Abstract

A transfer of chirality in an intramolecular Rh(I)-catalyzed allenic Pauson-Khand reaction (APKR) to access tetrahydroazulenones, tetrahydrocyclopenta[c]azepinones and dihydrocyclopenta[c]oxepinones enantioselectively (22 – 99% ee) is described. The substitution pattern of the allene affected the transfer of chiral information. Complete transfer of chirality was obtained for all trisubstituted allenes, but loss of chiral information was observed for disubstituted allenes. This work constitutes the first demonstration of a transfer of chiral information from an allene to the 5-position of a cyclopentenone using a cyclocarbonylation reaction. The absolute configuration of the corresponding cyclocarbonylation product was also established, something that is rarely done.
Introduction
Cyclopentanes serve as valuable building blocks in organic synthesis and are present in many naturally occurring compounds. Among the various polycyclic skeletons possessing five-membered carbocycles, the fused 5,5-, 5,6- and 5,7-bicyclic ring systems are common structural features found in many biologically relevant compounds.1 For many natural products, the five-membered rings are characterized by a relatively high oxidation state (Figure 1). Swartzianin D (1) is an excellent example of this, where every carbon of the five-membered ring is occupied with a double bond, a carbonyl or a hydroxyl group.2 There are a number of synthetic methods available to prepare these highly oxidized rings, but the options narrow rapidly when constructing five-membered carbocycles with stereogenic centers enantioselectively.3
Figure 1.

Bioactive and Natural Compounds Containing Cyclopentanes with Stereogenic Centers
There is a diverse array of methods to create stereogenic centers of cyclopentanes in a stereoselective manner. A common synthetic strategy is to take advantage of the chiral pool as a source of enantiopure materials. One example is the asymmetric synthesis of (+)-chinensiolide B (2), where the cyclopentane moiety of this compound is generated via a Favorskii rearrangement of (R)-carvone.4 Moreover, the cyclopentane ring of tecomanine (3), possessing a tetrahydrocyclopentapyridinone ring system, was also prepared from (−)-carvone.5 Other approaches to install stereogenic centers utilize existing stereocenters on rings adjacent to the five-membered carbocycle. For instance, the methyl group located at the C4 position of grosshemin (4) was installed by classic alkylation of an enol acetate of a 5,7-bicyclic ring system to yield a single product diastereoselectively.6 However, this diastereoselective approach is not always reliable for cyclopentenone-containing 5,7-ring systems. For example, in the synthesis of (+)-achalensolide (5), reduction of the C1-C11 double bond of a dienone group via a hydrogenation reaction afforded a 1:1 mixture of diastereomers.7 In some cases, asymmetric reactions are used to introduce stereogenic atoms but in less direct ways. For example, the five-membered carbocycle of arglabin (6) was obtained via an enantioselective cyclopropanation of a furan followed by a rearrangement to produce a protected hydroxy-2-cyclopentenone.8 The key stereodetermining step for C4 and C5 stereocenters of connatusin B (7) were established using a Diels-Alder reaction starting with cyclopentenone and enantiomerically pure cis-1,2-dihydrocatechol.9
Other synthetic methods implemented for the preparation of chiral non-racemic 2-cyclopentenones are the Nazarov cyclization and the Pauson-Khand reaction (PKR). The asymmetric Nazarov cyclization has been made possible by the use of chiral auxiliaries, chiral Lewis acids and transfer of axial to tetrahedral chirality when using allenes.10 However, the electrocyclization process requires an enantioselective formation of the stereogenic carbon beta to the carbonyl controlled by torquoselectivity, followed by a diastereoselective addition of an electrophile to the intermediate enol to form a second stereogenic center adjacent to the newly formed carbonyl.11 The asymmetric PKR has been performed using chiral metal complexes of rhodium,12 titanium,13 iridium14 and cobalt15 to provide cyclopentenone-containing bicyclic ring systems in good enantioselectivities. Nevertheless, like the Nazarov cyclization, all reactions establish a stereogenic carbon beta to the carbonyl. There are exceptions for both intra- and intermolecular PKRs where a stereocenter adjacent to the carbonyl is set. These reactions typically involve functionalization of the alkene component with a chiral auxiliary and for nearly all cases this chiral auxiliary is removed and the stereogenic center is lost.16 While there are methods to synthesize 2-cyclopentenones possessing a stereogenic center at the C5 position stereoselectively, the scope of these protocols is limited.17
Previously, we demonstrated that a complete transfer of chirality is obtained for chiral, non-racemic allenes 10 in the molybdenum- or zirconium-mediated PKR to afford 2-cyclopentenones 11 enantioselectively (Scheme 1).18 For both of these transformations, the reaction occurs with the proximal double bond of the allene to provide E-α-alkylidene or E-α-silylidene 2-cyclopentenones with the stereogenic center beta to the carbonyl group.19 Since a general protocol for the asymmetric synthesis of C5-substituted bicyclic cyclopentenones is valuable, we considered employing chiral allenes in a PKR to gain access to this motif. Based on these prior results, we envisioned that transfer of chiral information from allene 10 to a stereogenic center adjacent to the carbonyl group of 2-cyclopentenone 12 should also be possible for the Rh(I)-catalyzed cyclocarbonylation reaction, a transformation performed by the selective reaction with the distal π-bond of allene 10 (Scheme 1). Herein are reported studies directed toward a Rh(I)-catalyzed allenic Pauson-Khand reaction (APKR) to afford 5,7-bicyclic ring systems enantioselectively.
Scheme 1.

APKR: Transfer of Axial Chirality
Results and Discussion
Investigations into the scope and limitations of the transfer of allene axial chirality in the Rh(I)-catalyzed cyclocarbonylation reaction began with the synthesis of a series of enantiomerically enriched allene-ynes. The substitution pattern of the allene was varied to include trisubstituted allenylsilane 13, trisubstituted non-silylated allenes 14 – 16 and disubstituted allenes 17 – 19 (Figure 2). The terminus of the alkyne was substituted with either hydrogen, alkyl, aryl, heterocyclic or silyl groups (R3 = H, Me, cyclopropyl, Ph, 2- and 3-thiophene and TMS) and three different tethers were prepared, two heteroatom-containing (X = NTs, O) and one all carbon-containing (X = C(CO2Et)2).
Figure 2.
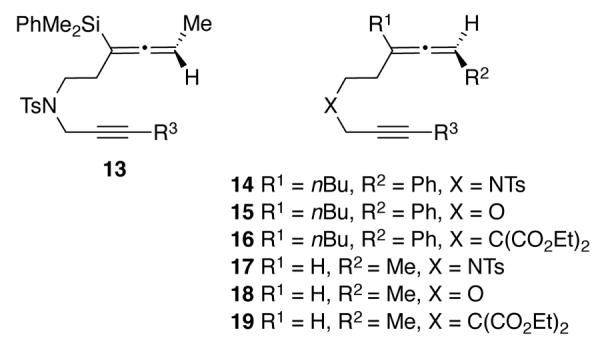
Allene-yne Precursors
Synthesis of enantiomerically enriched allenes
Synthesis of trisubstituted chiral allenyl alcohol (Ra)-22 was performed in four steps starting from commercially available (±)-3-butyn-2-ol (20) in a manner analogous to that reported by Brawn and Panek (Scheme 2, eq 1).20 Addition of n-butyllithium to alkyne 20 in the presence of lithium chloride followed by addition of phenyldimethylchlorosilane afforded silylated alkyne 21 in 90% yield. Kinetic resolution of racemic propargyl alcohol 21 using lipase AK “Amano” provided enantioenriched propargyl alcohol (S)-21 (47% yield, >98% ee) and the corresponding propargyl acetate (43% yield, >99% ee, not shown). Enantioenriched propargyl alcohol (S)-21 was reacted with trimethylorthoacetate and propionic acid to produce the corresponding allenyl ester via a Johnson-Claisen rearrangement. The methyl ester was reduced with lithium borohydride to afford enantioenriched allenylsilane (Ra)-22 in >93% ee. The enantiomeric excess was determined by chiral HPLC analysis. Synthesis of racemic allenyl alcohol 25 involved silylation of hept-2-yn-1-ol (23) followed by a retro-Brook rearrangement to afford propargyl alcohol 24 in 55% yield for the two steps (Scheme 2, eq 2). A Johnson-Claisen rearrangement performed on alcohol 24 followed by reduction of the methyl ester with lithium aluminum hydride provided allene 25. Disubstituted allene (Ra)-26 was prepared by a Johnson-Claisen rearrangement of commercially available (R)-3-butyn-2-ol (20) followed by lithium aluminum hydride reduction of the corresponding methyl ester (Scheme 2, eq 3). The enantiomeric excess of (Ra)-26 was based upon chiral HPLC analysis of p-nitrobenzoyl ester 27, for which a >99% ee was obtained. Allenyl alcohol (Ra)-29 was prepared from 1-hexyne by a palladium-catalyzed carbonylation reaction followed by reduction of the corresponding ynone carbonyl with (R)-alpine borane (Scheme 2, eq 4).21 The resulting propargyl alcohol (R)-28 was converted into a propargyl vinyl ether with ethyl vinyl ether and mercury acetate. A gold(I)-catalyzed [3,3]-sigmatropic rearrangement of the vinyl ether followed by in situ reduction of the intermediate aldehyde afforded allenyl alcohol (Ra)-29 in >79% ee.22 The enantiomeric excess was determined by chiral HPLC analysis.
Scheme 2.
Synthesis of Racemic and Nonracemic Allenes
Preparation of the Allenic Pauson-Khand Precursors
Allene-ynes 13a – 13e, 13g, 14c – 14e, 14g, 17c, 17d and 17f were all prepared using a Mitsunobu reaction of the corresponding allenols and the appropriately substituted propargyl tosylamides 30a – 30g (Scheme 3). Reacting (Ra)-22 with 3- and 2-thiophene-substituted propargyl tosylamides 30a and 30b gave allene-ynes 13a and 13b in 90% and 70% yield, respectively. Similarly, high yields were obtained for the Mitsunobu reaction between (Ra)-22 and 30c, 30d, 30e and 30g giving 13c, 13d, 13e and 13g in 92%, 92%, 90% and 73% yield, respectively. Allene-ynes 14c, 14d, 14e and 14g were obtained in a similar manner in high yields (77% – 93%) from the reaction of trisubstituted allene (Ra)-29 and propargyl tosylamides 30c, 30d, 30e and 30g, respectively. Reacting (Ra)-26 with propargyl tosylamides 30c, 30d and 30e afforded 17c, 17d and 17e in 74%, 85% and 84% yield, respectively. The trimethylsilyl-substituted alkynes 13f, 14f and 17f were prepared by deprotonation of allene-ynes 13e, 14e and 17e with lithium hexamethyldisilylazide followed by addition of trimethylsilyl chloride to provide the corresponding silylated alkynes in 87%, 80% and 84% yield, respectively.
Scheme 3.
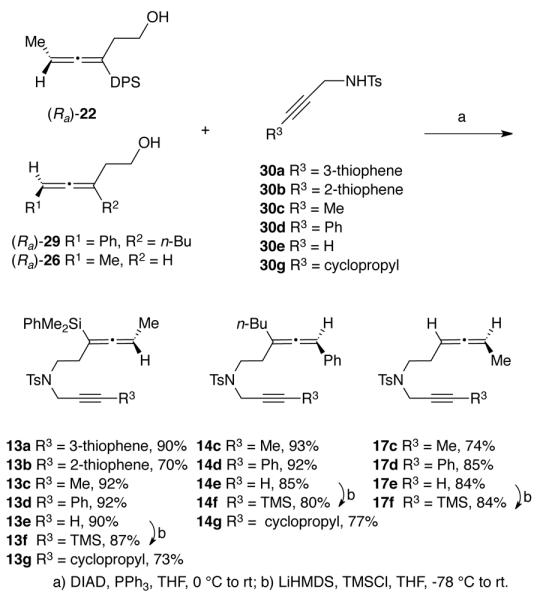
Synthesis of Allene-ynes 13, 14 and 17 Containing a Tosylamide Tether
Allene-ynes 15c, 15d, 15f and 18c, 18d, 18f containing an oxygen atom in the tether were synthesized by a Williamson etherification reaction (Scheme 4). Reacting (Ra)-26 with propargyl bromides 31c, 31d and 31e afforded allene-ynes 18c, 18d and 18e in 83%, 63% and 41% yield, respectively. Reacting (Ra)-29 with propargyl bromides 31c, 31d and 31e produced ethers 15c, 15d and 15e in 27%, 35% and 20% yield, respectively. Low yields for this series of compounds were attributed to the sterically congested environment at the proximal double bond of the allene. This hypothesis is supported by the complete recovery of starting material for the reaction of trisubstituted allenyl alcohol (Ra)-22 with 31d. The trimethylsilyl-substituted alkynes 15f and 18f were prepared by deprotonation of allene-ynes 15e and 18e using the same conditions as for allene-ynes 13f, 14f and 17f to provide the corresponding silylated alkynes in 65% and 69% yield, respectively.
Scheme 4.

Synthesis of Allene-ynes 15 and 18 Containing an Oxygen Tether
Preparation of allene-ynes 16c – 16e was accomplished by reaction of alcohol (Ra)-26 with methanesulfonyl chloride to produce mesylate 32. Addition of the mesylate to the sodium salt of malonates 34c – 34e provided the desired allene-ynes in 42 – 75% yields (Scheme 5). Allene-ynes 19c – 19e were obtained in an analogous manner from alcohol (Ra)-29 with yields ranging from 57% to 63%. Reaction of 16e and 19e with lithium hexamethyldisilylazide and trimethylsilylchloride afforded allene-ynes 16f and 19f in 84% and 80% yield, respectively. Attempts to convert alcohol (Ra)-22 to the corresponding allene-ynes using these conditions resulted in an inseparable mixture of silylated and desilylated products. For this reason, the silylated allene series of compounds bearing the oxygen tether was not further pursued.
Scheme 5.

Synthesis of Allene-ynes 16 and 19 Containing a Malonate Tether
Optimization of the Cyclocarbonylation Reaction
A brief examination of the cyclocarbonylation reaction conditions was performed using 13c and it was shown that the standard conditions developed in our group for the Rh(I)-catalyzed APKR provided compound 35 in 95% yield (Scheme 6 and Table 1, entry 1). Attempts to lower the catalyst loading to 5 mol % decreased the yield of the cyclocarbonylation product 35 to 75% and resulted in a 9% yield of cross-conjugated triene 36 (Scheme 6 and Table 1, entry 2). Cross-conjugated triene 36 results from a competing β-hydride elimination of rhodium metallocycle 37 to produce the alkene of intermediate 39. The rhodium hydride of 39 then undergoes a reductive elimination to generate the exocyclic alkene of 36 as a single diastereomer. This side product has previously been observed for the formation of seven-membered rings from allenes bearing a phenylsulfonyl group.23 Increasing the reaction temperature to 120 °C afforded dienone 35 in 61% yield and triene 36 in 35% yield (Table 1, entry 3). Decreasing the catalyst loading and increasing the reaction temperature afforded dienone 35 and triene 36 in a 1:1 ratio (Table 1, entries 4 and 5). Interestingly, the addition of silver tetrafluoroborate and triphenylphosphine afforded cyclocarbonylation product 35 in only 72% yield based upon recovered starting material. While the generality of this reaction could benefit from the lower reaction temperatures possible for the cationic Rh(I), our inability to take this reaction to completion is deemed problematic.
Scheme 6.

Cyclocarbonylation Reaction and Triene Formation
Table 1.
Optimization of the Cyclocarbonylation Reaction Conditions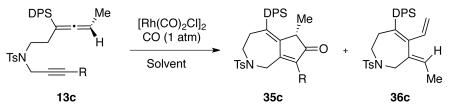
| Entry | [Rh(CO)2CI]2 (mol %) | Time (h) | T(°C) | Product and yield (%) | Solvent |
|---|---|---|---|---|---|
| 1 | 10 | 3 | 90 | 35c (95); 36c (0) | PhMe |
| 2 | 5 | 9 | 90 | 35c (75); 36c (9) | PhMe |
| 3 | 10 | 7 | 120 | 35c (61); 36c (35) | PhMe |
| 4 | 5 | 7 | 120 | 35c (42); 36c (48) | PhMe |
| 5 | 1 | 24 | 120 | 35c (29); 36c (26) | PhMe |
| 6a | 10 | 24 | 40 | 35cb (72); 36c (0) | DCE |
PPh3 (30 mol %), AgBF4 (22 mol %);
Yield based on recovered starting material (conversion : 54%)
Transfer of Chirality in the Rh(I)-catalyzed APKR
Each of the silyl-substituted allene-ynes 13a – 13g was subjected to the optimized cyclocarbonylation reaction conditions. High yields of the cyclocarbonylation products were obtained for the allene-ynes bearing an internal alkyne (Table 2, entries 1 – 4, 6 and 7). The presence of heterocyclic-containing systems on the alkyne did not affect the reaction (Table 2, entries 1 and 2). Terminal alkyne 13e was converted to cyclocarbonylation product 35e in 66% yield, along with a 13% yield of triene 36 (Table 2, entry 5). The enantiomeric purity of each of the allene-ynes 13a – 13g was based upon the enantiomeric excess obtained for allenylsilane (Ra)-22, which was determined to be greater than 93%. Each of the cycloadducts 35a – 35g was examined for their enantiomeric purity by HPLC analysis using a ChiralCel OD column. For each of these compounds, the enantiomeric excesses were found to be greater than 96%, constituting a complete transfer of chirality from the allene-ynes to the cyclocarbonylation products. While the transfer of chiral information may not be surprising, the high configurational stability of the dienone to the Rh(I) conditions was not anticipated.
Table 2.
Transfer of Chirality using Trisubstituted Silylated Allene-ynes
| Entry | Allene-yne | R | Time (h) | Product, % yield | % eea |
|---|---|---|---|---|---|
| 1 | 13a |

|
2.5 | 35a, 72 | >96 |
| 2 | 13b |

|
2.5 | 35b, 85 | >96 |
| 3 | 13c | Me | 3 | 35c, 95 | >96 |
| 4 | 13d | Ph | 3 | 35d, 92 | >96 |
| 5 | 13e | H | 2.5 | 35e, 66b | >96 |
| 6 | 13f | TMS | 3 | 35f, 89 | >99 |
| 7 | 13g |

|
2.3 | 35g, 83 | >99 |
Enantiomeric ratios were determined by HPLC analysis using a ChiralCel OD column;
Cross-conjugated triene was obtained in 13% yield.
Performing the APKR on trisubstituted allene 40 resulted in the formation the desilylated cyclocarbonylation product 41 in quantitative yield (Scheme 7).24 Desilylation of α-silyl ketones have been reported to occur upon purification by silica gel chromatography.25 However, it appears that desilylation of the resulting cyclocarbonylation product took place during the reaction as evidenced by crude 1H NMR spectroscopy. Thus, the loss of the stereogenic center precluded further examination of this series of compounds.
Scheme 7.

Desilylation During the Cyclocarbonylation Reaction
A series a trisubstituted allenes 14, 15 and 16 were examined in the cyclocarbonylation reaction, each possessing a phenyl group on the terminus of the allene but differing in their tether (X = NTs, O, C(CO2Et)2) and the substitution on the terminus of the alkyne (R = Me, Ph, TMS, cyclopropyl). The enantiomeric purity of each allene-yne was based upon the enantiomeric excess of the starting homoallenyl alcohol (Ra)-29, which was determined to be greater than 79%. For all cases examined, a complete transfer of chirality was obtained (Table 3, entries 1 – 10). For allene-ynes 14c, 14d, 14f and 14g possessing a tosylamide tether the reactions were complete in 30 minutes affording dienones 42c, 42d, 42f and 42g in 75 – 88% yield (Table 3, entries 1 – 4). The highest yielding substrate was found to be allene-yne 14g possessing a cyclopropyl group on the terminus of the alkyne and the lowest yield was obtained with aryl-substituted substrate 14d. For the oxygen containing tethers, the yields varied considerably (Table 3, entries 5 – 7). Methyl-substituted alkyne 15c produced 43c in 46% yield, phenyl-substituted alkyne 15d afforded 43d in 69% yield and trimethylsilyl-substituted alkyne 15f provided 43f in 91% yield. Furthermore, all the reactions in the oxygen-containing series were complete in less than 45 minutes. Finally, for the malonate-containing series, the yields were high for all allene-ynes and ranged from 88% to 92%. For this series (allene-ynes 16c, 16d and 16f) the reactions required 120 – 150 minutes for completion, but the longer reaction time did not affect the enantioselectivity of the products. Thus, for each of the cases examined, the enantioselectivity of the products was not dependent upon the nature of the tether, the substituent on the alkyne terminus, or the reaction time.
Table 3.
Transfer of chirality of Trisubstituted Allene-ynes
| Entry | Allene-ynea | X | R | Time (min) | Product, % yield | % eeb |
|---|---|---|---|---|---|---|
| 1 | 14c | NTs | Me | 30 | 42c, 80 | 74 |
| 2 | 14d | NTs | Ph | 30 | 42d, 75 | 79 |
| 3 | 14f | NTs | TMS | 30 | 42f, 78 | 79 |
| 4 | 14g | NTs |

|
30 | 42g, 88 | 79 |
| 5 | 15c | O | Me | 45 | 43c, 46 | 78 |
| 6 | 15d | O | Ph | 30 | 43d, 69 | 77 |
| 7 | 15f | O | TMS | 45 | 43f, 91 | 77 |
| 8 | 16c | C(CO2Et)2 | Me | 150 | 44c, 88 | 80 |
| 9 | 16d | C(CO2Et)2 | Ph | 120 | 44d, 92 | 76 |
| 10 | 16f | C(CO2Et)2 | TMS | 120 | 44f, 90 | 76 |
Enantiomeric excess of the disubstituted allenic alcohol >79% ee;
Determined by HPLC analysis using a ChiralCel OD column.
A series of allene-ynes containing disubstituted allenes were also examined for transfer of chiral information in the cyclocarbonylation reaction. The reaction was performed using three different tethers (X = NTs, O, C(CO2Et)2) and varying the substitution on the terminus of the alkyne (R = Me, Ph, H, TMS). For each of the allene-ynes reported in Table 4 the enantiomeric purity was based upon the enantiomeric excess obtained for allenyl ester (Ra)-27, which was determined to be greater than 99%. Contrary to the trisubstituted allenes, the degree of chiral information transferred from the disubstituted allenes to the cyclopentenones was dependent upon the tether and alkyne substitution. For example, allene-ynes 17 – 19d containing a phenyl group on the alkyne afforded the corresponding cyclocarbonylation products 45 – 47d in 88% ee for the tosylamide tether (Table 4, entry 2); 52% ee for the oxygen tether (Table 4, entry 5); and 72% ee for the malonate tether (Table 4, entry 9). Furthermore, the decrease in enantiomeric excess was also dependent on the substituent on the alkyne. This was especially prevalent in the ether series of allene-ynes where the enantiomeric excesses of the cycloadducts ranged from 22 – 52% for trimethylsilyl, hydrogen, methyl or phenyl substituents (Table 4, entries 4 – 7). Determination of the enantiomeric excesses of the cyclocarbonylation products 45 – 47 proved to be more difficult than in the trisubstituted series, in that a different chiral column was needed for nearly every product.
Table 4.
Transfer of chirality using Disubstituted Allene-ynes
| Entry | Allene-ynea | X | R | Time (h) | Product, % yield | % ee |
|---|---|---|---|---|---|---|
| 1 | 17c | NTs | Me | 4.6 | 45c, 73 | 81b |
| 2 | 17d | NTs | Ph | 3 | 45d, 84 | 88c |
| 3 | 17f | NTs | TMS | 3 | 45f, 83 | 84d |
| 4 | 18c | O | Me | 6.5 | 46c, 40 | 45c |
| 5 | 18d | O | Ph | 0.5 | 46d, 90 | 52c |
| 6 | 18e | O | H | 1.5 | 46e, 24 | 30e |
| 7 | 18f | O | TMS | 7 | 46f, 76 | 22d |
| 8 | 19c | C(CO2Et)2 | Me | 22 | 47c, 71 | 58f |
| 9 | 19d | C(CO2Et)2 | Ph | 1.5 | 47d, 96 | 72c |
| 10 | 19f | C(CO2Et)2 | TMS | 10 | 47f, 94 | 50d |
The enantiomeric excess of the disubstituted allenic alcohol was >99% ee;
Determined using HPLC analysis (ChiralPak IA-3);
Determined using HPLC analysis (ChiralCel OD);
Determined using SFC analysis (ChiralPak IC);
Determined using SFC analysis (ChiralPak IA);
Determined using HPLC analysis (Whelk O-1).
Insight regarding the partial racemization in the disubstituted series was obtained by reacting allene-yne 18c to the standard conditions and allowing it to reach 60% conversion. The enantiomeric excess of the cyclocarbonylation product 46c was measured to be 43% after purification by silica gel chromatography. The enantiomeric excess of 46c was 45% when the reaction was allowed to go to completion, indicating that racemization of the product was not occurring after the product is formed (Table 4, entry 4). Next, the enantiomeric excess of allene-ynes 17d and 18d were measured during the reaction. Stopping the cyclocarbonylation reaction of 18d at 50% conversion afforded the unreacted allene-yne in 11% ee. Similarly, stopping the reaction of allene-yne 17c at 29% conversion afforded the allene in 15% ee. The enantiomeric excesses (ee) of the allenes were determined by HPLC analysis (ChiralPak AS-H). These three experiments provide strong evidence to support the hypothesis that loss of enantiomeric excess is occurring prior to product formation and at the allene-yne stage of the reaction.
A mechanism is proposed to account for the erosion of enantiomeric excesses of the disubstituted allenes. Inspired by the work of Backvall, addition of an internal nucleophile to a rhodium-allene complex is postulated (Scheme 8).26 For the disubstituted allene A, coordination of the rhodium catalyst to the proximal double bond of the allene affords the rhodium complex B. Nucleophilic attack of the tethered heteroatom (oxygen in the example depicted) to the central carbon initially affords the corresponding η1-rhodium species that isomerizes to the η3 complex C that in turn isomerizes to the η1-rhodium species D. Free rotation around the designated carbon-carbon bond of D leads to the η3 rhodium-complex E. Reductive elimination from intermediate E leads to the formation allene-yne F, the enantiomer of allene-yne A. Erosion in the enantiomeric excesses correlates well with the relative nucleophilicities of the heteroatoms in the tether. For example, the highest enantiomeric excesses were obtained for the cyclocarbonylation products with a tosylamide tether whereas the lowest enantiomeric excesses were obtained for the oxygen tether. In the case of the malonate tether, formation of an intermediate seven-membered ring would explain the racemization process.
Scheme 8.

Postulated Mechanism for the Erosion of Enantiomeric Excesses of Disubsubstituted Allenes in the Cyclocarbonylation Reaction
For the trisubstituted allene series, the absence of racemization is explained by selective complexation of the rhodium catalyst with the less substituted distal double bond of the allene and complexation from the face opposite of the DPS group (intermediate G, Scheme 8). Moreover, the rhodium metallocycle of intermediate G is not set up for an internal nucleophilic addition but instead is well positioned to undergo complexation with alkyne, ultimately affording the desired cyclocarbonylation product with no loss in enantiomeric excesses.
Determination of the Absolute Configuration of the Cyclocarbonylation Product
The fortuitous crystallization of 35c at −20 °C allowed for the determination of the absolute configuration by X-ray diffraction using the anomalous scattering effect of the silicon and is in agreement with the absolute configuration of that established for allenyl alcohol (Ra)-22 (Scheme 9).27
Scheme 9.

Absolute Configuration of Cyclocarbonylation Product 35c
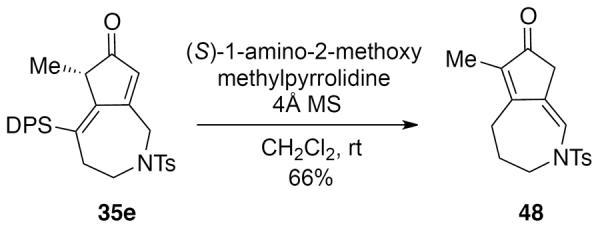
Furthermore, during attempts to establish the absolute configuration of cyclocarbonylation product 35e by derivatization with (S)-(−)-1-amino-2-(methoxymethyl)pyrrolidine (SAMP), compound 48 was isolated instead of the expected hydrazone (Scheme 10). Structurally similar compounds are usually synthesized by performing the APKR on allenamides.28 This type of isomerization has previously been observed during an APKR.28 The silicon group is shown to have no effect on this unusual isomerization since compound 48 was also obtained when the reaction was performed on the dienone lacking the silicon group.
Scheme 10.
Synthesis of Compound 48
Conclusion
In summary, we have successfully performed a transfer of chirality from a chiral non-racemic allene to a 4-alkylidene cyclopentenone in a Rh(I)-catalyzed APKR. Complete transfer of chirality was obtained for every trisubstituted allene. Investigations regarding the loss of enantiomeric excess observed when the APKR was performed with disubstituted allene-ynes demonstrated that partial racemization of the allenes was occurring during the reaction. Moreover, the mildness of the reaction conditions are highlighted by the absence of epimerization of the stereogenic center during the reaction. The absolute configuration of cyclocarbonylation product 35c was unambiguously established by X-ray crystallographic analysis. The absolute configuration of this dienone corresponds to the predicted assignment, based upon the absolute configuration of the propargylic alcohol precursor. Finally, an unusual isomerization of the dienone was observed during attempts to functionalize the carbonyl group, affording a presumably more stable vinylogous amide.
Experimental Section
Unless otherwise noted, all reactions were performed under N2 in flame-dried glassware using standard syringe, cannula, and septum techniques. All commercially available compounds were used as received unless otherwise noted. The reaction solvents tetrahydrofuran (THF) and dichloromethane (CH2Cl2) were obtained by passing commercially available predried, oxygen-free formulations through activated alumina columns. Toluene, acetonitrile, 2,6-lutidine and triethylamine (Et3N) were freshly distilled from CaH2 prior to use. N,N-dimethylacetamide was dried over 4 Å molecular sieves for 12 h prior to use. Flash chromatography was carried out on silica. Thin layer chromatography (TLC) analysis was performed on precoated silica gel F254 aluminium plates. 1H NMR and 13C NMR spectra were recorded in deuterated solvents and referenced to residual chloroform (7.26 ppm, 1H, 77.0 ppm, 13C). Chemical shifts are reported in ppm, multiplicities are indicated by s (singlet), d (doublet), t (triplet), q (quartet) and m (multiplet). Coupling constants, J, are reported in hertz. All NMR spectra were obtained at rt. Infrared spectra were recorded neat and are reported in cm−1. HRMS data were collected using a TOF mass spectrometer.
General Procedure for Preparation of Allene-yne via Mitsunobu Reaction
To a solution of the alcohol in THF cooled to 0 °C (ice bath) was added triphenylphosphine (1.2 equiv), the tosylamide (1.2 equiv) and diisopropylazodicarboxylate (1.2 equiv). The reaction mixture was stirred until completion as judged by TLC analysis. Concentration of the reaction mixture under reduced pressure afforded the crude residue. The crude material was purified by flash chromatography.
General Procedure for Preparation of Allene-yne via Williamson Etherification
A solution of the alcohol in THF was cooled to 0 °C (ice bath) and sodium hydride (2 equiv, 60% dispersion in mineral oil) was added portionwise. The mixture was stirred for 30 min at rt. The propargyl bromide (1.5 equiv) was added and the reaction was stirred at rt until completion as judged by TLC analysis. The reaction was quenched by careful dropwise addition of water over 5 min. The two layers were separated and the aqueous layer was extracted with Et2O. The organic layers were combined, dried (Na2SO4), filtered and concentrated under reduced pressure. The crude material was purified by flash chromatography.
General Procedure for Preparation of Allene-yne via Alkylation of the Malonate Moiety
The diethyl malonate23 (2 equiv) was added dropwise to a stirred suspension of sodium hydride (2 equiv, 60% dispersion in mineral oil) in a DMF/THF (1:1) mixture cooled to 0 °C (ice bath). After 1 h at 0 °C, hydrogen evolution had ceased and the mesylate (1 equiv) in THF was added, followed by potassium iodide (1.5 equiv). The reaction mixture was stirred at 70 °C for 12 h. The solution was cooled to rt, diluted with Et2O and quenched by careful dropwise addition of a saturated solution of NH4Cl. The layers were separated and the aqueous layer was extracted with Et2O. The combined organic phases were washed with brine, dried (Na2SO4), filtered and concentrated under reduced pressure. The crude material was purified by flash chromatography.
General Procedure for Preparation of Allene-yne via Silylation of the Terminal Alkyne
To a solution of the allene-yne (1 equiv) in THF cooled to −78 °C was added lithium hexamethyldisilylamide (1 M in THF, 1.3 equiv) and the resulting mixture was stirred at −78 °C for 1 h. Trimethylsilyl chloride (2 equiv) was then added and the reaction was stirred for 1 h at −78 °C. The mixture was quenched with a saturated solution of NH4Cl and diluted with Et2O. The layers were separated and the aqueous layer was extracted with Et2O. The combined organic phases were washed with brine, dried (Na2SO4), filtered and concentrated under reduced pressure. The crude material was purified by flash chromatography.
General Procedure for the [Rh(CO)2Cl]2-Catalyzed Cyclocarbonylation Reaction
A flame dried vial (15 × 45 mm) equipped with a Teflon-coated stir-bar and a septa cap was charged with allene-yne and toluene (0.1 M). The tube was evacuated for 3–5 sec. and refilled with CO (g) (3 ×) using a balloon. To the allene-yne solution was added [Rh(CO)2Cl]2 (0.1 equiv) in one portion, and the vial was evacuated and refilled with CO (g) (3 ×). The vial was placed in a preheated 90 °C oil bath and stirred under CO (g). After the reaction was complete as judged by TLC analysis, the mixture was cooled to rt, passed through a short plug of Celite using Et2O, and concentrated under reduced pressure. The crude material was purified by flash chromatography.
(Ra)-Hexa-3,4-dien-1-yl 4-nitrobenzoate (27)
(Ra)-hexa-3,4-dien-1-ol3226 (48 mg, 0.49 mmol) was dissolved in N,N-dimethylacetamide (0.9 mL) and 2,6-lutidine (150 μL, 1.32 mmol). This solution was added dropwise to a solution of p-nitrobenzoyl chloride (118 mg, 0.64 mmol), 4-N,N-dimethylaminopyridine (12 mg, 0.098 mmol) in acetonitrile (0.7 mL) at 0 °C (ice bath). After completion of the addition, the reaction mixture was stirred for 1 h at 0 °C. After quenching with a saturated solution of sodium bicarbonate (3 mL), the mixture was extracted with Et2O (20 mL), washed with water (5 mL), brine (5 mL), dried (Na2SO4), filtered and concentrated under reduced pressure. Purification of the crude residue using a Biotage normal phase automated purification system (4 g SNAP column, gradient of 1–10% Et2O/hexanes) afforded compound 27 (73.4 mg, 61%) as a colorless oil. 1H NMR (700 MHz, CDCl3) δ 8.29 (d, J = 8.7 Hz, 2H), 8.22 (d, J = 8.7 Hz, 2H), 5.12 – 5.10 (m, 2H), 4.45 – 4.43 (m, 2H), 2.48 – 2.43 (m, 2H), 1.62 – 1.61 (m, 3H) ; 13C NMR (175 MHz, CDCl3) δ 205.5, 164.6, 150.5, 135.7, 130.6 (2C), 123.5 (2C), 86.6, 85.8, 64.9, 28.2, 14.3 ; IR (thin film) 3113, 2954, 2901, 2852, 1965, 1720, 1532, 1352, 1274, 1103, 1111, 1013, 874; HRMS (ES+) C2H13NO4 [M+] Calculated: 247.0845; Found: 247.0867; (c 0.55, CH2Cl2).
(Ra)-N-(hexa-3,4-dien-1-yl)-4-methyl-N-(3-phenylprop-2-yn-1-yl)benzene sulfonamide (17d)
Following the general procedure for preparation of allene-yne via Mitsunobu reaction, (Ra)-hexa-3,4-dien-1-ol 26 (26.4 mg, 0.27 mmol) in THF (1.9 mL) was reacted with triphenylphosphine (85 mg, 0.32 mmol), 4-methyl-N-(3-phenylprop-2-yn-1-yl)benzenesulfonamide32 (92.1 mg, 0.32 mmol) and diisopropylazodicarboxylate (64 μL, 0.32 mmol) for 12 h. Purification of the crude residue using a Biotage normal phase automated purification system (25 g SNAP column, gradient of 0 – 20% Et2O/hexanes) afforded compound 17d (83.9 mg, 85%) as a colorless oil.1H NMR (600 MHz, CDCl3) δ 7.77 (d, J = 8.0 Hz, 2H), 7.28 – 7.22 (m, 5H), 7.08 (d, J = 7.3 Hz, 2H), 5.11 – 5.08 (m, 1H), 5.08 – 5.03 (m, 1H), 4.36 (s, 2H), 3.33 (t, J = 7.3 Hz, 2H), 2.34 (s, 3H), 2.32 – 2.31 (m, 2H), 1.66 – 1.64 (m, 3H) ; 13C NMR (150 MHz, CDCl3) δ 205.4, 143.3, 136.0, 131.5 (2C), 129.5 (2C), 128.4, 128.1 (2C), 127.7 (2C), 122.2, 86.6, 86.4, 85.6, 81.9, 46.1, 37.3, 27.6, 21.4, 14.4 ; IR (thin film) 3060, 3031, 2987, 2921, 2856, 2235, 1973, 1597, 1487, 1438, 1344, 1168, 1095, 1025, 907; HRMS (ES+) C22H23NO2NaS [M+Na] Calculated: 388.1347; Found: 388.1364; (c 1.45, CH2Cl2).
(S)-6-methyl-8-phenyl-2-tosyl-1,2,3,4-tetrahydrocyclopenta[c]azepin-7(6H)-one (45d)
Following the general procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 17d (40.4 mg, 0.11 mmol) and [Rh(CO)2Cl]2 (4.3 mg, 0.011 mmol) were reacted in toluene (3.4 mL) for 180 min. Purification of the crude residue using a Biotage normal phase automated purification system (10 g SNAP column, 70% Et2O/hexanes) afforded compound 45d (36.5 mg, 84%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.54 – 7.53 (m, 2H), 7.46 – 7.45 (m, 2H), 7.41 – 7.39 (m, 1H), 7.29 (d, J = 6.9 Hz, 2H), 7.22 (d, J = 8.0 Hz, 2H), 5.79 – 5.77 (m, 1H), 4.60 – 4.53 (m, 2H), 3.67 – 3.63 (m, 1H), 3.63 – 3.61 (m, 1H), 2.78 – 2.77 (m, 1H), 2.70 – 2.68 (m, 2H), 2.40 (s, 3H), 1.17 (d, J = 7.5 Hz, 3H) ; 13C NMR (150 MHz, CDCl3) δ 205.3, 160.2, 143.5, 141.2, 140.7, 136.5, 130.4, 129.6 (2C), 129.3 (2C), 128.7, 128.5 (2C), 127.0 (2C), 125.5, 49.1, 49.0, 45.2, 30.8, 21.5, 15.0 ; IR (thin film) 3060, 2974, 2925, 2876, 1699, 1601, 1499, 1450, 1336, 1160, 1095, 952; HRMS (ES+) C23H24NO3S [M+H+] Calculated: 394.1477; Found: 394.1473; (c 1.4, CH2Cl2).
(Ra)-N-(hexa-3,4-dien-1-yl)-4-methyl-N-(prop-2-yn-1-yl)benzenesulfonamide (17e)
Following the general procedure for preparation of allene-yne via Mitsunobu reaction, (Ra)-hexa-3,4-dien-1-ol 26 (42.1 mg, 0.43 mmol) in THF (3 mL) was reacted with triphenylphosphine (135 mg, 0.51 mmol), 4-methyl-N-(prop-2-yn-1-yl)benzenesulfonamide32 (108 mg, 0.51 mmol) and diisopropylazodicarboxylate (101 μL, 0.51 mmol) for 6 h. Purification of the crude residue using a Biotage normal phase automated purification system (12 g SNAP column, gradient of 5 – 10% Et2O/hexanes) afforded compound 17e (105 mg, 84%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 7.72 (d, J = 8.2 Hz, 2H), 7.29 (d, J = 8.2 Hz, 2H), 5.10 – 5.07 (m, 1H), 5.02 – 4.99 (m, 1H), 4.14 (d, J = 2.5 Hz, 2H), 3.26 (t, J = 7.4 Hz, 2H), 2.42 (s, 3H), 2.26 (m, 2H), 2.03 (t, J = 2.5 Hz, 1H), 1.65 – 1.63 (m, 3H) ; 13C NMR (175 MHz, CD2Cl2) δ 205.2, 143.7, 136.0, 129.5 (2C), 127.6 (2C), 86.5, 86.3, 76.7, 73.4, 46.1, 36.4, 27.5, 21.3, 14.2 ; IR (thin film) 3289, 2974, 2925, 2856, 1960, 1593, 1454, 1344, 1160, 1102; HRMS (ES+) C16H20NO2S [M+H+] Calculated: 290.1215; Found: 290.1237; (c 1.25, CH2Cl2).
(Ra)-N-(hexa-3,4-dien-1-yl)-4-methyl-N-(3-(trimethylsilyl)prop-2-yn-1-yl)benzene sulfonamide (17f)
Following the general procedure for preparation of allene-yne via silylation of the terminal alkyne, allene-yne 17e (70 mg, 0.24 mmol) in THF (1.8 mL) was reacted with lithium hexamethyldisilylamide (314 μL, 1M in THF, 0.31 mmol) and trimethylsilylchloride (61 μL, 0.48 mmol). Purification of the crude residue using a Biotage normal phase automated purification system (10 g SNAP column, gradient of 2 – 7% Et2O/hexanes) afforded compound 17f (73.2 mg, 84%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.72 (d, J = 8.3 Hz, 2H), 7.28 (d, J = 7.9 Hz, 2H), 5.09 – 5.02 (m, 2H), 4.15 (s, 2H), 3.25 (t, J = 7.4 Hz, 2H), 2.42 (s, 3H), 2.28 – 2.25 (m, 2H), 1.66 – 1.63 (m, 3H), −0.01 (s, 9H) ; 13C NMR (150 MHz, CDCl3) δ 205.3, 143.2, 135.9, 129.4 (2C), 127.7 (2C), 97.9, 90.8, 86.5, 86.3, 45.8, 37.3, 27.4, 21.5, 14.4, −0.5 (3C) ; IR (thin film) 2965, 2916, 2857, 2178, 1594, 1455, 1249, 1346, 115536, 1095; HRMS (ES+) C19H28NO2SSi [M+H+] Calculated: 362.1610; Found: 362.1628; (c 1.05, CH2Cl2).
(S)-6-methyl-2-tosyl-8-(trimethylsilyl)-1,2,3,4-tetrahydrocyclopenta[c]azepin-7(6H)-one (45f)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 17f (42 mg, 0.12 mmol) and [Rh(CO)2Cl]2 (4.5 mg, 0.012 mmol) were reacted in toluene (3.6 mL) for 180 min. Purification of the crude residue using a Biotage normal phase automated purification system (4 g SNAP column, 65% Et2O/hexanes) afforded compound 45f (37.5 mg, 83%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.64 (d, J = 8.3 Hz, 2H), 7.27 (d, J = 8.3 Hz, 2H), 5.70 – 5.67 (m, 1H), 4.53 (s, 2H), 3.56 – 3.52 (m, 2H), 2.64 – 2.59 (m, 3H), 2.41 (s, 3H), 1.07 (d, J = 7.5 Hz, 3H), 0.30 (s, 9H) ; 13C NMR (150 MHz, CDCl3) δ 211.3, 172.6, 143.6, 143.5, 141.9, 136.2, 129.7 (2C), 127.1 (2C), 124.8, 50.6, 48.8, 46.1, 31.0, 21.5, 14.9, −0.5 (3C) ; IR (thin film) 2958, 2921, 2864, 1691, 1536, 1340, 1164, 1091; HRMS (ES+) C20H28NO3SSi [M+H+] Calculated: 390.1559; Found: 390.1549; (c 0.8, CH2Cl2).
(Ra)-N-(but-2-yn-1-yl)-N-(hexa-3,4-dien-1-yl)-4-methylbenzenesulfonamide (17c)
Following the general procedure for preparation of allene-yne via Mitsunobu reaction, (Ra)-hexa-3,4-dien-1-ol 26 (25 mg, 0.26 mmol) in THF (1.8 mL) was reacted with triphenylphosphine (80.2 mg, 0.31 mmol), N-(but-2-yn-1-yl)-4-methylbenzenesulfonamide32 (68.3 mg, 0.31 mmol) and diisopropylazodicarboxylate (61 μL, 0.31 mmol) for 12 h. Purification of the crude residue using a Biotage normal phase automated purification system (10 g SNAP column, gradient of 10% Et2O/hexanes) afforded compound 17c (57 mg, 74%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.73 (d, J = 8.4 Hz, 2H), 7.29 (d, J = 8.0 Hz, 2H), 5.08 – 5.04 (m, 1H), 5.04 – 4.99 (m, 1H), 4.07 – 4.06 (m, 2H), 3.26 – 3.21 (t, J = 7.3 Hz, 2H), 2.42 (s, 3H), 2.26 – 2.23 (m, 2H), 1.66 – 1.63 (m, 3H), 1.57 – 1.55 (t, J = 2.4 Hz, 3H) ; 13C NMR (150 MHz, CDCl3) δ 205.3, 143.1, 136.1, 129.2 (2C), 127.8 (2C), 86.6, 86.3, 81.4, 71.8, 45.9, 36.9, 27.6, 21.5, 14.4, 3.2 ; IR (thin film) 2925, 2852, 1961, 1597, 1442, 1348, 1160, 1099; HRMS (ES+) C17H22NO2S [M+H+] Calculated: 304.1371; Found: 304.1363; (c 2.6, CH2Cl2).
(S)-6,8-Dimethyl-2-tosyl-1,2,3,4-tetrahydrocyclopenta[c]azepin-7(6H)-one (45c)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 17c (16.9 mg, 0.056 mmol) and [Rh(CO)2Cl]2 (2.2 mg, 0.0056 mmol) were reacted in toluene (1.7 mL) for 280 min. Purification of the crude residue using a Biotage normal phase automated purification system (4 g SNAP column, 70% Et2O/hexanes) afforded compound 45c (13.5 mg, 73%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.66 (d, J = 8.3 Hz, 2H), 7.29 – 7.26 (d, J = 8.3 Hz, 2H), 5.63 (t, J = 5.0 Hz, 1H), 4.43 – 4.41 (m, 2H), 3.55 – 3.51 (m, 2H), 2.70 – 2.59 (m, 3H), 2.42 (s, 3H), 1.81 (s, 3H), 1.10 (d, J = 7.4 Hz, 3H) ; 13C NMR (175 MHz, CDCl3) 207.0, 159.8, 143.6, 140.8, 138.0, 136.2, 129.7 (2C), 127.0 (2C), 123.7, 49.0, 48.9, 44.6, 31.3, 21.5, 14.9, 8.4 ; IR (thin film) 2062, 2921, 2856, 2002, 1691, 1597, 1446, 1340, 1164, 1095; HRMS (ES+) C18H22NO3S [M+H+] Calculated: 332.1320; Found: 332.1347; (c 0.85, CH2Cl2).
(Ra)-Hexa-3,4-dien-1-yl methanesulfonate (32)
To a stirred solution of (Ra)-hexa-3,4-dien-1-ol 26 (60.8 mg, 0.62 mmol) in THF (8.7 mL), cooled to 0 °C (ice bath), was added triethylamine (95 μL, 0.68 mmol) followed by methanesulfonyl chloride (53 μL, 0.681 mmol). The resulting solution was stirred at rt for 12 h. The mixture was diluted with Et2O (60 mL), washed with water (2 × 10 mL), dried (Na2SO4), filtered and concentrated under reduced pressure. Purification of the crude residue using a Biotage normal phase automated purification system (12 g SNAP column, 35% Et2O/hexanes) afforded compound 32 (84.1 mg, 77%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 5.22 – 5.12 (m, 1H), 5.05 – 5.03 (m, 1H), 4.25 (t, J = 6.8 Hz, 2H), 3.00 (s, 3H), 2.44 – 2.39 (m, 2H), 1.65 (dd, J = 7.0, 3.2 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 205.4, 86.9, 84.8, 69.0, 37.2, 28.5, 14.1; IR (thin film) 3032, 2987, 2942, 1965, 1462, 1344, 1172, 964, 911; HRMS (ES+) C7H13O3S [M+H+] Calculated: 177.0585; Found: 177.0588; (c 1.45, CH2Cl2).
(Ra)-Diethyl 2-(hexa-3,4-dien-1-yl)-2-(3-phenylprop-2-yn-1-yl)malonate (19d)
Following the General Procedure for Preparation of Allene-yne via alkylation of the malonate moiety, diethyl malonate 34d (79.3 mg, 0.29 mmol) was reacted with sodium hydride (8.7 mg, 60 wt% in oil, 0.22 mmol), mesylate 32 (25.5 mg, 0.15 mmol) and potassium iodide (36 mg, 0.22 mmol) in DMF-THF (1 : 1, 4 mL). Purification of the crude residue using a Biotage normal phase automated purification system (25 g SNAP column, gradient of 3 – 7% Et2O/hexanes) afforded compound 19d (35.4 mg, 69%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.38 – 7.35 (m, 2H), 7.29 – 7.27 (m, 3H), 5.09 – 5.05 (m, 2H), 4.22 (q, J = 7.2 Hz, 4H), 3.05 (s, 2H), 2.25 – 2.20 (m, 2H), 1.98 – 1.95 (m, 2H), 1.65 – 1.62 (m, 3H), 1.26 (t, J = 7.1 Hz, 6H) ; 13C NMR (175 MHz, CDCl3) δ 204.6, 170.3 (2C), 131.6 (2C), 128.1 (2C), 127.9, 123.3, 89.4, 86.4, 84.4, 83.4, 61.5 (2C), 56.9, 31.6, 23.8, 23.7, 14.4, 14.1 (2C) ; IR (thin film) 3465, 2987, 2921, 2856, 1965, 1732, 1597, 1487, 1446, 1368, 1270, 1197, 1091, 1030; HRMS (ES+) C22H27O4 [M+H+] Calculated: 355.1909; Found: 355.1885; (c 2.5, CH2Cl2).
(S)-Diethyl 1-methyl-2-oxo-3-phenyl-1,2,6,7-tetrahydroazulene-5,5(4H)-dicarboxylate (47d)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 19d (22.6 mg, 0.064 mmol) and [Rh(CO)2Cl]2 (2.5 mg, 0.0064 mmol) were reacted in toluene (2 mL) for 100 min. Purification of the residue by flash chromatography (45% Et2O/hexanes) afforded the title compound 47d (23.3 mg, 96%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.44 – 7.26 (m, 5H), 6.04 – 5.92 (m, 1H), 4.15 – 4.02 (m, 4H), 3.39 (s, 2H), 2.94 – 2.87 (q, J = 7.4 Hz, 1H), 2.56 – 2.52 (m, 2H), 2.45 – 2.41 (m, 2H), 1.27 (d, J = 7.5 Hz, 3H), 1.13 (q, J = 7.1 Hz, 6H) ; 13C NMR (175 MHz, CDCl3) δ 206.0, 171.1, 170.9, 161.3, 143.2, 141.9, 131.3, 129.4 (2C), 128.3 (2C), 128.2, 127.9, 61.7, 61.6, 56.3, 44.9, 34.5, 33.8, 25.1, 15.3, 13.9, 13.8 ; IR (thin film) 2966, 2929, 2852, 2365, 1732, 1699, 1450, 1364, 1242, 1176, 1078; HRMS (ES+) C23H27O5 [M+H+] Calculated: 383.1858; Found: 383.1860; (c 0.95, CH2Cl2).
(Ra)-Diethyl 2-(but-2-yn-1-yl)-2-(hexa-3,4-dien-1-yl)malonate (19c)
Following the General Procedure for Preparation of Allene-yne via alkylation of the malonate moiety, diethyl malonate 34c (94.4 mg, 0.45 mmol) was reacted with sodium hydride (15.3 mg, 60 wt% in oil, 0.38 mmol), mesylate 32 (56 mg, 0.32 mmol) and potassium iodide (79.1 mg, 0.48 mmol) in DMF-THF (1 : 1, 6 mL). Purification of the crude residue using a Biotage normal phase automated purification system (25 g SNAP column, gradient of 4 – 6% Et2O/hexanes) afforded compound 19c (59.6 mg, 63%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 5.07 – 5.02 (m, 2H), 4.18 (q, J = 7.1 Hz, 4H), 2.75 (s, 2H), 2.12 – 2.10 (m, 2H), 1.90 – 1.88 (m, 2H), 1.73 (s, 3H), 1.64 – 1.62 (m, 3H), 1.23 (t, J = 7.2 Hz, 6H) ; 13C NMR (175 MHz, CDCl3) δ 204.6, 170.4 (2C), 89.4, 86.3, 78.5, 73.4, 61.3 (2C), 56.8, 31.4, 23.7, 23.1, 14.4, 14.0 (2C), 3.4 ; IR (thin film) 2983, 2934, 2859, 1736, 1438, 1360, 1275, 1225, 1193; HRMS (ES+) C17H25O4 [M+H+] Calculated: 293.1753; Found:293.1778; (c 0.85, CH2Cl2).
(S)-diethyl 1,3-dimethyl-2-oxo-1,2,6,7-tetrahydroazulene-5,5(4H)-dicarboxylate (47c)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 19c (33.8 mg, 0.11 mmol) and [Rh(CO)2Cl]2 (4.4 mg, 0.011 mmol) were reacted in toluene (3.6 mL) for 22 h. Purification of the residue by flash chromatography (40% Et2O/hexanes) afforded compound 47c (25.7 mg, 71%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 5.81 – 5.79 (m, 1H), 4.23 – 4.15 (m, 4H), 3.21 (s, 2H), 2.76 – 2.68 (m, 1H), 2.51 – 2.47 (m, 2H), 2.39 – 2.33 (m, 2H), 1.84 (s, 3H), 1.27 – 1.22 (m, 6H), 1.16 (d, J = 7.5 Hz, 3H) ; 13C NMR (150 MHz, CDCl3) δ 208.1, 171.3, 171.2, 161.0, 143.6, 139.2, 125.9, 61.7 (2C), 56.2, 44.2, 33.9 (2C), 25.0, 15.0, 14.0 (2C), 8.3 ; IR (thin film) 2978, 2921, 1732, 1703, 1446, 1364, 1287, 1234, 1062, 1095; HRMS (ES+) C18H25O5 [M+H+] Calculated: 321.1702; Found: 321.1723; (c 0.5, CH2Cl2).
(Ra)-Diethyl 2-(hexa-3,4-dien-1-yl)-2-(prop-2-yn-1-yl)malonate (19e)
Following the General Procedure for Preparation of Allene-yne via alkylation of the malonate moiety, diethyl malonate 34e (189 mg, 0.95 mmol) was reacted with sodium hydride (28.6 mg, 60 wt% in oil, 0.71 mmol), mesylate 32 (84 mg, 0.48 mmol) and potassium iodide (119 mg, 0.72 mmol) in DMF-THF (1 : 1, 13 mL). Purification of the crude residue using a Biotage normal phase automated purification system (50 g SNAP column, gradient of 2 – 7% Et2O/hexanes) afforded compound 19e (75.5 mg, 57%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 5.09 – 5.03 (m, 2H), 4.24 – 4.17 (m, 4H), 2.83 (s, 2H), 2.20 – 2.14 (m, 2H), 2.00 (t, J = 2.7 Hz, 1H), 1.96 – 1.87 (m, 2H), 1.66 – 1.63 (m, 3H), 1.25 (t, J = 7.1 Hz, 6H); 13C NMR (100 MHz, CD2Cl2) δ 204.5, 170.0 (2C), 89.3, 86.3, 78.9, 71.0, 61.6 (2C), 56.4, 31.3, 23.7, 22.7, 14.2, 13.8 (2C) ; IR (thin film) 3293, 2892, 2938, 1736, 1454, 1270, 1193, 1095, 1030. HRMS (ES+) C16H23O4 [M+H+] Calculated: 279.1596; Found: 279.1623; (c 2.2, CH2Cl2).
(Ra)-Diethyl 2-(hexa-3,4-dien-1-yl)-2-(3-(trimethylsilyl)prop-2-yn-1-yl)malonate (19f)
Following the general procedure for preparation of allene-yne via silylation of the terminal alkyne, allene-yne 19e (67.6 mg, 0.24 mmol) in THF (1.8 mL) was reacted with lithium hexamethyldisilylamide (365 μL, 1M in THF, 0.36 mmol) and trimethylsilylchloride (62 μL, 0.49 mmol). Purification of the crude residue using a Biotage normal phase automated purification system (4 g SNAP column, 5% Et2O/hexanes) afforded compound 19f (74.4 mg, 88%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 5.08 – 5.03 (m, 2H), 4.20 – 4.17 (m, 4H), 2.84 (s, 2H), 2.14 – 2.12 (m, 2H), 1.93 – 1.90 (m, 2H), 1.65 – 1.62 (m, 3H), 1.24 (t, J = 7.1 Hz, 6H), 0.12 (s, 9H) ; 13C NMR (175 MHz, CDCl3) δ 204.6, 170.1 (2C), 101.4, 89.4, 88.0, 86.3, 61.5 (2C), 56.7, 31.4, 24.2, 23.7, 14.4, 14.0 (2C), −0.1 (3C) ; IR (thin film) 2958, 2925, 2860, 2186, 1740, 1471, 1442, 1254, 1197, 1103, 1034, 854; HRMS (ES+) C19H31O4Si [M+H+] Calculated: 351.1992; Found: 351.1990; (c 1.85, CH2Cl2).
(S)-Diethyl 1-methyl-2-oxo-3-(trimethylsilyl)-1,2,6,7-tetrahydroazulene-5,5(4H)-dicarboxylate (47f)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 19f (33.9 mg, 0.097 mmol) and [Rh(CO)2Cl]2 (2.5 mg, 0.0097 mmol) were reacted in toluene (3.2 mL) for 10 h. Purification of the crude residue using a Biotage normal phase automated purification system (4 g SNAP column, 25% Et2O/hexanes) afforded compound 47f (34.5 mg, 94%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 5.88 – 5.83 (m, 1H), 4.23 – 4.16 (m, 4H), 3.37 – 3.36 (m, 2H), 2.75 – 2.68 (m, 1H), 2.50 – 2.46 (m, 2H), 2.40 – 2.34 (m, 2H), 1.24 (td, J = 7.1, 0.9 Hz, 6H), 1.14 (t, J = 7.4 Hz, 3H), 0.27 (s, 9H) ; 13C NMR (100 MHz, CDCl3) δ 212.3, 173.4, 171.1, 171.0, 145.6, 142.8, 127.3, 61.7, 61.6, 56.3, 46.1, 36.5, 33.7, 25.2, 15.0, 14.0 (2C), −0.3 (3C) ; IR (Thin film) 2970, 2929, 2905, 1736, 1691, 1540, 1446, 1368, 1291, 1230, 1078, 1037, 841; HRMS (ES+) C20H31O5Si [M+H+] Calculated: 379.1941; Found: 379.1967; (c 2.2, CH2Cl2).
(Ra)-6-(But-2-yn-1-yloxy)hexa-2,3-diene (18c)
Following the general procedure for preparation of allene-yne via Williamson etherification, (Ra)-hexa-3,4-dien-1-ol 26 (32.3 mg, 0.33 mmol) in THF (0.8 mL) was reacted with sodium hydride (26.5 mg, 60% dispersion in mineral oil 0.66 mmol) and 1-bromobut-2-yne (61.3 mg, 0.46 mmol) for 12 h. Purification of the crude residue using a Biotage normal phase automated purification system (12 g SNAP column, gradient of 0 – 3% Et2O/hexanes) afforded compound 18c (37.4 mg, 83%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 5.09 – 5.04 (m, 2H), 4.10 – 4.08 (m, 2H), 3.56 – 3.51 (m, 2H), 2.29 – 2.25 (m, 2H), 1.86 – 1.84 (m, 3H), 1.66 – 1.62 (m, 3H); 13C NMR (150 MHz, CDCl3) δ 205.2, 86.6, 85.8, 82.1, 75.2, 69.3, 58.5, 29.1, 14.3, 3.5 ; IR (thin film) 2925, 2860, 1446, 1356, 1140, 1095; HRMS (ES+) C10H15O [M+H+] Calculated: 151.1123; Found: 151.1150; (c 1.85, CH2Cl2).
(S)-6,8-Dimethyl-3,4-dihydro-1H-cyclopenta[c]oxepin-7(6H)-one (46c)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 18c (27.1 mg, 0.20 mmol) and [Rh(CO)2Cl]2 (7.6 mg, 0.020 mmol) were reacted in toluene (6.4 mL) for 390 min. Purification of the crude residue using a Biotage normal phase automated purification system (4 g SNAP column, 60% Et2O/hexanes) afforded compound 46c (14.1 mg, 40%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 5.79 – 5.76 (m, 1H), 4.74 (s, 2H), 3.94 (t, J = 5.3 Hz, 2H), 2.87 – 2.74 (m, 1H), 2.63 – 2.58 (m, 2H), 1.74 (s, 3H), 1.23 (d, J = 7.5 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 207.2, 164.0, 140.6, 136.2, 124.6, 71.6, 71.0, 44.6, 33.8, 15.2, 8.1; IR (thin film) 2966, 2925, 2872, 1695, 1597, 1458, 1372, 1328, 1225, 1205, 1140; HRMS (ES+) C11H15O2 [M+H+] Calculated: 179.1072; Found: 179.1051; (c 1.05, CH2Cl2).
(Ra)-6-(Prop-2-yn-1-yloxy)hexa-2,3-diene (18e)
Following the general procedure for preparation of allene-yne via Williamson etherification (Ra)-hexa-3,4-dien-1-ol 26 (70.7 mg, 0.72 mmol) in THF (1.7 mL) was reacted with sodium hydride (58 mg, 60% dispersion in mineral oil 1.44 mmol) and propargyl bromide (161 mg, 80 wt% in toluene, 1.08 mmol) for 11 h. Purification of the crude residue using a Biotage normal phase automated purification system (12 g SNAP column, gradient of 0 – 2% Et2O/hexanes) afforded compound 18e (43.4 mg, 44%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 5.09 – 5.06 (m, 2H), 4.16 (d, J = 2.4 Hz, 2H), 3.58 (t, J = 6.8 Hz, 2H), 2.42 (t, J = 2.4 Hz, 1H), 2.30 – 2.26 (m, 2H), 1.66 – 1.64 (m, 3H) ; 13C NMR (150 MHz, CD2Cl2) δ 205.1, 86.6, 85.8, 80.0, 73.8, 69.4, 57.9, 29.2, 14.2 ; IR (thin film) 2933, 2852, 1454, 1356, 1099; HRMS (ES+) C9H11O [M−H+] Calculated: 135.0810; Found: 135.0835; (c 1.35, CH2Cl2).
(S)-6-Methyl-3,4-dihydro-1H-cyclopenta[c]oxepin-7(6H)-one (46e)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 18e (45.5 mg, 0.33 mmol) and [Rh(CO)2Cl]2 (13 mg, 0.033 mmol) were reacted in toluene (10.9 mL) for 105 min. Purification of the crude residue using a Biotage normal phase automated purification system (10 g SNAP column, 20 – 65% Et2O/hexanes) afforded compound 46e (13.3 mg, 24%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 6.00 (s, 1H), 5.91 – 5.89 (m, 2H), 4.78 – 4.77 (m, 2H), 3.96 – 3.92 (m, 2H), 2.88 – 2.84 (m, 1H), 2.63 – 2.61 (m, 1H), 1.25 (d, J = 7.6 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 207.6, 171.4, 141.6, 127.9, 127.6, 71.9, 71.8, 45.8, 33.8, 15.0; IR (thin film) 2925, 2864, 1699, 1565, 1458, 1368, 1201, 1120, 1070; HRMS (ES+) C10H13O2 [M+H+] Calculated: 165.0916; Found: 165.0897; (c 0.85, CH2Cl2).
(Ra)-(3-(Hexa-3,4-dien-1-yloxy)prop-1-yn-1-yl)trimethylsilane (18f)
Following the general procedure for preparation of allene-yne via silylation of the terminal alkyne, allene-yne 18e (72.4 mg, 0.53 mmol) in THF (4.2 mL) was reacted with lithium hexamethyldisilylamide (798 μL, 1M in THF, 0.80 mmol) and trimethylsilyl chloride (135 μL, 1.06 mmol). Purification of the crude residue using a Biotage normal phase automated purification system (10 g SNAP column, gradient of 0 – 1% Et2O/hexanes) afforded compound 18f (76 mg, 69%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 5.10 – 5.05 (m, 2H), 4.15 (s, 2H), 3.56 (t, J = 6.8 Hz, 2H), 2.30 – 2.26 (m, 2H), 1.64 – 1.63 (m, 3H), 0.18 (s, 9H); 13C NMR (150 MHz, CDCl3) δ 205.3, 101.7, 91.1, 86.7, 85.9, 69.5, 58.8, 29.1, 14.4, −0.2 (3C); IR (thin film) 2954, 2897, 2178, 1258, 1107, 919, 850; HRMS (ES+) C12H20OSi [M−H]+ Calculated: 207.1205; Found: 207.1189; (C 1.35, CH2Cl2).
(S)-6-Methyl-8-(trimethylsilyl)-3,4-dihydro-1H-cyclopenta[c]oxepin-7(6H)-one (46f)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed cyclocarbonylation Reaction, allene-yne 18f (29.8 mg, 0.14 mmol) and [Rh(CO)2Cl]2 (5.6 mg, 0.014 mmol) were reacted in toluene (4.7 mL) for 7 h. Purification of the crude residue using a Biotage normal phase automated purification system (4 g SNAP column, 40% Et2O/hexanes) afforded compound 46f (25.6 mg, 76%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 5.85 – 5.84 (m, 1H), 4.84 (s, 2H), 3.94 (t, J = 5.4 Hz, 2H), 2.80 – 2.78 (m, 1H), 2.63 – 2.60 (m, 2H), 1.21 (d, J = 7.5 Hz, 3H), 0.23 (s, 9H); 13C NMR (150 MHz, CDCl3) δ 211.5, 177.1, 142.9, 139.8, 126.1, 73.0, 71.5, 46.2, 33.8, 15.3, −0.4 (3C); IR (thin film) 2958, 2925, 2869, 1687, 1528, 1250, 1193, 1136; HRMS (ES+) C13H21O2Si [M+H+] Calculated: 237.1311; Found: 237.1283; (c 1.6, CH2Cl2).
(Ra)-(3-(hexa-3,4-dien-1-yloxy)prop-1-yn-1-yl)benzene (18d)
Following the general procedure for preparation of allene-yne via Williamson etherification, (Ra)-hexa-3,4-dien-1-ol 26 (62.8 mg, 0.64 mmol) in THF (1.5 mL) was reacted with sodium hydride (51.2 mg, 60% dispersion in mineral oil, 1.27 mmol) and (3-bromoprop-1-yn-1-yl)benzene (187.2 mg, 0.96 mmol) for 3 h. Purification of the crude residue using a Biotage normal phase automated purification system (10 g SNAP column, gradient of 0 – 3% Et2O/hexanes) afforded compound 18d (85.2 mg, 63%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 7.46 – 7.45 (m, 2H), 7.32 – 7.30 (m, 3H), 5.12 – 5.08 (m, 2H), 4.38 (s, 2H), 3.65 (t, J = 6.8 Hz, 2H), 2.34 – 2.31 (m, 2H), 1.67 – 1.64 (m, 3H); 13C NMR (150 MHz, CDCl3) δ 205.3, 131.7 (2C), 128.3, 128.2 (2C), 122.7, 86.7, 86.0, 85.9, 85.3, 69.5, 58.8, 29.2, 14.4; IR (thin film) 3060, 2917, 2845, 1969, 1491, 1442, 1360, 1099; HRMS (ES+) C15H17O [M+H+] Calculated: 213.1279; Found: 213.1280; (c 0.65, CH2Cl2).
(S)-6-Methyl-8-phenyl-3,4-dihydro-1H-cyclopenta[c]oxepin-7(6H)-one (46d)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 18d (29.6 mg, 0.14 mmol) and [Rh(CO)2Cl]2 (5.4 mg, 0.014 mmol) were reacted in toluene (4.4 mL) for 30 min. Purification of the crude residue using a Biotage normal phase automated purification system (4 g SNAP column, 45% Et2O/hexanes) afforded compound 46d (30 mg, 90%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.41 – 7.34 (m, 3H), 7.25 – 7.24 (m, 2H), 5.96 – 5.94 (m, 1H), 4.85 (s, 2H), 4.03 – 3.98 (m, 2H), 3.01 – 2.98 (m, 1H), 2.69 – 2.67 (m, 2H), 1.34 (d, J = 7.5 Hz, 3H); 13C NMR (175 MHz, CDCl3) δ 205.6, 164.4, 140.7, 139.1, 130.9, 129.3 (2C), 128.4, 128.3 (2C), 126.8, 71.9, 71.5, 45.3, 33.6, 15.5; IR (thin film) 2962, 2925, 2868, 1669, 1446, 1131; HRMS (ES+) C16H17O2 [M+H+] Calculated: 241.1229; Found: 241.1204; (c 0.25, CH2Cl2).
(Ra)-N-(But-2-yn-1-yl)-N-(3-(dimethyl(phenyl)silyl)hexa-3,4-dien-1-yl)-4-methyl benzenesulfonamide (13c)
Following the general procedure for preparation of allene-yne via Mitsunobu reaction, alcohol (Ra)-22 (157 mg, 0.675 mmol) in THF (4.4 mL) was reacted with triphenylphosphine (213 mg, 0.81 mmol), N-(but-2-yn-1-yl)-4-methylbenzenesulfonamide3230c (181 mg, 0.81 mmol) and diisopropylazodicarboxylate (160 μL, 0.81 mmol) for 3 h. Purification of the crude residue using a Biotage normal phase automated purification system (25 g SNAP column, gradient of 0 – 30% Et2O/hexanes) afforded compound 13c (272 mg, 92%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.65 (d, J = 7.8 Hz, 2H), 7.51 (d, J = 7.6 Hz, 2H), 7.38 – 7.32 (m, 3H), 7.24 (d, J = 7.8 Hz, 2H), 4.92 – 4.80 (m, 1H), 3.96 (d, J = 2.5 Hz, 2H), 3.22 – 3.10 (m, 2H), 2.41 (s, 3H), 2.16 (m, 2H), 1.63 (d, J = 6.9 Hz, 3H), 1.52 (t, J = 2.5 Hz, 3H), 0.36 (s, 6H); 13C NMR (150 MHz, CDCl3) δ 2078.0, 142.9, 137.9, 136.2, 133.7 (2C), 129.1 (2C), 129.0, 127.7 (2C), 127.7 (2C), 91.2, 81.2, 81.2, 72.0, 46.4, 37.1, 27.9, 21.4, 13.7, 3.2, −3.1, −3.2; IR (thin film) 3064, 2962, 2921, 2855, 2300, 2218, 1936, 1593, 1491; HRMS (ES+) C25H32NO2SSi [M+H+] Calculated: 438.1923; Found: 438.1926; (c 1.7, CH2Cl2).
(S)-5-(Dimethyl(phenyl)silyl)-6,8-dimethyl-2-tosyl-1,2,3,4-tetrahydrocyclo penta [c]azepin-7(6H)-one (35c)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 13c (22.5 mg, 0.051 mmol) and [Rh(CO)2Cl]2 (2 mg, 0.005 mmol) were reacted in toluene (1.6 mL) for 3 h. Purification of the crude residue by flash chromatography (55% Et2O/hexanes) afforded the title compound 35c (22.8 mg, 95%) as a yellow oil. 1H NMR (300 MHz, CDCl3) δ 7.55 (d, J = 8.1 Hz, 2H), 7.40 – 7.34 (m, 5H), 7.22 (d, J = 8.1 Hz, 2H), 4.60 (d, J = 17.0 Hz, 1H), 4.46 (d, J = 17.0 Hz, 1H), 3.45 (t, J = 6.1 Hz, 2H), 2.56 – 2.51 (m, 3H), 2.40 (s, 3H), 1.89 (s, 3H), 0.91 (d, J = 7.4 Hz, 3H), 0.38 (s, 6H); 13C NMR (150 MHz, CDCl3) δ 208.4, 160.9, 153.9, 143.5, 138.3, 137.1, 136.4, 133.8 (2C), 133.3, 129.5, 129.4 (2C), 128.0 (2C), 127.1 (2C), 48.0, 43.8, 43.0, 29.7, 21.4, 18.4, 8.2, −1.3, −1.6; IR (thin film) 2978, 2925, 2872, 1703, 1605, 1466, 1344, 1258, 1160, 1102; HRMS (ES+) C26H31NO3SSiNa [M+Na+] Calculated: 488.1692; Found: 488.1708; (c 1.0, CH2Cl2).
(Z)-5-(dimethyl(phenyl)silyl)-3-ethylidene-1-tosyl-4-vinyl-2,3,6,7-tetrahydro-1H-azepine (36)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 13c (32.5 mg, 0.074 mmol) and [Rh(CO)2Cl]2 (1.4 mg, 0.004 mmol) were reacted in toluene (2.3 mL) for 7 h. Purification of the crude residue by flash chromatography (55% Et2O/hexanes) afforded the cyclocarbonylation product 35 (14.4 mg, 42%) and triene 36 (15.6 mg, 48%) as yellow oils. 1H NMR (300 MHz, CDCl3) δ 7.63 (d, J = 8.3 Hz, 2H), 7.47 – 7.44 (m, 2H), 7.34 – 7.32 (m, 4H), 7.29 (s, 1H), 6.55 (dd, J = 16.9, 10.5 Hz, 1H), 5.52 – 5.45 (m, 1H), 5.15 (dd, J = 16.9, 1.9 Hz, 1H), 5.00 (dd, J = 10.5, 1.9 Hz, 1H), 3.82 (s, 2H), 3.07 – 3.03 (t, J = 5.9 Hz, 2H), 2.42 (s, 3H), 2.34 (t, J = 5.9 Hz, 2H), 1.78 (d, J = 7.0 Hz, 3H), 0.39 (s, 6H); 13C NMR (150 MHz, CDCl3) δ 153.7, 143.0, 138.9, 137.6, 135.7, 134.9, 133.9 (2C), 133.8, 129.6 (2C), 128.9, 127.9 (2C), 127.4 (2C), 127.3, 117.1, 46.6, 46.0, 31.7, 21.5, 13.3, −0.6 (2C); IR (thin film) 2966, 2921, 2847, 2010, 1515, 1462, 1429, 1336, 1254, 1160, 1103; HRMS (ES+) C25H32NO2SSi [M+H+] Calculated: 438.1923; Found: 438.1917.
(Ra)-N-(3-(Dimethyl(phenyl)silyl)hexa-3,4-dien-1-yl)-4-methyl-N-(prop-2-yn-1-yl)benzene sulfonamide (13e)
Following the general procedure for preparation of allene-yne via Mitsunobu reaction, alcohol (Ra)-22 (309 mg, 1.32 mmol) in THF (8.2 mL) was reacted with triphenylphosphine (415.4 mg, 1.58 mmol), 4-methyl-N-(prop-2-yn-1-yl)benzenesulfonamide3230e (331 mg, 1.58 mmol) and diisopropylazodicarboxylate (274 μL, 1.58 mmol) for 2 h. Purification of the crude residue using a Biotage normal phase automated purification system (25 g SNAP column, gradient of 0 – 30% Et2O/hexanes) afforded compound 13e (500 mg, 90%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.65 (d, J = 8.0 Hz, 2H), 7.51 (d, J = 8.0 Hz, 2H), 7.36 (d, J = 7.0 Hz, 3H), 7.25 – 7.24 (m, 2H), 4.92 – 4.82 (m, 1H), 4.03 (d, J = 2.6 Hz, 2H), 3.22 – 3.18 (m, 2H), 2.42 (s, 3H), 2.18 – 2.15 (m, 2H), 1.96 (t, J = 2.5 Hz, 1H), 1.63 (d, J = 7.0 Hz, 3H), 0.36 (s, 6H); 13C NMR (175 MHz, CD2Cl2) 208.0, 143.6, 138.0, 136.1, 133.8 (2C), 129.4 (2C), 129.1, 127.8 (2C), 127.5 (2C), 91.2, 81.4, 76.9, 73.3, 46.5, 36.6, 27.9, 21.3, 13.5, −3.3, −3.4; IR (thin film) 3289, 2962, 2917, 2860, 1936, 1597, 1495, 1450, 1348; HRMS (ES+) C24H30NO2SSi [M+H+] Calculated: 424.1767; Found: 424.1797; (c 1.65, CH2Cl2).
(S)-5-(Dimethyl(phenyl)silyl)-6-methyl-2-tosyl-1,2,3,4-tetrahydrocyclopenta[c] azepin-7(6H)-one (35e)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 13e (41 mg, 0.097 mmol) and [Rh(CO)2Cl]2 (4.2 mg, 0.009 mmol) were reacted in toluene (3 mL) for 2.5 h. Purification of the crude residue by flash chromatography (65% Et2O/hexanes) afforded the cyclocarbonylation product 35e (28.9 mg, 66%) and triene 49 (6.1 mg, 13%) as yellow oils. 1H NMR (400 MHz, CDCl3) δ 7.60 (d, J = 8.3 Hz, 2H), 7.39 – 7.35 (m, 5H), 7.34 – 7.24 (m, 2H), 6.04 (s, 1H), 4.54 (q, J = 16.9 Hz, 2H), 3.43 – 3.41 (m, 2H), 2.59 – 2.54 (m, 3H), 2.40 (s, 3H), 0.93 (d, J = 7.5 Hz, 3H), 0.39 (s, 6H); 13C NMR (175 MHz, CDCl3) 208.3, 167.4, 154.1, 143.6, 137.6, 136.7, 136.1, 133.8 (2C), 130.6, 129.7, 129.5 (2C), 128.1 (2C), 127.3 (2C), 47.8, 45.3, 45.3, 29.8, 21.5, 18.4, −1.5, −1.8; IR (thin film) 3056, 2974, 2925, 2868, 2014, 1699, 1573, 1462, 1340, 1164; HRMS (ES+) C25H30NO3SSi [M+H+] Calculated: 452.1716; Found: 452.1693; (c 2.5, CH2Cl2).
5-(Dimethyl(phenyl)silyl)-3-methylene-1-tosyl-4-vinyl-2,3,6,7-tetrahydro-1H-azepine (49)
1H NMR (300 MHz, CDCl3) δ 7.63 (d, J = 8.2 Hz, 2H), 7.50 – 7.44 (m, 2H), 7.35 – 7.33 (m, 3H), 7.29 – 7.26 (m, 2H), 6.55 (dd, J = 16.9, 10.5 Hz, 1H), 5.37 (d, J = 1.5 Hz, 1H), 5.26 – 5.16 (m, 1H), 5.03 (dd, J = 10.5, 1.7 Hz, 1H), 4.99 (s, 1H), 3.88 (s, 2H), 3.13 (t, J = 6.1 Hz, 2H), 2.41– 2.36 (m, 5H), 0.40 (s, 6H); 13C NMR (175 MHz, CDCl3) δ 151.9, 143.4, 143.0, 138.6, 136.9, 136.0, 135.3, 133.8 (2C), 129.5 (2C), 129.0, 127.9 (2C), 127.3 (2C), 117.1, 117.1, 51.1, 45.2, 31.8, 21.4, −0.7 (2C); IR (thin film) 3060, 2962, 2921, 2847, 1343, 1164, 1106; HRMS (ES+) C24H30NO2SSi [M+H+] Calculated: 424.1767; Found: 424.1787;
(Ra)-N-(3-(Dimethyl(phenyl)silyl)hexa-3,4-dien-1-yl)-4-methyl-N-(3-(trimethylsilyl) prop-2-yn-1-yl)benzenesulfonamide (13f)
Following the general procedure for preparation of allene-yne via silylation of the terminal alkyne, allene-yne 13e (92 mg, 0.22 mmol) in THF (2 mL) was reacted with lithium hexamethyldisilylamide (0.28 mL, 1 M in THF, 0.28 mmol) and trimethylsilyl chloride (55 μL, 0.434 mmol). Purification of the crude residue by silica gel chromatography using 10% Et2O/hexanes to afford compound 13f (93.3 mg, 87%) as a clear yellow oil. 1H NMR (600 MHz, CDCl3) δ 7.64 (d, J = 8.3 Hz, 2H), 7.52 (dd, J = 7.4, 2.0 Hz, 2H), 7.38 – 7.35 (m, 3H), 7.25 – 7.23 (d, J = 8.3 Hz, 2H), 4.87 (m, 1H), 4.05 (s, 2H), 3.22 – 3.18 (m, 2H), 2.40 (s, 3H), 2.19 – 2.16 (m, 2H), 1.63 (d, J = 7.0 Hz, 3H), 0.36 (d, J = 1.3 Hz, 6H), −0.02 (s, 9H); 13C NMR (150 MHz, CDCl3) δ 208.0, 143.1, 137.9, 136.1, 133.8 (2C), 129.4 (2C), 129.1, 127.8 (2C), 127.6 (2C), 98.2, 91.2, 90.6, 81.3, 46.3, 37.4, 27.8, 21.45, 13.7, −0.5 (3C), −3.0, −3.1; IR (thin film) 3064, 2958, 2913, 2173, 1940, 1601, 1348; HRMS (ES+) C27H38NO2SSi2 [M+H+] Calculated: 496.2162; Found: 496.2152; (c 1.65, CH2Cl2).
(S)-5-(Dimethyl(phenyl)silyl)-6-methyl-2-tosyl-8-(trimethylsilyl)-1,2,3,4-tetrahydro cyclopenta[c]azepin-7(6H)-one (35f)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 13f (25 mg, 0.050 mmol) and [Rh(CO)2Cl]2 (2 mg, 0.005 mmol) were reacted in toluene (1.6 mL) for 3 h. Purification of the crude residue by flash chromatography (55% Et2O/hexanes) afforded the title compound 35f (23.4 mg, 89%) as a yellow oil. 1H NMR (300 MHz, CDCl3) δ 7.58 (d, J = 8.1 Hz, 2H), 7.39 – 7.33 (m, 6H), 7.23 (s, 1H), 4.65 (d, J = 17.2 Hz, 1H), 4.56 (d, J = 17.2 Hz, 1H), 3.48 – 3.31 (m, 2H), 2.61 – 2.48 (m, 3H), 2.40 (s, 3H), 0.89 (d, J = 7.4 Hz, 3H), 0.36 (d, J = 9.0 Hz, 15H); 13C NMR (175 MHz, CDCl3) δ 212.5, 174.1, 156.1, 143.4, 142.1, 137.1, 136.5, 134.3, 133.9 (2C), 129.5 (3C), 128.0 (2C), 127.2 (2C), 47.3, 45.5, 45.1, 29.7, 21.5, 18.8, −0.5 (3C), −1.23, −1.6; IR (thin film) 3060, 2962, 2921, 2855, 2247, 1932, 1691, 1601, 1544, 1462, 1352, 1254, 1164; HRMS (ES+) C28H38NO2SSi [M+H+] Calculated: 524.2111; Found: 524.2101; (c 0.75, CH2Cl2).
(Ra)-N-(3-(dimethyl(phenyl)silyl)hexa-3,4-dien-1-yl)-4-methyl-N-(3-phenylprop-2-yn-1-yl) benzenesulfonamide (13d)
Following the general procedure for preparation of allene-yne via Mitsunobu reaction, alcohol (Ra)-22 (93.6 mg, 0.402 mmol) in THF (2.8 mL) was reacted with triphenylphosphine (127 mg, 0.48 mmol), 4-methyl-N-(3-phenylprop-2-yn-1-yl)benzenesulfonamide3230d (134 mg, 0.48 mmol) and diisopropylazodicarboxylate (95 μL, 0.48 mmol) for 3 h. Purification of the crude residue using a Biotage normal phase automated purification system (25 g SNAP column, gradient of 0 – 30% Et2O/hexanes) afforded compound 13d (185 mg, 92%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.67 (d, J = 8.4 Hz, 2H), 7.52 – 7.49 (m, 2H), 7.35 – 7.32 (m, 4H), 7.23 – 7.19 (m, 3H), 7.04 (dd, J = 8.0, 1.7 Hz, 2H), 4.90 – 4.86 (m, 1H), 4.24 (s, 2H), 3.29 – 3.23 (m, 2H), 2.33 (s, 3H), 2.23 (td, J = 8.1, 2.7 Hz, 2H), 1.63 (d, J = 7.0 Hz, 3H), 0.36 (d, J = 0.8 Hz, 6H); 13C NMR (175 MHz, CDCl3) δ 208.0, 143.2, 137.9, 136.0, 133.7 (2C), 131.4 (2C), 129.4 (2C), 129.1, 128.3, 128.0 (2C), 127.8 (2C), 127.6 (2C), 122.2, 91.2, 85.4, 82.0, 81.3, 46.5, 37.4, 27.9, 21.4, 13.7, −3.1, −3.1; IR (thin film) 3068, 2953, 2921, 2855, 2238, 1940, 1589, 1486; HRMS (ES+) C30H34NO2SSi [M+H+] Calculated: 500.2080; Found: 500.2086; (c 1.15, CH2Cl2).
(S)-5-(Dimethyl(phenyl)silyl)-6-methyl-8-phenyl-2-tosyl-1,2,3,4-tetrahydrocyclo penta[c]azepin-7(6H)-one (35d)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 13d (41.5 mg, 0.083 mmol) and [Rh(CO)2Cl]2 (3.2 mg, 0.003 mmol) were reacted in toluene (2.5 mL) for 3 h. Purification of the crude residue by flash chromatography (55% Et2O/hexanes) afforded the title compound 35d (40.1 mg, 92%) as a yellow oil. 1H NMR (300 MHz, CDCl3) δ 7.49 – 7.32 (m, 12H), 7.21 (d, J = 8.0 Hz, 2H), 4.63 (s, 2H), 3.51 (t, J = 6.1 Hz, 2H), 2.71 (q, J = 7.3 Hz, 1H), 2.65 – 2.58 (t, J = 5.4 Hz, 2H), 2.40 (s, 3H), 0.97 (t, J = 8.4 Hz, 3H), 0.45 (s, 6H); 13C NMR (175 MHz, CDCl3) δ 206.4, 161.4, 153.7, 143.4, 140.7, 137.0, 136.6, 135.7, 133.9 (2C), 129.9, 129.6 (3C), 129.4 (2C), 128.9, 128.6 (2C), 128.1 (2C), 127.1 (2C), 48.2, 44.6, 43.7, 29.8, 21.5, 18.7, −1.2, −1.5; IR (thin film) 2970, 2917, 2859, 2018, 1736, 1707, 1454, 1340, 1164; HRMS (ES+) C31H34NO3SSi [M+H+] Calculated: 528.2029; Found: 528.2034; (c 0.75, CH2Cl2).
4-Methyl-N-(3-(thiophen-3-yl)prop-2-yn-1-yl)benzenesulfonamide (30a)
A mixture of propargyltosylamide (444 mg, 1.97 mmol), 3-bromothiophene (963 mg, 5.91 mmol), palladium dichloride bistriphenylphosphine (27.7 mg, 0.039 mmol), copper iodide (15 mg, 0.079 mmol) in piperidine (3.9 mL) were heated at 80 °C for 17 h. The solution was concentrated under reduced pressure. Purification of the crude residue using a Biotage normal phase automated purification system (25 g SNAP column, gradient of 5 – 25% EtOAc/hexanes) afforded compound 30a (155.6 mg, 27%) as a white foam. 1H NMR (600 MHz, CDCl3) δ 7.81 (d, J = 8.2 Hz, 2H), 7.28 (d, J = 8.2 Hz, 2H), 7.24 – 7.15 (m, 2H), 6.84 (d, J = 4.4 Hz, 1H), 4.69 – 4.65 (m, 1H), 4.05 (d, J = 6.1 Hz, 2H), 2.38 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 143.7, 136.8, 129.6 (2C), 129.5, 129.1, 127.5 (2C), 125.2, 121.1, 82.8, 79.9, 33.8, 21.5; IR (thin film) 3265, 1593, 1430, 1324, 1156, 1091, 1042, 813, 788; HRMS (ES+) C14H12NO2S2 [M+H+] Calculated: 290.0276; Found: 290.0299.
(Ra)-N-(3-(Dimethyl(phenyl)silyl)hexa-3,4-dien-1-yl)-4-methyl-N-(3-(thiophen-3-yl)prop-2-yn-1-yl)benzenesulfonamide (13a)
Following the general procedure for preparation of allene-yne via Mitsunobu reaction, alcohol (Ra)-22 (39.8 mg, 0.171 mmol) in THF (1.2 mL) was reacted with triphenylphosphine (54 mg, 0.21 mmol), 4-methyl-N-(3-(thiophen-3-yl)prop-2-yn-1-yl)benzenesulfonamide 30a (60 mg, 0.21 mmol) and diisopropylazodicarboxylate (41 μL, 0.21 mmol) for 10 h. Purification of the crude residue using a Biotage normal phase automated purification system (10 g SNAP column, gradient of 2 – 4% EtOAc/hexanes) afforded compound 13a (77.6 mg, 90%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.67 (d, J = 8.3 Hz, 2H), 7.51 (dd, J = 7.8, 1.7 Hz, 2H), 7.37 – 7.34 (m, 3H), 7.22 – 7.19 (m, 3H), 7.09 (dd, J = 3.0, 1.1 Hz, 1H), 6.76 (dd, J = 5.0, 1.2 Hz, 1H), 4.90 – 4.86 (m, 1H), 4.22 (d, J = 1.6 Hz, 2H), 3.24 (dd, J = 8.9, 6.9 Hz, 2H), 2.35 (s, 3H), 2.23 – 2.20 (m, 2H), 1.63 (d, J = 7.0 Hz, 3H), 0.36 (d, J = 2.0 Hz, 6H); 13C NMR (150 MHz, CDCl3) δ 208.0, 143.2, 137.9, 136.1, 133.8 (2C), 129.6, 129.4 (2C), 129.1, 128.7, 127.8 (2C), 127.7 (2C), 125.1, 121.3, 91.2, 81.7, 81.4, 80.5, 46.6, 37.4, 28.0, 21.5, 13.7, −3.0 (2C); IR (thin film) 3109, 2958, 2921, 2864, 1936, 1732, 1593, 1458, 1434, 1352, 1164, 1111; HRMS (ES+) C28H31NO2NaS2Si [M+Na+] Calculated: 528.1463 ; Found: 528.1414; (c 1.75, CH2Cl2).
(S)-5-(Dimethyl(phenyl)silyl)-6-methyl-8-(thiophen-3-yl)-2-tosyl-1,2,3,4-tetrahydro cyclopenta[c]azepin-7(6H)-one (35a)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 13a (17.8 mg, 0.036 mmol) and [Rh(CO)2Cl]2 (1.4 mg, 0.003 mmol) were reacted in toluene (1.2 mL) for 2.5 h. Purification of the crude residue using a Biotage normal phase automated purification system (4 g SNAP column, gradient of 5 – 25% EtOAc/hexanes) afforded compound 35a (13.5 mg, 72%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.68 – 7.66 (m, 1H), 7.47 – 7.42 (m, 5H), 7.39 – 7.33 (m, 4H), 7.22 (d, J = 8.0 Hz, 2H), 4.73 (s, 2H), 3.52 (t, J = 6.1 Hz, 2H), 2.68 – 2.58 (m, 3H), 2.40 (s, 3H), 0.98 (d, J = 7.4 Hz, 3H), 0.43 (d, J = 1.3 Hz, 6H); 13C NMR (150 MHz, CDCl3) δ 206.4, 159.9, 153.8, 143.5, 137.1, 136.5, 135.6, 135.5, 133.9 (2C), 130.4, 129.6, 129.4 (2C), 128.1 (2C), 127.9, 127.1 (2C), 126.8, 125.9, 48.3, 44.5, 43.8, 29.9, 21.5, 18.8, −1.2, −1.5; IR (thin film) 2958, 2925, 1695, 1605, 1467, 1430, 1344, 1246, 1160, 1107, 964; HRMS (ES+) C29H31NO3NaS2Si [M+Na+] Calculated: 556.1412; Found: 556.1465; (c 1.15, CH2Cl2).
4-Methyl-N-(3-(thiophen-2-yl)prop-2-yn-1-yl)benzenesulfonamide (30b)
A mixture of propargyltosylamide (415.9 mg, 1.84 mmol), 2-iodothiophene (215 μL, 2.03 mmol), palladium dichloride bistriphenylphosphine (25.9 mg, 0.037 mmol), copper iodide (14.1 mg, 0.074 mmol) and triethylamine (1.29 mL, 0.092 mmol) in acetonitrile (3 mL) was stirred at rt for 19 h. The residue obtained after removal of acetonitrile was taken up in CH2Cl2 (30 mL) and washed with water (10 mL). The organic phase was separated, dried (Na2SO4), filtered and concentrated under reduced pressure. Purification of the crude residue by silica gel column chromatography (Hex/Et2O 50%), afforded compound 30b (316 mg, 59%) as a white foam. 1H NMR (300 MHz, CDCl3) δ 7.80 (d, J = 8.2 Hz, 2H), 7.29 (d, J = 8.0 Hz, 2H), 7.21 (dd, J = 5.1, 1.2 Hz, 1H), 6.97 (d, J = 3.6 Hz, 1H), 6.92 – 6.89 (m, 1H), 4.63 – 4.62 (m, 1H), 4.09 (d, J = 6.2 Hz, 2H), 2.38 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 143.9, 136.6, 132.4, 129.7 (2C), 127.4 (2C), 126.8, 121.9, 87.1, 78.1, 33.9, 21.6; IR (thin film) 3265, 2361, 1589, 1491, 1422, 1344, 1315, 1160, 1091, 1038, 845; HRMS (ES+) C14H12NO2S2 [M−H+] Calculated: 290.0309; Found: 290.0316.
(Ra)-N-(3-(dimethyl(phenyl)silyl)hexa-3,4-dien-1-yl)-4-methyl-N-(3-(thiophen-2-yl) prop-2-yn-1-yl)benzenesulfonamide (13b)
Following the general procedure for preparation of allene-yne via Mitsunobu reaction, alcohol (Ra)-22 (35.2 mg, 0.151 mmol) in THF (1.0 mL) was reacted with triphenylphosphine (48 mg, 0.18 mmol), 4-methyl-N-(3-(thiophen-2-yl)prop-2-yn-1-yl)benzenesulfonamide 30b (53 mg, 0.18 mmol) and diisopropylazodicarboxylate (36 μL, 0.18 mmol) for 11 h. Purification of the crude residue using a Biotage normal phase automated purification system (10 g SNAP column, gradient of 2 – 4% EtOAc/hexanes) afforded compound 13b (69.3 mg, 90%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.67 (d, J = 8.0 Hz, 2H), 7.52 – 7.50 (m, 2H), 7.36 – 7.33 (m, 3H), 7.24 – 7.20 (m, 3H), 6.90 (d, J = 3.3 Hz, 2H), 4.90 – 4.86 (m, 1H), 4.25 (s, 2H), 3.25 – 3.20 (m, 2H), 2.36 (s, 3H), 2.24 – 2.18 (m, 2H), 1.63 (d, J = 7.0 Hz, 3H), 0.36 (s, 6H); 13C NMR (150 MHz, CDCl3) δ 208.0, 143.3, 137.9, 135.8, 133.8 (2C), 132.1, 129.5 (2C), 129.1, 127.8 (2C), 127.6 (2C), 127.2, 126.7, 122.2, 91.2, 86.1, 81.4, 78.6, 46.5, 37.5, 27.9, 21.5, 13.7, −3.1 (2C); IR (thin film) 2954, 2925, 2852, 2353, 2329, 1936, 1593, 1430, 1352, 1250, 1185, 1168, 1107; HRMS (ES+) C28H31NO2NaS2Si [M+H+] Calculated: 528.1463; Found: 528.1494; (c 1.8, CH2Cl2).
(S)-5-(Dimethyl(phenyl)silyl)-6-methyl-8-(thiophen-2-yl)-2-tosyl-1,2,3,4-tetrahydro cyclopenta[c]azepin-7(6H)-one (35b)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 13b (28.3 mg, 0.056 mmol) and [Rh(CO)2Cl]2 (2.2 mg, 0.006 mmol) were reacted in toluene (1.8 mL) for 3 h. Purification of the crude residue using a Biotage normal phase automated purification system (4 g SNAP column, gradient of 5 – 25% EtOAc/hexanes) afforded compound 35b (24.9 mg, 84%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.56 (d, J = 5.2 Hz, 2H), 7.45 – 7.35 (m, 8H), 7.21 – 7.19 (m, 3H), 4.89 – 4.82 (m, 2H), 3.55 – 3.53 (m, 2H), 2.63 – 2.60 (m, 2H), 2.39 (s, 3H), 0.95 (d, J = 7.4 Hz, 3H), 0.43 (d, J = 5.6 Hz, 6H); 13C NMR (150 MHz, CDCl3) δ 205.3, 158.9, 153.5, 143.5, 136.9, 136.5, 135.8, 133.9 (2C), 133.7, 130.9, 129.6, 129.4 (2C), 129.2, 129.0, 128.1 (2C), 127.4, 127.0 (2C), 48.6, 44.3, 43.9, 30.0, 21.5, 18.8, −1.2, −1.5; IR (thin film) 3060, 2954, 2929, 2872, 1704, 1589, 1434, 1344, 1246, 1152, 1103, 1017; HRMS (ES+) C29H30NO3S2Si [M−H+] Calculated: 532.1436; Found: 532.1453; (c 1.80, CH2Cl2).
tert-Butyl (3-cyclopropylprop-2-yn-1-yl)(tosyl)carbamate (50)
Following the general procedure for preparation of allene-yne via Mitsunobu reaction, 3-cyclopropylprop-2-yn-1-ol (283 mg, 2.94 mmol) in THF (21 mL) was reacted with triphenylphosphine (927 mg, 3.53 mmol), tert-butyl tosylcarbamate (959 mg, 3.53 mmol) and diisopropylazodicarboxylate (0.7 mL, 3.53 mmol) for 11 h. Purification of the crude residue by silica gel chromatography using 20% Et2O/hexanes afforded compound 50 (535 mg, 52%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 1H NMR 7.93 (d, J = 8.3 Hz, 2H), 7.35 – 7.28 (m, 2H), 4.57 (d, J = 2.0 Hz, 2H), 2.45 (s, 3H), 1.34 (s, 9H), 1.27 – 1.23 (m, 1H), 0.78 – 0.74 (m, 2H), 0.67 – 0.64 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 150.3, 144.2, 136.9, 129.0 (2C), 128.2 (2C), 87.5, 84.5, 70.5, 36.4, 27.8 (3C), 21.6, 7.9 (2C), −0.6; IR (thin film) 2974, 2925, 2251, 2235, 1724, 1597, 1356, 1311, 1279, 1091, 1070; HRMS (ES+) C14H16NO4S [M-C5H10O2+H ] Calculated: 294.0800; Found: 294.0810.
N-(3-Cyclopropylprop-2-yn-1-yl)-4-methylbenzenesulfonamide (30g)
To a solution of tert-butyl (3-cyclopropylprop-2-yn-1-yl)(tosyl)carbamate 50 (102.7 mg, 0.293 mmol) in methanol (1.7 mL) and THF (0.7 mL) was added lithium hydroxide (88 mg, 1.17 mmol). After stirring for 12 h at rt, the mixture was diluted with CH2Cl2 (40 mL) and HCl 1 M (10 mL). The organic layer was separated, washed with brine (10 mL) and dried (Na2SO4). The solvent was removed under reduced pressure. Purification of the crude residue by silica gel chromatography using 40% Et2O/hexanes afforded compound 30g (52.3 mg, 71%) as a white foam. 1H NMR (300 MHz, CDCl3) δ 7.76 (d, J = 8.3 Hz, 2H), 7.29 (d, J = 8.0 Hz, 2H), 4.55 (br. s, 1H), 3.76 – 3.76 (m, 2H), 2.43 (s, 3H), 1.03 (m, 1H), 0.65 – 0.62 (m, 2H), 0.42 – 0.40 (m, 2H); 13C NMR (150 MHz, CDCl3) δ 143.5, 136.8, 129.5 (2C), 127.4 (2C), 88.4, 69.3, 33.4, 21.5, 7.8 (2C), −0.9; IR (thin film) 3265, 1438, 1315, 1160, 1095, 1058, 1030; HRMS (ES+) C13H16NO2S [M+H+] Calculated: 250.0902; Found: 250.0888.
(Ra)-N-(3-Cyclopropylprop-2-yn-1-yl)-N-(3-(dimethyl(phenyl)silyl)hexa-3,4-dien-1-yl)-4-methylbenzenesulfonamide (13g)
Following the general procedure for preparation of allene-yne via Mitsunobu reaction, alcohol (Ra)-22 (45 mg, 0.19 mmol) in THF (1.4 mL) was reacted with triphenylphosphine (61.1 mg, 0.23 mmol), N-(3-cyclopropylprop-2-yn-1-yl)-4-methylbenzenesulfonamide 30g (58.3 mg, 0.23 mmol) and diisopropylazodicarboxylate (46 μL, 0.23 mmol) for 11 h. Purification of the crude residue using a Biotage normal phase automated purification system (12 g SNAP column, gradient of 1 – 7% EtOAc/hexanes) afforded compound 13g (63.7 mg, 73%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 7.66 (d, J = 8.3 Hz, 2H), 7.56 – 7.54 (m, 2H), 7.40 – 7.38 (m, 3H), 7.29 – 7.27 (dd, J = 8.8, 1.2 Hz, 2H), 4.92 – 4.89 (m, 1H), 4.00 (d, J = 2.0 Hz, 2H), 3.22 – 3.18 (m, 2H), 2.45 (s, 3H), 2.21 – 2.16 (m, 2H), 1.67 (d, J = 7.0 Hz, 3H), 1.00 – 0.96 (m, 1H), 0.65 – 0.61 (m, 2H), 0.40 (d, J = 1.0 Hz, 6H), 0.33 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 208.0, 142.9, 137.9, 136.3, 133.8 (2C), 129.2 (2C), 129.1, 127.8 (2C), 127.6 (2C), 91.3, 88.9, 81.2, 67.9, 46.3, 37.0, 27.9, 21.5, 13.7, 7.8 (2C), −0.9, −3.0, −3.1; IR (thin film) 2954, 2921, 2251, 1932, 1601, 1454, 1430, 1348, 1254, 1164, 1107, 1030; HRMS (ES+) C27H34NO2SSi [M+H+] Calculated: 464.2080; Found: 464.2053; (c 0.70, CH2Cl2).
(S)-8-Cyclopropyl-5-(dimethyl(phenyl)silyl)-6-methyl-2-tosyl-1,2,3,4-tetrahydro cyclopenta[c]azepin-7(6H)-one (35g)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 13g (24.5 mg, 0.053 mmol) and [Rh(CO)2Cl]2 (2.1 mg, 0.0053 mmol) were reacted in toluene (1.8 mL) for 135 min. Purification of the crude residue by silica gel chromatography using 40% Et2O/hexanes afforded compound 35g (22.1 mg, 83%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.62 (d, J = 8.0 Hz, 2H), 7.36 – 7.32 (m, 5H), 7.25 – 7.21 (m, 2H), 4.65 – 4.64 (m, 2H), 3.43 – 3.43 (m, 2H), 2.55 – 2.51 (m, J = 4.8 Hz, 2H), 2.41 – 2.38 (m, 4H), 1.62 – 1.57 (m, 1H), 1.30 – 1.25 (m, 2H), 0.93 – 0.90 (m, 2H), 0.81 (d, J = 7.4 Hz, 3H), 0.36 – 0.35 (t, 3H); 13C NMR (150 MHz, CDCl3) δ 207.5, 160.6, 153.2, 143.4, 141.4, 137.2, 136.3, 133.8 (2C), 132.2, 129.5, 129.3 (2C), 127.9 (2C), 127.3 (2C), 48.1, 44.3, 42.5, 29.5, 21.5, 18.4, 8.1, 6.1, 6.0, −1.2, −1.5; IR (thin film) 2962, 2925, 2868, 2018, 1695, 1589, 1467, 1348, 1250, 1160, 1103; HRMS (ES+) C28H34NO3SSi [M+H+] Calculated: 492.2029; Found: 492.2042; (c 1.45, CH2Cl2).
(Ra)-N-(Hexa-3,4-dien-1-yl)-4-methyl-N-(3-phenylprop-2-yn-1-yl)benzene sulfonamide (14d)
Following the general procedure for preparation of allene-yne via Mitsunobu reaction, alcohol (Ra)-29 (45 mg, 0.21 mmol) in THF (1.5 mL) was reacted with triphenylphosphine (65.5 mg, 0.25 mmol), 4-methyl-N-(3-phenylprop-2-yn-1-yl)benzenesulfonamide3030d (71.2 mg, 0.25 mmol) and diisopropylazodicarboxylate (49 μL, 0.25 mmol) for 11 h. Purification of the crude residue by silica gel chromatography using 1% Et2O/hexanes afforded compound 14d (80.2 mg, 92%) as a colorless oil. 1H NMR (700 MHz, CDCl3) δ 7.70 (d, J = 8.3 Hz, 2H), 7.27 – 7.25 (m, 5H), 7.20 – 7.16 (m, 5H), 7.00 (d, J = 7.0 Hz, 2H), 6.15 – 6.13 (m, 1H), 4.34 (d, J = 18.5 Hz, 2H), 4.27 (d, J = 18.5 Hz, 2H), 3.47 – 3.43 (m, 1H), 3.34 – 3.30 (m, 1H), 2.44 – 2.41 (m, 2H), 2.29 (s, 3H), 2.15 – 2.09 (m, 2H), 1.45 – 1.44 (m, 1H), 1.35 – 1.32 (m, 2H), 0.86 (t, J = 7.3 Hz, 3H); 13C NMR (175 MHz, CDCl3) δ 202.3, 143.4, 135.6, 135.4, 131.4 (2C), 129.4 (2C), 128.6 (2C), 128.3, 128.1 (2C), 127.7 (2C), 126.6, 126.5 (2C), 122.1, 105.4, 96.0, 85.7, 81.6, 44.8, 37.31, 32.1, 31.2, 29.7, 22.4, 21.4, 13.9; IR (thin film) 2958, 2925, 2855, 1597, 1495, 1458, 1352, 1160, 1095, 918; HRMS (ES+) C31H34NO2S [M+H+] Calculated: 484.2310; Found: 484.2283; (c 1.15, CH2Cl2).
(S)-6-Methyl-8-phenyl-2-tosyl-1,2,3,4-tetrahydrocyclopenta[c]azepin-7(6H)-one (42d)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 14d (22.7 mg, 0.054 mmol) and [Rh(CO)2Cl]2 (2.1 mg, 0.0054 mmol) were reacted in toluene (1.7 mL) for 30 min. Purification of the crude residue by silica gel chromatography using 60% Et2O/hexanes afforded compound 42d (21.6 mg, 76%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.50 – 7.32 (m, 7H), 7.24 – 7.20 (m, 5H), 6.96 – 6.93 (m, 2H), 4.83 (d, J = 16.9 Hz, 1H), 4.61 (d, J = 16.8 Hz, 1H), 3.91 (s, 1H), 3.72 – 3.65 (m, 2H), 2.76 – 2.71 (m, 1H), 2.59 – 2.50 (m, 1H), 2.41 (s, 3H), 1.93 – 1.87 (m, 2H), 1.02 – 0.98 (m, 2H), 0.88 – 0.86 (m, 2H), 0.69 (t, J = 7.2 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 201.8, 162.8, 143.5, 141.4, 139.4, 137.8, 136.8, 136.0, 130.3, 129.6 (2C), 129.5 (2C), 128.7 (2C), 128.6, 128.5 (2C), 127.6 (2C), 127.2 (2C), 126.9, 55.4, 47.9, 44.3, 37.1, 31.1, 29.2, 22.7, 21.6, 13.7; IR (thin film) 2954, 2929, 2864, 1699, 1499, 1339, 1164, 1095; HRMS (ES+) C32H34NO3S [M+H+] Calculated: 512.2259; Found: 512.2232; (c 0.90, CH2Cl2).
(Ra)-N-(Hexa-3,4-dien-1-yl)-4-methyl-N-(3-phenylprop-2-yn-1-yl)benzene sulfonamide (14c)
Following the general procedure for preparation of allene-yne via Mitsunobu reaction, alcohol (Ra)-29 (50.7 mg, 0.23 mmol) in THF (1.7 mL) was reacted with triphenylphosphine (74 mg, 0.28 mmol), N-(but-2-yn-1-yl)-4-methylbenzenesulfonamide30 (63 mg, 0.28 mmol) and diisopropylazodicarboxylate (56 μL, 0.28 mmol) for 12 h. Purification of the crude residue by silica gel chromatography using 10% Et2O/hexanes afforded compound 14c (91.9 mg, 93%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.69 – 7.68 (d, J = 8.3 Hz, 2H), 7.30 – 7.26 (m, 4H), 7.23 – 7.22 (m, 3H), 6.14 (t, J = 2.9 Hz, 1H), 4.08 – 3.99 (m, 2H), 3.36 – 3.32 (m, 1H), 3.28 – 3.23 (m, 1H), 2.39 (s, 3H), 2.37 – 2.36 (m, 2H), 2.13 – 2.07 (m, 2H), 1.51 (t, J = 2.4 Hz, 3H), 1.46 – 1.43 (m, 2H), 1.37 – 1.33 (m, 2H), 0.88 (t, J = 7.3 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 202.2, 143.1, 135.9, 135.5, 129.2 (2C), 128.5 (2C), 127.7 (2C), 126.6, 126.5 (2C), 105.4, 95.9, 81.5, 71.7, 44.7, 37.0, 32.1, 31.2, 29.7, 22.4, 21.5, 13.9, 3.2; IR (thin film) 2954, 2925, 2864, 1593, 1499, 1458, 1344, 1164, 1091, 915; HRMS (ES+) C26H32NO2S [M+H+] Calculated: 422.2154; Found: 422.2120; (c 1.15, CH2Cl2).
(S)-5-Butyl-8-methyl-6-phenyl-2-tosyl-1,2,3,4-tetrahydrocyclopenta[c]azepin-7(6H)-one (42c)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 14c (33.5 mg, 0.079 mmol) and [Rh(CO)2Cl]2 (3.1 mg, 0.0079 mmol) were reacted in toluene (2.5 mL) for 90 min. Purification of the crude residue using a Biotage normal phase automated purification system (12 g SNAP column, gradient of 5 – 30% EtOAc/hexanes) afforded compound 42c (29.1 mg, 82%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.68 (d, J = 8.2 Hz, 2H), 7.29 (d, J = 8.1 Hz, 2H), 7.19 – 7.18 (m, 3H), 6.89 – 6.88 (m, 2H), 4.67 (d, J = 16.9 Hz, 1H), 4.50 (d, J = 16.9 Hz, 1H), 3.77 (s, 1H), 3.61 – 3.60 (m, 2H), 2.61 – 2.53 (m, 2H), 2.42 (s, 3H), 1.86 (s, 3H), 1.83 – 1.78 (m, 2H), 1.12 – 1.10 (m, 1H), 1.02 – 0.95 (m, 2H), 0.84 – 0.83 (m, 1H), 0.67 (t, J = 7.3 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 203.6, 162.3, 143.7, 139.2, 137.9, 136.4, 136.3, 135.9, 129.7 (2C), 128.6 (2C), 127.5 (2C), 127.1 (2C), 126.8, 54.9, 47.8, 44.6, 36.9, 31.8, 29.3, 22.6, 21.6, 13.7, 8.6; IR (thin film) 2954, 2925, 2860, 2361, 2333, 1699, 1597, 1487, 1454, 1344, 1266, 1152, 1095; HRMS (ES+) C27H32NO3S [M+H+] Calculated: 450.2103; Found: 450.2058; (c 0.90, CH2Cl2).
(Ra)-4-Methyl-N-(3-(2-phenylvinylidene)heptyl)-N-(prop-2-yn-1-yl)benzene sulfonamide (14e)
Following the general procedure for preparation of allene-yne via Mitsunobu reaction, alcohol (Ra)-29 (114.4 mg, 0.53 mmol) in THF (3.8 mL) was reacted with triphenylphosphine (166 mg, 0.63 mmol), 4-methyl-N-(prop-2-yn-1-yl)benzenesulfonamide3030e (133 mg, 0.63 mmol) and diisopropylazodicarboxylate (125 μL, 0.63 mmol) for 12 h. Purification of the crude residue using a Biotage normal phase automated purification system (25 g SNAP column, gradient of 0 – 7% EtOAc/hexanes) afforded compound 14e (182.1 mg, 85%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.66 (d, J = 8.1 Hz, 2H), 7.29 – 7.24 (m, 4H), 7.22 – 7.19 (m, 3H), 6.13 – 6.11 (m, 1H), 4.10 – 4.07 (m, 2H), 3.37 – 3.26 (m, 2H), 2.42 – 2.33 (m, 5H), 2.09 – 2.07 (m, 2H), 1.98 (t, J = 2.5 Hz, 1H), 1.43 – 1.31 (m, 4H), 0.86 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CD2Cl2) δ 202.2, 143.7, 135.8, 135.5, 129.5 (2C), 128.5 (2C), 127.6 (2C), 126.7, 126.5 (2C), 105.4, 95.9, 76.7, 73.6, 44.8, 36.6, 32.1, 31.1, 29.7, 22.5, 21.3, 13.7; IR (thin film) 3289, 2958, 2929, 2872, 1949, 1712, 1593, 1491, 1462, 1352, 1160, 1091 1001; HRMS (ES+) C25H29NO2S [M+] Calculated: 407.1919; Found: 407.1888; (c 0.80, CH2Cl2).
(Ra)-4-methyl-N-(3-(2-phenylvinylidene)heptyl)-N-(3-(trimethylsilyl)prop-2-yn-1-yl) benzenesulfonamide (14f)
Following the general procedure for preparation of allene-yne via silylation of the terminal alkyne, allene-yne 14e (81.7 mg, 0.20 mmol) in THF (1.6 mL) was reacted with lithium hexamethyldisilylamide (301 μL, 1M in THF, 0.30 mmol) and trimethylsilylchloride (51 μL, 0.40 mmol). Purification of the crude product using a Biotage normal phase automated purification system (12 g SNAP column, gradient of 0 – 8% EtOAc/hexanes) afforded compound 14f (76.8 mg, 80%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.70 (d, J = 8.1 Hz, 2H), 7.33 – 7.26 (m, 4H), 7.23 – 7.21 (m, 3H), 6.17 (t, J = 3.0 Hz, 1H), 4.22 – 4.06 (m, 2H), 3.44 – 3.37 (m, 1H), 3.33 – 3.26 (m, 1H), 2.44 – 2.39 (m, 5H), 2.16 – 2.14 (m, 2H), 1.51 – 1.37 (m, 4H), 0.91 (t, J = 7.1 Hz, 3H), −0.02 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 202.2, 143.2, 135.7, 135.4, 129.4 (2C), 128.5 (2C), 127.7 (2C), 126.6, 126.5 (2C), 105.3, 97.8, 95.9, 90.9, 44.6, 37.4, 32.1, 31.1, 29.7, 22.4, 21.5, 13.9, −0.5 (3C); IR (thin film) 3031, 2958, 2929, 2868, 2118, 1953, 1598, 1503, 1458, 1356, 1246, 1164, 1103, 1021, 919; HRMS (ES+) C28H37NO2SSi [M+] Calculated: 479.2314; Found: 479.2278; (c 1.70, CH2Cl2).
(S)-5-Butyl-6-phenyl-2-tosyl-8-(trimethylsilyl)-1,2,3,4-tetrahydrocyclopenta [c]azepin-7(6H)-one (42f)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 14f (32.8 mg, 0.068 mmol) and [Rh(CO)2Cl]2 (2.7 mg, 0.068 mmol) were reacted in toluene (2.2 mL) for 30 min. Purification of the crude product using a Biotage normal phase automated purification system (4 g SNAP column, gradient of 5 – 25% EtOAc/hexanes) afforded compound 42f (26.9 mg, 78%) as a white foam. 1H NMR (300 MHz, CDCl3) δ 7.68 (d, J = 8.1 Hz, 2H), 7.30 (d, J = 8.0 Hz, 2H), 7.20 – 7.18 (m, 3H), 6.92 – 6.89 (m, 2H), 4.80 (d, J = 16.9 Hz, 1H), 4.53 (d, J = 16.9 Hz, 1H), 3.76 (s, 1H), 3.59 – 3.57 (m, 2H), 2.59 – 2.50 (m, 2H), 2.43 (s, 3H), 1.84 – 1.80 (m, 2H), 1.02 – 0.90 (m, 4H), 0.66 (t, J = 7.1 Hz, 3H), 0.31 (s, 9H); 13C NMR (175 MHz, CDCl3) δ 207.8, 175.4, 143.5, 140.5 (2C), 139.4, 138.3, 136.0, 129.7 (2C), 128.7 (2C), 127.4 (2C), 127.2 (2C), 126.6, 56.6, 47.3, 46.1, 37.4, 31.5, 29.2, 22.6, 21.6, 13.7, −0.4 (3C); IR (thin film) 3024, 2954, 2921, 2864, 1683, 1593, 1540, 1491, 1454, 1348, 1242, 1164, 1099; HRMS (ES+) C29H37NO3SSi [M+] Calculated: 507.2263; Found: 507.2297; (c 1.15, CH2Cl2).
(Ra)-3-(2-Phenylvinylidene)heptyl methanesulfonate (33)
To a stirred solution of alcohol (Ra)-29 (197.2 mg, 0.911 mmol) in THF (19 mL), cooled to 0 °C (ice bath), was added triethylamine (152 μL, 1.09 mmol) followed by methanesulfonyl chloride (85 μL, 1.09 mmol). The resulting solution was stirred at rt for 18 h. The mixture was diluted with Et2O (60 mL) and washed with water (2 × 10 mL). The organic phase was dried (Na2SO4), filtered and concentrated under reduced pressure. Purification of the crude residue by silica gel chromatography using 40% Et2O/hexanes afforded compound 33 (247 mg, 92%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.37 – 7.32 (m, 4H), 7.26 – 7.25 (m, 1H), 6.29 – 6.28 (m, 1H), 4.43 – 4.36 (m, 2H), 2.90 (s, 3H), 2.61 – 2.59 (m, 2H), 2.19 – 2.18 (m, 2H), 1.55 – 1.53 (m, 2H), 1.45 – 1.41 (m, 2H), 0.96 (t, J = 7.3 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 201.7, 134.7, 128.4 (2C), 126.6, 126.3 (2C), 103.9, 96.6, 67.6, 36.7, 32.3, 31.8, 29.3, 22.1, 13.6; IR (thin film) 3027, 2958, 2925, 2864, 1953, 1597, 1495, 1462, 1364, 1181, 960, 899; HRMS (ES+) C16H22O3S [M+] Calculated: 294.1290; Found: 294.1284; (c 0.95, CH2Cl2).
(Ra)-Diethyl 2-(3-phenylprop-2-yn-1-yl)-2-(3-(2-phenylvinylidene)heptyl) malonate (16d)
Following the General Procedure for Preparation of Allene-yne via alkylation of the malonate moiety, diethyl malonate 34d (87.2 mg, 0.32 mmol) was reacted with sodium hydride (10.6 mg, 60 wt% in oil, 0.26 mmol), mesylate 33 (62.5 mg, 0.18 mmol) and potassium iodide (44 mg, 0.27 mmol) in DMF-THF (1 : 1, 3.6 mL). Purification of the crude residue by silica gel chromatography using 5% Et2O/hexanes afforded compound 16d (58.7 mg, 70%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.31 – 7.27 (m, 5H), 7.25 – 7.14 (m, 5H), 6.17 (t, J = 2.9 Hz, 1H), 4.23 (q, J = 7.2 Hz, 4H), 3.08 (s, 2H), 2.37 – 2.31 (m, 2H), 2.16 – 2.06 (m, 4H), 1.50 – 1.38 (m, 2H), 1.36 – 1.31 (m, 2H), 1.26 (t, J = 7.1 Hz, 6H), 0.87 (t, J = 7.2 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 202.0, 170.2 (2C), 135.8, 131.6 (2C), 128.4 (2C), 128.1 (2C), 127.8, 126.4 (2C), 126.3, 123.2, 107.8, 96.0, 84.2, 83.5, 61.6 (2C), 56.9, 32.4, 30.5, 29.8, 27.5, 23.9, 22.5, 14.1 (2C), 13.9; IR (thin film) 2966, 2925, 2864, 1736, 1499, 1462, 1438, 1266, 1189, 1099, 1074, 1025; HRMS (ES+) C31H31O4 [M+H+] Calculated: 473.2692; Found: 473.2734; (c 1.85, CH2Cl2).
(S)-Diethyl 8-butyl-2-oxo-1,3-diphenyl-1,2,6,7-tetrahydroazulene-5,5(4H)-dicarboxylate (44d)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 16d (36.8 mg, 0.078 mmol) and [Rh(CO)2Cl]2 (3 mg, 0.0078 mmol) were reacted in toluene (2.6 mL) for 2 h. Purification of the crude residue by flash chromatography (40% Et2O/hexanes) afforded the title compound 44d (35.6 mg, 92%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.37 – 7.34 (m, 2H), 7.30 – 7.28 (m, 3H), 7.21 – 7.19 (m, 5H), 4.15 – 4.10 (m, 2H), 4.09 (s, 1H), 4.02 – 3.99 (m, 1H), 3.96 – 3.93 (m, 1H), 3.59 (d, J = 14.9 Hz, 1H), 3.50 (d, J = 14.9 Hz, 1H), 2.58 – 2.42 (m, 4H), 1.97 – 1.93 (m, 2H), 1.28 – 1.25 (m, 2H), 1.10 – 1.08 (td, J = 7.1, 2.1 Hz, 6H), 1.01 – 0.96 (m, 2H), 0.71 (t, J = 7.3 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 202.2, 171.3, 171.2, 164.4, 146.3, 140.9, 138.3, 137.1, 131.4, 129.4 (2C), 128.7 (2C), 128.1 (2C), 127.8, 127.6 (2C), 126.8, 61.8, 61.7, 56.1, 55.7, 37.5, 33.5, 32.7, 29.1, 28.6, 22.7, 13.8 (3C); IR (thin film) 2962, 2925, 2856, 1728, 1699, 1605, 1577, 1503, 1454, 1373, 1274, 1234, 1181; HRMS (ES+) C32H37O5 [M+H+] Calculated: 501.2641; Found: 501.2623; (c 1.60, CH2Cl2).
(Ra)-Diethyl 2-(3-(2-phenylvinylidene)heptyl)-2-(prop-2-yn-1-yl)malonate (16e)
Following the General Procedure for Preparation of Allene-yne via alkylation of the malonate moiety, diethyl malonate 34e (98 mg, 0.49 mmol) was reacted with sodium hydride (16.5 mg, 60 wt% in oil, 0.41 mmol), mesylate 33 (97 mg, 0.27 mmol) and potassium iodide (68.3 mg, 0.41 mmol) in DMF-THF (1 : 1, 5.6 mL). Purification of the crude residue using a Biotage normal phase automated purification system (25 g SNAP column, gradient of 0 – 5% EtOAc/hexanes) afforded compound 16e (45.5 mg, 42%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.28 – 7.26 (m, 4H), 7.17 – 7.15 (m, 1H), 6.15 (t, J = 3.0 Hz, 1H), 4.21 – 4.18 (q, J = 7.1 Hz, 4H), 2.84 (d, J = 2.7 Hz, 2H), 2.27 – 2.24 (m, 2H), 2.10 – 2.08 (m, 2H), 2.04 – 1.99 (m, 1H), 1.97 (t, J = 2.7 Hz, 1H), 1.45 – 1.42 (m, 2H), 1.36 – 1.32 (m, 2H), 1.23 (td, J = 7.1, 2.8 Hz, 6H), 0.87 (t, J = 7.3 Hz, 3H); 13C NMR (150 MHz, CD2Cl2) δ 201.9, 169.9 (2C), 135.8, 128.4 (2C), 126.5 (2C), 126.4, 107.9, 95.9, 78.8, 71.1, 61.6 (2C), 56.4, 32.4, 30.2, 29.8, 27.2, 22.7, 22.5, 13.8 (2C), 13.7; IR (thin film) 3297, 2962, 2925, 2868, 1740, 1467, 1438, 1270, 1193, 1099, 1070; HRMS (ES+) C25H32O4 [M+H+] Calculated: 397.2379; Found: 397.2376; (c 1.5, CH2Cl2).
(Ra)-diethyl 2-(3-(2-phenylvinylidene)heptyl)-2-(3-(trimethylsilyl)prop-2-yn-1-yl)malonate (16f)
Following the general procedure for preparation of allene-yne via silylation of the terminal alkyne, allene-yne 16e (44.1 mg, 0.11 mmol) in THF (1 mL) was reacted with lithium hexamethyldisilylamide (200 μL, 1M in THF, 0.20 mmol) and trimethylsilylchloride (35 μL, 0.28 mmol). Purification of the crude product using a Biotage normal phase automated purification system (4 g SNAP column, gradient of 1 – 3% EtOAc/hexanes) to afford compound 16f (43 mg, 84%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.28 – 7.26 (m, 4H), 7.18 – 7.14 (m, 1H), 6.16 – 6.14 (m, 1H), 4.22 – 4.15 (m, 4H), 2.85 (s, 2H), 2.27 – 2.21 (m, 2H), 2.12 – 1.99 (m, 4H), 1.46 – 1.33 (m, 4H), 1.23 (td, J = 7.1, 1.8 Hz, 6H), 0.88 (t, J = 7.2 Hz, 3H), 0.08 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 202.1, 170.1 (2C), 135.8, 128.5 (2C), 126.5 (2C), 126.4, 107.8, 101.2, 95.9, 88.1, 61.5 (2C), 56.8, 32.3, 30.4, 29.8, 27.5, 24.2, 22.5, 14.0 (2C), 13.9, −0.1 (3C); IR (thin film) 2950, 2921, 2856, 2178, 1941, 1728, 1458, 1437, 1266, 1193, 1030; HRMS (ES+) C28H41O4Si [M+H+] Calculated: 469.2774; Found: 469.2762; (c 2.65, CH2Cl2).
(S)-Diethyl 8-butyl-2-oxo-1-phenyl-3-(trimethylsilyl)-1,2,6,7-tetrahydroazulene-5,5(4H)-dicarboxylate (44f)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 16f (26.1 mg, 0.056 mmol) and [Rh(CO)2Cl]2 (2.2 mg, 0.0056 mmol) were reacted in toluene (1.9 mL) for 2 h. Purification of the crude residue by flash chromatography (15% Et2O/hexanes) afforded compound 44f (24.8 mg, 90%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.29 – 7.21 (m, 2H), 7.20 – 7.18 (m, 1H), 7.12 – 7.10 (m, 2H), 4.31 – 4.18 (m, 4H), 3.93 (s, 1H), 3.56 (d, J = 14.8 Hz, 1H), 3.46 (d, J = 14.8 Hz, 1H), 2.56 – 2.54 (m, 1H), 2.50 – 2.46 (m, 1H), 2.42 – 2.39 (m, 2H), 1.94 – 1.87 (m, 2H), 1.29 (td, J = 7.1, 2.4 Hz, 6H), 1.26 – 1.22 (m, 1H), 1.11 – 0.94 (m, 3H), 0.71 (t, J = 7.3 Hz, 3H), 0.25 (s, 9H); 13C NMR (150 MHz, CDCl3) δ 208.5, 176.3, 171.4, 171.3, 145.6, 141.4, 139.9, 138.9, 128.6 (2C), 127.5 (2C), 126.5, 61.8, 61.7, 57.1, 56.3, 37.8, 34.7, 33.6, 29.1, 28.9, 22.7, 14.0, 14.0, 13.8, −0.3 (3C); IR (thin film) 2954, 2921, 2856, 1732, 1683, 1540, 1454, 1266, 1242, 1185, 1050; HRMS (ES+) C29H41O5Si [M+H+] Calculated: 497.2723; Found: 497.2733; (c 2.1, CH2Cl2).
(Ra)-Diethyl 2-(but-2-yn-1-yl)-2-(3-(2-phenylvinylidene)heptyl)malonate (16c)
Following the General Procedure for Preparation of Allene-yne via alkylation of the malonate moiety, diethyl malonate 34c (96 mg, 0.35 mmol) was reacted with sodium hydride (11.7 mg, 60 wt% in oil, 0.29 mmol), mesylate 33 (68.7 mg, 0.19 mmol) and potassium iodide (48.3 mg, 0.29 mmol) in DMF-THF (1 : 1, 4 mL). Purification of the crude residue by flash chromatography (5% Et2O/hexanes) afforded compound 16c (60.1 mg, 75%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 7.26 – 7.24 (m, 4H), 7.16 – 7.13 (m, 1H), 6.14 (t, J = 2.8 Hz, 1H), 4.17 (q, J = 7.1 Hz, 4H), 2.76 – 2.75 (m, 2H), 2.24 – 2.19 (m, 2H), 2.10 – 2.07 (m, 2H), 2.06 – 1.97 (m, 2H), 1.66 (t, J = 2.6 Hz, 3H), 1.44 – 1.42 (m, 2H), 1.36 – 1.32 (m, 2H), 1.21 (td, J = 7.1, 1.0 Hz, 6H), 0.86 (t, J = 7.2 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 202.1, 170.5 (2C), 135.9, 128.4 (2C), 126.5 (2C), 126.4, 107.9, 95.9, 78.8, 73.2, 61.4 (2C), 56.8, 32.4, 30.2, 29.8, 27.3, 23.1, 22.5, 14.0 (2C), 13.9, 3.4; IR (thin film) 2958, 2933, 2860, 1728, 1462, 1446, 1266, 1193, 1103, 1066; HRMS (ES+) C26H35O4 [M+H+] Calculated: 411.2535; Found: 411.2528; (c 1.35, CH2Cl2).
(S)-Diethyl 8-butyl-3-methyl-2-oxo-1-phenyl-1,2,6,7-tetrahydroazulene-5,5(4H)-dicarboxylate (44c)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 16c (33.2 mg, 0.081 mmol) and [Rh(CO)2Cl]2 (3.2 mg, 0.0081 mmol) were reacted in toluene (2.7 mL) for 2.5 h. Purification of the crude residue by flash chromatography (30% Et2O/hexanes) afforded compound 44c (31.2 mg, 88%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.29 – 7.27 (m, 3H), 7.22 – 7.19 (m, 1H), 7.13 – 7.12 (m, 2H), 4.26 – 4.23 (m, 4H), 3.95 (s, 1H), 3.39 (m, 2H), 2.50 – 2.47 (m, 3H), 2.39 – 2.36 (m, 1H), 1.90 – 1.89 (m, 2H), 1.85 (s, 3H), 1.30 (td, J = 7.1, 2.5 Hz, 6H), 1.26 – 1.22 (m, 2H), 1.10 – 0.87 (m, 2H), 0.72 (t, J = 7.3 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 204.3, 171.5, 171.4, 164.1, 143.4, 138.5, 137.7, 137.3, 128.6 (2C), 127.5 (2C), 126.6, 61.8 (2C), 55.9, 55.1, 37.1, 33.8, 32.6, 29.1, 28.6, 22.6, 14.0 (2C), 13.8, 8.5; IR (thin film) 2950, 2921, 2856, 1732, 1691, 1597, 1450, 1381, 1361, 1270, 1230, 1185; HRMS (ES+) C27H35O5 [M+H+] Calculated: 439.2484; Found: 439.2467; (c 2.0, CH2Cl2).
(Ra)-(3-(2-((3-Phenylprop-2-yn-1-yl)oxy)ethyl)hepta-1,2-dien-1-yl)benzene (15d)
Following the general procedure for preparation of allene-yne via Williamson etherification, alcohol (Ra)-29 (95.8 mg, 0.44 mmol) in THF (1 mL) was reacted with sodium hydride (35.4 mg, 60% dispersion in mineral oil, 0.89 mmol) and (3-bromoprop-1-yn-1-yl)benzene (130 mg, 0.66 mmol) for 11 h. Purification of the crude residue by flash chromatography (1% Et2O/hexanes) afforded compound 15d (51.3 mg, 35%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.39 – 7.38 (m, 2H), 7.29 – 7.26 (m, 7H), 7.17 – 7.15 (m, 1H), 6.17 – 6.16 (m, 1H), 4.35 – 4.29 (m, 2H), 3.76 – 3.70 (m, 2H), 2.44 – 2.42 (m, 2H), 2.13 – 2.12 (m, 2H), 1.50 – 1.44 (m, 2H), 1.39 – 1.34 (m, 2H), 0.88 (t, J = 7.3 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 202.3, 135.8, 131.7 (2C), 128.5 (2C), 128.3, 128.2 (2C), 126.5 (2C), 126.4, 122.7, 105.5, 95.9, 86.1, 85.2, 68.3, 58.8, 32.8, 32.7, 29.8, 22.5, 13.9; IR (thin film) 3060, 3019, 2962, 2929, 2864, 1949, 1675, 1605, 1495, 1462, 1442, 1352, 1099, 1030; HRMS (ES+) C24H27O1 [M+H+] Calculated: 331.2062; Found: 331.2025; (c 1.05, CH2Cl2).
(S)-5-Butyl-6,8-diphenyl-3,4-dihydro-1H-cyclopenta[c]oxepin-7(6H)-one (43d)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 15d (41 mg, 0.124 mmol) and [Rh(CO)2Cl]2 (4.8 mg, 0.0124 mmol) were reacted in toluene (4.1 mL) for 30 min. Purification of the crude residue by flash chromatography (30% Et2O/hexanes) afforded compound 43d (30.6 mg, 69%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.38 – 7.28 (m, 7H), 7.25 – 7.22 (m, 3H), 4.88 (s, 2H), 4.20 (s, 1H), 4.08 – 4.04 (m, 2H), 2.84 – 2.75 (m, 1H), 2.63 – 2.56 (m, 1H), 2.06 – 1.99 (m, 2H), 1.30 – 1.26 (m, 2H), 1.13 – 1.06 (m, 3H), 0.73 (t, J = 7.1 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 201.8, 167.6, 141.8, 138.3, 138.2, 136.1, 130.9, 129.4 (2C), 128.7 (2C), 128.2 (2C), 128.1, 127.6 (2C), 126.9, 70.0, 67.3, 56.2, 36.9, 35.1, 29.7, 22.7, 13.8; IR (thin film) 3064, 3031, 2958, 2925, 2860, 2030, 1695, 1605, 1499, 1458, 1373, 1275, 1140; HRMS (ES+) C25H27O2 [M+H+] Calculated: 359.2011; Found: 359.1980; (c 1.2, CH2Cl2).
(Ra)-(3-(2-(But-2-yn-1-yloxy)ethyl)hepta-1,2-dien-1-yl)benzene (15c)
Following the general procedure for preparation of allene-yne via Williamson etherification, alcohol (Ra)-29 (105 mg, 0.442 mmol) in THF (1.1 mL) was reacted with sodium hydride (38.8 mg, 60% dispersion in mineral oil, 0.97 mmol) and 1-bromobut-2-yne (97 mg, 0.73 mmol) for 11 h. Purification of the crude residue by flash chromatography (1% Et2O/hexanes) afforded compound 15c (33 mg, 27%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.29 – 7.27 (m, 4H), 7.19 – 7.14 (m, 1H), 6.16 – 6.12 (m, 1H), 4.04 – 4.03 (m, 2H), 3.65 – 3.58 (m, 2H), 2.40 – 2.35 (m, 2H), 2.10 – 2.07 (m, 2H), 1.80 (t, J = 2.3 Hz, 3H), 1.46 – 1.32 (m, 4H), 0.87 (t, J = 7.2 Hz, 3H); 13C NMR (175 MHz, CDCl3) δ 202.2, 135.8, 128.5 (2C), 126.6 (2C), 126.5, 105.5, 95.8, 82.3, 75.2, 68.2, 58.7, 32.8, 32.6, 29.7, 22.5, 13.9, 3.6; IR (thin film) 2954, 2929, 2852, 1495, 1462, 1356, 1140, 1095; HRMS (ES+) C19H25O [M+H+] Calculated: 269.1905; Found: 269.1889; (c 2.3, CH2Cl2).
(S)-5-butyl-8-methyl-6-phenyl-3,4-dihydro-1H-cyclopenta[c]oxepin-7(6H)-one (43c)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 15c (19 mg, 0.0719 mmol) and [Rh(CO)2Cl]2 (2.8 mg, 0.00719 mmol) were reacted in toluene (2.5 mL) for 45 min. Purification of the crude residue by flash chromatography (20% Et2O/hexanes) afforded compound 43c (10 mg, 46%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.44 – 7.27 (m, 2H), 7.27 – 7.15 (m, 3H), 4.82 – 4.80 (m, 2H), 4.03 – 3.94 (m, 3H), 2.59 (t, J = 5.5 Hz, 2H), 1.98 – 1.89 (m, 2H), 1.74 (s, 3H), 1.18 – 0.97 (m, 4H), 0.70 (t, J = 7.1 Hz, 3H); 13C NMR (175 MHz, CDCl3) δ 203.3, 166.8, 140.4, 138.5, 134.9, 134.8, 128.7 (2C), 127.5 (2C), 126.8, 70.4, 69.6, 55.7, 37.1, 36.8, 29.9, 22.7, 13.8, 8.3; IR (thin film) 2950, 2921, 2852, 1691, 1458, 1270, 1152; HRMS (ES+) C20H25O2 [M+H+] Calculated: 297.1855; Found: 297.1834; (c 1.0, CH2Cl2).
(Ra)-(3-(2-(Prop-2-yn-1-yloxy)ethyl)hepta-1,2-dien-1-yl)benzene (15e)
Following the general procedure for preparation of allene-yne via Williamson etherification, alcohol (Ra)-29 (221.6 mg, 2.05 mmol) in THF (2.4 mL) was reacted with sodium hydride (82 mg, 60% dispersion in mineral oil, 0.97 mmol) and propargyl bromide (229 mg, 1.54 mmol) for 11 h. Purification of the crude residue by flash chromatography (0.7% Et2O/hexanes) afforded compound 15e (51.9 mg, 20%) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 7.26 – 7.22 (m, 4H), 7.16 – 7.11 (m, 1H), 6.11 (m, 1H), 4.05 (t, J = 2.0 Hz, 2H), 3.66 – 3.58 (m, 2H), 2.38 – 2.33 (m, 3H), 2.10 – 2.04 (m, 2H), 1.43 – 1.30 (m, 4H), 0.84 (t, J = 7.2 Hz, 3H); 13C NMR (175 MHz, CD2Cl2) δ 202.2, 135.8, 128.4 (2C), 126.5 (2C), 126.4, 105.5, 95.5, 79.9, 73.8, 68.2, 57.9, 32.6, 32.6, 29.7, 22.5, 13.7; IR (thin film) 3309, 2954, 2921, 2856, 1728, 1663, 1593, 1467, 1360, 1254, 1160, 1103; HRMS (ES+) C18H23O [M+H+] Calculated: 255.1749; Found: 255.1756; (c 1.0, CH2Cl2).
(Ra)-Trimethyl(3-((3-(2-phenylvinylidene)heptyl)oxy)prop-1-yn-1-yl)silane (15f)
Following the general procedure for preparation of allene-yne via silylation of the terminal alkyne, allene-yne 15e (48 mg, 0.19 mmol) in THF (1.7 mL) was reacted with lithium hexamethyldisilylamide (340 μL, 1M in THF, 0.34 mmol) and trimethylsilylchloride (60 μL, 0.47 mmol). Purification of the crude residue using a Biotage normal phase automated purification system (4 g SNAP column, gradient of 0 – 2% EtOAc/hexanes) to afford compound 15f (39.9 mg, 65%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.30 – 7.28 (m, 4H), 7.20 – 7.18 (m, 1H), 6.17 – 6.16 (m, 1H), 4.12 – 4.11 (m, 2H), 3.70 – 3.62 (m, 2H), 2.43 – 2.40 (m, 2H), 2.14 – 2.12 (m, 2H), 1.50 – 1.46 (m, 2H), 1.40 – 1.34 (m, 2H), 0.90 (t, J = 7.3 Hz, 3H), 0.17 (s, 9H); 13C NMR (150 MHz, CDCl3) δ 202.2, 135.7, 128.5 (2C), 126.5 (2C), 126.4, 105.4, 101.6, 95.7, 91.2, 68.2, 58.9, 32.7, 32.6, 29.7, 22.5, 13.9, −0.2 (3C); IR (thin film) 2954, 2925, 2856, 2169, 1949, 1716, 1597, 1491, 1458, 1356, 1250, 1099; HRMS (ES+) C21H31OSi [M+H+] Calculated: 327.2144; Found: 327.2128; (c 0.85, CH2Cl2).
(S)-5-butyl-6-phenyl-8-(trimethylsilyl)-3,4-dihydro-1H-cyclopenta[c]oxepin-7(6H)-one (43f)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 15f (25.1 mg, 0.077 mmol) and [Rh(CO)2Cl]2 (3 mg, 0.0077 mmol) were reacted in toluene (2.5 mL) for 45 min. Purification of the crude residue by flash chromatography (15% Et2O/hexanes) afforded compound 43f (24.9 mg, 91%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 7.29 – 7.25 (m, 2H), 7.21 – 7.14 (m, 3H), 4.94 – 4.82 (m, 2H), 4.02 – 3.97 (m, 3H), 2.72 – 2.65 (m, 1H), 2.60 – 2.52 (m, 1H), 2.00 – 1.91 (m, 2H), 1.27 – 1.26 (m, 1H), 1.08 – 1.03 (m, 3H), 0.71 (t, J = 7.1 Hz, 3H), 0.21 (s, 9H); 13C NMR (175 MHz, CDCl3) δ 207.5, 180.1, 141.8, 138.8, 138.4, 138.2, 128.7 (2C), 127.4 (2C), 126.6, 69.8, 69.7, 57.4, 37.3, 36.0, 29.7, 22.7, 13.8, −0.3 (3C); IR (thin film) 2954, 2925, 2964, 1695, 1544, 1446, 1250, 1152, 841; HRMS (ES+) C22H31O2Si [M+H+] Calculated: 355.2093; Found: 355.2114; (c 2.3, CH2Cl2).
(Ra)-N-(3-Cyclopropylprop-2-yn-1-yl)-4-methyl-N-(3-(2-phenylvinylidene) heptyl) benzenesulfonamide (14g)
Following the general procedure for preparation of allene-yne via Mitsunobu reaction, alcohol (Ra)-29 (37.8 mg, 0.18 mmol) in THF (1.3 mL) was reacted with triphenylphosphine (55 mg, 0.21 mmol), N-(3-cyclopropylprop-2-yn-1-yl)-4-methylbenzenesulfonamide 30g (52.3 mg, 0.21 mmol) and diisopropylazodicarboxylate (42 μL, 0.21 mmol) for 11 h. Purification of the crude residue using a Biotage normal phase automated purification system (12 g SNAP column, gradient of 0 – 6% EtOAc/hexanes) afforded compound 14g (78.2 mg, 78%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 7.64 (d, J = 7.9 Hz, 6H), 7.29 – 7.24 (m, 4H), 7.21 – 7.16 (m, 3H), 6.12 (s, 1H), 4.07 – 3.96 (m, 2H), 3.36 – 3.29 (m, 1H), 3.25 – 3.18 (m, 1H), 2.37 (s, 3H), 2.36 – 2.34 (m, 2H), 2.15 – 2.06 (m, 2H), 1.45 – 1.41 (m, 2H), 1.36 – 1.30 (m, 2H), 0.91 – 0.90 (m, 1H), 0.86 (t, J = 7.2 Hz, 3H), 0.56 – 0.53 (m, 2H), 0.26 – 0.24 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 202.3, 143.1, 135.9, 135.5, 129.3 (2C), 128.5 (2C), 127.7 (2C), 126.6, 126.5 (2C), 105.4, 95.9, 89.3, 67.7, 44.6, 37.0, 32.1, 31.2, 29.7, 22.5, 21.5, 13.9, 7.8 (2C), −0.9; IR (thin film) 3027, 2962, 2940, 2860, 2251, 1945, 1593, 1491, 1458, 1348, 1164, 1099; HRMS (ES+) C28H34NO2S [M+H+] Calculated: 448.2310; Found: 448.2329; (c 0.55, CH2Cl2).
(S)-5-butyl-8-cyclopropyl-6-phenyl-2-tosyl-1,2,3,4-tetrahydrocyclopenta[c]azepin -7(6H)-one (42g)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 14g (31 mg, 0.069 mmol) and [Rh(CO)2Cl]2 (2.7 mg, 0.0069 mmol) were reacted in toluene (2.2 mL) for 35 min. Purification of the crude residue by silica gel chromatography using 40% Et2O/hexanes afforded compound 42g (29.1 mg, 88%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.75 (d, J = 8.3 Hz, 2H), 7.28 (d, J = 8.2 Hz, 2H), 7.15 (m, 3H), 6.75 – 6.73 (m, 2H), 4.88 (d, J = 16.9 Hz, 1H), 4.61 (d, J = 16.9 Hz, 1H), 3.67 – 3.65 (m, 1H), 3.64 (s, 1H), 3.57 – 3.53 (m, 1H), 2.68 – 2.63 (m, 1H), 2.53 – 2.48 (m, 1H), 2.41 (s, 3H), 1.78 – 1.72 (m, 2H), 1.62 – 1.59 (m, 1H), 1.30 – 1.27 (m, 1H), 1.21 – 1.18 (m, 1H), 1.08 – 1.06 (m, 1H), 1.01 – 0.96 (m, 1H), 0.94 – 0.90 (m, 1H), 0.87 – 0.85 (m, 2H), 0.80 – 0.77 (m, 1H), 0.66 (t, J = 7.3 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 202.7, 162.1, 143.6, 139.4, 138.4, 137.9, 136.0, 135.7, 129.6 (2C), 128.6 (2C), 127.4 (2C), 127.3 (2C), 126.7, 55.3, 47.9, 43.8, 36.9, 31.2, 29.2, 22.6, 21.6, 13.7, 8.3, 5.9, 5.7; IR (thin film) 2962, 2917, 2860, 1695, 1454, 1344, 1172, 1087, 1021; HRMS (ES+) C29H34NO3S [M+H+] Calculated: 476.2259; Found: 476.2271; (c 0.75, CH2Cl2).
1-(Dimethyl(phenyl)silyl)hept-2-yn-1-ol (24)
To a solution of hept-2-yn-1-ol 23 (5.48 g, 48.9 mmol) in THF (74 mL) cooled to −78 °C was added n-BuLi (33.6 mL, 1.0 M in hexanes, 53.7 mmol) and chlorodimethylphenylsilane (9.17 g, 53.7 mmol). After the addition was complete, the bath was removed and the reaction mixture was stirred at rt for 20 h. The solution was cooled to −78 °C and tert-butyllithium (34.5 mL, 1.7 M in hexanes, 58.6 mmol) was added dropwise. The mixture was then stirred at −45 °C for 2 h. The solution was cooled to −78 °C and quenched slowly with a 10% solution of acetic acid in THF (20 mL). The mixture was diluted with NaHCO3 (100 mL) and extracted with Et2O (400 mL). The organic phase was separated and extracted with NaHCO3 (4 × 150 mL). The organic phase was washed with brine, dried (Na2SO4), filtered and concentrated under reduced pressure. Purification of the crude residue using a Biotage normal phase automated purification system (120 g SNAP column, gradient of 1 – 25% EtOAc/hexanes) afforded compound 24 (6.65 g, 55%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.63 – 7.62 (m, 2H), 7.40 – 7.37 (m, 3H), 4.25 (t, J = 2.4 Hz, 1H), 2.25 – 2.23 (m, 2H), 1.49 – 1.45 (m, 2H), 1.40 – 1.37 (m, 2H), 0.94 – 0.93 (m, 1H), 0.90 (t, J = 7.3 Hz, 3H), 0.43 (d, J = 7.0 Hz, 6H); 13C NMR (150 MHz, CDCl3) δ 135.5, 134.3 (2C), 129.6, 127.8 (2C), 89.0, 79.9, 56.2, 30.9, 21.9, 18.7, 13.6, −5.5, −5.9; IR (thin film) 3440, 2954, 2929, 2860, 1248, 1120, 968; HRMS (ES+) C15H23OSi [M+H+] Calculated: 247.1518; Found: 247.1496.
Methyl 3-(2-(dimethyl(phenyl)silyl)vinylidene)heptanoate (51)
A 25 mL round bottom flask was charged with propargyl alcohol 24 (1.01 g, 4.1 mmol), trimethylorthoacetate (3.13 mL, 24.5 mmol) and propionic acid (52 μL, 0.69 mmol). The resulting solution was stirred at 160 °C for 12 h using a Dean-Stark apparatus. After cooling to rt, the mixture was concentrated under a 10 mmHg vacuum for 2 h. Purification of the crude residue by silica gel chromatography using 2% Et2O/hexanes afforded compound 51 (940 mg, 76%) as a colorless oil. 1H NMR (700 MHz, CDCl3) δ 7.55 – 7.54 (m, 2H), 7.36 – 7.35 (m, 3H), 5.15 (t, J = 3.4 Hz, 1H), 3.66 (s, 3H), 2.96 (qd, J = 15.1, 3.0 Hz, 2H), 1.99 – 1.98 (m, 2H), 1.37 – 1.31 (m, 4H), 0.88 (t, J = 7.0 Hz, 3H), 0.36 (s, 6H); 13C NMR (150 MHz, CDCl3) 209.8, 172.0, 138.6, 133.7 (2C), 129.0, 127.7 (2C), 91.5, 82.9, 51.7, 38.2, 31.0, 29.8, 22.4, 13.9, −2.28, −2.32; IR (thin film) 2958, 2921, 2860, 1940, 1744, 1434, 1368, 1168; HRMS (ES+) C18H27O2Si [M+H ] Calculated: 303.1780; Found: 303.1755.
3-(2-(Dimethyl(phenyl)silyl)vinylidene)heptan-1-ol (25)
A 25 mL round bottom flask was charged with lithium aluminum hydride (4.1 mL of a 1 M solution in Et2O, 4.02 mmol) and THF (4 mL). The resulting mixture was cooled to 0 °C and a solution of ester 51 (810.5 mg, 2.68 mmol) in THF (4.7 mL) was added dropwise. The solution was stirred for 1 h at rt. The reaction mixture was cooled to 0 °C (ice bath), diluted with Et2O (30 mL) and quenched very slowly with water (2 mL). The phases were separated, the organic phase was washed with brine (10 mL), dried (Na2SO4), filtered and concentrated under reduced pressure. Purification of the crude residue by silica gel chromatography using 30% Et2O/hexanes afforded compound 25 (541 mg, 74%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.56 – 7.54 (m, 2H), 7.38 – 7.36 (m, 3H), 5.15 – 5.14 (m, 1H), 3.67 – 3.65 (m, 2H), 2.19 – 2.17 (m, 3H), 1.94 – 1.92 (m, 2H), 1.38 – 1.34 (m, 4H), 0.90 (m, 3H), 0.37 – 0.36 (m, 6H); 13C NMR (150 MHz, CDCl3) δ 209.0, 138.4, 133.6 (2C), 129.0, 127.7 (2C), 93.8, 82.8, 60.8, 34.9, 31.4, 29.9, 22.4, 13.9, −2.2, −2.3; IR (thin film) 3342, 2962, 2933, 2844, 1945, 1458, 1453, 1381, 1242; HRMS (ES+) C17H25OSi [M−H+] Calculated: 273.1675; Found: 273.1653.
N-(but-2-yn-1-yl)-N-(3-(2-(dimethyl(phenyl)silyl)vinylidene)heptyl)-4-methyl benzenesulfonamide (40)
Following the general procedure for preparation of allene-yne via Mitsunobu reaction, alcohol 25 (54.8 mg, 0.2 mmol) in THF (1.5 mL) was reacted with triphenylphosphine (63 mg, 0.24 mmol), N-(but-2-yn-1-yl)-4-methylbenzenesulfonamide3030c (54 mg, 0.24 mmol) and NaHCO3 (48 μL, 0.24 mmol) for 2.5 h. Purification of the crude residue by silica gel chromatography using 5% Et2O/hexanes afforded compound 40 (46.7 mg, 49%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.74 (d, J = 8.1 Hz, 2H), 7.57 (d, J = 5.0 Hz, 2H), 7.39 – 7.37 (m, 3H), 7.29 (d, J = 7.4 Hz, 2H), 5.15 – 5.14 (m, 1H), 4.09 (d, J = 2.6 Hz, 2H), 3.24 (t, J = 8.0 Hz, 2H), 2.44 (s, 3H), 2.22 – 2.20 (m, 2H), 1.96 – 1.95 (m, 2H), 1.56 (t, J = 2.3 Hz, 3H), 1.38 – 1.35 (m, 5H), 0.92 (t, J = 7.0 Hz, 3H), 0.37 (s, 6H); 13C NMR (150 MHz, CDCl3) δ 208.9, 143.0, 138.5, 136.0, 133.6 (2C), 129.2 (2C), 129.0, 127.7 (2C), 127.6 (2C), 94.1, 82.9, 81.4, 71.7, 44.9, 37.0, 31.3, 29.8 (2C), 22.4, 21.4, 13.9, 3.2, −2.2, −2.3; IR (thin film) 3395, 3064, 2954, 2860, 2165, 1669, 1593, 1438, 1361, 1254, 1160; HRMS (ES+) C28H36NO2SSi [M−H+] Calculated: 478.2236; Found: 478.2238.
5-Butyl-8-methyl-2-tosyl-1,2,3,4-tetrahydrocyclopenta[c]azepin-7(6H)-one (41)
Following the General Procedure for the [Rh(CO)2Cl]2 Catalyzed Cyclocarbonylation Reaction, allene-yne 40 (33.5 mg, 0.07 mmol) and [Rh(CO)2Cl]2 (2.7 mg, 0.007 mmol) were reacted in toluene (2.3 mL) for 6 h. Purification of the crude residue by silica gel chromatography using 65% Et2O/hexanes afforded compound 41 (25.1 mg, 96%) as a colorless oil. 1H NMR (700 MHz, CDCl3) δ 7.58 (d, J = 8.2 Hz, 2H), 7.23 (d, J = 8.0 Hz, 2H), 4.45 (s, 2H), 3.57 (t, J = 14.0 Hz, 2H), 2.70 (s, 2H), 2.54 (t, J = 6.0 Hz, 2H), 2.39 (s, 3H), 2.00 (t, J = 7.4 Hz, 2H), 1.82 (s, 3H), 1.27 – 1.24 (m, 4H), 0.88 (t, J = 6.9 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 203.9, 161.5, 143.5, 138.6, 137.0, 136.1, 131.1, 129.4 (2C), 127.0 (2C), 47.9, 45.4, 39.2, 37.0, 32.6, 30.0, 22.6, 21.5, 14.0, 8.2; IR (thin film) 2954, 2929, 2856, 1703, 1462, 1352, 1160; HRMS (ES+) C21H28NO3S [M+H+] Calculated: 374.1790; Found: 374.1806.
6-Methyl-2-tosyl-2,3,4,5-tetrahydrocyclopenta[c]azepin-7(1H)-one (48)
To a solution of cyclocarbonylation product 35e (30 mg, 0.067 mmol) in CH2Cl2 (0.1 mL) were added 4 Å molecular sieves (12 mg, activated by heating at 170 °C under vacuum for 12 h) and (S)-1-amino-2-methoxymethylpyrrolidine (SAMP) (14 μl, 0.1 mmol). The resulting mixture was stirred at rt for 15 h. The solution was diluted with Et2O (3 mL), filtered on a Celite plug, rinsed with Et2O (10 mL) and concentrated under reduced pressure. Purification of the crude residue using a Biotage normal phase automated purification system (4 g SNAP column, gradient of 50 – 70% Et2O/hexanes) afforded compound 48 (14 mg, 66%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 7.69 (d, J = 8.3 Hz, 2H), 7.33 (d, J = 8.1 Hz, 2H), 6.84 (s, 1H), 3.66 (m, 2H), 3.04 (s, 2H), 2.65 (t, J = 6.2 Hz, 2H), 2.43 (s, 3H), 1.91 – 1.86 (m, 2H), 1.73 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 203.9, 163.7, 144.3, 139.5, 135.4, 130.1 (2C), 126.9 (2C), 121.3, 116.1, 49.3, 40.9, 30.1, 24.4, 21.6, 8.5; IR (thin film) 2921, 2860, 1683, 1634, 1589, 1450, 1344, 1303, 1164, 1095; HRMS (ES+) C17H20NO3S [M+H+] Calculated: 318.1164; Found: 318.1162.
Supplementary Material
Acknowledgements
We greatly acknowledge the National Institute of Health (P50GM067982 and GM54161) for funding this project. We also thank Chiral Technologies, Inc., Pr. Peter Wipf, Dr. Erin M. Skoda and Lotus Separation for their help in the HPLC and SFC analysis. Finally we also thank Dr. Steve Geib for crystallographic studies.
Footnotes
Supporting Information Copies of 1H and 13C NMR spectra for all new compounds; HPLC chromatogram of the racemic and enantioenriched compounds; coordinates for the crystallographic analysis (CIF) for compounds 35c and 48. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
References
- (1).For [5,5] ring systems: Amouzou E, Ayer WA, Browne LM. J. Nat. Prod. 1989;52:1042–1054.. Ban DJ-YD, Banwell MG, Cade IA, Willis AC. Tetrahedron. 2011;67:8348–8352.. For [5,6] ring systems: Jones G, Fales HM, Wildman WC. Tetrahedron Lett. 1963;4:397–400.. For [5,7] ring systems: Bovi Mitre G, Kamiya N, Bardón A, Asakawa Y. J. Nat. Prod. 2004;67:31–36. doi: 10.1021/np030074z.. Tori M, Nakashima K, Takeda T, Kan Y, Takaoka S, Asakawa Y. Tetrahedron. 1996;52:6339–6354.. Choudhary MI, Batool I, Atif M, Hussain S, Atta-ur R. J. Nat. Prod. 2007;70:849–852. doi: 10.1021/np068052a.. Dorfelt H, Schlegel B, Grafe U. J. Antibiot. 2000;53:838–843. doi: 10.7164/antibiotics.53.839..
- (2).Tori M, Nakashima K, Takeda T, Kan Y, Takaoka S, Asakawa Y. Tetrahedron. 1996;52:6339–6354. [Google Scholar]
- (3).For a general review discussing the different methods to access cyclopentenones, see: Gibson SE, Lewis SE, Mainolfi N. J. Organomet. Chem. 2004;689:3873–3890..
- (4).Elford TG, Hall DG. J. Am. Chem. Soc. 2010;132:1488–1489. doi: 10.1021/ja9104478. [DOI] [PubMed] [Google Scholar]
- (5).Kametani T, Suzuki Y, Ban C, Honda T. Heterocycles. 1987;26:1491–1493. [Google Scholar]
- (6).Rigby JH, Senanayake C. J. Am. Chem. Soc. 1987;109:3147–3149. [Google Scholar]
- (7).Hirose T, Miyakoshi N, Mukai C. J. Org. Chem. 2008;73:1061–1066. doi: 10.1021/jo702330y. [DOI] [PubMed] [Google Scholar]
- (8).Kalidindi S, Jeong WB, Schall A, Bandichhor R, Nosse B, Reiser O. Angew. Chem. Int. Ed. 2007;46:6361–6363. doi: 10.1002/anie.200701584. [DOI] [PubMed] [Google Scholar]
- (9).Ban DJ-YD, Banwell MG, Willis AC. Tetrahedron. 2010;66:7807–7814. [Google Scholar]
- (10).Shimada N, Tius MA. Tetrahedron. 2011;67:5851–5870. doi: 10.1016/j.tet.2011.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).He W, Herrick IR, Atesin TA, Caruana PA, Kellenberger CA, Frontier AJ. J. Am. Chem. Soc. 2008;130:1003–1011. doi: 10.1021/ja077162g. [DOI] [PubMed] [Google Scholar]
- (12).(a) Lee HW, Kwong FY, Chan ASC. Synlett. 2008:1553–1556. [Google Scholar]; (b) Lee HW, Lee LN, Chan ASC, Kwong FY. Eur. J. Org. Chem. 2008:3403–3406. [Google Scholar]; (c) Lee HW, Chan ASC, Kwong FY. Chem. Commun. 2007:2633–2635. doi: 10.1039/b702718d. [DOI] [PubMed] [Google Scholar]; (d) Fan B-M, Xie J-H, Li S, Tu Y-Q, Zhou Q-L. Adv. Synth. Catal. 2005;347:759–762. [Google Scholar]; (e) Kwong FY, Li YM, Lam WH, Qiu L, Lee HW, Yeung CH, Chan KS, Chan ASC. Chem. Eur. J. 2005;11:3872–3880. doi: 10.1002/chem.200401237. [DOI] [PubMed] [Google Scholar]; (f) Kwong FY, Lee HW, Qiu L, Lam WH, Li Y-M, Kwong HL, Chan ASC. Adv. Synth. Catal. 2005;347:1750–1754. [Google Scholar]; (g) Jeong N, Kim DH, Choi JH. Chem. Commun. 2004:1134–1135. doi: 10.1039/b401288g. [DOI] [PubMed] [Google Scholar]; (h) Schmid TM, Consiglio G. Chem. Commun. 2004:2318–2319. doi: 10.1039/b407843h. [DOI] [PubMed] [Google Scholar]; (i) Morimoto T, Kakiuchi K, Fuji K. Tetrahedron Lett. 2004;45:9163–9166. [Google Scholar]; (j) Suh WH, Choi M, Lee SI, Chung YK. Synthesis. 2003:2169–2172. [Google Scholar]; (k) Shibata T, Toshida N, Takagi K. J. Org. Chem. 2002;67:7446–7450. doi: 10.1021/jo0262661. [DOI] [PubMed] [Google Scholar]; (l) Shibata T, Toshida N, Takagi K. Org. Lett. 2002;4:1619–1621. doi: 10.1021/ol025836g. [DOI] [PubMed] [Google Scholar]; (m) Jeong N, Sung BK, Choi YK. J. Am. Chem. Soc. 2000;122:6771–6772. [Google Scholar]
- (13).(a) Mandal SK, Amin SR, Crowe WE. J. Am. Chem. Soc. 2001;123:6457–6458. doi: 10.1021/ja005568m. [DOI] [PubMed] [Google Scholar]; (b) Hicks FA, Buchwald SL. J. Am. Chem. Soc. 1999;121:7026–7033. [Google Scholar]; (c) Hicks FA, Buchwald SL. J. Am. Chem. Soc. 1996;118:11688–11689. [Google Scholar]
- (14).(a) Kwong FY, Lee HW, Lam WH, Qiu L, Chan ASC. Tetrahedron Asymmetry. 2006;17:1238–1252. [Google Scholar]; (b) Shibata T, Toshida N, Yamasaki M, Maekawa S, Takagi K. Tetrahedron. 2005;61:9974–9979. [Google Scholar]; (c) Shibata T, Takagi K. J. Am. Chem. Soc. 2000;122:9852–9853. [Google Scholar]
- (15).(a) Revés M, Achard T, Solà J, Riera A, Verdaguer X. J. Org. Chem. 2008;73:7080–7087. doi: 10.1021/jo800710n. [DOI] [PubMed] [Google Scholar]; (b) Lledó A, Solà J, Verdaguer X, Riera A, Maestro MA. Adv. Synth. Catal. 2007;349:2121–2128. [Google Scholar]; (c) Solà J, Revés M, Riera A, Verdaguer X. Angew. Chem. Int. Ed. 2007;46:5020–5023. doi: 10.1002/anie.200701040. [DOI] [PubMed] [Google Scholar]; (d) Solà J, Riera A, Verdaguer X, Maestro MA. J. Am. Chem. Soc. 2005;127:13629–13633. doi: 10.1021/ja053653u. [DOI] [PubMed] [Google Scholar]; (e) Gibson SE, Kaufmann KAC, Loch JA, Steed JW, White AJP. Chem. Eur. J. 2005;11:2566–2576. doi: 10.1002/chem.200401210. [DOI] [PubMed] [Google Scholar]; (f) Gimbert Y, Greene AE, Robert F, Konya D. Tetrahedron Lett. 2004;45:6975–6978. [Google Scholar]; (g) Verdaguer X, Lledó A, López-Mosquera C, Maestro MA, Pericàs MA, Riera A. J. Org. Chem. 2004;69:8053–8061. doi: 10.1021/jo0486894. [DOI] [PubMed] [Google Scholar]; (h) Gibson SE, Lewis SE, Loch JA, Steed JW, Tozer MJ. Organometallics. 2003;22:5382–5384. [Google Scholar]; (i) Verdaguer X, Pericàs MA, Riera A, Maestro MA, Mahía J. Organometallics. 2003;22:1868–1877. [Google Scholar]; (j) Sturla SJ, Buchwald SL. J. Org. Chem. 2002;67:3398–3403. doi: 10.1021/jo016038r. [DOI] [PubMed] [Google Scholar]; (k) Hiroi K, Watanabe T, Kawagishi R, Abe I. Tetrahedron Lett. 2000;41:891–895. [Google Scholar]; (l) Hiroi K, Watanabe T, Kawagishi R. Tetrahedron Asymmetry. 2000;11:797–808. [Google Scholar]
- (16).(a) Adrio J, Carretero JC. J. Am. Chem. Soc. 1999;121:7411–7412. [Google Scholar]; (b) Adrio J, Rodríguez Rivero M, Carretero JC. Angew. Chem. Int. Ed. 2000;39:2906–2909. doi: 10.1002/1521-3773(20000818)39:16<2906::aid-anie2906>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]; (c) Günter M, Gais H-J. J. Org. Chem. 2003;68:8037–8041. doi: 10.1021/jo030171x. [DOI] [PubMed] [Google Scholar]
- (17).(a) Shi X, Gorin DJ, Toste FD. J. Am. Chem. Soc. 2005;127:5802–5803. doi: 10.1021/ja051689g. [DOI] [PubMed] [Google Scholar]; (b) Davie CP, Danheiser RL. Angew. Chem. Int. Ed. 2005;44:5867–5870. doi: 10.1002/anie.200501579. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Qi X, Ready JM. Angew. Chem. Int. Ed. 2008;47:7068–7070. doi: 10.1002/anie.200801957. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yan B, Spilling CD. J. Org. Chem. 2008;73:5385–5396. doi: 10.1021/jo8004028. [DOI] [PubMed] [Google Scholar]; (e) Revés M, Lledó A, Ji Y, Blasi E, Riera A, Verdaguer X. Org. Lett. 2012;14:3534–3537. doi: 10.1021/ol301545e. [DOI] [PubMed] [Google Scholar]; (f) Jurkauskas V, Buchwald SL. J. Am. Chem. Soc. 2002;124:2892–2893. doi: 10.1021/ja025603k. [DOI] [PubMed] [Google Scholar]; (g) Barluenga J, Álvarez-Fernández A, Suárez-Sobrino ÁL, Tomás M. Angew. Chem. Int. Ed. 2012;51:183–186. doi: 10.1002/anie.201105362. [DOI] [PubMed] [Google Scholar]
- (18).Brummond KM, Kerekes AD, Wan H. J. Org. Chem. 2002;67:5156–5163. doi: 10.1021/jo016305t. [DOI] [PubMed] [Google Scholar]
- (19).Sato also showed that allene-ynes could undergo a titanium-mediated [2 + 2 + 1] cycloaddition reaction to afford enantioenriched 2-cyclopentenones with complete transfer of chirality. Urabe H, Takeda T, Hideura D, Sato F. J. Am. Chem. Soc. 1997;119:11295–11305..
- (20).Brawn RA, Panek JS. Org. Lett. 2009;11:4362–4365. doi: 10.1021/ol901692n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Liang B, Huang M, You Z, Xiong Z, Lu K, Fathi R, Chen J, Yang Z. J. Org. Chem. 2005;70:6097–6100. doi: 10.1021/jo050498t. [DOI] [PubMed] [Google Scholar]
- (22).Sherry BD, Toste FD. J. Am. Chem. Soc. 2004;126:15978–15979. doi: 10.1021/ja044602k. [DOI] [PubMed] [Google Scholar]
- (23).The stereochemistry of the exocyclic double bond of alkene 36 is inferred based upon previous results obtained in our group in the formal Alder ene process. See: Brummond KM, Chen H, Sill P, You L. J. Am. Chem. Soc. 2002;124:15186–15187. doi: 10.1021/ja027588p..
- (24).For the preparation of allene-yne 40, see Supporting Information.
- (25).For examples of desilylation of α-silyl ketones upon silica gel chromatography, see: Dalton AM, Zhang Y, Davie CP, Danheiser RL. Org. Lett. 2002;4:2465–2468. doi: 10.1021/ol026014m.. Leost F, Doutheau A. Tetrahedron Lett. 1999;40:847–848..
- (26).Horvath A, Backvall J-E. Chem. Commun. 2004:964–965. doi: 10.1039/b316482a. [DOI] [PubMed] [Google Scholar]
- (27).X-ray crystallography diffraction allows to establish the absolute configuration of a chiral molecule containing a heavy atom such as Silicon through the use of the Flack parameter. For a recent review regarding the determination of the absolute configuration of a molecule using X-ray crystallography, see: Flack HD, Bernardinelli G. Chirality. 2008;20:681–690. doi: 10.1002/chir.20473.
- (28).Xiong H, Hsung RP, Wei L-L, Berry CR, Mulder JA, Stockwell B. Org. Lett. 2000;2:2869–2871. doi: 10.1021/ol000181+. [DOI] [PubMed] [Google Scholar]; For a general review on allenamides, see: Wei L.-l., Xiong H, Hsung RP. Acc. Chem. Res. 2003;36:773–782. doi: 10.1021/ar030029i.
- (29).Alcaide B, Almendros P, Aragoncillo C. Chem. Eur. J. 2002;8:1719–1729. doi: 10.1002/1521-3765(20020402)8:7<1719::aid-chem1719>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- (30).(Ra)-hexa-3,4-dien-1-ol was synthesized according to a literature procedure: Stoll AH, Blakey SB. J. Am. Chem. Soc. 2009;132:2108–2109. doi: 10.1021/ja908538t..
- (31).Artok L, Kus M, Ziyanak F. Adv. Synth. Catal. 2011;353:897–902. [Google Scholar]
- (32).Achard T, Lepronier A, Gimbert Y, Clavier H, Giordano L, Tenaglia A, Buono G. Angew. Chem. Int. Ed. 2011;50:3552–3556. doi: 10.1002/anie.201007992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



