Abstract
BACKGROUND
Spinal cord stimulation (SCS) has been shown to modulate atrial electrophysiology and confer protection against ischemia and ventricular arrhythmias in animal models.
OBJECTIVE
To determine whether SCS reduces the susceptibility to atrial fibrillation (AF) induced by tachypacing (TP).
METHODS
In 21 canines, upper thoracic SCS systems and custom cardiac pacing systems were implanted. Right atrial and left atrial effective refractory periods were measured at baseline and after 15 minutes of SCS. Following recovery in a subset of canines, pacemakers were turned on to induce AF by alternately delivering TP and searching for AF. Canines were randomized to no SCS therapy (CTL) or intermittent SCS therapy on the initiation of TP (EARLY) or after 8 weeks of TP (LATE). AF burden (percent AF relative to total sense time) and AF inducibility (percentage of TP periods resulting in AF) were monitored weekly. After 15 weeks, echocar-diography and histology were performed.
RESULTS
Effective refractory periods increased by 21 ±14 ms (P ±.001) in the left atrium and 29 ±12 ms (P ±.002) in the right atrium after acute SCS. AF burden was reduced for 11 weeks in EARLY compared with CTL (P ±.05) animals. AF inducibility remained lower by week 15 in EARLY compared with CTL animals (32% ±10% vs 91% ±6%; P <.05). AF burden and inducibility were not significantly different between LATE and CTL animals. There were no structural differences among any groups.
CONCLUSIONS
SCS prolonged atrial effective refractory periods and reduced AF burden and inducibility in a canine AF model induced by TP. These data suggest that SCS may represent a treatment option for AF.
Keywords: Atrial fibrillation, Spinal cord stimulation, Atrial tachypacing animal models
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia and is associated with increased mortality and morbidity.1–5 Pharmacologic management of AF remains a first line of therapy, and although radiofrequency ablation is effective at restoring sinus rhythm and reducing the symptoms of AF, a large percentage of patients require more than 1 procedure to maintain sinus rhythm, because of recurrent AF or atrial tachycardia.6,7 Better treatment options are needed for the long-term management of AF.
Spinal cord stimulation (SCS) delivers electrical stimuli to segments of the spinal cord through implanted electrodes and is used to treat a variety of painful conditions including chronic back pain and refractory angina perctoris.8,9 The mechanism of action of SCS is based on the gate-control theory of pain perception.10 SCS induces negative feedback by stimulation of the cells of the substantia gelatinosa, inhibiting nociception. In addition to inhibiting pain, SCS modulates afferent and efferent connections between target organs and the autonomic nervous system (ANS).
Numerous studies have established that the ANS modulates cardiac electrophysiology. In particular, autonomic imbalance contributes to the initiation and maintenance of AF.11 Vagal stimulation shortens the atrial effective refractory period (ERP) and in some regions of the atria increases dispersion of refractoriness, while stimulation of the stellate ganglion does not alter atrial refractory periods.12 Excessively unbalanced ANS stimulation causes atrial arrhyth-mias in animal models, particularly with stimulation of the vagus nerves.13–17 In humans, paroxysmal AF can be precipitated by autonomic triggers that may be vagal, adrener-gic, or mixed.18 Unlike direct unbalanced stimulation of the ANS, acute modulation of the ANS with SCS reduces the incidence of neuronally induced atrial arrhythmias.19 However, it is unknown whether SCS is effective at preventing atrial arrhythmias induced by tachypacing (TP) and whether it can suppress arrhythmias in chronic ambulatory animal models of AF. The purpose of this study was to test the hypothesis that SCS reduces AF in a chronic TP animal model.
Results
All 21 animals were implanted successfully. Three animals were removed from the study shortly after the implant procedure, and 4 additional animals were removed from the study before week 15. Attrition was due to cardiac lead dislodgment (n = 4), device pocket infection (n = 2), and gastrointestinal bleed unrelated to the experimental protocol (n = 1). Three CTL animals experienced SCS lead dislocation noted radiographically and were maintained in the study. SCS motor thresholds remained constant in all remaining animals over the course of the study.
Electrophysiology study
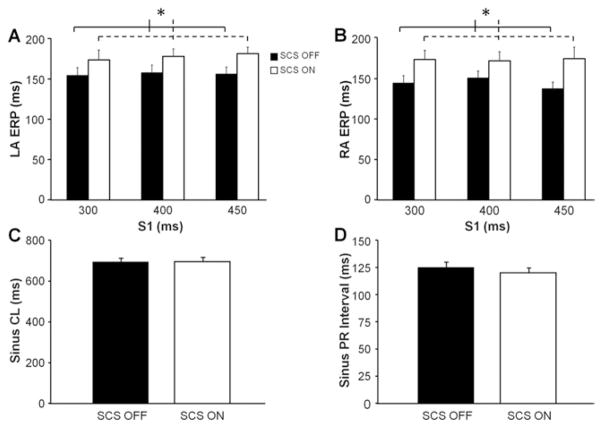
LA ERP and RA ERP were significantly prolonged with SCS at all 3 cycle lengths (Figures 3A and 3B). Following 15 minutes of SCS ON, LA ERP increased by an average of 21 ± 14 ms (P <.001) and RA ERP increased by an average of 29 ± 12 ms (P <.002) compared with baseline (SCS OFF). Sinus cycle length and PR intervals were unchanged during SCS stimulation compared with baseline measurements (Figures 3C and 3D). No sustained arrhythmias were induced during the electrophysiology study in any of the animals. Finally, QT intervals were also unchanged during SCS stimulation.
Figure 3.
Acute spinal cord stimulation (SCS) increases atrial refractory periods. A: Average left atrial effective refractory periods (LA ERPs) obtained at different S1 cycle lengths (CLs) before (SCS OFF) and after 15 minutes of SCS (SCS OFF). B: Average right atrial effective refractory periods (RA ERPs) obtained by using the same protocol. Significant differences between SCS ON and SCS OFF for LA ERP and RA ERP were found with 2-factor analysis of variance. C: Average sinus CL obtained during SCS ON and SCS OFF. D: Average sinus PR interval obtained during SCS ON and SCS OFF. n = 12 for each group. *Significant difference of P <.05.
AF burden, onset, and inducibility
The mean AF burden in the CTL group after 1 week of atrial TP was 77.5% ± 17.6% and 93.1% ± 4.2% by week 4 (Figure 4A). There was a significant decrease in the AF burden (P = .0045) for the EARLY group than for the CTL group. Specifically, the EARLY group showed an average AF burden of 2.6% ± 0.8% after 1 week of TP and remained below 50% until week 10. Post hoc analysis of the AF burden indicated that the EARLY group was lower for weeks 1–11 compared with the CTL group. The AF burden for the LATE group was not significantly different from that for the CTL group during weeks 1–8 (SCS not active; P = .67) or weeks 9–15 (SCS active; P = .57).
Figure 4.
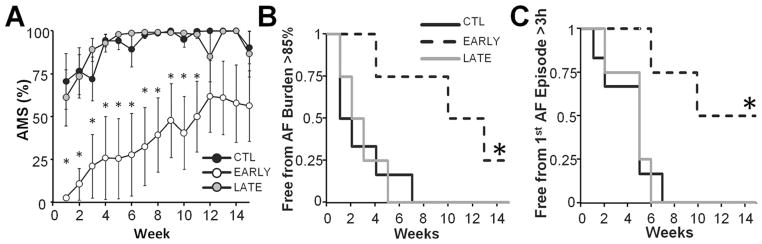
Atrial fibrillation (AF) burden is decreased in response to spinal cord stimulation. A: Average AF burden (percentage of time per week in automatic mode switch [AMS]) values plotted as a function of time. Significant differences between treatment groups were found with a 2-factor analysis of variance. The AF burden in the early (EARLY) group was significantly decreased compared with that in the control group (CTL). No differences in the AF burden were found between the control (CTL) and late (LATE) groups. B: Kaplan-Meier plots for freedom from persistent AF, defined as weekly AF burden >85%. C: Kaplan-Meier plots for freedom from first prolonged AF episode lasting at least 3 hours. *Significant difference of P <.05 compared with CTL.
SCS significantly prolonged the freedom from persistent AF (Figure 4B; P <.03) and the freedom from first prolonged AF episode (Figure 4C; P <.02). The median freedom from persistent AF for the CTL, LATE, and EARLY groups was 1, 2, and 13 weeks, respectively. All untreated animals were in persistent AF by week 7, while through the 15-week follow-up 2 EARLY animals remained below 85% AF burden. The median time to the first prolonged AF episode was 4.5 weeks for the CTL and LATE groups and 10 weeks for the EARLY group. All untreated animals experienced a prolonged AF episode by week 7. Notably, 2 animals in the EARLY group did not experience any AF episodes lasting 3 hours during the entire study period.
The AF inducibility tended to increase throughout the follow-up period, doing so more quickly in CTL and LATE groups than in the EARLY group (Figure 5A). The mean AF inducibility in the CTL group after 1 week of atrial TP was 39.0% ± 13.8%, which progressively increased to 94.8% ± 5.2% at week 8, remaining above 85% through the end of the study (Figure 5B). There was a significant decrease in AF inducibility (P = .0065) for the EARLY group compared with the CTL group. The AF inducibility for the EARLY group was 0.2% ± 0.04% after 1 week of TP and remained below 44% for the duration of the study. The AF inducibility for the LATE group was not significantly different from that for the CTL group for weeks 1–8 (P = .31) or for weeks 9–15 (P = .094).
Figure 5.
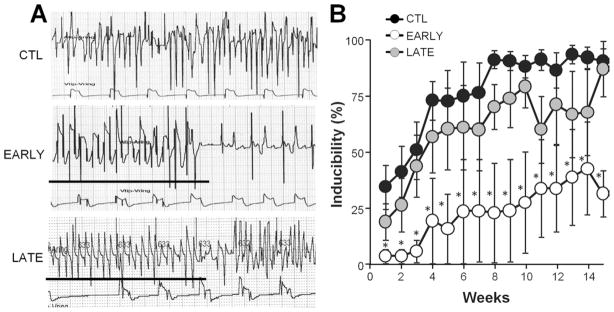
Spinal cord stimulation reduces arrhythmia inducibility. A: Representative electrograms from a control (CTL), early (EARLY), and late (LATE) animal recorded during week 15. Bar indicates tachypacing episode. B: Average arrhythmia inducibility plotted as a function of time. Significant differences between treatment groups were found with a 2-factor analysis of variance. *Significant difference of P <.05 compared with CTL.
Echocardiography
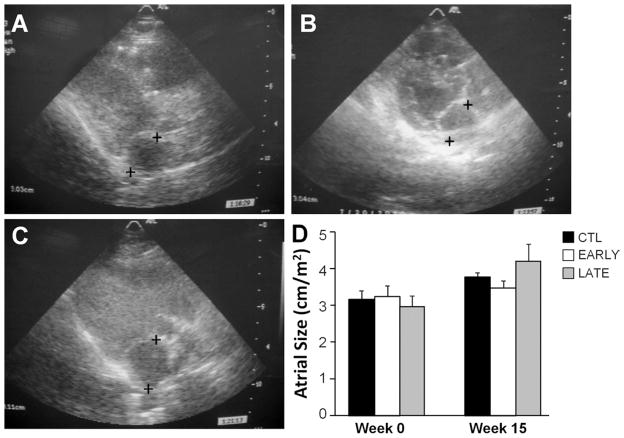
LV wall motion and systolic function were normal for all animals both at baseline and at 15 weeks. LA size was significantly increased, by 24% ± 10% at week 15 compared with baseline for all animals (P <.03) (Figure 6). Group assignment had no significant effect on LA size (20% ± 8% for CTL, 9% ± 10% for EARLY, and 48% ± 33% for LATE).
Figure 6.
Left atrial (LA) size is unchanged with spinal cord stimulation treatment. A–C: Representative echocardiographic images of the LA parallel to the mitral valve annulus in the parasternal long-axis view for a control (CTL), early (EARLY), and late (LATE) animal, respectively. + = locations used to measure the maximum end-systolic dimension. D: Average LA size normalized to body surface area. n = 4 (CTL and EARLY) and n = 3 (LATE).
Tissue histology and nerve staining
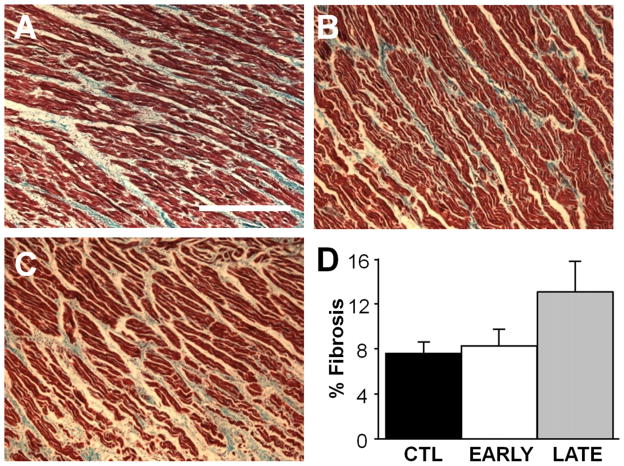
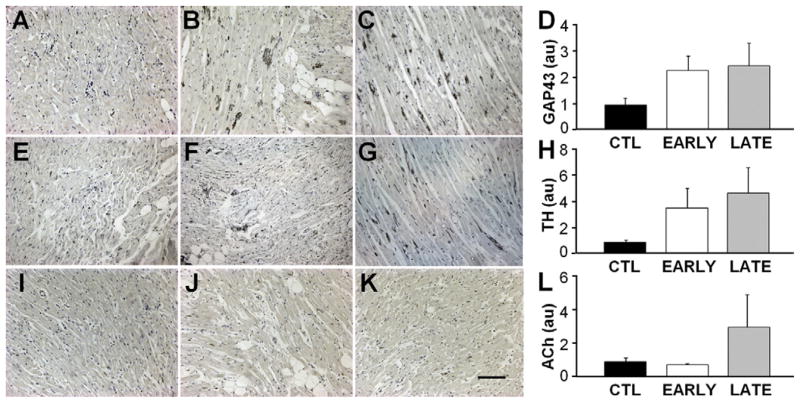
There was no significant difference in fibrosis content between the 3 groups (Figure 7). Analysis of variance indicated no significant differences between the 3 groups for any of the neural histological markers, but several nonsignificant differences were quite pronounced. SCS increased markers of atrial cardiac nerve sprouting (GAP43; Figures 8A–8C) more than 2-fold compared with CTL for both EARLY and LATE delivery of the therapy (Figure 8D). Similarly, SCS increased sympathetic nerve density (tyrosine hydroxylase; Figures 8E–8G) more than 3-fold for both EARLY and LATE groups compared with the CTL group (Figure 8H). The EARLY group showed no clear difference from the CTL group in parasympathetic nerve density (Figures 8I–8L).
Figure 7.
Atrial fibrosis is unchanged with spinal cord stimulation treatment. A–C: Representative right atrial trichrome stained sections from a control (CTL), early (EARLY), and late (LATE) animal, respectively. Bar = 250 μm. D: Average atrial fibrosis measurements. n = 4 for each group.
Figure 8.
Markers of nerve sprouting and sympathetic are elevated in response to spinal cord stimulation. A–C: Representative right atrial sections stained for Gap43 from a control (CTL), early (EARLY), and late (LATE) animal, respectively. Bar = 200 μm. D: Average Gap43 density. E–G: Representative right atrial sections stained for tyrosine hydroxylase (TH) from a CTL, EARLY, and LATE animal, respectively. H: Average TH density. I–K: Representative right atrial sections stained for acetylcholine (ACh) from a CTL, EARLY, and LATE animal, respectively. L: Average ACh density.
Discussion
SCS and cardiac electrophysiology
The major new findings of this study are that thoracic SCS prolongs atrial refractory periods and has a beneficial effect on the AF burden and inducibility in a canine model of AF induced by TP. Previous studies have illustrated some direct effects of acute SCS on cardiac electrophysiology.19,21 The mechanism of action of SCS on atrial electrophysiology is unknown but appears to arise from a number of complex interactions of central and local circuit processing within the nervous system. Modulation of the ANS alters the heart rate, systemic blood pressure, and cellular electrophysiology.22 Autonomic neuronal input to the heart is coordinated by the intrinsic cardiac nervous system, groups of epicardial ganglia that integrate sympathetic and parasympathetic sensory information. Synapses within these ganglia allow for crosstalk and complex interactions between the branches of the extrinsic nervous system.13 Consequently, stimulus from a single branch of the extrinsic ANS may have both parasympathetic and sympathetic effects on the heart.
We showed that 15 minutes of SCS in the T1–T5 region increased both LA ERP and RA ERP but had no effect on sinus cycle length or the PR interval and did not result in inducible atrial tachyarrhythmias. Prolongation of atrial ERP (AERP) suggests a withdrawal of vagal tone, while lack of other atrial electrophysiologic changes indicates that autonomic balance is likely still at least partly intact. Olgin et al21 studied the effects of acute SCS delivered to the T1–T2 region on sinus cycle length and atrium-to-His interval in canines. They showed that SCS prolonged sinus cycle length and atrium-to-His interval during normal sinus rhythm and that transection of the vagus nerve eliminated these effects, suggesting that the SCS-induced electrophysiological changes are mediated at least in part by the parasympathetic branch of the ANS.21 The contrast between our findings and those of Olgin et al suggests that SCS delivered over a broader region may provide more balanced autonomic modulation of the sinoatrial node and AV conduction. Recent studies by Shen et al23 support the conclusion that modulation of the parasympathetic branch of the ANS can have arrhythmic effects. These studies demonstrated that subthreshold vagal stimulation suppresses TP-induced atrial arrhythmias. Taken together, our data and those of Olgin et al support the concept that SCS has sympathetic and parasympathetic effects on atrial electrophysiology. It is possible that such acute effects, particularly the SCS-induced prolongation of atrial ERP, contribute to a reduction in atrial arrhythmia inducibility and chronic AF burden.
In this study, the effect of chronic SCS on TP-induced atrial arrhythmias was investigated, and we showed that simultaneous activation of SCS with atrial TP lowered the AF burden. In addition, once AF had been induced by atrial TP, subjects receiving SCS therapy at the same time TP was initiated were less prone to experiencing prolonged episodes. This observed decrease in AF burden accompanied by reduced inducibility and reduced susceptibility to prolonged episodes may be due to the prolongation of AERP by SCS. Smeets et al24 demonstrated that the wavelength of AF wavelets is determined by the AERP and conduction velocity. Prolongation of AERP by antiarrhythmic drugs or other techniques prevents or terminates AF episodes by reducing the ability of the atrium to sustain AF.24 One possibility is that the increase in AERP could transiently or chronically alter atrial capture thresholds. Rapid ectopic activation originating from the pulmonary veins is a well-described triggering event for AF. Therapeutic approaches that affect the rate of atrial capture have the potential to limit remodeling effects of TP.
Studies have shown that unbalanced autonomic input to the cardiac intrinsic nervous system can lead to atrial ar-rhythmias13–17 while restoration of normal ANS balance is antiarrhythmic.25–27 Cardinal et al19 studied the effects of acute SCS on neuronally induced atrial arrhythmias, showing that stimulation of the mediastinal nerve induced bradycardia followed by atrial tachycardia in the majority of animals and that after SCS the number of stimulation sites along the nerve that could induce tachycardia were significantly reduced. The effect of SCS on atrial arrhythmia inducibility was reduced following bilateral sympathetic stellectomy, suggesting that the SCS effect is mediated at least in part by the sympathetic ANS. That SCS reduces the inducibility of atrial arrhythmias arising from both unbalanced neural stimulation and direct cardiac TP and that decentralization of either the sympathetic branch or the vagal branch of the ANS alters the effects of SCS intimate that SCS operates with one or several complex mechanisms.
LA dimension increased throughout the follow-up, though there were no differences in the dilation across the 3 groups. Similarly, there were no significant differences between the 3 groups for fibrosis or histological markers of neural remodeling. It is possible that these findings reflect the effect of the AF model rather than of the ability of SCS to mitigate atrial remodeling. In another atrial TP model with ablated AV node reported by Avitall et al,28 the fibrosis found in the LA was 10.7%, similar to that which we report here. Avitall et al further reported that atrial tachypaced canines with intact AV conduction had increased LA fibro-sis of 14.2%, suggesting that fast atrial rate is only one contributing factor to the development of LA dilation and fibrosis, while other factors such as high ventricular rate and LV dysfunction also contribute. In our model, canines had high atrial rates all the time, whether they were in AF or were receiving TP while not in AF. The use of different TP parameters or following subjects for a longer duration might uncover differences in remodeling associated with SCS. The pronounced but statistically nonsignificant changes in GAP43 and acetylcholine associated with EARLY SCS may be a response from a suggested withdrawal of sympathetic input to the heart, but further study must be performed to confirm these results and elucidate the mechanism.
The transition from paroxysmal to persistent AF is characterized by structural and electrical remodeling of the atria. These findings include increase in LA size, shortened mono-phasic action potentials, and shortened AERP in humans.29,30 Wijffels et al31 were the first to demonstrate in an animal model that atrial TP leads to electrophysiological remodeling and AF. In chronically instrumented goats, shortening of AERP was associated with the prolongation of induced paroxysms of AF. Other investigators subsequently found that chronic atrial TP is associated with decreased atrial conduction velocities.32 Shortening of ERP and decreased conduction velocity are thought to contribute to a profibrillatory state in the atria. Our data show rapid progression to chronic AF after 4 weeks of TP. In our investigation, both CTL and LATE subjects did not receive SCS during the first 8 weeks of atrial TP, and among LATE subjects, activation of SCS following induction of AF with atrial TP did not affect AF burden, inducibility, or LA structure compared with untreated canines. Importantly, the TP model used for this study represents a considerably more rapid progression to persistent AF than what is observed in the clinical population. It is possible that SCS therapy initiated after the establishment of AF may have a more pronounced effect on AF burden in a less aggressive model or in many clinical situations. Furthermore, the optimization of SCS parameters and location may improve the effectiveness of SCS treatment once AF has been established.
SCS has been used clinically for decades in a variety of clinical conditions including chronic pain, peripheral vascular disease, and angina. Recently, it has been suggested that SCS therapy may also be effective in improving LV function and reducing ventricular tachyarrhythmias in congestive heart failure.20 Clinical trials on SCS are underway to determine whether these effects are observed in patients with heart failure.33–35 The current study is the first to evaluate the chronic effects of SCS therapy in an animal model of AF. The clinical implications of this study are that SCS exerts powerful antiarrhythmic effects in an atrial TP model. Future studies are warranted to evaluate the potential effectiveness of SCS therapy for the management of clinical AF.
There are several limitations of this study. First, the electrophysiological study was performed under general anesthesia. Anesthesia has been shown to alter cardiac electrophysiology parameters. Second, the study did not include healthy (not tachypaced) animals and animals that were treated with SCS therapy alone in the absence of TP-induced AF. As a result, the chronic effects of SCS stimulation on normal atrial electrophysiology were not studied. We also did not study the effect of SCS in the presence of conventional drug therapy for AF.
Conclusions
This study demonstrated that SCS therapy significantly decreases the AF burden and inducibility in a canine atrial TP model. This finding was associated with an acute increase in AERP, consistent with the observed antifibrillatory effects. Although SCS therapy modulates atrial electrophysiology and susceptibility to atrial arrhythmia, it is unclear whether SCS can reverse atrial electrical and structural remodeling associated with chronic fibrillation. These data suggest that SCS therapy may become a new treatment option for AF; however, additional studies are needed to further understand the antiarrhythmic mechanisms and long-term effects of SCS.
Expanded Methods
Experimental animals
The New York University School of Medicine Institutional Animal Care and Use Committee approved all experiments described with these animals. Canines were housed in an AAALAC-accredited animal facility, in compliance with the Animal Welfare Act and adhering to the principles of the Guide for the Care and Use of Laboratory Animals. Twenty-one purpose-bred, male mongrel canines (Marshall Bioresources, North Rose, NY) of age 7–11 months and weight 19–25 kg were used in this study. They received ad libitum water and food (Hill’s® Science and Prescription Diets, Topeka, KS). The canines were housed in compatible pairs prior to the surgery and post-operatively after 30 days. They were maintained under a 12:12 light:dark cycle at 68–74°F, 30–70% relative humidity, and 12–15 air exchanges per hour.
Protocol
The study period was 15 weeks. At the beginning of the study, animals were weighed and transthoracic echocardiograms were performed. Animals were randomized into three groups: SCS pulse generators were not to be activated (CTL), SCS pulse generators to be activated at the time of the initiation of atrial TP (EARLY), and SCS pulse generators to be activated 8 weeks after initiation of atrial TP (LATE). The animals were next implanted with cardiac pulse generators (Model 5386 or 2215–36, St. Jude Medical, Sylmar CA) with custom firmware, SCS pulse generators (EonC™ Model 3688, St. Jude Medical, Plano TX), and SCS percutaneous leads (Octrode™ Model 3186, St. Jude Medical, Plano TX). During the implant surgery an electrophysiology study and an AV node ablation were also performed. Atrial TP was initiated in all animals 3 to 21 days after the implant procedure. The time of initiation of atrial TP was designated as week 0. Interrogation of the cardiac and SCS pulse generators was performed weekly. SCS lead position was confirmed with fluoroscopy 8 weeks after the initiation of TP. Fifteen weeks after the initiation of TP, animals were weighed, SCS lead position was confirmed, an echocardiogram was performed, and the hearts were extracted for histological analysis.
Initial procedure
Animals were anesthetized with 6 mg/kg Diprivan®/ propofol (Astra Zeneca, Wilmington, DE) intravenously and inhaled Aerrane/isofluorane (Baxter, Deerfield, IL) 1–5% in oxygen. Transthoracic echocardiography was performed as described below, after which the animals were placed in the prone position with spinal flexion. Under fluoroscopic guidance, epidural access was obtained with a Tuohy needle. Epidural SCS leads were positioned in the upper thoracic region (T1–T5). The leads were tunneled to a subcutaneous pocket in the rear flank and connected to the SCS pulse generator. SCS lead electrodes were configured as an alternating bipolar array. Motor threshold testing was performed as to ensure proper lead placement and functioning of the SCS system. Motor threshold was determined at a frequency of 2 Hz and using 200 μs pulse width while incrementing the output current until noticeable muscle twitch was observed. SCS pulse generators were inactivated after motor threshold testing.
Animals were next placed in the supine position. Venous access was obtained with a right femoral vein and right internal jugular vein cutdown. A 7 French ablation catheter (Therapy CoolPath™, St. Jude Medical, St. Paul, MN) was advanced under fluoroscopic guidance from the femoral vein to the right atrium. A 6 French decapolar CSL catheter (Response™, St. Jude Medical, St. Paul, MN) was advanced from the left internal jugular vein to the distal coronary sinus for left atrial pacing and recording. Active fixation permanent pacing leads (Model 1688 or 1888, St. Jude Medical, Sylmar, CA) were advanced into the right atrial appendage (RAA) and right ventricle (RV) apex. Leads were secured to the fascia and connected to the cardiac pulse generator. Cardiac pulse generators were implanted in a subcutaneous pocket in the lateral neck.
An electrophysiology study was conducted in a subset of animals (n = 12) with the SCS pulse generators off (SCS OFF) and then on (SCS ON). Sinus cycle lengths and PR intervals were determined from consecutive beats over 2 complete respiratory cycles. Measurements of the right and left atrial effective refractory periods (RA ERP, LA ERP) were obtained using premature extrastimuli at S1 cycle lengths of 300, 400 and 450 ms. Pacing was performed from the right atrial appendage (RAA) and distal coronary sinus with a multi-channel stimulator (Programmable Stimulator Model 5328, Medtronic, Minneapolis, MN). The extrastimulus (S2) coupling interval was decreased by 10 ms until the S2 failed to capture the atria. ERP was defined as the longest premature coupling interval which failed to capture the atrium. ERP measurements were repeated three times for each animal and averaged. Atrial arrhythmia inducibility was evaluated with burst pacing at decreasing cycle lengths in 20 ms intervals until 2:1 atrial capture or sustained atrial ar-rhythmias (duration greater than 30 s) were induced. Recordings were analyzed using the EnSite NavX™ system (St. Jude Medical, St. Paul MN). For the SCS ON measurements, SCS pulse generators were activated to deliver 200 μs pulses at a frequency 50 Hz and 90% of motor threshold amplitude. Measurements were made after 15 minutes of stimulation while SCS remained ON. SCS pulse generators were inactivated after electrophysiological study was completed.
AV node ablation was performed following the electro-physiological study by positioning an ablation catheter near the AV node and applying radiofrequency energy (Model IBI-1500-T9, St. Jude Medical, Irvine CA) until complete AV block was achieved. AV block was confirmed 30 minutes later. The ablation catheter was withdrawn and the femoral vein was ligated. All skin incisions were closed with absorbable suture (2–0, 3–0 Vicryl, Ethicon Inc). Cardiac pulse generators were programmed to DDD mode with a base rate of 95 beats per minute (bpm) at twice diastolic threshold on both channels. Animals were extubated and moved to a recovery area. For the first two weeks after the surgical procedure, animals were sedated with buprenorphine (0.01–0.02 mg/kg, twice daily) and provided daily leash walks to minimize activity and provide exercise opportunity. The dogs were housed singly for 30 days post-operatively.
Echocardiography
Echocardiograms (HDI 5000 SonoCT, Philips Healthcare, Andover, MA) were performed with atrial TP turned off to record the apical and parasternal long-axis views of the heart. Echocardiographic images were stored on VHS tape for offline analysis. Left ventricular wall motion was assessed in multiple views to evaluate left ventricular ejection fraction. Left atrial (LA) dimension was measured using the maximum end-systolic dimension parallel to the mitral valve annulus in the parasternal long-axis view. The measurements were repeated three times and averaged. The LA dimension was normalized to body surface area (BSA) using the formula .1
Ambulatory atrial fibrillation model
The cardiac pulse generators were programmed with custom research firmware designed for ambulatory AF induction. Briefly, atrial TP at a rate of 600 bpm (2 times diastolic threshold) was delivered along with VVI pacing to the RV at 95 bpm. The devices delivered 30 s cycles of atrial TP followed by 6 s windows to sense the intrinsic atrial rate. An intrinsic atrial rate above 250 bpm triggered an automatic mode switch (AMS) and suspended TP while maintaining ventricular pacing. TP resumed upon the exit from AMS with return to sinus rhythm.
SCS stimulation protocol
SCS therapy was delivered three times daily for 2 hour cycles with the pulse parameters described above for the electrophysiological study. These parameters are similar to the settings used clinically for the treatment of angina and in previous animal studies of heart failure.2 SCS systems were interrogated weekly to confirm motor threshold, measure lead impedance, and ensure proper function.
AF model analysis
Cardiac pulse generators were interrogated weekly to download data and ensure normal function. Confirmation of tachypacing was performed by review of local electrograms during high rate atrial pacing cycles. Reports retrieved from the devices included the number of 30 s TP cycles delivered, total number of AMS events, total time spent in AMS, histograms of AMS episode duration, and stored intracardiac electrograms showing AMS transitions. The AF Burden was defined as the percent time in AMS relative to the total sense time and Persistent AF as weekly AF burden greater than 85%. The 1st Prolonged AF Episode was marked by finding the first AMS episode longer than 3 hours in the weekly AMS duration histograms. AF inducibility was defined as the percent of atrial TP cycles that resulted in an AMS episode.
Terminal procedure
Fifteen weeks after the initiation of atrial TP, animals were returned to the operating room, anesthetized as with the initial surgery, and placed in the supine position. Transthoracic echocardiography was repeated in the same manner as the baseline. Animals were given an intravenous bolus of 10,000 Units of heparin. A midline sternotomy was performed and the pericardium was opened. The great vessels were identified. Following euthanasia with an overdose of sodium pentobarbital (Sleepaway, Fort Dodge, Inc.) the great vessels were cross-clamped and cut. The heart was excised and flushed with ice cold (32°C) Tyrode’s solution containing (in mM): CaCl2 1.8, MgCl2 1.0, KH2PO4 1.2, NaCl 130.0, KCl 4.7, glucose 11.1, NaHCO3 24.0, albumin 0.052 g/L, equilibrated with a 95% O2, 5% CO2 gas mixture. Atrial samples were obtained for histology and immunohistochemistry.
Histology and immunohistochemistry
Atrial samples were fixed in 4% paraformaldehyde and kept at 4°C for 8 hours. Samples were then washed in phosphate-buffered saline (PBS) for 1 hour (4°C), dehydrated in ethanol (20°C), fixed in xylene (20°C), and paraffin-embedded. Tissue was sectioned at 5 μm parallel to the epicardium. For analysis of tissue fibrosis, sections were stained with Gomori’s Trichrome according to manufacturer’s instructions (Sigma). The free walls of the left (LA) and right atria (RA) were examined. Fibrosis content was determined in a blinded fashion using Image-Pro Plus 5.0 software (Media Cybernetics, Bethesda, MD). Data were acquired from fifteen sections per heart (5 regions/sample), which were analyzed by quantifying blue pixel content as a percentage of total tissue area. For immunohistochemical studies, slides were deparaffinized in xylene and rehydrated with graded alcohol. Antigen retrieval was carried out by immersion in citrate buffer placed in a steamer for 30 minutes. Slides were allowed to cool to room temperature, washed several times in PBS, and incubated with 1% bovine serum albumin to block unspecific binding for 60 minutes. Sections were incubated with primary antibodies overnight at 4°C in a humidified chamber. The following day, the slides were rinsed several times with PBS, and then incubated with a 3% hydrogen peroxide/methanol solution for 10 minutes to inactivate endogenous peroxidase. After another series of PBS washes, the slides were incubated with anti per-oxidase complex for 45 minutes at room temperature (1:200 in PBS; Santa Cruz). The immunoreactive products were visualized through incubation of tissue sections for 2–10 minutes with 3,3′-diaminobenzidine (DAB). The sections were counterstained with dilute hematoxylin followed by bluing solution. The slides were dehydrated with a graded alcohol series, cleared in xylene, and mounted. Primary antibodies used were rabbit anti-GAP43 polyclonal antibody (1:800 dilution; Millipore), rabbit anti-tyrosine hydroxylase antibody (1:1000 dilution; Millipore), and rabbit anti-acetylcholine polyclonal antibody (1:1000; Abcam).
Supplementary Material
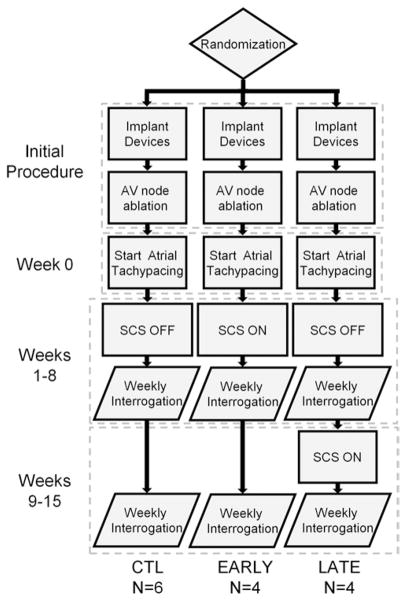
Figure 1.
Animal randomization and experimental protocol. AV = atrio-ventricular; CTL = no SCS therapy; EARLY = intermittent SCS therapy on the initiation of TP; LATE = intermittent SCS therapy after 8 weeks of TP; SCS = spinal cord stimulation; TP = tachypacing.
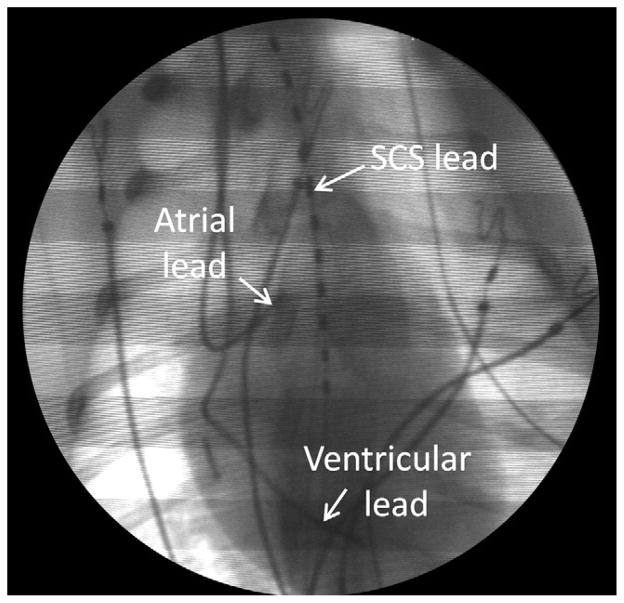
Figure 2.
Fluoroscopic image showing the position of the SCS and cardiac pacing leads. SCS = spinal cord stimulation. end-systolic dimension parallel to the mitral valve annulus in the parasternal long-axis view.
Acknowledgments
We acknowledge Steven Ullery, MS, for his support with statistical analysis.
This work was supported by grants from St Jude Medical, Inc (to Dr Morley) and from the National Institutes of Health grants HL076751 (to Dr Morley) and T32HL098129 (to Dr Vasquez).
ABBREVIATIONS
- AERP
atrial effective refractory period
- AF
atrial fibrillation
- AMS
automatic mode switch
- ANS
autonomic nervous system
- AV
atrioventricular
- EARLY
intermittent SCS therapy on the initiation of TP
- ERP
effective refractory period
- LA
left atrial
- LATE
intermittent SCS therapy after 8 weeks of TP
- LV
left ventricular
- RA
right atrial
- SCS
spinal cord stimulation
- TP
tachypacing
References
- 1.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 2.Vidaillet H, Granada JF, Chyou PH, et al. A population-based study of mortality among patients with atrial fibrillation or flutter. Am J Med. 2002;113:365–370. doi: 10.1016/s0002-9343(02)01253-6. [DOI] [PubMed] [Google Scholar]
- 3.Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 4.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 5.Hamer ME, Blumenthal JA, McCarthy EA, Phillips BG, Pritchett EL. Quality-of-life assessment in patients with paroxysmal atrial fibrillation or paroxysmal supraventricular tachycardia. Am J Cardiol. 1994;74:826–829. doi: 10.1016/0002-9149(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 6.Callans DJ, Gerstenfeld EP, Dixit S, et al. Efficacy of repeat pulmonary vein isolation procedures in patients with recurrent atrial fibrillation. J Cardiovasc Electrophysiol. 2004;15:1050–1055. doi: 10.1046/j.1540-8167.2004.04052.x. [DOI] [PubMed] [Google Scholar]
- 7.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibril-lation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 8.Stojanovic MP, Abdi S. Spinal cord stimulation. Pain Phys. 2002;5:156–166. [PubMed] [Google Scholar]
- 9.Deer TR, Raso LJ. Spinal cord stimulation for refractory angina pectoris and peripheral vascular disease. Pain Phys. 2006;9:347–352. [PubMed] [Google Scholar]
- 10.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Wasmund SL, Hamdan MH. Back to the future: the role of the autonomic nervous system in atrial fibrillation. Pacing Clin Electrophysiol. 2006;29:413–421. doi: 10.1111/j.1540-8159.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 12.Zipes DP, Mihalick MJ, Robbins GT. Effects of selective vagal and stellate ganglion stimulation of atrial refractoriness. Cardiovasc Res. 1974;8:647–655. doi: 10.1093/cvr/8.5.647. [DOI] [PubMed] [Google Scholar]
- 13.Armour JA. Cardiac neuronal hierarchy in health and disease. Am J Physiol Regul Integr Comp Physiol. 2004;287:R262–R271. doi: 10.1152/ajpregu.00183.2004. [DOI] [PubMed] [Google Scholar]
- 14.Armour JA, Hageman GR, Randall WC. Arrhythmias induced by local cardiac nerve stimulation. Am J Physiol. 1972;223:1068–1075. doi: 10.1152/ajplegacy.1972.223.5.1068. [DOI] [PubMed] [Google Scholar]
- 15.Hageman GR, Goldberg JM, Armour JA, Randall WC. Cardiac dysrhythmias induced by autonomic nerve stimulation. Am J Cardiol. 1973;32:823–830. doi: 10.1016/s0002-9149(73)80012-8. [DOI] [PubMed] [Google Scholar]
- 16.Armour JA, Randall WC, Sinha S. Localized myocardial responses to stimulation of small cardiac branches of the vagus. Am J Physiol. 1975;228:141–148. doi: 10.1152/ajplegacy.1975.228.1.141. [DOI] [PubMed] [Google Scholar]
- 17.Armour JA, Richer LP, Page P, et al. Origin and pharmacological response of atrial tachyarrhythmias induced by activation of mediastinal nerves in canines. Auton Neurosci. 2005;118:68–78. doi: 10.1016/j.autneu.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 18.de Vos CB, Nieuwlaat R, Crijns HJ, et al. Autonomic trigger patterns and anti-arrhythmic treatment of paroxysmal atrial fibrillation: data from the Euro Heart Survey. Eur Heart J. 2008;29:632–639. doi: 10.1093/eurheartj/ehn025. [DOI] [PubMed] [Google Scholar]
- 19.Cardinal R, Page P, Vermeulen M, et al. Spinal cord stimulation suppresses bradycardias and atrial tachyarrhythmias induced by mediastinal nerve stimulation in dogs. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1369–R1375. doi: 10.1152/ajpregu.00056.2006. [DOI] [PubMed] [Google Scholar]
- 20.Lopshire JC, Zhou X, Dusa C, et al. Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model. Circulation. 2009;120:286–294. doi: 10.1161/CIRCULATIONAHA.108.812412. [DOI] [PubMed] [Google Scholar]
- 21.Olgin JE, Takahashi T, Wilson E, et al. Effects of thoracic spinal cord stimulation on cardiac autonomic regulation of the sinus and atrioventricular nodes. J Cardiovasc Electrophysiol. 2002;13:475–481. doi: 10.1046/j.1540-8167.2002.00475.x. [DOI] [PubMed] [Google Scholar]
- 22.Kapa S, Venkatachalam KL, Asirvatham SJ. The autonomic nervous system in cardiac electrophysiology: an elegant interaction and emerging concepts. Cardiol Rev. 2010;18:275–284. doi: 10.1097/CRD.0b013e3181ebb152. [DOI] [PubMed] [Google Scholar]
- 23.Shen MJ, Shinohara T, Park HW, et al. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachy-arrhythmias in ambulatory canines. Circulation. 123:2204–2212. doi: 10.1161/CIRCULATIONAHA.111.018028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smeets JL, Allessie MA, Lammers WJ, Bonke FI, Hollen J. The wavelength of the cardiac impulse and reentrant arrhythmias in isolated rabbit atrium: the role of heart rate, autonomic transmitters, temperature, and potassium. Circ Res. 1986;58:96–108. doi: 10.1161/01.res.58.1.96. [DOI] [PubMed] [Google Scholar]
- 25.Tan AY, Li H, Wachsmann-Hogiu S, et al. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol. 2006;48:132–143. doi: 10.1016/j.jacc.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 26.Po SS, Nakagawa H, Jackman WM. Localization of left atrial ganglionated plexi in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:1186–1189. doi: 10.1111/j.1540-8167.2009.01515.x. [DOI] [PubMed] [Google Scholar]
- 27.Oral H, Chugh A, Scharf C, et al. Pulmonary vein isolation for vagotonic, adrenergic, and random episodes of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2004;15:402–406. doi: 10.1046/j.1540-8167.2004.03432.x. [DOI] [PubMed] [Google Scholar]
- 28.Avitall B, Bi J, Mykytsey A, Chicos A. Atrial and ventricular fibrosis induced by atrial fibrillation: evidence to support early rhythm control. Heart Rhythm. 2008;5:839–845. doi: 10.1016/j.hrthm.2008.02.042. [DOI] [PubMed] [Google Scholar]
- 29.Olsson SB, Cotoi S, Varnauskas E. Monophasic action potential and sinus rhythm stability after conversion of atrial fibrillation. Acta Med Scand. 1971;190:381–387. doi: 10.1111/j.0954-6820.1971.tb07446.x. [DOI] [PubMed] [Google Scholar]
- 30.Boutjdir M, Le Heuzey JY, Lavergne T, et al. Inhomogeneity of cellular refractoriness in human atrium: factor of arrhythmia? Pacing Clin Electrophysiol. 1986;9:1095–1100. doi: 10.1111/j.1540-8159.1986.tb06676.x. [DOI] [PubMed] [Google Scholar]
- 31.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation: a study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 32.Gaspo R, Bosch RF, Talajic M, Nattel S. Functional mechanisms underlying tachycardia-induced sustained atrial fibrillation in a chronic dog model. Circulation. 1997;96:4027–4035. doi: 10.1161/01.cir.96.11.4027. [DOI] [PubMed] [Google Scholar]
- 33.DEFEAT-HF: determining the feasibility of spinal cord neuromodulation for the treatment of chronic heart failure. [Accessed February 10, 2012.];2010 doi: 10.1016/j.jchf.2015.10.006. Available at: http://clinicaltrials.gov/ct2/show/NCT01112579. [DOI] [PubMed]
- 34. [Accessed February 10, 2012.];Neurostimulation of spinal nerves that affect the heart. Available at: http://clinicaltrials.gov/ct2/show/NCT01124136.
- 35. [Accessed February 10, 2012.];SCS HEART: spinal cord stimulation for heart failure. Available at: http://clinicaltrials.gov/ct2/show/NCT01362725.
References
- 1.Birchard SJ, Sherding RG. Saunders manual of small animal practice. Philadelphia: WB Saunders; 1994. [Google Scholar]
- 2.Lopshire JC, Zhou X, Dusa C, et al. Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model. Circulation. 2009;120:286–294. doi: 10.1161/CIRCULATIONAHA.108.812412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.