvr1c, which were both significantly up-regulated in Dicer-KO adrenals. Nr6a1 (Gcnf) and Acvr1c (Alk7) are implicated in developmental processes in other tissues and organs. Most provocative are the known roles of these genes and associated pathways in gonadal development. Because the gonad and adrenal share their embryonic origin and the activation of unique genes specify adrenal versus gonadal fate, it is intriguing to speculate a role for these genes in fate determination during organogenesis. Although Nr6a1 and Acvr1c have both been shown to be expressed in the developing adrenal gland at E14.5 (49), their function in the embryonic adrenal is not known. Nr6a1 is important in germ cell and neuronal development (50) and is a paralog of Sf1, residing a mere 13 kb downstream of Sf1 on chromosome 2 (51). Despite the close proximity to Sf1, the expression pattern of Nr6a1 is relatively distinct, and an insulator defining a transcriptional boundary between Sf1 and Nr6a1 has been previously described (52). Nr6a1 is transiently up-regulated after retinoic acid-induced differentiation of embryonic stem cells (53) and is a potent transcriptional repressor of the stem cell pluripotency factor Oct4 (54).
Acvr1c (Alk7) is a member of the TGF-β receptor superfamily and is the preferred receptor for activin AB, activin B, and Nodal (55, 56). Nodal is a secreted ligand belonging to the TGF-β superfamily and is responsible for mesendoderm formation, node formation, and left-right patterning in the mouse (57, 58). It is also able to induce caspase 3-dependent apoptosis by activating Alk7 signaling in a variety of cell types, including the ovary and placental trophoblast cells during follicular atresia and placentation (59–63). There are no published reports of Acvr1c expression or function in the adrenal cortex, although its role in the ovary, an organ with a common development origin with the adrenal cortex, has been described (64). Our laboratory and other groups have detailed a role of Tgfβ2 and inhibin in the specification of adrenal (versus gonadal) fate of progenitor cells. In the absence of inhibin and unopposed Tgfβ2 signaling, peripheral adrenocortical progenitor cells assume a gonadal fate, replete with theca and granulosa lineage and follicular structure (65). Although intriguing, it is unknown whether Acvr1c (Activin A receptor, type IC), despite functioning as a receptor for certain activin family ligands, has a direct role in this previously observed phenomenon.
Interestingly, the literature provides circumstantial evidence of cross talk between the Nr6a1, Acvr1c, and Wnt/β-catenin pathways. It is known that Nr6a1 represses the expression of Cripto1, a member of the epidermal growth factor-Cripto1/FRL1/cryptic family, which is capable of significantly enhancing Nodal-mediated signaling through Acvr1c (66). In contrast, Cripto1 is a target of the canonical Wnt/β-catenin signaling pathway and is activated by lymphoid enhancer factor/T-cell factor transcription factors (67). Our previous studies have demonstrated that active β-catenin is present in the peripheral cortical cells of the adrenal gland as early as E14.5; these cells are believed to receive Wnt signals from the adrenal capsule (2). Further study would be required to evaluate the potential interaction of these signaling pathways in the normal developing adrenal.
The miRNAs found to be significantly down-regulated in E15.5 and E16.5 Dicer-KO adrenals provide several interesting avenues for further study. miR-34c, miR-21, miR-10a, and let-7d play roles in a variety of physiological processes including tumorigenesis. Let-7, the second miRNA to be described after lin-4, regulates developmental timing in nematodes (68). In addition, it is known to regulate the oncogenes RAS (69), HMGA2 (70), and MYC (71) as well as proliferation pathways in human cells (72). miR-34 can act as a tumor suppressor downstream of p53 and promote cell cycle arrest, apoptosis, and senescence (73, 74). In contrast, evidence supports a role of miR-10a in retinoic acid-induced differentiation of neuroblastoma cells (75) and regulation of Bcl-6, a gene involved in the development of diffuse large B cell lymphoma (76). miR-21 is implicated in the regulation of aldosterone synthesis in the H295 human adrenocortical carcinoma cell line (77) and is believed to promote tumor metastasis and tumorigenesis by targeting phosphatase and tensin homolog (PTEN) (78). Because the pathways responsible for organism development and the pathology of cancer often coincide, knowledge of the role of these miRNAs in cancer should be helpful in elucidating their function in developmental processes.
We found that Nr6a1 and Acvr1c are predicted targets of a subset of miRNAs that are significantly down-regulated in Dicer-KO adrenals, including let-7, miR-10a, miR-21, miR-182, miR-101b, and miR-362. These associations suggested that after the loss of Dicer function, underexpression of specific miRNAs may result in derepression of Nr6a1 and Acvr1c. When we compared the predicted let-7 binding sites in both Nr6a1 and Acvr1c, we found that these sequences were highly conserved between mouse, human, and other organisms, suggesting these sites may be evolutionarily conserved to maintain functional miRNA-mRNA interactions. Multiple examples of miRNA regulation of nuclear receptors can be found in the literature. Let7 not only regulates Dauer Formation 12 (DAF12) in C. elegans but also has been shown to regulate mammalian homolog of taillness (TLX) (Nr2e1), thereby affecting balance between neural stem cell proliferation and differentiation (79, 80). The estrogen receptor-α is regulated by miR-22, -206, -221, and -222, and the vitamin D receptor is negatively regulated by miR-27b and -125b (81, 82). Specifically in the adrenal gland, it has been reported that miRNAs 96, -101a, -142-3p, and -433 are elevated upon ACTH stimulation and in turn down-regulate the glucocorticoid receptor (Nr2e1) (83). Additional experiments are required to empirically confirm the miRNA-target associations reported here and to establish whether derepression of Nr6a1 and Acvr1c by these miRNAs is a result of the Dicer-KO phenotype or is contributory to it.
This study provides evidence for the requirement of Dicer in the developing mouse adrenal cortex. Although the work builds upon a similar phenotypic report of adrenocortical demise in the absence of Dicer (18), our work uncovers a set of miRNAs enriched in the developing adrenal and down-regulated in the absence of Dicer that potentially control critical milestones of adrenal organogenesis. The correlated changes in mRNA expression provide insight into potential roles of adrenal-expressed miRNAs and their target genes in adrenocortical development. Further efforts that focus on these miRNA-mRNA networks will be essential next steps in understanding the role of miRNA-mediated gene expression in adrenocortical cell fate, specification, and homeostasis.
Acknowledgments
We thank Drs Deborah Gumucio, Andrzej Dlugosz, Eric Fearon, and John Kim for valuable advice with this project.
This work was supported by National Institutes of Health Grants R01-DK062027 (to G.D.H.), T32 HD007505 to the University of Michigan's Training Program in Organogenesis (to K.T.K), and T32 DK094775 to the University of Michigan Training in Basic and Translational Digestive Sciences (to K.G.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Acvr1c
- Activin A receptor, type IC
- AGP
- adrenogonadal primordium
- BAC
- bacterial artificial chromosome
- DAB
- 3,3′-diaminobenzidine
- DAPI
- 4′,6-diamidino-2-phenylindole
- E9.0
- embryonic day 9
- FDR
- false discovery rate
- KO
- knockout
- miRNA
- microRNA
- PCNA
- proliferating cell nuclear antigen
- Sf1
- steroidogenic factor 1
- UTR
- untranslated region.
References
- 1. Hammer GD, Parker KL, Schimmer BP. Minireview: transcriptional regulation of adrenocortical development. Endocrinology. 2005;146:1018–1024 [DOI] [PubMed] [Google Scholar]
- 2. Kim AC, Barlaskar FM, Heaton JH, et al. In search of adrenocortical stem and progenitor cells. Endo Rev. 2009;30:241–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lalli E. Adrenal cortex ontogenesis. Best Pract Res Clin Endocrinol Metab. 2010;24:853–864 [DOI] [PubMed] [Google Scholar]
- 4. Parker KL, Schimmer BP. The roles of the nuclear receptor steroidogenic factor 1 in endocrine differentiation and development. Trends Endocrinol Metab. 1996;7:203–207 [DOI] [PubMed] [Google Scholar]
- 5. Wood MA, Hammer GD. Adrenocortical stem and progenitor cells: unifying model of two proposed origins. Mol Cell Endocrinol. 2011;336:206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim AC, Hammer GD. Adrenocortical cells with stem/progenitor cell properties: recent advances. Mol Cell Endocrinol. 2007;265–266:10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854 [DOI] [PubMed] [Google Scholar]
- 8. Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610 [DOI] [PubMed] [Google Scholar]
- 9. Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12:99–110 [DOI] [PubMed] [Google Scholar]
- 10. Bernstein E, Kim SY, Carmell MA, et al. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217 [DOI] [PubMed] [Google Scholar]
- 11. Zhao Y, Ransom JF, Li A, et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1–2. Cell. 2007;129:303–317 [DOI] [PubMed] [Google Scholar]
- 12. Chen JF, Murchison EP, Tang R, et al. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci U S A. 2008;105:2111–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harris KS, Zhang Z, McManus MT, Harfe BD, Sun X. Dicer function is essential for lung epithelium morphogenesis. Proc Natl Acad Sci U S A. 2006;103:2208–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yi R, Poy MN, Stoffel M, Fuchs E. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature. 2008;452:225–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Rourke JR, Georges SA, Seay HR, et al. Essential role for Dicer during skeletal muscle development. Dev Biol. 2007;311:359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hong X, Luense LJ, McGinnis LK, Nothnick WB, Christenson LK. Dicer1 is essential for female fertility and normal development of the female reproductive system. Endocrinology. 2008;149:6207–6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayashi K, Chuva de Sousa Lopes SM, Kaneda M, et al. MicroRNA biogenesis is required for mouse primordial germ cell development and spermatogenesis. PloS One. 2008;3:e1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang CC, Yao HH. Inactivation of Dicer1 in steroidogenic factor 1-positive cells reveals tissue-specific requirement for Dicer1 in adrenal, testis, and ovary. BMC Dev Biol. 2010;10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kanellopoulou C, Muljo SA, Kung AL, et al. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005;19:489–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hatfield SD, Shcherbata HR, Fischer KA, Nakahara K, Carthew RW, Ruohola-Baker H. Stem cell division is regulated by the microRNA pathway. Nature. 2005;435:974–978 [DOI] [PubMed] [Google Scholar]
- 21. Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44:419–424 [DOI] [PubMed] [Google Scholar]
- 22. Kim AC, Reuter AL, Zubair M, et al. Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008;135:2593–2602 [DOI] [PubMed] [Google Scholar]
- 23. Harfe BD, McManus MT, Mansfield JH, Hornstein E, Tabin CJ. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci U S A. 2005;102:10898–10903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 25. Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264 [DOI] [PubMed] [Google Scholar]
- 26. Smyth GK. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article3 [DOI] [PubMed] [Google Scholar]
- 27. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Ser B. 1995;57:289–300 [Google Scholar]
- 28. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57 [DOI] [PubMed] [Google Scholar]
- 29. Schulte DM, Shapiro I, Reincke M, Beuschlein F. Expression and spatio-temporal distribution of differentiation and proliferation markers during mouse adrenal development. Gene Expr Patterns. 2007;7:72–81 [DOI] [PubMed] [Google Scholar]
- 30. Berthon A, Martinez A, Bertherat J, Val P. Wnt/β-catenin signalling in adrenal physiology and tumour development. Mol Cell Endocrinol. 2012;351:87–95 [DOI] [PubMed] [Google Scholar]
- 31. Heaton JH, Wood MA, Kim AC, et al. Progression to adrenocortical tumorigenesis in mice and humans through insulin-like growth factor 2 and β-catenin. Am J Pathol. 2012;181:1017–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mudhasani R, Zhu Z, Hutvagner G, et al. Loss of miRNA biogenesis induces p19Arf-p53 signaling and senescence in primary cells. J Cell Biol. 2008;181:1055–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murchison EP, Partridge JF, Tam OH, Cheloufi S, Hannon GJ. Characterization of Dicer-deficient murine embryonic stem cells. Proc Natl Acad Sci U S A. 2005;102:12135–12140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muljo SA, Ansel KM, Kanellopoulou C, Livingston DM, Rao A, Rajewsky K. Aberrant T cell differentiation in the absence of Dicer. J Exp Med. 2005;202:261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Andl T, Murchison EP, Liu F, et al. The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Curr Biol. 2006;16:1041–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Soukup GA, Fritzsch B, Pierce ML, et al. Residual microRNA expression dictates the extent of inner ear development in conditional Dicer knockout mice. Dev Biol. 2009;328:328–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schaefer A, O'Carroll D, Tan CL, et al. Cerebellar neurodegeneration in the absence of microRNAs. J Exp Med. 2007;204:1553–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zubair M, Parker KL, Morohashi K. Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol Cell Biol. 2008;28:7030–7040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grimm D, Streetz KL, Jopling CL, et al. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541 [DOI] [PubMed] [Google Scholar]
- 40. Snøve O, Rossi JJ. Toxicity in mice expressing short hairpin RNAs gives new insight into RNAi. Genome Biol. 2006;7:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaneko H, Dridi S, Tarallo V, et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471:325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997;88:747–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang S, Krinks M, Lin K, Luyten FP, Moos M., Jr Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997;88:757–766 [DOI] [PubMed] [Google Scholar]
- 44. Gaujoux S, Grabar S, Fassnacht M, et al. β-Catenin activation is associated with specific clinical and pathologic characteristics and a poor outcome in adrenocortical carcinoma. Clin Cancer Res. 2011;17:328–336 [DOI] [PubMed] [Google Scholar]
- 45. Nishimoto K, Rigsby CS, Wang T, et al. Transcriptome analysis reveals differentially expressed transcripts in rat adrenal zona glomerulosa and zona fasciculata. Endocrinology. 2012;153:1755–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Palermo M, Marazzi MG, Hughes BA, Stewart PM, Clayton PT, Shackleton CH. Human Δ4-3-oxosteroid 5β-reductase (AKR1D1) deficiency and steroid metabolism. Steroids. 2008;73:417–423 [DOI] [PubMed] [Google Scholar]
- 47. Haselbeck RJ, Ang HL, Deltour L, Duester G. Retinoic acid and alcohol/retinol dehydrogenase in the mouse adrenal gland: a potential endocrine source of retinoic acid during development. Endocrinology. 1997;138:3035–3041 [DOI] [PubMed] [Google Scholar]
- 48. Haselbeck RJ, Duester G. ADH1 and ADH4 alcohol/retinol dehydrogenases in the developing adrenal blastema provide evidence for embryonic retinoid endocrine function. Dev Dyn. 1998;213:114–120 [DOI] [PubMed] [Google Scholar]
- 49. Visel A, Thaller C, Eichele G. GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res. 2004;32:D552–D556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zechel C. The germ cell nuclear factor (GCNF). Mol Reprod Dev. 2005;72:550–556 [DOI] [PubMed] [Google Scholar]
- 51. Kuo MW, Postlethwait J, Lee WC, Lou SW, Chan WK, Chung BC. Gene duplication, gene loss and evolution of expression domains in the vertebrate nuclear receptor NR5A (Ftz-F1) family. Biochem J. 2005;389:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ishihara SL, Morohashi K. A boundary for histone acetylation allows distinct expression patterns of the Ad4BP/SF-1 and GCNF loci in adrenal cortex cells. Biochem Biophys Res Commun. 2005;329:554–562 [DOI] [PubMed] [Google Scholar]
- 53. Gu P, LeMenuet D, Chung AC, Mancini M, Wheeler DA, Cooney AJ. Orphan nuclear receptor GCNF is required for the repression of pluripotency genes during retinoic acid-induced embryonic stem cell differentiation. Mol Cell Biol. 2005;25:8507–8519 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 54. Fuhrmann G, Chung AC, Jackson KJ, et al. Mouse germline restriction of Oct4 expression by germ cell nuclear factor. Dev Cell. 2001;1:377–387 [DOI] [PubMed] [Google Scholar]
- 55. Reissmann E, Jörnvall H, Blokzijl A, et al. The orphan receptor ALK7 and the Activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development. Genes Dev. 2001;15:2010–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tsuchida K, Nakatani M, Yamakawa N, Hashimoto O, Hasegawa Y, Sugino H. Activin isoforms signal through type I receptor serine/threonine kinase ALK7. Mol Cell Endocrinol. 2004;220:59–65 [DOI] [PubMed] [Google Scholar]
- 57. Schier AF, Shen MM. Nodal signalling in vertebrate development. Nature. 2000;403:385–389 [DOI] [PubMed] [Google Scholar]
- 58. Jörnvall H, Reissmann E, Andersson O, Mehrkash M, Ibáñez CF. ALK7, a receptor for nodal, is dispensable for embryogenesis and left-right patterning in the mouse. Mol Cell Biol. 2004;24:9383–9389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Munir S, Xu G, Wu Y, Yang B, Lala PK, Peng C. Nodal and ALK7 inhibit proliferation and induce apoptosis in human trophoblast cells. J Biol Chem. 2004;279:31277–31286 [DOI] [PubMed] [Google Scholar]
- 60. Xu G, Zhong Y, Munir S, Yang BB, Tsang BK, Peng C. Nodal induces apoptosis and inhibits proliferation in human epithelial ovarian cancer cells via activin receptor-like kinase 7. J Clin Endocrinol Metab. 2004;89:5523–5534 [DOI] [PubMed] [Google Scholar]
- 61. Wang H, Tsang BK. Nodal signalling and apoptosis. Reproduction. 2007;133:847–853 [DOI] [PubMed] [Google Scholar]
- 62. Zhong Y, Xu G, Ye G, Lee D, Modica-Amore J, Peng C. Nodal and activin receptor-like kinase 7 induce apoptosis in human breast cancer cell lines: Role of caspase 3. Int J Physiol Pathophysiol Pharmacol. 2009;1:83–96 [PMC free article] [PubMed] [Google Scholar]
- 63. Zhao F, Huang F, Tang M, et al. Nodal induces apoptosis through the activation of ALK7 signaling pathways in pancreatic INS-1 β-cells. Am J Physiol Endocrinol Metab. 2012;303:E132–E143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang H, Jiang JY, Zhu C, Peng C, Tsang BK. Role and regulation of nodal/activin receptor-like kinase 7 signaling pathway in the control of ovarian follicular atresia. Mol Endocrinol. 2006;20:2469–2482 [DOI] [PubMed] [Google Scholar]
- 65. Looyenga BD, Hammer GD. Origin and identity of adrenocortical tumors in inhibin knockout mice: implications for cellular plasticity in the adrenal cortex. Mol Endocrinol. 2006;20:2848–2863 [DOI] [PubMed] [Google Scholar]
- 66. Hentschke M, Kurth I, Borgmeyer U, Hübner CA. Germ cell nuclear factor is a repressor of CRIPTO-1 and CRIPTO-3. J Biol Chem. 2006;281:33497–33504 [DOI] [PubMed] [Google Scholar]
- 67. Morkel M, Huelsken J, Wakamiya M, et al. β-Catenin regulates Cripto- and Wnt3-dependent gene expression programs in mouse axis and mesoderm formation. Development. 2003;130:6283–6294 [DOI] [PubMed] [Google Scholar]
- 68. Reinhart BJ, Slack FJ, Basson M, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906 [DOI] [PubMed] [Google Scholar]
- 69. Johnson SM, Grosshans H, Shingara J, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647 [DOI] [PubMed] [Google Scholar]
- 70. Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science. 2007;315:1576–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sampson VB, Rong NH, Han J, et al. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res. 2007;67:9762–9770 [DOI] [PubMed] [Google Scholar]
- 72. Johnson CD, Esquela-Kerscher A, Stefani G, et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–7722 [DOI] [PubMed] [Google Scholar]
- 73. Bommer GT, Gerin I, Feng Y, et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol. 2007;17:1298–1307 [DOI] [PubMed] [Google Scholar]
- 74. Ji Q, Hao X, Zhang M, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PloS one. 2009;4:e6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Meseguer S, Mudduluru G, Escamilla JM, Allgayer H, Barettino D. MicroRNAs-10a and -10b contribute to retinoic acid-induced differentiation of neuroblastoma cells and target the alternative splicing regulatory factor SFRS1 (SF2/ASF). J Biol Chem. 2011;286:4150–4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Takahashi H, Kanno T, Nakayamada S, et al. TGF-β and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells. Nat Immunol. 2012;13:587–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Romero DG, Plonczynski MW, Carvajal CA, Gomez-Sanchez EP, Gomez-Sanchez CE. Microribonucleic acid-21 increases aldosterone secretion and proliferation in H295R human adrenocortical cells. Endocrinology. 2008;149:2477–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hammell CM, Karp X, Ambros V. A feedback circuit involving let-7-family miRNAs and DAF-12 integrates environmental signals and developmental timing in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2009;106:18668–18673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Eendebak RJ, Lucassen PJ, Fitzsimons CP. Nuclear receptors and microRNAs: who regulates the regulators in neural stem cells? FEBS Lett. 2011;585:717–722 [DOI] [PubMed] [Google Scholar]
- 81. Adams BD, Furneaux H, White BA. The micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen receptor-α (ERα) and represses ERα messenger RNA and protein expression in breast cancer cell lines. Mol Endocrinol. 2007;21:1132–1147 [DOI] [PubMed] [Google Scholar]
- 82. Mohri T, Nakajima M, Takagi S, Komagata S, Yokoi T. MicroRNA regulates human vitamin D receptor. Int J Cancer. 2009;125:1328–1333 [DOI] [PubMed] [Google Scholar]
- 83. Riester A, Issler O, Spyroglou A, Rodrig SH, Chen A, Beuschlein F. ACTH-dependent regulation of microRNA as endogenous modulators of glucocorticoid receptor expression in the adrenal gland. Endocrinology. 2012;153:212–222 [DOI] [PubMed] [Google Scholar]