A V600E mutation in the BRAF oncogene found in ~2% of lung adenocarcinomas leads to constitutive kinase activity, triggering downstream pathways regulating cancer cell proliferation and survival.1, 2 Mechanisms of acquired resistance to BRAF-targeted therapy in BRAF-mutant lung cancer have not been described.
CASE REPORT
A 63-year-old never-smoker presented with dyspnea and a pleural effusion. Cytology was consistent with lung adenocarcinoma. Over the next three years, therapies included carboplatin/paclitaxel, erlotinib, pemetrexed/bevacizumab, gemcitabine, vinorelbine, and radiation. The patient was referred to Johns Hopkins for consideration of clinical trial options. The patient was consented to a multicenter Lung Cancer Mutation Consortium study (NCT101014286), and an omental metastasis was biopsied and profiled for potential driver mutations. The mutational profile revealed multiple alterations including the oncogenic BRAFV600E mutation (Table 1). The patient was enrolled in a phase 2 clinical trial of the selective BRAF inhibitor, dabrafenib, in patients with metastatic lung cancer with BRAFV600E (NCT01336634).
Table 1.
Tumor mutational profiles pre-treatment and upon disease progression
| Gene | Mutations | |
|---|---|---|
| Pre-study† | Progressive disease† | |
| BRAF | V600E | V600E |
| CCND3 | amplification | amplification |
| ARID1A | S90fs*11 | S90fs*11 |
| RB1 | S807* | S807* |
| KRAS | - | G12D |
| TP53 | - | R175H |
| CDKN2A | - | R24fs*20 |
Both tumor biopsies were profiled using the FoundationOne™ next-generation sequencing assay.
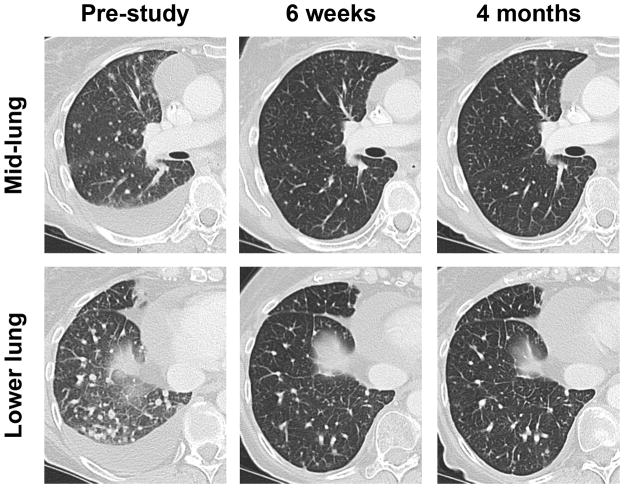
Within 3 weeks of starting dabrafenib, the patient reported improvement in both dyspnea and cough. A CT scan at 6 weeks confirmed partial response (Figure 1). Her response persisted for 8 months, at which time she was found to have disease progression, including marked enlargement of the omental mass. This mass was re-biopsied. Mutational profiling revealed the full spectrum of mutations present at baseline, confirming clonogenicity, and revealed three new mutations, including a known oncogenic mutation in KRAS, encoding the immediate upstream regulator of the RAF kinases (Table 1).
Figure 1. Metastatic lung cancer response to dabrafenib.
Serial CT scans of the right lung are shown, pre-treatment, at first on-treatment assessment (6 weeks) and after 4 months of therapy. Multiple pulmonary nodules and a malignant effusion are evident at baseline. The partial response at 6 weeks was confirmed on multiple subsequent scans, before disease progression as described.
DISCUSSION
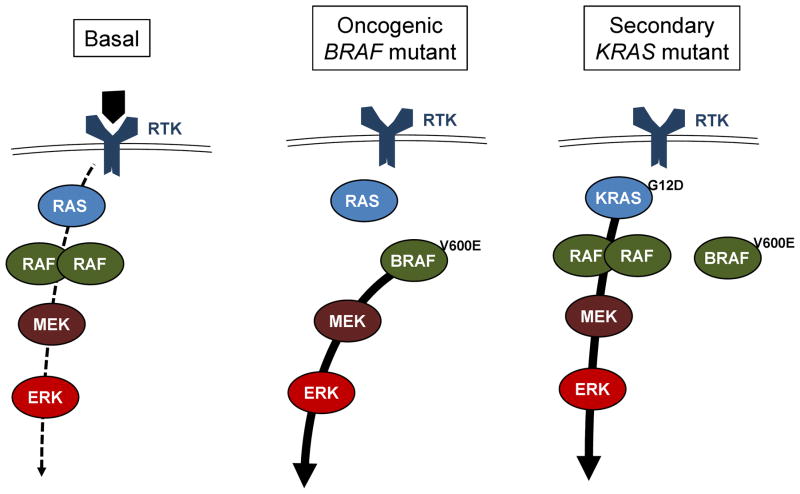
Activity of wild-type BRAF is regulated by dimerization, and is dependent on upstream signaling through one of three isoforms of RAS (Figure 2). Oncogenic BRAF mutation leads to a constitutively active monomeric kinase, independent of these upstream regulatory controls. The same oncogenic mutation is found in ~50% of melanomas. Multiple mechanisms of acquired resistance to targeted BRAF inhibitors have been described in melanoma, including upregulation of receptor tyrosine kinases, activation of the AKT pathway, and acquired mutation in NRAS.3 NRAS mutations are rare in lung adenocarcinoma, whereas KRAS is a frequently mutated oncogenic driver.4 Recent data suggest that inhibition of BRAFV600E can activate feedback leading to increased activity of and dependence on RAS.5 We hypothesize that the acquired KRASG12D mutation was primarily responsible for acquired dabrafenib resistance in this patient.
Figure 2. MAPK signaling in non-transformed and cancer cells.
Left: wild-type signaling. In most cells the Mitogen Activated Protein Kinase (MAPK) pathway is tightly regulated by ligand-dependent activation of receptor tyrosine kinases, which signal through any of 3 RAS family GTPases (N-, K-, and H-RAS, depending on the cell type). RAS-GTP in turn triggers dimerization and activation of RAF family tyrosine kinases (A-, B-, and C-RAF), leading to activation of the downstream proliferative signaling pathway. Middle: Oncogenic BRAF signaling. The BRAFV600E mutation promotes kinase activity as a monomer and independent of RAS regulation, leading to potent and constitutive activation of the pathway. Such cells may be highly responsive to BRAF inhibitor therapy. Right: Secondary KRAS mutation. A KRASG12D mutation locks KRAS in the GTP-bound (ON) configuration, independent of receptor tyrosine kinase regulation. Oncogenic KRAS activates multiple downstream pathways regulating proliferation and survival (not shown here). Among other effects, it may bypass inhibition of BRAFV600E by BRAF inhibitors, through stimulating dimerization of other RAF family members, leading to downstream activation of the MAPK pathway.
Secondary mutations in two other oncogenes, TP53 and CDKN2A, were identified as well. While these are both commonly mutated genes in lung cancer and can facilitate cancer progression, neither is directly implicated in RAF-dependent signaling, and their contributions to acquired resistance are unclear. Tumor heterogeneity could also contribute to the changes seen; serial biopsies of the same omental mass limit the extent to which this is a probable factor.
More broadly, this case underscores therapeutic insights gained through serial biopsies and mutational profiling in lung cancer. Identification of the driver BRAF mutation prompted use of a targeted therapy that led to a major objective response and substantial, though transient, 8-month symptomatic improvement. The second biopsy defined a probable mechanism of acquired resistance of particular relevance to lung cancer. Characterization of the molecular changes in progressive tumors initially responsive to targeted therapy may indicate further therapeutic opportunities, and may identify approaches to prevent emergence of drug resistance.
Acknowledgments
Acknowledgement of Research Support: GlaxoSmithKline and NIH 1RC2CA148394-01
References
- 1.Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol. 2011;29:2046–2051. doi: 10.1200/JCO.2010.33.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ji H, Wang Z, Perera SA, et al. Mutations in BRAF and KRAS converge on activation of the mitogen-activated protein kinase pathway in lung cancer mouse models. Cancer Res. 2007;67:4933–4939. doi: 10.1158/0008-5472.CAN-06-4592. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan RJ, Flaherty KT. Resistance to BRAF-targeted therapy in melanoma. Eur J Cancer. 2013 doi: 10.1016/j.ejca.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Imielinski M, Berger AH, Hammerman PS, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150:1107–1120. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lito P, Pratilas CA, Joseph EW, et al. Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell. 2012;22:668–682. doi: 10.1016/j.ccr.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]