Abstract
The decades-old terminology of androgen independence has been replaced in recent years with castration-resistant prostate cancer. Biological and clinical evidence have together conspired to support the use of this revised terminology by demonstrating that in the vast majority of cases tumors are neither truly depleted of androgens, nor are they free of the requirement for androgens to sustain growth and progression. Abiraterone acetate, an androgen synthesis inhibitor, and enzalutamide, a potent androgen receptor antagonist, both exploit the continued requirement for androgens. A central question, given the therapeutic gains enabled by further suppression of the androgen axis with these newer agents, is whether there may be additional clinical benefit gained by moving the goal posts of androgen suppression even further. The answer lies in part with the mechanisms utilized by tumors that enable resistance to these therapies. The aims of this review were to give a broad outline of steroidogenesis in prostate cancer and to highlight recent developments in understanding resistance to hormonal therapies.
In the human male, steroidogenesis occurs in the Leydig cell of the testes, resulting in the production of testosterone (T) (1). Synthesis occurs with the use of cholesterol as the initial substrate and ends with the conversion of Δ4-androstendione (AD) by 17β-hydroxysteroid dehydrogenase-3 (17βHSD3) to T (2). Release of T into circulation allows physiological exposure to this steroid that binds the androgen receptor (AR) (3). However, the physiological effects of T bound to AR are insufficient for masculinization. Males with loss-of-function mutations in steroid-5α-reductase (SRD5A)-2, the dominant isoenzyme in the prostate that converts T to DHT, develop with pseudohermaphroditism and do not form a normal prostate gland (4). The more potent effects of DHT on AR are therefore required for normal male physiology. On the other hand, the androgen signaling axis defined by DHT bound to AR is commandeered in the pathophysiological setting of prostate cancer to drive tumor progression, both in early disease and with disease that is resistant to hormonal therapy (5). The deflection of the androgen signaling axis from its normal physiological role to its role in tumor development and progression is due at least in part to chromosomal rearrangements, resulting in ETS-family oncogenes driven by androgen-regulatory elements (6, 7).
Androgen deprivation therapy
Advanced prostate cancer requires systemic treatment (8). Metastatic disease is treated with androgen deprivation therapy (ADT) by gonadal T depletion and is administered by medical or surgical castration (9). In most cases, the initiation of ADT is followed by an initial response, often with declines in prostate-specific antigen (PSA), radiographic regression and symptomatic relief. Clinical responses to ADT are due to a block on the effects of intratumoral T and its product, DHT, on AR-dependent transcription. Nonetheless, studies assessing intraprostatic concentrations of T and DHT after the initiation of ADT have shown that androgens in benign prostate and prostate cancer are far from deplete (10, 11). These studies indicate that the testes are but one source of intraprostatic androgens and that persistent androgens are supplied by alternative means. The presence of tumor-dependent processes that permit the reconstitution of extragonadal androgens suggests that the tumors may acquire the metabolic capability to increase the local concentration of biologically active androgens. Evidence for the presence of residual androgens after commencing ADT has been used to argue for concomitant upfront initiation of an AR antagonist to block the effects of the remaining intratumoral androgens (12). However, the clinical benefit of upfront addition of an AR antagonist to ADT over multiple trials has not been impressive (13). This issue will undoubtedly be revisited with the newer and more potent agents that are now available.
Castration-resistant prostate cancer (CRPC)
Frequently the first clinical indication that tumors treated with and responding to ADT are morphing toward a resistant phenotype is a serum PSA value that has begun to rise after reaching its nadir (14). At the cellular level, this represents a surge in AR stimulation, resulting in increased transcription of the androgen-responsive PSA gene and secretion of its encoded protein. Heightened AR activity may be due to enhanced response of the receptor to a stimulus (eg, ligand sensitization) or due to increased expression, mutation, or other fundamental AR alteration (15). Alternatively, this may be due to a higher level of stimulation upstream of AR. In other words, the fundamental change may be in prereceptor regulation.
Geller et al (16) were the first to describe detectable and physiologically significant concentrations of DHT in CRPC tissues. These studies were done by immunoassay, and the results were received with some skepticism, at least in part due to the possibility that the method of detection may have been susceptible to nonspecific signal detection. A quarter century later, locally recurrent CRPC tissues were again assessed for intratumoral androgen concentrations, this time using mass spectrometry (17). Again, DHT concentrations were found to be at physiologically significant concentrations. Subsequently, another group measured androgens in distant metastatic sites using mass spectrometry from a rapid autopsy study and found similar androgen concentrations, showing that these findings are not limited to locally recurrent disease (18). This study also describes the up-regulation of various transcripts encoding for steroidogenic enzymes, which may be involved in the generation of these androgens. DHT concentrations in these studies are approximately 1 nM, which is ample for binding AR and inducing the expression of androgen-responsive genes (19). The extension of survival conferred by agents that deplete intratumoral androgens serves as the most definitive evidence that androgen synthesis plays a central role in the progression of CRPC (20, 21).
Steroidogenic enzymes
Much of the work on steroidogenesis has been appropriately focused on organs and tissues that synthesize steroids abundantly, such as the testes, ovaries, adrenals, and placenta (22). The function of many of the requisite enzymes involved has been discovered through loss-of-function mutations in humans that lead to phenotypic abnormalities caused by deficient and/or excessive steroid production. The mutations discovered in steroidogenic enzymes in humans with congenital adrenal hyperplasia typify these investigations (23).
All steroids originate from cholesterol as the initial 27-carbon substrate. Rapid initiation of steroidogenesis is regulated by the steroidogenic acute regulatory protein, which is required for transporting cholesterol to the inner mitochondrial membrane, in which it undergoes further processing (24). The first substrate modification is catalyzed by cholesterol side-chain cleavage enzyme (CYP11A1), which resides in the inner mitochondrial membrane and cleaves 27-carbon cholesterol to 21-carbon pregnenolone (Figure 1). From this point, the possible pathways of steroid modification diverge.
Figure 1.
Pathways of androgen metabolism. Cholesterol is the initial substrate with de novo steroidogenesis via the back door pathway, which entails conversion by 3βHSD, SRD5A, and 3αHSD, followed by 2 sequential reactions (17α-hydroxylase and 17,20-lyase) by CYP17A1 to yield androsterone, which is converted to DHT in 2 further steps via 5α-androstane-3α,17β-diol. The canonical adrenal pathway entails conversion from DHEA→AD→T→DHT. The major adrenal pathway is the 5α-androstanedione pathway and requires conversion from DHEA→AD→5α-androstanedione→DHT. Inset, Chemical structure of DHEA. Steroid rings A–D and carbons 1–19 are indicated. The double bond between C5 and C6 defines the Δ5-structure of DHEA, which undergoes isomerization to between C4 and C5 when it is converted by 3βHSD to a Δ4-structure.
Cholesterol and pregnenolone both have a Δ5 (carbon-carbon double bond between C5 and C6), 3β-hydroxyl-structure. The enzyme 3β-hydroxysteroid dehydrogenase/Δ5→Δ4 isomerase (3βHSD) is encoded by 2 distinct genes, HSD3B1 and HSD3B2, which are mainly expressed in peripheral tissues and steroidogenic organs, respectively (25–27). Both isoenzymes oxidize 3β-hydroxy- to 3-keto-steroids and isomerize the double bond between C5 and C6 to between C4 and C5 (Δ4-structure; see Figure 1, inset). These reactions by 3βHSD isoenzymes result in 3-keto, Δ4-steroid products, for example resulting in the conversion from pregnenolone to progesterone and from dehydroepiandrosterone (DHEA) to AD. In turn, the 3-keto, Δ4-structure permits these steroids to be substrates for 5α-reduction by the SRD5A isoenzymes, including conversion of T to DHT, in the final step of the gonadal pathway, for the production of the most potent androgen. In addition to SRD5A1 and SRD5A2 (28), a related enzyme, SRD5A3, has also been implicated in prostate cancer (29, 30) and is up-regulated in CRPC (31). Humans with loss-of-function mutations in SRD5A3 have been described. However, afflicted patients have mental retardation and a glycosylation defect instead of a detectable deficiency in sexual differentiation or steroid metabolism, suggesting that the major biochemical function of this enzyme is not in androgen metabolism (32, 33).
Instead of 3βHSD, the alternative enzyme that can act on pregnenolone is 17α-hydroxylase/17,20-lyase (CYP17A1). CYP17A1 has 2 enzymatic activities that are required for the conversion of 21-carbon steroids, such as pregnenolone, to 19-carbon androgens. 17α-Hydroxylation is followed by 17,20-lyase activity, which cleaves off C20 and C21. The second reaction frees C17 of its bond with C20, resulting in an oxidized (17-keto) androgen. For pregnenolone, these sequential reactions result in the synthesis of DHEA, which, along with its sulfated form (DHEA sulfate), is the major product of the human adrenal reticularis (34). 17-Keto-androgens are reversibly reduced to 17β-hydroxyandrogens by 17β-hydroxysteroid dehydrogenase (17βHSD) isoenzymes (35). Notably, both androgens (T and DHT) that bind AR appreciably have a 17β-hydroxyl structure, illustrating the necessity of the reductive reaction for the synthesis of biologically active androgens that stimulate AR-responsive gene expression (36). 17βHSD3 is the specific isoenzyme required for 17-keto reduction of AD in the final step of T synthesis in the testes (2, 37). 17βHSD5, also known as aldo-keto reductase 1C3, is another isoenzyme with a reductive preference and also converts AD to T (38–40). On the other hand, 17βHSD2 has an oxidative preference and rapidly converts T and DHT to their 17-keto congeners that do not appreciably bind AR (41).
Initial substrates and metabolic pathways
There are at least 2 possibilities for the initial substrates that are converted to intratumoral DHT in CRPC. The use of cholesterol for de novo steroidogenesis would require the same components of the steroidogenic machinery present in the adrenals and gonads, including steroidogenic acute regulatory protein, CYP11A1, and CYP17A1 (42), which may be operative in a model of prostate cancer (43). Comparisons between untreated prostate cancer and CRPC demonstrate that transcripts encoding these proteins are up-regulated in CRPC (18) and CYP17A1 protein is detectable in a subset of metastatic CRPC cases (44). Other studies have instead described limited expression of transcripts encoding both CYP17A1 and 3βHSD1, which are both required for de novo steroidogenesis, in metastatic tumors (45). In contrast to steroidogenesis in the adrenals and gonads, CRPC expresses SRD5A1 and clearly possesses 5α-reductase enzymatic activity (46). One of the implications of robust SRD5A enzyme activity is that any de novo steroidogenesis would likely occur through the back door pathway that bypasses the requirement for T and involves 5α-reduction of a 21-carbon steroid (progesterone or 17α-hydroxyprogesterone) instead of a 19-carbon androgen (Figure 1) (47, 48). Although this biochemical pathway may be engaged in CRPC, the relatively lengthy 8 enzymatic steps required for conversion from cholesterol to DHT, the abundance of adrenal precursors present in serum, and the much closer pathway proximity of adrenal precursors to DHT, together suggest that adrenal precursors serve as the major substrate pool. Experiments directly comparing these pathways support the dominant use of adrenal precursor steroids (22).
There are 2 possible pathways from adrenal precursor steroids to DHT (5, 49). The canonical or conventional adrenal pathway is the route that results in T synthesis as the penultimate metabolite, which undergoes 5α-reduction to DHT (15, 50–52). In this pathway, DHEA is converted by 3βHSD to AD, which is then 17-keto reduced by aldo-keto reductase 1C3 or 17βHSD3 to T, the immediate precursor to DHT. This pathway is probably favored in the field because of the general notion that T must be the precursor to DHT, as occurs with gonadal androgen physiology, and because T is frequently detectable at concentrations greater than DHT in CRPC (17, 18). With the presence of so much T, surely this is the requisite precursor on the route taken to DHT.
On the other hand, AD, like T, is a 3-keto, Δ4-steroid, making it also a potential substrate for SRD5A (28, 53). This suggests the alternative possibility that AD is 5α-reduced to 5α-androstanedione (5α-dione), which then becomes the immediate precursor to DHT. The 5α-dione pathway is the major pathway for the synthesis of DHT in CRPC (46). Similar to the back door pathway, the 5α-dione pathway also completely circumvents T as an intermediate metabolite, but it uses abundant adrenal precursors present in serum and requires only 3 enzymatic steps. The uniform finding across 6 different human CRPC cell lines and 2 metastatic tumors freshly biopsied from men with CRPC is that the 5α-dione pathway dominates over the conventional pathway requiring T (5, 46). Furthermore, in vivo studies demonstrate that instead of the conventional pathway, the 5α-dione pathway drives CRPC progression and requires SRD5A1 expression (46). Engagement of the 5α-dione pathway is probably due in large part to the suppression of SRD5A2 expression and SRD5A1 up-regulation that occurs in CRPC (18, 31, 54, 55). SRD5A1 is better at converting AD to 5α-dione than it is T to DHT (56). Selective SRD5A1 expression in CRPC allows its substrate preference for AD to direct flux through the 5α-dione pathway (56, 57). SRD5A1 probably also uses AD in part because it is synthesized and made available prior to T in the metabolic pathway.
T and DHT concentrations in CRPC
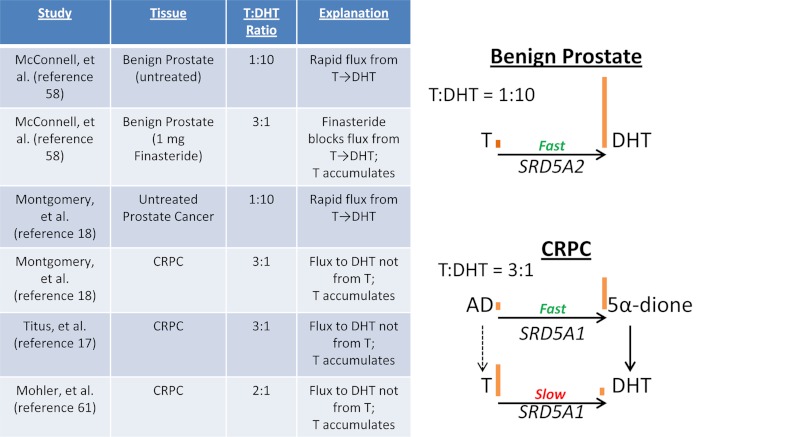
Is the dominance of the 5α-dione pathway consistent with the findings of elevated T concentrations in CRPC? It may initially seem counterintuitive that with abundant concentrations of T, CRPC tumors use an alternative precursor for DHT synthesis. The following model may reconcile these seemingly disparate findings. Benign prostate and untreated localized prostate cancer both have been consistently found to have levels of T that are much lower than DHT, typically with T/DHT concentration ratios of about 1:10 (Figure 2) (18, 58). In this clinical setting, sustained SRD5A2 expression allows rapid and efficient flux from gonadal T→DHT. In the setting of CRPC, adrenal precursors are converted to AD (59), most of which is effectively 5α-reduced by SRD5A1 to 5α-dione, and processed on to DHT (46, 60). However, a minor portion of the synthesized AD still trickles down to T (46). This T is not efficiently converted to DHT because SRD5A1 instead favors AD as its substrate and ends up accumulating in the tumor tissue to steady-state concentrations greater than DHT (17, 18, 61). In other words, although there is a modicum of T synthesis in CRPC, over time it accumulates to higher concentrations, essentially as a reservoir, precisely because it is not readily converted to DHT. In a comparable situation, clinical studies of 1 mg finasteride (which blocks SRD5A2) in benign prostatic tissue yields the same 3:1 ratio of T to DHT that is present in CRPC in the absence of SRD5A inhibition (58). The 5α-reduction of T is impeded pharmacologically in the former setting and because of SRD5A1 expression in the latter.
Figure 2.
T to DHT ratios in the prostate in various clinical states. Left, T to DHT ratios reported from independent studies. Benign prostate and untreated localized prostate cancer have concentrations of T ≪ DHT, with a ratio of approximately 1:10. In contrast, CRPC has concentrations of T > DHT, with an approximate ratio of 3:1. Pharmacological inhibition of SRD5A2 similarly increases the T to DHT ratio in benign prostate. Right, A model that explains these results in the context of the 5α-dione pathway in CRPC. In benign prostate, SRD5A2 robustly converts T to DHT. In CRPC, SRD5A1 robustly converts AD to 5α-dione. Although minor flux exists from AD to T, T is inefficiently converted to DHT by SRD5A1 and accumulates to concentrations above DHT. 5α-Dione concentrations have not been directly measured in CRPC and may also be determined in part by flux from 5α-dione to androsterone (not shown). Orange bars indicate the relative levels of the designated androgen.
Δ5-Androstenediol (A5diol)
A5diol is formed by 17-keto reduction of DHEA and is not involved in either the conventional or the 5α-dione pathway (Figure 1). A5diol has been implicated in the direct activation of both mutant and wild-type AR in prostate cancer (62, 63), as well as other steroid receptors, in particular estrogen receptor-β (64). In fact, A5diol has also been termed hermaphrodiol because of its reported androgenic and estrogenic activities (65, 66). However, the effects of A5diol on AR (or estrogen receptor) may also be dependent on metabolism by 3βHSD, which converts A5diol to T (T can in turn be converted by aromatase to estrogens). Although A5diol robustly activates AR, our studies using [3H]-A5diol and detection by HPLC show that CRPC cell lines clearly metabolize A5diol to T (67) and that pharmacological inhibition of 3βHSD blocks the major effects of A5diol on both mutant and wild-type AR (59). The metabolism-dependent effects, as opposed to direct effects of A5diol on AR, might initially be surprising given that A5diol has much more profound effects than DHEA on AR in cells (59, 63). However, in the setting of robust 3βHSD enzymatic activity, A5diol is immediately converted in 1 step to an AR agonist (A5diol→T), whereas DHEA undergoes 3 steps before it is converted to a potent agonist (DHEA→AD→5α-dione→DHT). The much shorter route from A5diol, as opposed to DHEA, to a potent AR binding androgen, probably explains some of the seemingly potent effects of A5diol on AR in intact cells.
Abiraterone and enzalutamide
Abiraterone acetate is a potent inhibitor of both 17α-hydroxylase and 17,20-lyase enzyme activity (68). Treatment with abiraterone acetate in men with CRPC leads to a profound depletion of serum adrenal androgens and frequent declines in PSA (69–73). Phase III clinical trials comparing abiraterone acetate plus prednisone vs placebo plus prednisone have now been reported, both prior to and after docetaxel chemotherapy (20, 21). The survival benefit conferred by abiraterone acetate in these trials is probably the strongest evidence that the generation of intratumoral androgens is a central and critical driver of disease progression in CRPC. Other CYP17A1 inhibitors, including TAK-700, TOK-001, and VT-464, are currently in clinical trials at various stages (74). Although these are all very potent inhibitors, some residual urinary androgens persist with pharmacological CYP17A1 inhibition with abiraterone, suggesting that sustained steroidogenesis is a possible mechanism of resistance (75). Blocking a second step in the pathway for cooperative inhibition of steroidogenesis would counter such a mechanism of resistance. Increased abiraterone drug exposure may block 3βHSD and is one possibility for dual CYP17A1 and 3βHSD inhibition (67). Furthermore, abiraterone may also directly inhibit AR (76). Clinical trials are currently underway that will test the hypothesis that increased drug exposure may increase clinical efficacy.
Enzalutamide (formerly known as MDV3100) is an antagonist that has more potent activity compared with other AR antagonists that are used in clinical practice (77). The phase I and II clinical trials of enzalutamide demonstrated PSA declines comparable with the clinical activity of abiraterone (77, 78). A phase III trial of enzalutamide vs placebo in docetaxel-treated CRPC showed a survival extension (79). The results of a second phase III clinical trial in docetaxel-naïve CRPC is pending, but it is anticipated to show a similar benefit. ARN-509 is another potent AR antagonist that is in clinical trials (80).
Conclusions
Androgen metabolism is a central factor in response and resistance to hormonal therapies in prostate cancer. Recent clinical gains have been achieved with recognition of the importance of CYP17A1 and the continued reliance of CRPC tumors on AR. However, the underlying mechanisms of resistance through metabolism that sustain androgens by promoting synthesis and possibly preventing loss have not yet been fully exploited. A precise context-dependent understanding of the required enzymes, intermediate metabolites, metabolic pathways, and interactions with other signaling pathways is necessary to develop the next generation of treatments and to identify biomarkers of response and resistance.
Acknowledgments
This work was supported in part by a Howard Hughes Medical Institute Physician-Scientist Early Career Award, the Prostate Cancer Foundation, Grant 1R01CA172382 from the National Cancer Institute, an American Cancer Society Research Scholar Award, and Grant PC080193 from the US Army Medical Research and Materiel Command.
Disclosure Summary: The author has received research funding from Janssen and has been consultant to Janssen and Medivation.
Footnotes
- AD
- Δ4-androstendione
- A5diol
- Δ5-androstenediol
- ADT
- androgen deprivation therapy
- AR
- androgen receptor
- CRPC
- castration-resistant prostate cancer
- CYP17A1
- 17α-hydroxylase/17,20-lyase
- Δ4
- double bond between C4 and C5
- Δ5
- carbon-carbon double bond between C5 and C6
- DHEA
- dehydroepiandrosterone
- 5α-dione
- 5α-androstanedione
- 3βHSD
- 3β-hydroxysteroid dehydrogenase
- 17βHSD
- 17β-hydroxysteroid dehydrogenase
- 17βHSD3
- 17β-hydroxysteroid dehydrogenase-3
- PSA
- prostate-specific antigen
- SRD5A
- steroid-5α-reductase.
References
- 1. Sharifi N, Auchus RJ. Steroid biosynthesis and prostate cancer. Steroids. 2012;77:719–726 [DOI] [PubMed] [Google Scholar]
- 2. Andersson S, Geissler WM, Wu L, et al. Molecular genetics and pathophysiology of 17β-hydroxysteroid dehydrogenase 3 deficiency. J Clin Endocrinol Metab. 1996;81:130–136 [DOI] [PubMed] [Google Scholar]
- 3. Bagatell CJ, Bremner WJ. Androgens in men—uses and abuses. N Engl J Med. 1996;334:707–714 [DOI] [PubMed] [Google Scholar]
- 4. Andersson S, Berman DM, Jenkins EP, Russell DW. Deletion of steroid 5α-reductase 2 gene in male pseudohermaphroditism. Nature. 1991;354:159–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang KH, Sharifi N. Prostate cancer-from steroid transformations to clinical translation. Nat Rev Urol. 2012;9:721–724 [DOI] [PubMed] [Google Scholar]
- 6. Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648 [DOI] [PubMed] [Google Scholar]
- 7. Tomlins SA, Mehra R, Rhodes DR, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39:41–51 [DOI] [PubMed] [Google Scholar]
- 8. Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381 [DOI] [PubMed] [Google Scholar]
- 9. Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244 [DOI] [PubMed] [Google Scholar]
- 10. Page ST, Lin DW, Mostaghel EA, et al. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab. 2006;91:3850–3856 [DOI] [PubMed] [Google Scholar]
- 11. Nishiyama T, Hashimoto Y, Takahashi K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin Cancer Res. 2004;10:7121–7126 [DOI] [PubMed] [Google Scholar]
- 12. Labrie F. Blockade of testicular and adrenal androgens in prostate cancer treatment. Nat Rev Urol. 2011;8:73–85 [DOI] [PubMed] [Google Scholar]
- 13. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Prostate Cancer Trialists' Collaborative Group. Lancet. 2000;355:1491–1498 [PubMed] [Google Scholar]
- 14. Scher H, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261 [DOI] [PubMed] [Google Scholar]
- 16. Geller J, Albert J, Loza D, Geller S, Stoeltzing W, de la Vega D. DHT concentrations in human prostate cancer tissue. J Clin Endocrinol Metab. 1978;46:440–444 [DOI] [PubMed] [Google Scholar]
- 17. Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–4657 [DOI] [PubMed] [Google Scholar]
- 18. Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deslypere JP, Young M, Wilson JD, McPhaul MJ. Testosterone and 5α-dihydrotestosterone interact differently with the androgen receptor to enhance transcription of the MMTV-CAT reporter gene. Mol Cell Endocrinol. 1992;88:15–22 [DOI] [PubMed] [Google Scholar]
- 20. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in Metastatic Prostate Cancer without Previous Chemotherapy. N Engl J Med. 2013;368(2):138–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sharifi N, McPhaul MJ, Auchus RJ. “Getting from here to there”—mechanisms and limitations to the activation of the androgen receptor in castration-resistant prostate cancer. J Invest Med. 2010;58:938–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krone N, Arlt W. Genetics of congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab. 2009;23:181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stocco DM. Clinical disorders associated with abnormal cholesterol transport: mutations in the steroidogenic acute regulatory protein. Mol Cell Endocrinol. 2002;191:19–25 [DOI] [PubMed] [Google Scholar]
- 25. Lorence MC, Murry BA, Trant JM, Mason JI. Human 3β-hydroxysteroid dehydrogenase/Δ5-4isomerase from placenta: expression in nonsteroidogenic cells of a protein that catalyzes the dehydrogenation/isomerization of C21 and C19 steroids. Endocrinology. 1990;126:2493–2498 [DOI] [PubMed] [Google Scholar]
- 26. Thomas JL, Mason JI, Brandt S, Spencer BR, Jr, Norris W. Sstructure/function relationships responsible for the kinetic differences between human type 1 and type 2 3β-hydroxysteroid dehydrogenase and for the catalysis of the type 1 activity. J Biol Chem. 2002;277:42795–42801 [DOI] [PubMed] [Google Scholar]
- 27. Simard J, Ricketts ML, Gingras S, Soucy P, Feltus FA, Melner MH. Molecular biology of the 3β-hydroxysteroid dehydrogenase/Δ5-4 isomerase gene family. Endocr Rev. 2005;26:525–582 [DOI] [PubMed] [Google Scholar]
- 28. Russell DW, Wilson JD. Steroid 5α-reductase: two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61 [DOI] [PubMed] [Google Scholar]
- 29. Godoy A, Kawinski E, Li Y, et al. 5α-Reductase type 3 expression in human benign and malignant tissues: a comparative analysis during prostate cancer progression. Prostate. 2011;71:1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Uemura M, Tamura K, Chung S, et al. Novel 5α-steroid reductase (SRD5A3, type-3) is overexpressed in hormone-refractory prostate cancer. Cancer Sci. 2008;99:81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mitsiades N, Sung CC, Schultz N, et al. Distinct patterns of dysregulated expression of enzymes involved in androgen synthesis and metabolism in metastatic prostate cancer tumors. Cancer Res. 72(23):6142–6152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cantagrel V, Lefeber DJ, Ng BG, et al. SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder. Cell. 2010;142:203–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stiles AR, Russell DW. SRD5A3: A surprising role in glycosylation. Cell. 2010;142:196–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Auchus RJ. Overview of dehydroepiandrosterone biosynthesis. Semin Reprod Med. 2004;22:281–288 [DOI] [PubMed] [Google Scholar]
- 35. Moeller G, Adamski J. Integrated view on 17β-hydroxysteroid dehydrogenases. Mol Cell Endocrinol. 2009;301:7–19 [DOI] [PubMed] [Google Scholar]
- 36. DePrimo SE, Diehn M, Nelson JB, et al. Transcriptional programs activated by exposure of human prostate cancer cells to androgen. Genome Biol. 2002;3:RESEARCH0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Andersson S, Russell DW, Wilson JD. 17β-Hydroxysteroid dehydrogenase 3 deficiency. Trends Endocrinol Metab. 1996;7:121–126 [DOI] [PubMed] [Google Scholar]
- 38. Penning TM, Jin Y, Rizner TL, Bauman DR. Pre-receptor regulation of the androgen receptor. Mol Cell Endocrinol. 2008;281:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Penning TM, Steckelbroeck S, Bauman DR, et al. Aldo-keto reductase (AKR) 1C3: role in prostate disease and the development of specific inhibitors. Mol Cell Endocrinol. 2006;248:182–191 [DOI] [PubMed] [Google Scholar]
- 40. Knudsen KE, Penning TM. Partners in crime: deregulation of AR activity and androgen synthesis in prostate cancer. Trends Endocrinol Metab. 2010;21:315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nagasaki S, Miki Y, Akahira J, Suzuki T, Sasano H. 17β-Hydroxysteroid dehydrogenases in human breast cancer. Ann NY Acad Sci. 2009;1155:25–32 [DOI] [PubMed] [Google Scholar]
- 42. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Locke JA, Guns ES, Lubik AA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415 [DOI] [PubMed] [Google Scholar]
- 44. Efstathiou E, Titus M, Tsavachidou D, et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J Clin Oncol. 2012;30:637–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hofland J, van Weerden WM, Dits NF, et al. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Res. 2010;70:1256–1264 [DOI] [PubMed] [Google Scholar]
- 46. Chang KH, Li R, Papari-Zareei M, et al. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci USA. 2011;108:13728–13733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004;15:432–438 [DOI] [PubMed] [Google Scholar]
- 48. Shaw G, Renfree MB, Leihy MW, Shackleton CH, Roitman E, Wilson JD. Prostate formation in a marsupial is mediated by the testicular androgen 5α-androstane-3α,17β-diol. Proc Natl Acad Sci USA. 2000;97:12256–12259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Luu-The V, Belanger A, Labrie F. Androgen biosynthetic pathways in the human prostate. Best Pract Res Clin Endocrinol Metab. 2008;22:207–221 [DOI] [PubMed] [Google Scholar]
- 50. Ryan CJ, Tindall DJ. Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. J Clin Oncol. 2011;29:3651–3658 [DOI] [PubMed] [Google Scholar]
- 51. Stein MN, Goodin S, Dipaola RS. Abiraterone in prostate cancer: a new angle to an old problem. Clin Cancer Res. 2012;18:1848–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hofland J, van Weerden WM, Steenbergen J, Dits NF, Jenster G, de Jong FH. Activin A stimulates AKR1C3 expression and growth in human prostate cancer. Endocrinology. 2012;153:5726–5734 [DOI] [PubMed] [Google Scholar]
- 53. Tomkins GM. The enzymatic reduction of Δ4–3-ketosteroids. J Biol Chem. 1957;225:13–24 [PubMed] [Google Scholar]
- 54. Titus MA, Gregory CW, Ford OH, 3rd, Schell MJ, Maygarden SJ, Mohler JL. Steroid 5α-reductase isozymes I and II in recurrent prostate cancer. Clin Cancer Res. 2005;11:4365–4371 [DOI] [PubMed] [Google Scholar]
- 55. Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825 [DOI] [PubMed] [Google Scholar]
- 56. Thigpen AE, Cala KM, Russell DW. Characterization of Chinese hamster ovary cell lines expressing human steroid 5α-reductase isozymes. J Biol Chem. 1993;268:17404–17412 [PubMed] [Google Scholar]
- 57. Sharifi N. Clinical implications of the 5α-androstanedione pathway for castration-resistant prostate cancer. Future Oncol. 2011;7:1239–1241 [DOI] [PubMed] [Google Scholar]
- 58. McConnell JD, Wilson JD, George FW, Geller J, Pappas F, Stoner E. Finasteride, an inhibitor of 5α-reductase, suppresses prostatic dihydrotestosterone in men with benign prostatic hyperplasia. J Clin Endocrinol Metab. 1992;74:505–508 [DOI] [PubMed] [Google Scholar]
- 59. Evaul K, Li R, Papari-Zareei M, Auchus RJ, Sharifi N. 3β-Hydroxysteroid dehydrogenase is a possible pharmacological target in the treatment of castration-resistant prostate cancer. Endocrinology. 2010;151:3514–3520 [DOI] [PubMed] [Google Scholar]
- 60. Sharifi N. The 5α-androstanedione pathway to dihydrotestosterone in castration-resistant prostate cancer. J Invest Med. 2012;60:504–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mohler JL, Gregory CW, Ford OH., 3rd The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448 [DOI] [PubMed] [Google Scholar]
- 62. Mizokami A, Koh E, Fujita H, et al. The adrenal androgen androstenediol is present in prostate cancer tissue after androgen deprivation therapy and activates mutated androgen receptor. Cancer Res. 2004;64:765–771 [DOI] [PubMed] [Google Scholar]
- 63. Miyamoto H, Yeh S, Lardy H, Messing E, Chang C. Δ5-Androstenediol is a natural hormone with androgenic activity in human prostate cancer cells. Proc Natl Acad Sci USA. 1998;95:11083–11088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Saijo K, Collier JG, Li AC, Katzenellenbogen JA, Glass CK. An ADIOL-ERβ-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell. 2011;145:584–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sharifi N. New agents and strategies for the hormonal treatment of castration-resistant prostate cancer. Expert Opin Investig Drugs. 2010;19:837–846 [DOI] [PubMed] [Google Scholar]
- 66. Adams JB. Control of secretion and the function of C19-Δ 5-steroids of the human adrenal gland. Mol Cell Endocrinol. 1985;41:1–17 [DOI] [PubMed] [Google Scholar]
- 67. Li R, Evaul K, Sharma KK, et al. Abiraterone inhibits 3β-hydroxysteroid dehydrogenase: a rationale for increasing drug exposure in castration-resistant prostate cancer. Clin Cancer Res. 2012;18:3571–3579 [DOI] [PubMed] [Google Scholar]
- 68. Potter GA, Barrie SE, Jarman M, Rowlands MG. Novel steroidal inhibitors of human cytochrome P45017α (17α-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. J Med Chem. 1995;38:2463–2471 [DOI] [PubMed] [Google Scholar]
- 69. Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–4571 [DOI] [PubMed] [Google Scholar]
- 70. Attard G, Reid AH, A'Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28:1481–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Reid AH, Attard G, Danila DC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28:1489–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Danila DC, Morris MJ, de Bono JS, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ferraldeschi R, Sharifi N, Auchus RJ, Attard G. Molecular pathways: inhibiting steroid biosynthesis in prostate cancer [published online March 7, 2013]. Clin Cancer Res. doi:10.1158/1078-0432.CCR-12-0931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Attard G, Reid AH, Auchus RJ, et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab. 2012;97:507–516 [DOI] [PubMed] [Google Scholar]
- 76. Richards J, Lim AC, Hay CW, et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res. 2012;72:2176–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375:1437–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197 [DOI] [PubMed] [Google Scholar]
- 80. Clegg NJ, Wongvipat J, Joseph JD, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72:1494–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]