Abstract
Aims: We performed a meta-analysis to assess the possible association between the MTHFR gene C677T polymorphism and hemorrhagic stroke. Methods: A comprehensive search was conducted to identify all case–control or cohort design studies of the associations between C677T and HS. The fixed or random effect pooled measure was selected on the basis of a homogeneity test among studies. Heterogeneity among studies was evaluated using the I2. Meta-regression and the “leave-one-out” sensitive analysis of Patsopoulos et al. were used to explore potential sources of between-study heterogeneity. Publication bias was estimated using the Begg's test. Results: Fifteen case–control studies corresponded to the inclusion criteria, including 2034 cases and 4485 controls for the present meta-analysis. After excluding articles that deviated from the Hardy–Weinberg equilibrium in controls and the key contributors to between-study heterogeneity, significant associations between the MTHFR C677T genetic polymorphism and the risk of hemorrhagic stroke were observed in dominant (Odds ratio [OR] 1.611, 95% confidence interval [CI] 1.336–1.942), codominant (OR 1.500, 95% CI 1.330–1.692), and recessive (OR 1.695, 95% CI 1.409–2.038) models. Conclusions: The meta-analysis suggests that the MTHFR C667T genetic polymorphism was associated with increased risk of hemorrhagic stroke, and the T allele may be an important risk factor for hemorrhagic stroke. The findings are of importance to the genetic epidemiology of hemorrhagic stroke, and to explore genetic diagnosis, treatment, and prevention of hemorrhagic stroke.
Introduction
Stroke is one of the common neurological disorders, which is the third most common leading cause of death worldwide (WHO, 2002). Hemorrhagic stroke accounts for 10% to 15% of all cases of stroke. The world-wide incidence of hemorrhagic stroke ranges from 10 to 20 cases per 100,000 population, and increases with age (Qureshi et al., 2001).
Case–control and prospective studies have demonstrated an association between moderate hyperhomocystinemia and risk of hemorrhagic stroke (Verhoef et al., 1994; Aronis et al., 2002; Li et al., 2003). As an important enzyme in homocysteine metabolism, mutations in methylenetetrahydrofolate reductase (MTHFR) play a critical role in modulating the levels of plasma homocysteine (Toyoda et al., 2004). A common mutation in the MTHFR gene, involving C-to-T substitution at nucleotide 677 (C677T), results in the conversion of alanine to valine. And this mutation leads to reduction in the enzyme activity and ensuing elevation of the plasma homocysteine level (Munshi et al., 2008). Apparently, most previous studies are limited to ischemic stroke, with less research available on hemorrhagic stroke. In recent years, several studies had been performed to evaluate the relationship between the C677T polymorphism in the MTHFR gene and hemorrhagic stroke risk. However, the results in the publications remain controversial, because individual studies have relatively small numbers of participants with underpower to detect the effect. In the present study, therefore, we perform the current meta-analysis to examine whether the MTHFR C677T polymorphism is associated with patients with hemorrhagic stroke.
Methods
Search strategy
A search was conducted for relevant available articles published in English or Chinese from four databases: (1) PubMed (1990–2012); (2) China National Knowledge Infrastructure (CNKI) (1990–2012); (3) China Biology Medical literature database (CBM) (1990–2012); and (4) Web of Science (ISI) (1990–2012). The search strategy used the following keywords: “MTHFR polymorphism,” “stroke,” “hemorrhagic stroke,” “cerebral hemorrhage,” and “C677T.” Additional studies not captured by our database searches were identified by reviewing the bibliographies of relevant articles as well as those of relevant studies.
Inclusion criteria
The inclusion criteria were as follows: (1) case–control study as original published to evaluate the association between the C677T polymorphism in the MTHFR gene and risk of hemorrhagic stroke; (2) confirmed hemorrhagic stroke with clinical, magnetic resonance imaging or computer tomography; (3) numbers in case and control groups or exposed and unexposed groups reported for each genotype, or data provided from which numbers could be calculated; (4) The most recent and complete article was chosen if a study had been published more than once. All identified studies were carefully reviewed independently by two investigators to identify and determine whether an individual study was eligible for inclusion in this meta-analysis. If the two reviewers could not reach a consensus about the eligibility of an article, it was resolved using a third investigator.
Data extraction
Data were extracted from all eligible publications by two investigators independently according to the inclusion criteria listed above. The following information was collected from each study: name of the first author, the publication date, country, ethnic origin of the studied population, total number of cases and controls, distributions of genotype and allele, mean age, male sex percentage for both case (exposed) and control (unexposed) groups.
Statistical analyses
Departure from the Hardy–Weinberg equilibrium (HWE) for the C677T genotype distribution of the MTHFR gene both in the case and control groups was tested by χ2 analysis with exact probability, and p<0.05 was considered as departure from HWE. Pooled measure was calculated as the inverse variance -weighted mean of the logarithm of the odds ratio (OR) with 95% confidence interval (CI) to assess the strength of association between the MTHFR gene C677T polymorphism and risk of hemorrhagic stroke for dominant (CT+TT vs. CC), recessive (TT vs. CT+CC), and codominant (T vs. C) models, respectively. Heterogeneity among studies was assessed by using the I2 statistic of Higgins and Thompson (2002), which describes the percentage of total variation attributable to between-study heterogeneity as opposed to random error or chance. In the presence of substantial heterogeneity (I2>50%) (Higgins et al., 2003), the DerSimonian and Laird random effect model (REM) was used as the pooling method; otherwise, the fixed effect model (FEM) was adopted as the pooling method. Meta-regression with restricted maximum likelihood estimation was performed to assess the potentially important covariates: region (categorized as Asia and Europe), age (the ratio of age or mean age in the case group to that in the control group), and sex (the ratio of male percent in the case group to that in the control group) that might exert substantial impacts on between-study heterogeneity. The leave-one-out sensitive analysis (Patsopoulos et al., 2008) was carried out to evaluate the key studies with substantial impact on between-study heterogeneity. An analysis of influence was conducted (Tobias, 1999) to describe how robust the pooled estimator is to removal of individual studies. If the main estimate of an individual study's omitted analysis lies outside the 95% CI of the combined analysis, it is suspected of excessive influence. Publication bias was estimated using the Begg's test. All statistical analyses were performed by using STATA version 10.0 (Stata Corporation, College Station, TX). All reported probabilities (p-values) were two-sided, with the p-value less than 0.05 considered representative of statistical significance.
Results
Study characteristics
We identified 15 published articles (Zhao et al., 2001; Li et al., 2003; Fang et al., 2004; Hu et al., 2004; Ye et al., 2004; Zhang et al., 2004a; Zhang et al., 2004b; Cai et al., 2005; Dikmen et al., 2006; Sazci et al., 2006; Xiao et al., 2006; Zhang et al., 2008; Ruigrok et al., 2010; Hultdin et al., 2011; Somarajan et al., 2011) that were eligible for this meta-analysis on the relation of the C677T polymorphism in the MTHFR gene to hemorrhagic stroke. All 15 eligible studies were case–control designs. General characteristics, the C677T allele and genotype distributions in the published articles included in this meta-analysis are showed in Table 1.
Table 1.
Characteristics of MTHFR Gene C677T Polymorphism Genotype Distributions in Studies Included in the Meta-Analysis
| |
|
|
|
Genotype CC/CT/TT |
Allelic frequency T of 677, (%) |
|
|
|
|
|
|
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Country | Ethnicity | Case | Control | Case | Control | Mean age(case/control) | Percentage of male(case/control) | P for HWEa | ORc | Pd | χ2 |
| Dikmen et al. | 2006 | Turkey | European | 28/18/3 | 32/21/2 | 24.49 | 22.73 | N/56.8 | N/29 | 0.601 | 1.73 | 0.765 | 0.089 |
| Sazci et al. | 2006 | Turkey | European | 10/11/7 | 115/119/25 | 44.64 | 32.63 | N/56.7 | N/56 | 0.572 | 3.12 | 0.071 | 3.262 |
| Ynte et al. | 2010 | Dutch | European | 185b/N/22 | 695b/N/69 | N | N | 59.5/N | 28/N | N | 1.20 | N | N |
| Hultdin et al. | 2011 | Sweden | European | 21/30/8 | 401/306/59 | 38.98 | 27.68 | 54.8/55 | 73/60 | 0.928 | 1.88 | 0.009 | 6.876 |
| Fang et al. | 2004 | China | Asian | 7/10/10 | 40/37/19 | 55.56 | 39.06 | 57.4/N | 59/N | 0.085 | 2.38 | 0.030 | 4.686 |
| Xiao et al. | 2006 | China | Asian | 12/33/16 | 49/41/10 | 53.29 | 30.5 | N/53 | N/58 | 0.813 | 3.20 | 0.000 | 16.507 |
| Ye et al. | 2004 | China | Asian | 63/27/10 | 192/87/21 | 23.5 | 21.5 | 62/63 | N/67 | 0.017 | 1.48 | 0.554 | 0.3497 |
| Zhang et al. | 2004 | China | Asian | 94/21/59 | 40/49/11 | 39.94 | 35.5 | 69.68/57.7 | 63/51 | 0.662 | 1.42 | 0.031 | 4.662 |
| Zhao et al. | 2001 | China | Asian | 28/85/88 | 48/87/55 | 64.93 | 51.84 | 60.19/60.15 | 62/63 | 0.248 | 1.90 | 0.000 | 13.129 |
| Zhang et al. | 2004 | China | Asian | 37/68/51 | 65/123/51 | 54.49 | 47.07 | 59/57 | 64/38 | 0.697 | 1.79 | 0.042 | 4.153 |
| Cai et al. | 2005 | China | Asian | 35/24/18 | 35/19/11 | 38.96 | 31.54 | 65.69/62.25 | 57/51 | 0.010 | 1.50 | 0.193 | 1.694 |
| Hu et al. | 2007 | China | Asian | 11/12/9 | 61/42/12 | 46.88 | 28.7 | 57.9/55.3 | 56/45 | 0.257 | 3.36 | 0.006 | 7.525 |
| Li et al. | 2003 | China | Asian | 145/245/113 | 610/824/398 | 46.82 | 44.21 | 58.2/59.6 | 63/57 | 0.000 | 1.04 | 0.141 | 2.166 |
| Zhang et al. | 2008 | China | Asian | 57/103/62 | 74/140/68 | 51.26 | 48.94 | 63.5/59.4 | N/N | 0.906 | 1.22 | 0.284 | 1.148 |
| Somarajan et al. | 2011 | Indian | Asian | 164/44/9 | 129/54/5 | 14.29 | 17.02 | 57/55.25 | 69/67 | 1.000 | 1.58 | 0.490 | 0.477 |
P for Hardy–Weinberg equilibrium (HWE) in control group.
The number of (CC+CT).
Odds ratio of individual study in recessive model (TT vs. CC+CT).
P for allelic (T vs. C) association.
N, not available; χ2, chi-square for allelic (T vs. C) association.
Quantitative synthesis
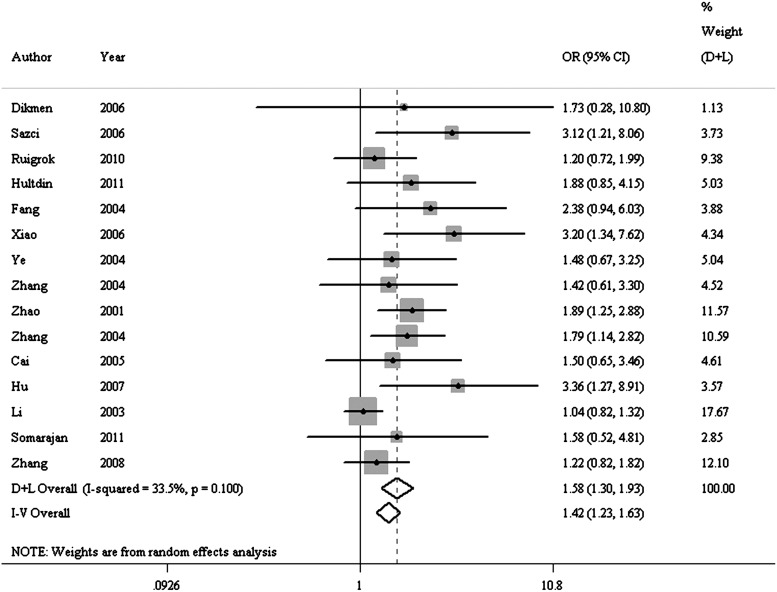
Results of pooled analysis are summarized in detail in Table 2. In the overall analysis, this meta-analysis showed a significant association of the T allele and increased risk of hemorrhagic stroke in the codominant (REM: OR=1.395, 95% CI=1.194–1.630), dominant (REM: OR=1.425, 95% CI=1.149–1.767) and recessive (FEM: OR=1.416, 95% CI=1.230–1.631) models (Fig. 1). After excluding articles (Li et al., 2003; Ye et al., 2004; Cai et al., 2005) that deviated from HWE in controls, all the associations were not altered significantly.
Table 2.
Pooled Measures on the Relations of MTHFR Gene C677T Polymorphisms to Hemorrhagic Stroke Risk
| |
|
Before HETRED analysis |
After HETRED analysis |
|||
|---|---|---|---|---|---|---|
| Data | Inherited model | FEM pooled OR (95% CI) | REM pooled OR (95% CI) | I2(%) | Pooled OR (95% CI) | I2(%) |
| All relevant articles | Dominant | 1.321 (1.165–1.499)b | 1.425 (1.149–1.767)b | 56 | 1.274 (1.120–1.449)a | 42.7 |
| Recessive | 1.416 (1.230–1.631)a | 1.583 (1.296–1.933)a | 33.5 | — | — | |
| Codominant | 1.280 (1.176–1.394)b | 1.395 (1.194–1.630)b | 61 | 1.279 (1.170–1.398)b | 42.1 | |
| Excluded for DHWE | Dominant | 1.418 (1.196–1.682)a | 1.551 (1.147–2.097)a | 63.9 | 1.611 (1.336–1.942)b | 45 |
| Recessive | 1.695 (1.409–2.038)b | 1.695 (1.409–2.040)b | 0.3 | — | — | |
| Codominant | 1.419 (1.265–1.591)b | 1.488 (1.225–1.808)b | 61.1 | 1.500 (1.330–1.692)b | 45.9 | |
p<0.05, bp<0.01.
CI, confidence interval; dominant model, TT+CT versus CC; recessive model, TT versus CT+CC; codominant model, T versus C; DHWE, deviated from HWE in controls; FEM, fixed effect model; REM, random effect model; HETRED, sensitivity analysis for reducing heterogeneity by omitting study using the STATA module of HETRED when I2>50%.
FIG. 1.
Forest plot of odds ratios (ORs) for hemorrhagic stroke in the recessive model (TT vs. CT+CC) of the MTHFR gene C677T polymorphism.
Sources of heterogeneity and sensitivity analysis
As seen in Table 2, before the leave-one-out sensitive analysis, strong evidence of heterogeneity among studies was demonstrated in the domiant and codomiant inherited models. However, univariate meta-regression with the covariates of a region (categorized as Asia and Europe), age (the ratio of mean age in the case group to that in the control group), and sex (the ratio of male percent in the case group to that in the control group) for the above-mentioned polymorphisms, showed that no covariates had a significant impact on between-study heterogeneity (data not shown).
After excluding articles that deviated from HWE in controls (Li et al., 2003; Ye et al., 2004; Cai et al., 2005), no substantial variation of I2 was found in the above-mentioned inherited models based on the tentative categorization of I2 values (Higgins et al., 2003). The key contributor of the article to this low between-study heterogeneity assessed by the leave-one-out sensitive analysis was Somarajan et al. (2011). After further excluding this article, low heterogeneity (I2<50%) was found, and the association of the C677T polymorphism in the MTHFR gene to hemorrhagic risk altered only slightly in dominant (OR=1.611, 95% CI=1.336–1.942), codominant (OR=1.500, 95% CI=1.330–1.692), and recessive (OR=1.695, 95% CI=1.409–2.038) models (detailed data shown in Table 2).
Influence analysis
No individual study was found to have excessive influence on the pooled effect in the three above-mentioned inherited models before and after excluding the article that deviated from HWE in controls (Li et al., 2003; Ye et al., 2004; Cai et al., 2005), and further, the key contributor (Somarajan et al., 2011) to low between-study heterogeneity.
Publication bias
After exclusion of articles deviating from HWE in controls and sensitivity analysis, no significant publication bias was detected in any of the above-mentioned inherited models (data not shown).
Discussion
Homocysteine plays an important role in vascular function and its levels are determined by the interaction of genetic and environmental factors. A mutation in the human MTHFR gene at 677 C- to- T leads to reduction in the enzyme activity and ensuing elevation of the plasma homocysteine level (Munshi et al., 2008). In recent years, several studies had been performed to evaluate the relationship between the C677T polymorphism in the MTHFR gene and hemorrhagic stroke risk. However, the results in the publications remain controversial. Because individual studies have relatively small numbers of participants with underpower to detect the effect, a meta-analysis may be the appropriate approach to obtain a more definitive conclusion regarding the role of the MTHFR gene C677T polymorphism on risk of hemorrhagic stroke. In the present meta-analysis, we finally retrieved 15 studies (2034 cases and 4485 controls) to evaluate this association. As far as we know, this is the first meta-analysis carried out to investigate the relationship between the MTHFR C677T genetic polymorphism and hemorrhagic stroke. In our meta-analysis, the combined evidence suggests that the MTHFR C677T polymorphism is significant associated with the risk of hemorrhagic stroke. According to Munafo and Flint (2004), between-study heterogeneity is common in meta-analysis for genetic association studies. Our meta-analysis also showed significant between-study heterogeneity in most of the inherited models. A number of characteristics, such as population stratification, characteristics of the sample, design quality, noncomparable measure of genotyping, variation of the covariate, and deviation from HWE in some studies, could be the cause of the between-study heterogeneity. Thus, we used both meta- regression and the leave-one-out sensitive analysis that aims to reduce the between-study heterogeneity to explore the potential important causes of the between-study heterogeneity for both covariates and studies. Our meta-analysis did not find any of the above-mentioned covariates as the important contributor to the between-study heterogeneity. After excluding articles that deviated from HWE in controls (Li et al., 2003; Ye et al., 2004; Cai et al., 2005), no substantial variation of I 2 was found in the above-mentioned inherited models based on the tentative categorization of I 2 values (Higgins et al., 2003). The key contributor of the article to this low between-study heterogeneity assessed by the leave-one-out sensitive analysis was by Somarajan et al., (2011). After further excluding this article, low heterogeneity (I2<50%) was found in the inherited models, and the result is not changed. In our meta-analysis, we could not find any significant publication bias in the above-mentioned inherited models; this may be due to the small sample size of studies in our meta-analysis.
In conclusion, this is the first meta-analysis to confirm the association between the T allele of the C677T polymorphism in the MTHFR gene and increased risk of hemorrhagic stroke. Further studies are needed to confirm the findings, and determine the utility of the C677T polymorphism in the MTHFR gene in the genetic diagnosis, treatment, and prevention of hemorrhagic stroke.
Acknowledgments
This report was supported by the Natural Science Foundation of Shandong Province (grant # ZR2009CM111 and ZR2010HM100).
Author Disclosure Statement
No competing financial interests exist.
References
- Aronis S. Bouza H. Pergantou H, et al. Prothrombotic factors in neonates with cerebral thrombosis and intraventricular hemorrhage. Acta Paediatr Suppl. 2002;91:87–91. doi: 10.1111/j.1651-2227.2002.tb02910.x. [DOI] [PubMed] [Google Scholar]
- Cai YM. Lin L. Zhou WZ. Lu Q. Analysis between plasma Homocysteine level and the gene polymorphism of MTHFR in cerebrovascular disease patients. J Neurol Neurorehabil. 2005;2:196–199. [Google Scholar]
- Dikmen M. Ozbabalik D. Gunes HV, et al. Acute stroke in relation to homocysteine and methylenetetrahydrofolate reductase gene polymorphisms. Acta Neurol Scand. 2006;113:307–314. doi: 10.1111/j.1600-0404.2005.00556.x. [DOI] [PubMed] [Google Scholar]
- Fang L. Wu YQ. Wu TW. Correlation of polymorphism 0f gene methylenetetrahydrofolate reductase and cystathionine beta-synthase with heredity of cerebral infaraction and cerebral hemorrhage in northern Chinese Han people. Chin J Clin Rehabil. 2004;8:4654–4656. [Google Scholar]
- Higgins JP. Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Higgins JP. Thompson SG. Deeks JJ. Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu RL. Zhao SG. Niu GM, et al. The association between gene ploymorphisms of N5,10-methylene-tetrahydrofolate reductase (MTHFR) and mongol nation patients with primarily hypertension disease and hypertension complicating cerebrovascular disease. J Neurol Sci. 2004;17:13–15. [Google Scholar]
- Hultdin J. Guelpen BV. Winkvist A, et al. Prospective study of first stroke in relation to plasma homocysteine and MTHFR 677C>T and 1298A>C genotypes and haplotypes–evidence for an association with hemorrhagic stroke. Clin Chem Lab Med. 2011;49:1555–1562. doi: 10.1515/CCLM.2011.234. [DOI] [PubMed] [Google Scholar]
- Li Z. Sun L. Zhang H, et al. Elevated plasma homocysteine was associated with hemorrhagic and ischemic stroke, but methylenetetrahydrofolate reductase gene C677T polymorphism was a risk factor for thrombotic stroke: a multicenter case–control study in China. Stroke. 2003;34:2085–2090. doi: 10.1161/01.STR.0000086753.00555.0D. [DOI] [PubMed] [Google Scholar]
- Munafo MR. Flint J. Meta-analysis of genetic association studies. Trends Genet. 2004;20:439–444. doi: 10.1016/j.tig.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Munshi A. Sultana S. Kaul S, et al. Angiotensin-converting enzyme insertion/deletion polymorphism and the risk of ischemic stroke in a South Indian population. J Neurol Sci. 2008;272:132–135. doi: 10.1016/j.jns.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Patsopoulos NA. Evangelou E. Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis:proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37:1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi AI. Tuhrim ST. Broderick JP, et al. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- Ruigrok YM. Slooter AJC. Rinkel GJE, et al. Genes influencing coagulation and the risk of aneurysmal Subarachnoid hemorrhage, and subsequent complications of secondary cerebral ischemia and rebleeding. Acta Neurochir. 2010;152:257–262. doi: 10.1007/s00701-009-0505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazci A. Ergul E. Tuncer N, et al. Methylenetetrahydrofolate reductase genepolymorphisms are associated with ischemic and hemorrhagic stroke: dual effect of MTHFR polymorphisms C677T and A1298C. Brain Res Bull. 2006;71:45–50. doi: 10.1016/j.brainresbull.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Somarajan BI. Kalita J. Mittal B. Misra UK. Evaluation of MTHFR C677T polymorphism in ischemic and hemorrhagic stroke patients. A case–control study in a Northern Indian population. J Neurol Sci. 2011;304:67–70. doi: 10.1016/j.jns.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull. 1999;47:15–17. [Google Scholar]
- Toyoda K. Uwatoko T. Shimada T, et al. Recurrent small-artery disease in hyperhomoc- ysteinemia: widowers' stroke syndrome? Intern Med. 2004;43:869–872. doi: 10.2169/internalmedicine.43.869. [DOI] [PubMed] [Google Scholar]
- Verhoef P. Hennekens CH. Malinow MR, et al. Aprospective study of plasma homocysteine and risk of ischemic stroke. Stroke. 1994;25:1924–1930. doi: 10.1161/01.str.25.10.1924. [DOI] [PubMed] [Google Scholar]
- WHO. WHO; Geneva: 2002. The world Health Report 2002: reducing risks, promoting healthy life. [DOI] [PubMed] [Google Scholar]
- Xiao YQ. Jiang LL. Lu Q, et al. The relationship between the gene polymorphism of methylene tetrahydrofolate reductase and plasma Homocysteine level with gene cerebrovascular disease. Lab Med. 2006;21:201–204. [Google Scholar]
- Ye H. Yan JT. Shao JM, et al. A case-control study on the relationship between stroke and plasma homocysteine level and the mutation of MTHFR gene. Chin Epidemiol. 2004;25:958–961. [PubMed] [Google Scholar]
- Zhang F. Lu L. Shi H, et al. The relationship between MTHFR gene polymorphism and cerebral hemorrhage. J Clin Neurol. 2004a;17:267–269. [Google Scholar]
- Zhang Y. Xie RP. Chen DF. Association of methylene tetrahydrofolate reductase polymorphism with cerebral hemorrhage; a case-control study. J Peking Univ. 2004b;26:219–221. [Google Scholar]
- Zhang Y. Xie RP. Shen Y. Fan DS. Interaction between methylene tetrahydrofolate reductase C667T gene polymorphism and sleep duration on risk of stroke pathogenesis. J Peking Univ. 2008;40:262–269. [PubMed] [Google Scholar]
- Zhao Y. Ma LY. Wang XY. Liu LS. Relationship between MTHFR gene C677T polymorphism and hemorrhagic stroke. Chin J Crit Care. 2001;21:577–579. [Google Scholar]