SUMMARY
Antigenic variation in Plasmodium falciparum malaria is mediated by transcriptional switches between different members of the multicopy var gene family. Each var gene encodes a member of a group of heterogeneous surface proteins collectively referred to as PfEMP1. Mutually exclusive expression ensures that an individual parasite only transcribes a single var gene at a time. In this work we studied var gene switching to determine if transcriptional switches favor expression of particular subgroups of var genes and if var gene activation within a clonal population of parasites follows a predetermined order. We show that in clonal parasite populations, expression of var genes located in the central regions of chromosomes is remarkably stable and that they rarely undergo transcriptional switches in the absence of selection. In contrast, parasites expressing subtelomerically located var genes readily switched to alternative var loci. We confirmed these observations by generating transgenic parasites carrying drug selectable markers in subtelomeric and central var loci and monitoring switching after release from selection. Our data show that different var genes have different intrinsic switching rates that correlate with var gene subtype, and that there is no pre-determined order of expression.
INTRODUCTION
Antigenic variation serves the purpose of evading the host immune response and thus is essential for the survival of many pathogens. In Plasmodium falciparum, the parasite causing the most severe form of human malaria, antigenic variation is mediated by switches in expression within the large heterogeneous family of surface proteins collectively referred to as Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) (Miller, Good, and Milon, 1994). Alternative forms of PfEMP1 are encoded by different var genes and each parasite contains approximately 60 genes located in subtelomeric or central regions of its chromosomes (Su et al., 1995;Baruch et al., 1995;Gardner et al., 2002).
Each individual parasite only expresses a single var gene at a time and thus only places one PfEMP1 variant on the surface of the infected erythrocyte (Scherf et al., 1998). This phenomenon is referred to as mutually exclusive expression and recent studies have revealed that it is transcriptionally regulated and independent of antigen production (Dzikowski, Frank, and Deitsch, 2006;Voss et al., 2006). This implies that changes in var gene expression do not occur in response to external signals, thus the parasite must be able to create transcriptional switches spontaneously in order to generate small subsets of parasites expressing different PfEMP1 proteins. These switches need to occur often enough to generate parasite subpopulations that escape the human immune response, yet be tightly controlled in order to avoid premature expenditure of the 60 different proteins encoded within the genome of each individual parasite. Consistent with this need for tightly controlled transcriptional switches, recent studies have revealed that the regulation of this large gene family consists of multiple layers (Horrocks et al., 2004). The first layer includes the two promoters found in virtually all var genes: the 5′ var promoter and a second promoter found within the var intron (Deitsch, Calderwood, and Wellems, 2001;Frank et al., 2006;Gannoun-Zaki et al., 2005). These genetic elements confer the ability to silence each individual var gene as an epigenetic unit. Recently a second layer of regulation has emerged consisting of chromatin modifications, specifically histone deacytelation mediated by the Plasmodium falciparum deacetylase PFSIR2 (Freitas-Junior et al., 2005). Knock out parasites for this gene exhibited transcriptional up regulation of a subset of subtelomerically located var genes (Duraisingh et al., 2005), however the majority of var genes were not affected, suggesting that this layer of regulation does not uniformly affect all var genes.
In this study, we attempt to outline the dynamics of antigenic variation in in vitro propagated P. falciparum parasites. We examined the switching pattern of bulk and clonal NF54 parasites grown without selection pressure and show that expression of var genes located in central areas of P. falciparum chromosomes is favored because of their low “off” rates. We verified this expression bias using transgenic parasites and show that subtelomeric var transgenes indeed have significantly higher off rates compared to a central var transgene, even if they are located on the same chromosome. In addition, we show that after selection for activation of different var loci in a clonal parasite background, parasite subpopulations with completely different var gene expression patterns arise over time, demonstrating that the direction and rate of switching is determined by the identity and off rate of the active var gene only, and that there is no pre-determined order of var gene expression. This type of stochastic mechanism ensures the generation of heterogenous var gene expression patterns despite the long-term bias toward expression of var genes with low off rates.
RESULTS
Heterogeneous cultures of wild type NF54 parasites exhibit biased var gene expression
Antigenic variation in P. falciparum malaria is achieved through switches in var gene transcription, resulting in expression of different PfEMP1 variants on the surface of the infected erythrocyte. Previous studies suggested that var gene switches are regulated exclusively at the transcriptional level (Scherf et al., 1998;Dzikowski, Frank, and Deitsch, 2006;Voss et al., 2006) and that the information determining the probability of activation or silencing of individual var genes is present in their surrounding DNA (Horrocks et al., 2004). Since in parasites cultured under standard conditions there is no selection for either antigenic or cytoadhesive phenotypes, we hypothesized that var genes possessing an intrinsically high “on” or low “off” switching rate would predominate over time, thus an unselected population would potentially be biased toward expression of such genes.
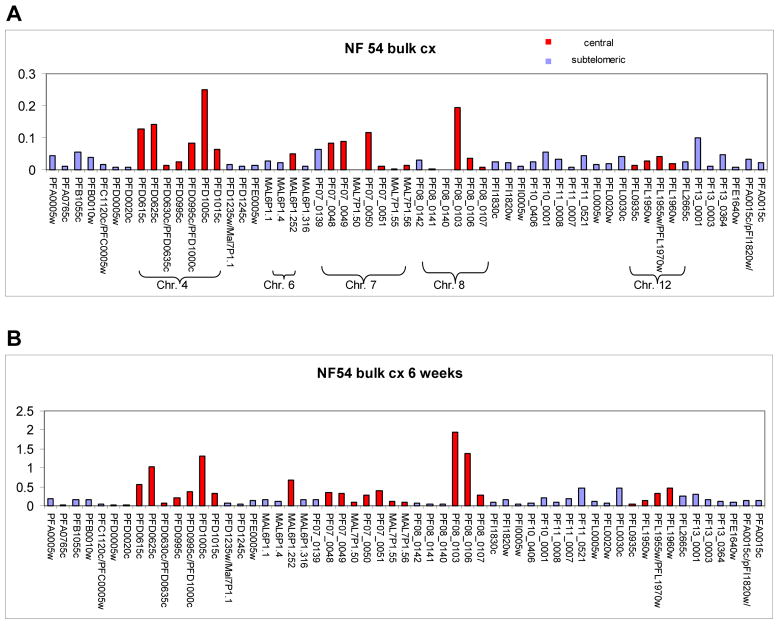
To investigate this possibility, we evaluated the var gene expression pattern in unselected NF54 bulk cultures. var gene expression was assayed by quantitative reverse transcriptase polymerase chain reaction (Q-RT-PCR) with a primer set that measures transcription from each individual var gene (Salanti et al., 2003). As expected, transcriptional analysis revealed that in an unselected bulk culture, transcripts from virtually all var genes were detected (Figure 1). However transcription of var genes was not uniform and in particular seemed to favor expression of var genes located in central chromosomal clusters. Ranking of all var genes according to their level of transcription revealed that of the 10 most highly expressed var genes, 9 were located in central regions of Plasmodium falciparum chromosomes (Figure 1A). A second transcriptional analysis performed on the same parasite culture after 6 weeks of continuous growth revealed a similar picture with a predominance of the same central var genes being expressed at the highest levels. In contrast, the previously highly expressed subtelomeric var genes were now expressed at a relatively low level (Figure 1B). These initial experiments suggested that var gene expression in our cultures was biased towards central var genes, possibly due to differences in off rates between subtelomeric and centrally located var genes.
Figure 1. Transcriptional analysis of bulk NF54 cultures by quantitative RT-PCR. Panel A.
Transcriptional analysis of an unselected NF 54 bulk culture. var genes located in central and subtelomeric regions of P.falciparum chromosomes are colored in red and blue respectively. The expression pattern of the var gene family favors expression of central var genes. Panel B. Transcriptional analysis of the same culture after 6 weeks of unselected growth. The var gene expression pattern of the parasite population continues to favor expression of central var genes.
Very low off rates of var genes correlate with location in central regions of chromosomes
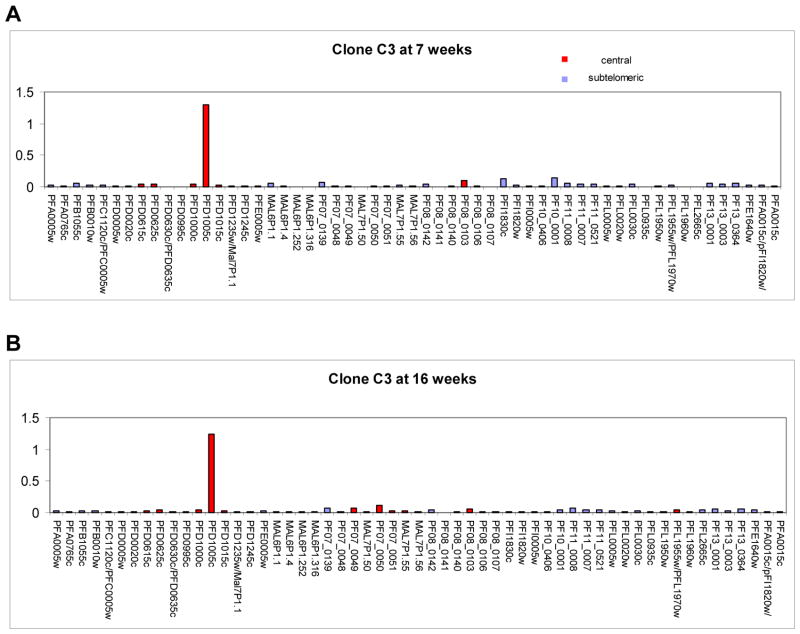
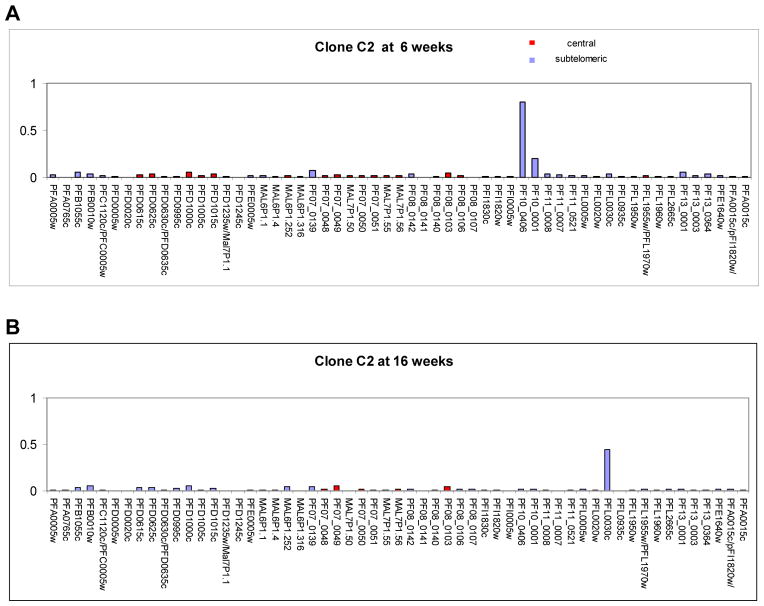
To examine if the bias towards expression of central var genes that we observed in our NF54 bulk cultures was due to differences in chromosomal position, we generated clonal parasite lines from the original NF54 bulk culture and followed these individual cultures for up to 28 weeks (Figure 2). We analyzed the var expression profile for 10 individual clones at several time points after cloning. var gene transcriptional profiling was first possible at 6 weeks, a time point that reflects the minimum time required for a single parasite to expand into a clonal culture large enough to enable transcriptional analysis of the entire var gene repertoire. As expected, we found a clear bias towards the expression of central var genes within these clones, despite the fact that they are a minority of the gene family (23 out of 59). Seven of the 10 clones were predominately expressing central var genes and the remaining 3 were expressing subtelomeric var genes (Table 1). Expression of central var genes favored loci located on chromosome 4, with 5 different clones expressing the gene PFD1005c. Interestingly, 2 of the 3 cultures expressing subtelomeric var genes exhibited a transcription pattern that showed 2 var genes expressed at almost identical levels, suggesting that they may have switched early during the 6 weeks of growth after the limiting dilution. To further investigate the switching behaviors of these individual clones, we followed them over a period of at least 16 weeks. All clones expressing central var genes exhibited very low off rates with continued expression of the dominant gene at the same levels until at least 16 and up to 28 weeks after the initial limiting dilution (Figure 3 and Table 2). In contrast, clonal populations initially expressing subtelomeric var genes had switched away from the original locus at 16 weeks and some as early as 10 weeks after the limiting dilution (Table 2 and Figure 4). We detected expression of both subtelomeric and internal var genes in the switched populations, indicating that both types of genes may have similar on rates. Taken together this data suggested that subtelomeric var genes have higher off rates than central var genes and may explain why, in an unselected culture, expression of central var genes is favored over time.
Figure 2. Overview of wild type NF 54 clone var transcriptional analysis.
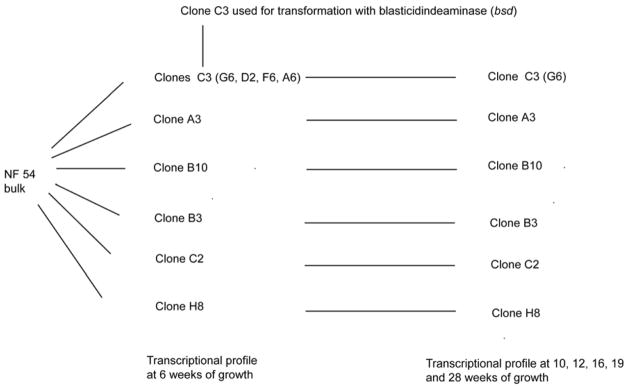
10 clones were generated from the original NF54 culture and transcriptional analysis was performed at time points ranging from 6 to 28 weeks after the initial cloning experiment. Clones G6, D2, F6 and A6 are in parenthesis because they expressed the same gene.
Table 1.
Dominant var genes in 10 clones generated from a NF54 bulk culture after 6 weeks of unselected growth.
| Clone | Dominant gene(s) | Chromosomal Location |
|---|---|---|
| A3 | MAL6p1.252 | 6, central |
| C3 | PFD1005c | 4, central |
| G6 | PFD1005c | 4, central |
| D2 | PFD1005c | 4, central |
| F6 | PFD1005c | 4, central |
| A6 | PFD1005c | 4, central |
| B10 | PFL1960, PFL1955/PFL1970 | 12, central |
| B3 | PFE0005w, PFL0005w | 5,12 subtelomeric |
| C2 | PF10_0406 | 10, subtelomeric |
| H8 | PF13 0001, MAL6P1.1 | 13,6 subtelomeric |
Figure 3. Transcriptional analysis of the var gene family in the C3 clone shows a very low off rate for a central var gene.
Panel A. Transcriptional profile of C3 7 weeks after the initial cloning experiment. The central var gene PFD1005c on chromosome 4 is the dominantly expressed gene. Panel B. Transcriptional profile at 16 weeks of unselected growth. The transcriptional activity of PFD1005c is unchanged suggesting a very low off rate of this var locus.
Table 2.
var gene expression in clonal NF54 cultures after 10–28 weeks of unselected growth.
| NF54 Clone | Dominant Gene(s) | Time from limiting Dilution | Switched away | Chromosomal Location |
|---|---|---|---|---|
| A3 | MAL6p1.252 | 19 weeks | No | 6, central |
| C3 | PFD1005c | 16 weeks | No | 4, central |
| G6 | PFD1005c | 28 weeks | No | 4, central |
| B10 | PFL1960, PFL1955/PFL 1970, PFL0030c | 16 weeks | No | 12, central, subtelomeric |
| B3 | PFE0005w | 12 weeks | Yes | 5, subtelomeric |
| C2 | PFL0030c | 16 weeks | Yes | 12, subtelomeric |
| H8 | MAL6P1.4 | 10 weeks | Yes | 6, subtelomeric |
Figure 4. Transcriptional analysis of the var gene family in the C2 clone shows a high off rate for a subtelomeric var gene.
Panel A. Transcriptional activity at 6 weeks. var gene transcription is dominated by the subtelomeric gene PF10_0406 on chromosome 10. Panel B. Transcriptional activity at 16 weeks. The originally transcriptionally active subtelomeric locus is now silent and var gene transcription has switched to the subtelomeric locus PFL_0030c on chromosome 12.
To quantify the off rates of central var loci, we calculated the decrease of the dominant gene over time. This revealed an off rate between 0–0.3% per generation for all central var genes examined (Table 3). In contrast, the clones predominately expressing subtelomeric var genes had completely switched away from the gene initially expressed, making it impossible to calculate specific off rates. Therefore, to measure off rates of subtelomeric loci, we utilized populations of parasites that can be selected to homogenously express a single var gene and followed their switching patterns over time.
Table 3.
off rate ranges for central and subtelomeric var loci in wild type NF54 parasites.
| Parasite | Gene | Off rate per generation | # of generations between analysis | Chromosomal Location |
|---|---|---|---|---|
| A3 | MAL6P1.252 | 0 | 45 | 6, central |
| C3 | PFD1005c | 0.02% | 35 | 4, central |
| G6 | PFD1005c | 0.27% | 77 | 4, central |
| NF 54 CSA sel. | PFL0030c | 1.0% | 41 | 12, subtelom. |
| C2 | PF10_0406 | Very high | 35 | 10, subtelom. |
NF 54 parasites expressing var2csa switch expression without selection
To further investigate if subtelomeric location is associated with an increased off rate, we monitored var switching by analyzing the transcription patterns of bulk NF54 parasites that were selected for binding to chondroitin sulfate A (CSA). This selection results in preferential expression of the var2csa gene, a var gene that is located in the subtelomeric region of chromosome 12 (Lavstsen et al., 2003). Interestingly however, while var2csa is located within a subtelomeric region, it does not share one of the specific promoter types typical of var genes located in these chromosomal regions, and its conserved sequence between parasite isolates implies that it does not undergo frequent recombination in a manner thought to be common among other var genes. We were therefore interested in whether var2csa would also have a high off rate similar to other subtelomeric var genes, and thus exhibit significant switches over time when its expression is not selected for.
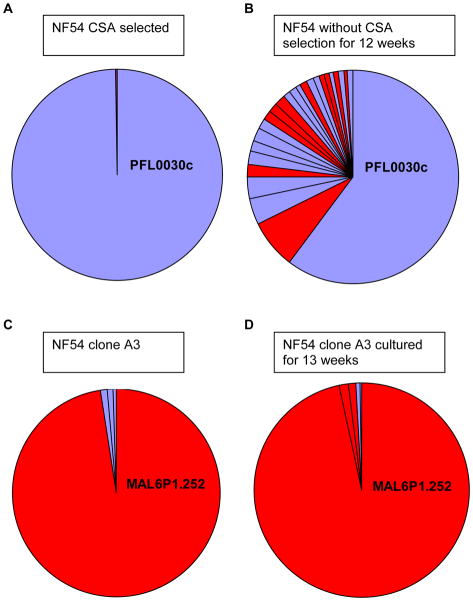
As expected, immediately after selection for binding to CSA, the parasites exclusively expressed the var2csa gene (Figure 5A). These parasites were then grown without CSA selection for 12 weeks. Consistent with what was observed for other subtelomeric genes, without selection the level of var2csa transcript had significantly decreased over time demonstrating that indeed a large part of the population had switched expression to different var genes (Figure 5B). In contrast, the clonal parasite population A3 that exclusively expresses the central var gene MAL6P1.252 showed no change in var gene expression over the same period of unselected growth (Figure 5C and D). These data indicate that the subtelomeric var2csa gene has an off rate of approximately 1 % (Table 3). However the var2csa signal was still the dominant signal after 12 weeks of growth without selection, suggesting that this subtelomeric locus behaved somewhat differently from the subtelomeric loci in the NF54 clones. Interestingly, var gene activation appeared to be evenly distributed between central and subtelomeric var genes, again suggesting that central and subtelomeric var genes have relatively similar on rates.
Figure 5. Comparison of var gene switching in NF54 parasites expressing the subtelomeric var2csa locus and in NF54 parasites expressing a central var gene.
The relative contribution of each var gene to total transcriptional activity of the var gene family is shown. Each slice of the pie represents transcription from an individual var gene, with the genes arranged in a clockwise direction from most highly expressed to least, starting from the top of the graph. Central var genes are colored in red and subtelomeric var genes are colored in light blue. Panel A. Expression pattern of an NF54 parasite population selected for CSA binding. Immediately after selection the culture exclusively expresses the locus PFL0030c located in the subtelomeric region of chromosome 12. Panel B. The same culture following 12 weeks of unselected growth. A large part of the population has switched away from the var2csa gene to other var loci. Panel C. var gene expression in the clonal culture A3 that exclusively expresses the central var gene MAL6P1.252 on chromosome 6. Panel D. After 13 weeks of continuous growth the expression of MAL6P1.252 is unchanged indicating an immeasurably low off rate for this locus.
Subtelomeric location of transgenic var genes is associated with high “off” rates
Taken together our data showed that central var genes consistently had very low off rates ranging from 0–0.3% per generation. In contrast to subtelomeric var genes that exhibited a dynamic range of off rates of at least 1 %, and in some cases much higher. This is consistent with data reported by Horrocks et al. (Horrocks et al., 2004) who described different “on” and “off” rates for individual var genes and suggested that these rates might be dependent on the different local chromatin environments at each var locus. To further test the hypothesis that var genes in different chromosomal regions have different switching rates, we generated transgenic parasites with the bsd selectable marker integrated into specific central or subtelomeric var genes (Dzikowski, Frank, and Deitsch, 2006). Under blasticidin pressure these parasite lines only express the recombinant var locus containing the transgene while all other var genes in the genome are transcriptionally silent, thereby generating a conditional knock out of the entire var gene family. Thus by growing parasites in the presence of blasticidin, homogenous populations of parasites all expressing the same transgenic var locus can be generated, and by subsequently removing blasticidin pressure, the rate at which parasites spontaneously switch to expressing different var genes can easily be assessed over time.
We targeted 2 integration events to chromosome 12 to specifically examine the effect of different locations on the same chromosome. The clone B15C2 carries the drug selectable marker bsd at the subtelomeric locus PFL0020c, directly adjacent to the locus PFL0030c, the previously described var2csa gene. The clone C7G12 contained the marker gene integrated into the locus PFL1960w in the central region of chromosome 12. The NF45 clone B10 expressed this var gene as one of the dominant genes and therefore enabled us to directly compare the transcriptional activity of wild type and transgene in the same chromosomal locus. To test if subtelomeric loci on different chromosomes exhibit similar switching behaviors, we also generated the clone B12E3 with an integration event at the subtelomeric locus PFB1055c on chromosome 2.
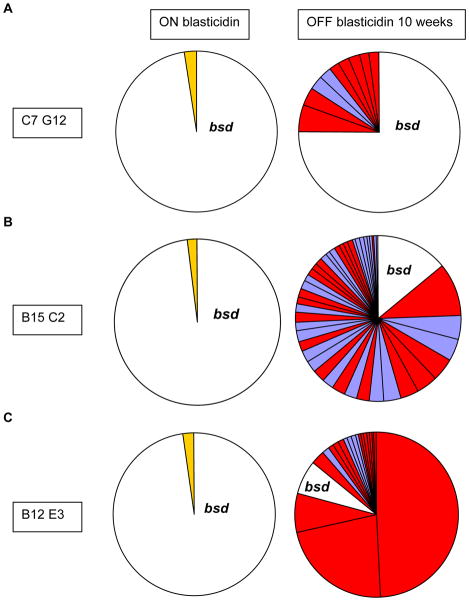
In order to evaluate if the position of the transgene influenced the switch rate, we initially grew all three transgenic parasite lines under blasticidin pressure, followed by growth without blasticidin and var gene transcription was assayed after 3 and 10 weeks of unselected growth (Figure 6). Additionally, after 10 weeks of unselected growth the individual transgenic cultures were then re-cloned by limiting dilution and transcription was again assayed in all sub-clones after another 6 weeks of unselected growth. After 10 weeks of unselected growth, the recombinant var gene in the central locus PFL1960w on chromosome 12 (clone C7G12) displayed a relatively low off rate of 0.7 % per generation suggesting that the majority of parasites were still expressing the transgene despite the absence of selection (Figure 7, Table 4). In marked contrast, the transgene at the subtelomeric locus PFL0020c on chromosome 12 showed a relatively high off rate of 2.5 % after 10 weeks of unselected growth (clone B15C2). Consistent with an association of subtelomeric location and high off rate, the transgene at the subtelomeric locus PFB1055c on chromosome 2 (clone B12E3) also displayed a high off rate of 2.7 % after 10 weeks of unselected growth (Table 4).
Figure 6. Overview of var gene transcriptional analysis in transgenic parasites carrying the blasticidin-S-deaminase selectable marker gene at different chromosomal locations.
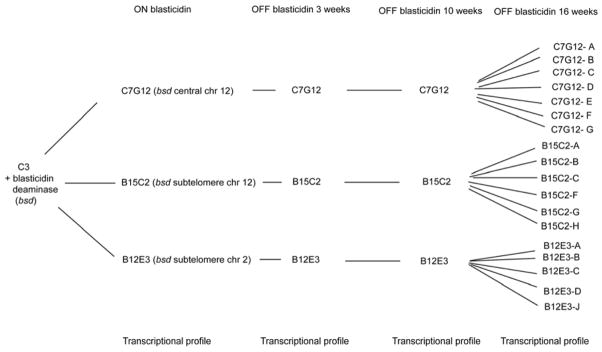
The diagram shows the strategy used to isolate different parasite populations, which were then grown for different time periods either with or without selection with blasticidin. Addition of blasticidin to the culture media selects for expression of a single, transgenic var gene and consequent silencing of all other var genes in the parasite genome. Removal of blasticidin pressure allows the parasites to switch var expression freely.
Figure 7. Change in var gene expression after release from selection pressure in the 3 different transgenic parasite lines generated in the C3 clonal background.
Transcriptional analysis of the var gene family under blasticidin selection and after 10 weeks of blasticidin free growth. Panel A. In the parasite line C7G12 (central integration on chr 12), even after 10 weeks of growth without drug pressure the marker gene is still the dominant transcript (in white). Panel B. In the parasite line B15C2 (subtelomeric integration on chromosome 12) after 10 weeks of drug free growth the majority of the population has switched away from the transgene and activated endogenous var loci. The expression profile is dominated by central var genes (in red). Panel C. In the transgenic parasite line B12E3 (subtelomeric integration on chromosome 2) the majority of the population has switched away from the marker gene and is now expressing endogenous var loci. The expression profile is again dominated by central var genes (in red).
Table 4.
off rate ranges for central and subtelomeric var transgenes.
| Parasite | Gene | Off rate per generation | # of generations between analysis | Chromosomal Location |
|---|---|---|---|---|
| C7G12 | PFL1960c/Blast | 0.7% | 35 | 12, central |
| B15C2 | PFL0020c/Blast | 2.5% | 35 | 12, subtelom. |
| B12E3 | PFB1055c/Blast | 2.7% | 35 | 2, subtelom. |
To confirm the switching bias conferred by the transgenic var loci that was detected in the bulk cultures, we re-cloned all parasites after 10 weeks of unselected growth and determined the dominant var gene in each subclone (Table 5). We had previously observed that clonal NF54 parasites stably expressed the central var locus PFL1960w on chromosome 12 for at least 35 generations (Table 2). Consistent with a very low switch rate for this central var locus, all clones of the transgenic parasites that carried the marker at PFL1960w (C7G12) predominantly expressed the transgene. In contrast, all clones of the parasite that carried the marker gene in the subtelomeric locus PFL0020w on the same chromosome (B15C2) had switched away from the transgene. Interestingly, 3 of these clones expressed var genes also located on chromosome 12, suggesting a possible bias towards expression of genes located on the same chromosome as the marker gene. Similarly, 3 of the 5 clones generated from the parasite line B12E3 had switched to expressing different var genes.
Table 5.
Dominant var genes in 18 recloned transgenic parasites after 10 weeks of drug free growth.
| Transgenic clone | Dominant Gene | Switched away from marker gene | Chromosomal Location |
|---|---|---|---|
| C7G12-A | PFL1960w/Blast | No | 12, central |
| C7G12-B | PFL1960w/Blast | No | 12, central |
| C7G12-C | PFL1960w/Blast | No | 12, central |
| C7G12-D | PFL1960w/Blast | No | 12, central |
| C7G12-E | PFL1960w/Blast | No | 12, central |
| C7G12-F | PFL1960w/Blast | No | 12, central |
| C7G12-G | PFL1960w/Blast | No | 12, central |
| B15C2-A | PF08_0142 | Yes | 8, subtelomeric |
| B15C2-B | PFE0005w | Yes | 5, subtelomeric |
| B15C2-C | PFA0005w | Yes | 1, subtelomeric |
| B15C2-F | PFL1950w | Yes | 12, central |
| B15C2-G | PFL1950w | Yes | 12, central |
| B15C2-H | PFL0935c | Yes | 12, central |
| B12E3-B | PFD0625c | Yes | 4, central |
| B12E3-J | PFD1015c | Yes | 4, central |
| B12E3-C | PF08_0142 | Yes | 1, subtelomeric |
| B12E3-A | PFB1055c/Blast | No | 2, subtelomeric |
| B12E3-D | PFB1055c/Blast | No | 2, subtelomeric |
Activation of different var loci in an isogenic background results in different switching patterns
Previous work by Horrocks et al. had suggested a role for the switching history of an individual parasite in providing a switching direction for the subsequent generations of parasites (Horrocks et al., 2004). To test this hypothesis, we generated all our transgenic parasites in the same clonal background (Figure 2). We chose the NF54 clone C3 because it expressed the dominant central var gene in our NF bulk culture: PFD1005c. This gene is located in a central region of chromosome 4 and displayed a very low switching rate (Table 3), thus allowing us to determine if the transgenic parasites derived from this line would maintain the original low switching rate. As shown in Figure 7, activation of the 3 different transgenic var loci appeared to result in complete erasing of the C3 background, and all three lines displayed different var gene expression profiles when given time to switch without selection. Consistent with this, the on rates of individual var loci ranged from 0 to 1.1% across the different parasite lines (Table 6). Overall this data show that transcriptional activation of different var loci in parasites with identical switching histories can confer a new switching direction on each resulting parasite line.
Table 6.
var locus on rates differ in all 3 transgenic parasite lines. The on rate per generation for the most active endogenous var gene after 10 weeks of unselected growth is displayed. The 3 transgenic parasites preferentially activated different var genes.
| Locus | On rate B15C2 | On rate B12E3 | On rate C7G12 |
|---|---|---|---|
| PF10_0001 | 1.1% | 0 | 0 |
| PF07_0139 | 0 | 0.2% | 0 |
| PFL0935c | 0 | 0 | 0.5% |
DISCUSSION
The ability of the human malaria parasite P. falciparum to switch expression to different variants within its repertoire of antigenic surface proteins enables it to evade the immune system of its host and to sustain long-term, chronic infections (Roberts et al., 1992). Therefore, understanding the molecular mechanism by which the parasite switches which var gene is expressed has important implications for both understanding the pathogenesis of the disease and for developing new intervention strategies. Our experiments show that unselected heterogeneous populations of wild type parasites preferentially express central var genes and that central loci display low off rates ranging from 0–0.3 % per generation. Conversely, subtelomeric wild type var and transgenic var loci exhibited higher off rates of at least 1%–2%, indicating that var genes in subtelomeric locations may be subject to additional constraints that result in a more dynamic switching pattern. The switching rates determined here using Q-RT-PCR to measure expression of each individual var gene are similar to those previously detected using either Northern analysis (Horrocks et al., 2004) or antibodies against specific PfEMP1 variants (Roberts et al., 1992). All of the work described here was performed in the NF54/3D7 genetic background due to the availability of a complete primer set to the entire var gene family and the transgenic parasite lines that allow manipulation of var gene expression. Once suitable reagents are available, it will be interesting to determine if similar switching bias is found in other parasite lines.
Interestingly, it appeared that in the transgenic line B15C2 release from drug pressure resulted in preferential activation of var genes on the same chromosome with 3 of 5 subclones expressing var genes located on chromosome 12. This supports a model where var genes can switch through the spreading of “loose” chromatin from the gene that was previously expressed into a nearby region of the chromosome. Similarly, spreading of chromatin structure might also explain the generally higher off rates displayed by subtelomeric var genes. Telomeres are known to consist of highly condensed, transcriptionally inactive chromatin, and the propagation of this structure into the nearby subtelomeric regions could influence the propensity of the resident genes to become transcriptionally active. It has been demonstrated in several organisms that the histone deacetylase SIR2 contributes to the propagation of telomeric heterochromatin and gene silencing (Moazed, 2001). The P. falciparum homolog, PfSIR2, has been shown to spread up to 55kb internally from the telomeric repeats, thus extending into the chromosomal regions where many subtelomeric var genes are located (Freitas-Junior et al., 2005). The high off rates of subtelomeric var genes therefore could be due to the epigenetic impact of PfSIR2 or similar subtelomere associated chromatin remodeling factors.
In cultured parasites, var gene expression will be biased towards genes with low off rates, offering a possible explanation for why we uniformly observed a long term bias towards expression of central var genes in our unselected cultures. It is not yet clear if the differences in switching rates detected in the experiments presented here are a result of chromosomal position (subtelomeric vs. central) or promoter type. Analysis of the 3D7 genome sequence has identified three basic var gene promoter types, termed Ups A, B and C (Lavstsen et al., 2003;Kraemer and Smith, 2003). While types A and B are primarily found in subtelomeric regions, type C dominates the internal var clusters. Therefore it is possible that the different on and off rates described here that correlate with chromosomal position are actually a result of the different promoter types. More extensive studies using transgenic parasite lines in which alternative promoter types are artificially placed in different chromosomal regions will more directly answer this question.
All the transgenic lines described here were derived from a single recently subcloned parasite population that expressed a single, dominant central var gene, indicating that they all had similar or identical recent var gene expression histories. Nonetheless, after forced activation of different var loci followed by release from drug pressure to allow var genes to switch freely, each parasite line displayed a completely different var gene expression pattern. The expression patterns were also different than that of the original wild-type parasite line. In a related study it was recently shown that transcription of a defined subset of type C var loci occurs during gametocyte development in vitro. This transcriptional program occurs in gametocytes regardless of the var expression phenotype of their asexual progenitors and therefore is subject to regulatory processes distinct from those that manage antigenic variation during asexual replication (Sharp et al., 2006). Therefore our study as well as the study of Sharp et al. both suggest that the influence of var expression history on subsequent var gene activation patterns is unlikely to extend back further than the gene that was active at the time of the switch, and that there is no pre-determined order of var gene expression. However, the fact that we detected preferential sequential activation of adjacent var genes may also imply that var gene switching might not be entirely random, thus increasing the likelihood that the parasites arising within a specific wave of parasitemia all express a single var gene.
The observation that the expression of certain subsets of var genes is favored over time implies that central var genes might be more readily expressed under conditions of relatively weak selection, whereas transcription of subtelomeric genes would be favored when selection for specific PfEMP1 phenotypes is very stringent. For example, the CSA selected parasite line expressing the var2csa gene readily switched away from this locus when cultured without CSA selection, suggesting that it can indeed only dominate a population if it is continuously selected for. This property might underlie the observation that expression of var genes with upsA and upsB promoters, located mainly in subtelomeric areas, is increased in parasites from patients with severe and symptomatic malaria (Jensen et al., 2004;Rottmann et al., 2006)while in asymptomatic infections the expression of central var genes with upsC promoters predominates, possibly because acquired host immunity prevents certain adhesion phenotypes and thus limits the population of parasites expressing upsA and upsB var genes (Kaestli et al., 2004;Kaestli et al., 2006). How this expression pattern impacts the development of semi-sterile immunity in adults living in endemic regions is not yet clear, but unveiling expression patterns in natural infections might have implications for the design of successful vaccine strategies.
The switches in var gene expression observed in cultured parasites in this study and others indicate that var gene expression switching readily occurs in the absence of an external immune response. In addition, recent studies showed that regulation of var gene expression is independent of PfEMP1 production (Dzikowski, Frank, and Deitsch, 2006;Voss et al., 2006). Together these observations indicate that var gene regulation and the rate of expression switching are intrinsic properties encoded at the level of the genome, and that they are likely independent of phenotypic changes based on the PfEMP1 variant that is expressed on the infected cell surface. The waves of parasitemia that have been described for P. falciparum infections (Miller, Good, and Milon, 1994) therefore are likely to arise simply from clonal expansion of parasites that have spontaneously undergone switches in var gene expression, and the subsequent selection of specific PfEMP1 variants by the host immune response. Understanding the details of immune recognition of PfEMP1 therefore is likely to provide the most significant insights into the dynamics of P. falciparum infections and the prospects for successful vaccine development.
Tight regulation of var gene expression is imperative for the survival of malaria parasites within their human hosts and their ability to be transmitted to their mosquito vector. Coordinating such a large gene family that is spread throughout the genome represents a fascinating and complex problem. Our work indicates that while there is no evidence of a pre-determined order of var gene expression, the probability of expressing any particular var gene is influenced both by its chromosomal position and by which var gene was most recently expressed. In conjunction with selection by the antibody response of the host and for specific cytoadhesion properties, the resulting var gene expression patterns generate the distinct waves of parasitemia typical of P. falciparum infections. A better understanding of these host-parasite dynamics is important for the design of new and innovative intervention strategies.
EXPERIMENTAL PROCEDURES
Parasite lines and tissue culture
The NF54 parasite isolate and transgenic parasites generated in the NF54 background were used for all experiments. Bulk cultures of parasites were cloned by limiting dilution to generate clonal parasites populations as previously described (Kirkman, Su, and Wellems, 1996). var gene expression patterns of 4 clones (A3, G6, C3 and B3) were determined at 6, 10, 12, 16, 19 and 28 weeks after the initial limiting dilution. Subsequently another NF54 bulk culture was cloned, generating the clones B10, C2, H8, C6, D2, F6 and A6. var gene expression patterns were analyzed for all clones at 6 weeks and the clones B10, C2 and H8 were chosen for additional transcriptional analysis at 10, 12 and 16 weeks. Bulk NF54 parasites selected on CSA were kindly provided by Ali Salanti (Salanti et al., 2003) and propagated for 12 weeks without selection pressure. Transcriptional analysis of this culture was performed directly after selection and subsequently at 4, 8 and 12 weeks of unselected growth.
The transgenic lines B12E3, B15C2, C7G12 have been previously described (Dzikowski, Frank, and Deitsch, 2006). They were generated in the NF54/C3 clonal background and carry the bsd gene encoding blasticidin S deaminase integrated into subtelomeric and central var loci. Blasticidin pressure activates the var-trans gene and results in a reversible knock out of the entire var gene family. To assess the switching behavior of the recombinant var loci all clones were initially cultured in the presence of 20 μg/ml blasticidin S HCL ® (Invitrogen) and subsequently cultured without blasticidin for 16 weeks. Transcriptional analysis was performed after 3, 10 and 16 weeks of drug free growth. To assess if growth without drug pressure resulted in different var gene activation patterns, all 3 cultures were re-cloned by limiting dilution after 10 weeks of drug free growth generating the clones C7G12-A,B,C,D,E,F,G, B15C2-A,B,C,F,G,H and B12E3-A,B,C,D,J. Transcriptional analysis was again performed on all clones 6 weeks after the re-cloning experiment at which point all parasites had been cultured without drug selection for 16 weeks.
DNA, RNA extraction and cDNA synthesis
DNA was extracted from selected NF54 clones as well as from the transgenic parasites as described (Frank et al., 2006). RNA was Trizol (Invitrogen) extracted from 20 ml tightly synchronized late ring stages parasites (18h post invasion) as previously described (Dzikowski, Frank, and Deitsch, 2006). cDNA synthesis was performed on approximately 800 ng of RNA in a 40 μl reaction with Superscript II RNase H reverse transcriptase® (Invitrogen) and random primers® (Invitrogen). cDNA was evaluated for DNA contamination with a PCR spanning the intron on locus PFD1155w with the primers PFD1155w-F2 GCA GGG AAA GGT TTT TCA AG and PFD1155w-R2a AAA GCT GAA TCT TGG CCC GTT.
Validation of the Real-time RT-PCR primer set on genomic DNA
We employed a real-time PCR primer set developed by Salanti et. al (Salanti et al., 2003) with few modifications as described (Dzikowski, Frank, and Deitsch, 2006). In addition we modified the primers for PF10_0001, PFL2665c, PF08_0140 and MAL7P1.50 as follows: PF10_0001 F (5′-GCGTTCTGTGCTCAAACAAA-3′), PF10_0001 R (5′-GTCCTCGTCATCATCGTCCT-3′), PFL2665c F (5′-GCGAGGTCTTCTCGTTCTTG-3″), PFL2665cR(5′-ATGACGAAGAAGCAGCAGGT-3′), PF08_140F(5′-GGAGGAGGAAGAGGAAAACG-3′), PF08_140R(5′-CCACCTCCTCTTGTTGTGGT3′), MAL7P1.50F(5′GGTGGAGGTAGTCCACAGGA3′), MAL7P1.50 R (5′-CAGCTATTTCCCCACCAGAA-3′). Since NF54 is non-clonal we tested the amplification efficiency of the entire primer set on genomic DNA of individual NF54 clones and the transgenic parasite lines. This revealed that the primer set amplified genomic DNA from the different cell lines with equal efficiency. The primer bias for all primer pairs was determined by calculating the ΔCT value for each primer pair with respect to the single copy housekeeping gene PF07_0073 (seryl-tRNA synthetase) and used to correct the ΔCT values from the transcriptional analysis.
Quantitative Reverse Transcription PCR for assaying transcription of the var gene family
All reactions were performed at a final primer concentration of 0.5 μM using Biorad ITAQ SYBR SUPERMIX ® in 20 μl reactions on an ABI Prism ® 7900HT real time PCR machine. The ΔCT for each individual primer pair was determined by substracting the individual CT value from the CT value of the control the seryl-tRNA synthetase (User bulletin 2, Applied Biosystems, http://www.appliedbiosystems.com) and corrected for primer bias as described above. Δ CTs were then converted to relative copy numbers with the formula 2 Δ Ct. The dominant var gene was defined as the highest expressed var gene. All dominant genes were confirmed by a second independent real-time PCR run on the same cDNA. In addition for a subset of clones a second and third cDNA synthesis 48 and 96 hours after the first one was performed to verify the reproducibility across different cDNA preparations.
Generation of pie graphs to visually display switches in var gene expression over time
Pie graphs were used in cultures selected to express a single var gene either through binding to CSA or drug pressure in transgenic lines. Previous work has shown that, while under selection, transgenic parasites lines only express the single, recombinant var gene containing the selectable marker (Dzikowski, Frank, and Deitsch, 2006). Q-RT-PCR analysis was performed on parasites under selection and the level of background var gene expression was designated as the highest transcript level of an unselected, off gene. To display changes in expression over time, the subsequent pie graphs include all transcripts that exceed the background level. The background of the CSA selected line was applied to generate pie charts for the NF54 clone A3.
Calculation of on and off rates for individual var loci
Rates were calculated for NF54 wild type and transgenic parasites lines. In analogy to Horrocks et al., we defined off rate (roff) as the decrease of transcript from a particular var locus per generation. However, we expressed the decrease of transcript from an individual var locus over time as a percentage of the total var transcript signal. roff= Δ T/n, where ΔT is expression of the dominant var gene at time point 1 (quantified as percentage of total var gene signal) minus the expression level of this locus at time point 2, and n is number of generations between two time points. In parasites that had been under selection pressure, background was defined as the highest signal of the endogenous var gene detected under selection, and the off rates were calculated were calculated excluding background levels. In the clonal NF54 parasite lines, it was impossible to determine if the small amount of transcriptional signal from the non-dominant var genes represented background or small numbers of parasites that had switched to alternative loci and in these examples the data represents the crude transcription level measured. Similarly the on rate of a particular var locus was defined as the increase in relative transcriptional signal from that locus between two time points divided by the number of generations between these two time points.
We only calculated off rates for NF54 parasites expressing a single dominant gene at the earliest time point and that still had detectable signal from the same locus at the time point of the second transcriptional analysis. For clones expressing two dominant var genes at the earliest time point or that were no longer expressing the initially dominant var locus at the second transcriptional analysis, it was not possible to calculate off rates. The off rates for the NF54 clones C3, G6 and A3, were calculated after, 35, 77 and 45 generations, the off rate of the subtelomeric var2csa gene was calculated after 41 generations. The off rate of all transgenic var loci was calculated after 35 generations of drug free growth.
Supplementary Material
Acknowledgments
We would like to thank Ali Salanti for supplying us with the CSA selected parasites. We are indebted to our colleagues in Tom Tempelton’s laboratory: Drue Thacker, Christina Moreira and Cathrine Lavazec for generating and culturing many of the wild type clones. We thank Laskshmi Schonbucher for the primers PFD1155w-F2 and PFD1155w-R2a. All authors and the all of the work described in this paper were supported by National Institutes of Health Grant AI 52390 and a grant from the Ellison Medical Foundation. The Department of Microbiology and Immunology at Weill Medical College of Cornell University acknowledges the support of the William Randolph Hearst Foundation. KWD is a Stavros S. Niarchos Scholar. None of these funding agencies played any role in the design so the study; in the collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
List of abbreviations
- P. falciparum
Plasmodium falciparum
- PfEMP1
Plasmodium falciparum erythrocyte membrane protein 1
- PFSIR2
Plasmodium falciparum Sir2
- Q-RT-PCR
quantitative reverse transcriptase polymerase chain reaction
- CSA
chondroitin sulfate A
- bsd
blasticidin-S-deaminase gene
Contributor Information
Matthias Frank, Email: Mfrank1966@aol.com.
Ron Dzikowski, Email: rrd2001@med.cornell.edu.
Borko Amulic, Email: boa@med.cornell.edu.
Reference List
- Baruch DI, Pasloske BL, Singh HB, Bi X, Ma XC, Feldman M, Taraschi TF, Howard RJ. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- Deitsch KW, Calderwood MS, Wellems TE. Malaria - Cooperative silencing elements in var genes. Nature. 2001;412:875–876. doi: 10.1038/35091146. [DOI] [PubMed] [Google Scholar]
- Duraisingh MT, Voss TS, Marty AJ, Duffy MF, Good RT, Thompson JK, Freitas-Junior LH, Scherf A, Crabb BS, Cowman AF. Heterochromatin silencing and locus repositioning linked to regulation of virulence genes in Plasmodium faiciparum. Cell. 2005;121:13–24. doi: 10.1016/j.cell.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Dzikowski R, Frank M, Deitsch K. Mutually Exclusive Expression of Virulence Genes by Malaria Parasites Is Regulated Independently of Antigen Production. PLoS Pathog. 2006;2:e22. doi: 10.1371/journal.ppat.0020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Dzikowski R, Constantini D, Amulic B, Burdougo E, Deitsch K. Strict pairing of var promoters and introns is required for var gene silencing in the malaria parasite plasmodium falciparum. J Biol Chem. 2006 doi: 10.1074/jbc.M513067200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas-Junior LH, Hernandez-Rivas R, Ralph SA, Montiel-Condado D, Ruvalcaba-Salazar OK, Rojas-Meza AP, Mancio-Silva L, Leal-Silvestre RJ, Gontijo AM, Shorte S, Scherf A. Telomeric heterochromatin propagation and histone acetylation control mutually exclusive expression of antigenic variation genes in malaria parasites. Cell. 2005;121:25–36. doi: 10.1016/j.cell.2005.01.037. [DOI] [PubMed] [Google Scholar]
- Gannoun-Zaki L, Jost A, Mu JB, Deitsch KW, Wellems TE. A silenced Plasmodium falciparum var promoter can be activated in vivo through spontaneous deletion of a silencing element in the intron. Eukaryotic Cell. 2005;4:490–492. doi: 10.1128/EC.4.2.490-492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DM, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM, Barrell B. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrocks P, Pinches R, Christodoulou Z, Kyes S, Newbold C. Variable var transition rates underlie antigenic variation in malaria. Proc Natl Acad Sci USA. 2004 doi: 10.1073/pnas.0402347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen ATR, Magistrado P, Sharp S, Joergensen L, Lavstsen T, Chiucciuini A, Salanti A, Vestergaard LS, Lusingu JP, Hermsen R, Sauerwein R, Christensen J, Nielsen MA, Hviid L, Sutherland C, Staalsoe T, Theander TG. Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes. Journal of Experimental Medicine. 2004;199:1179–1190. doi: 10.1084/jem.20040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestli M, Cockburn IA, Cortes A, Baea K, Rowe JA, Beck HP. Virulence of malaria is associated with differential expression of Plasmodium falciparum var gene subgroups in a case-control study. J Infect Dis. 2006;193:1567–1574. doi: 10.1086/503776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestli M, Cortes A, Lagog M, Ott M, Beck HP. Longitudinal assessment of Plasmodium falciparum var gene transcription in naturally infected asymptomatic children in Papua New Guinea. J Infect Dis. 2004;189:1942–1951. doi: 10.1086/383250. [DOI] [PubMed] [Google Scholar]
- Kirkman LA, Su XZ, Wellems TE. Plasmodium falciparum: isolation of large numbers of parasite clones from infected blood samples. Exp Parasitol. 1996;83:147–149. doi: 10.1006/expr.1996.0058. [DOI] [PubMed] [Google Scholar]
- Kraemer SM, Smith JD. Evidence for the importance of genetic structuring to the structural and functional specialization of the Plasmodium falciparum var gene family. Molecular Microbiology. 2003;50:1527–1538. doi: 10.1046/j.1365-2958.2003.03814.x. [DOI] [PubMed] [Google Scholar]
- Lavstsen T, Salanti A, Jensen ATR, Arnot DE, Theander TG. Subgrouping of Plasmodium falciparum 3D7 var genes based on sequence analysis of coding and non-coding regions. Malaria Journal. 2003;2:27. doi: 10.1186/1475-2875-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LH, Good MF, Milon G. Malaria Pathogenesis. Science. 1994;264:1878–1883. doi: 10.1126/science.8009217. [DOI] [PubMed] [Google Scholar]
- Moazed D. Common themes in mechanisms of gene silencing. Mol Cell. 2001;8:489–498. doi: 10.1016/s1097-2765(01)00340-9. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Craig AG, Berendt AR, Pinches R, Nash G, Marsh K, Newbold CI. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. 1992;357:689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottmann M, Lavstsen T, Mugasa JP, Kaestli M, Jensen AT, Muller D, Theander T, Beck HP. Differential expression of var gene groups is associated with morbidity caused by Plasmodium falciparum infection in Tanzanian children. Infect Immun. 2006;74:3904–3911. doi: 10.1128/IAI.02073-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanti A, Staalsoe T, Lavstsen T, Jensen ATR, Sowa MPK, Arnot DE, Hviid L, Theander TG. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Molecular Microbiology. 2003;49:179–191. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- Scherf A, Hernandez-Rivas R, Buffet P, Bottius E, Benatar C, Pouvelle B, Gysin J, Lanzer M. Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in. Plasmodium falciparum EMBO J. 1998;17:5418–5426. doi: 10.1093/emboj/17.18.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp S, Lavstsen T, Fivelman QL, Saeed M, McRobert L, Templeton TJ, Jensen ATR, Baker DA, Theander TG, Sutherland CJ. Programmed Transcription of the var Gene Family, but Not of stevor, in Plasmodium falciparum Gametocytes. Eukaryotic Cell. 2006;5:1206–1214. doi: 10.1128/EC.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Heatwole VM, Wertheimer SP, Guinet F, Herrfeldt JV, Peterson DS, Ravetch JV, Wellems TE. A large and diverse gene family (var) encodes 200–350 kD proteins implicated in the antigenic variation and cytoadherence of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- Voss TS, Healer J, Marty AJ, Duffy MF, Thompson JK, Beeson JG, Reeder JC, Crabb BS, Cowman AF. A var gene promoter controls allelic exclusion of virulence genes in Plasmodium falciparum malaria. Nature. 2006;439:1004–1008. doi: 10.1038/nature04407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.