Abstract
Background: Mitochondrial mutations have been shown to be responsible for syndromic and nonsyndromic hearing impairment. Aim: To assess the genotypic–phenotypic correlation of mitochondrial DNA mutations in three generations of a single family. Methods: A single family with maternally inherited diabetes and hearing loss was recruited. Genomic DNA was subject to polymerase chain reaction–restriction fragment length polymorphism analysis (ApaI) for A3243G mutation detection and confirmation with direct DNA sequencing. The degree of heteroplasmy for the A3243G mutation in blood DNA samples was quantified. In addition, we reviewed audiological data of A3243G-associated hearing loss cases from the literature to provide details of audiologic features. Results: Six of 11 family members were recruited. All affected members harbored the A3243G mutation. Four of six members had diabetes. Five of five affected members demonstrated hearing loss ranging from mild to severe. The degree of heteroplasmy ranged from 5.51% to 27.74%. Conclusions: Patients with a greater percentage of heteroplasmy have a trend toward more severe phenotypic presentations. Hearing loss is bilateral, sensorineural, and symmetric. The main audiogram shapes found were sloping. Additional studies are necessary to clarify the relationship between degree of heteroplasmy and phenotypic presentation.
Introduction
It is estimated that up to 20% of all postlingual hearing loss may be due to mitochondrial DNA (mtDNA) mutations (Estivill et al., 1998). Mitochondrial diseases are multisystem disorders with a wide spectrum of severity (Xing et al., 2007). The onset and severity of hearing loss, as well as other symptoms, vary greatly among patients with mitochondrial diseases and may manifest as syndromic or nonsyndromic forms, as seen in aminoglycoside-induced hearing loss (Gold and Rapin, 1994; Hutchin and Cortopassi, 2000; Guan, 2004; Hsu et al., 2005; Liu et al., 2008). Even in the same family, patients may have completely different levels of hearing loss (Harrison et al., 1997).
Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) is one of a handful of syndromic illnesses associated with hereditary hearing loss and mtDNA mutations (Hutchin and Cortopassi, 2000; Hirano and Pavlakis, 1994). More than 80% of patients with MELAS carry the A3243G mutation in the mitochondrial tRNAleu(UUR) gene (Goto et al., 1992). Hearing loss has been implicated in up to 75% of the cases and deafness as a clinical feature in 44% in a meta-analysis by Chinnery et al. (1997, 2000a). As with other mitochondrial diseases, while some patients with MELAS syndrome present asymptomatically, others may suffer severe central neurological complications (Hutchin and Cortopassi, 2000). To understand this variability, researchers have attempted to determine the relationship between genotype and phenotype using different methods.
Population-based studies performed in northeast England (Chinnery et al., 2000b) and in Finland (Majamaa et al., 1998) have shown that the mutation carrier frequencies of the A3243G mutation is estimated at 1.4 and 16.3 per 100,000 people, respectively. These incidence rates were obtained from patients referred for molecular diagnosis to catchment areas where population census data could be established. However, a study by Manwaring et al. (2007) reported a higher rate. Analyzing a large cohort of individuals within a defined urban area within the Australian population, the prevalence of the A3243G mutation has been determined at 236/100,000. This suggests that individuals with the 3243A>G mtDNA mutation could be markedly under-recognized. The frequency of the A3243G mutation was 0.5% and 1.7% in the United Kingdom and Japanese patients with nonsyndromic hearing loss (NSHL), respectively (Hutchin et al., 2001; Nagata et al., 2001). Only 0.1% of Iranian NSHL patients harbored the 3243A>G mutation (Montazer Zohour et al., 2012). Individuals with mtDNA point mutations carry both mutant and wild-type mtDNA within each cell, a condition called heteroplasmy. We studied a single family of European origin with maternally inherited diabetes and hearing loss, living in an urban area of Florida. We present clinical and audiologic data of this three-generation family associated with the A3243G mtDNA mutation to further evaluate the relationship between the genotype and phenotype using peripheral blood cell heteroplasmy levels. We reviewed audiological data of 32 cases with the A3243G mutation from the literature to better understand the audiologic profile of this disease process.
Materials and Methods
Subjects
All subjects participating in the study were recruited from the University of Miami Ear Institute following institutional review board approval. Written informed consent for research participation was obtained from all participants and from parents of patients younger than 18 years.
A three-generation patient cohort consisting of 11 patients with a maternal transmission pattern of hearing loss was recruited. Seven of 11 family members show evidence of hearing loss and/or diabetes. Blood samples were collected from six members. Nine members of this pedigree were interviewed at length to identify both personal or family medical histories, and other clinical abnormalities. The clinical history and physical examination of family members was performed by the senior investigator with special emphasis on identifying potential environmental causes of hearing loss such as infection, trauma, noise, or exposure to ototoxic drugs, including aminoglycosides. Audiometric measurements of hearing loss were obtained. Air conduction thresholds were measured at 250 Hz, 500 Hz, 1 kHz, 2 kHz, 4 kHz, 6 kHz, and 8 kHz. Bone conduction thresholds were determined to ascertain whether there was any evidence for a conductive component in patients with hearing loss. The audiological profiles from the present study were supplemented by data from the literature. These subjects were ascertained from a variety of sources.
Mutational analysis
Genomic DNA was extracted from peripheral blood using a standard extraction method. The mtDNA A3243G mutation was determined by polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) analysis. Primers were designed to amplify the 3243 region of the mtDNA gene with primers: 5′-GCC TTC CCC CGT AAA TGA TA-3′ (forward primer) and 5′-AGG TTG GCC ATG GGT ATG T-3′ (reverse primer) by using standard PCR conditions. Digestion of PCR product was subsequently carried out using the appropriate buffers and ApaI restriction enzyme. Equal amounts of digested samples were then analyzed by electrophoresis through 7% polyacrylamide gels. The proportions of digested and undigested PCR product were determined using the ImageJ program (U.S. National Institutes of Health, Bethesda, MD) after silver staining to determine the level of heteroplasmy for the mitochondrial A3243G mutation in these subjects.
DNA sequencing
Bi-directional sequencing was carried out using genomic DNA extracted from subjects with the A3243G mutation (results from PCR-RFLP analysis), and was performed using the ABI PRISM Big Dye Terminator Sequencing kit (Applied Biosystems, Carlsbad, CA) on a 3100 ABI DNA-sequencer (Applied Biosystems).
Results
Clinical features
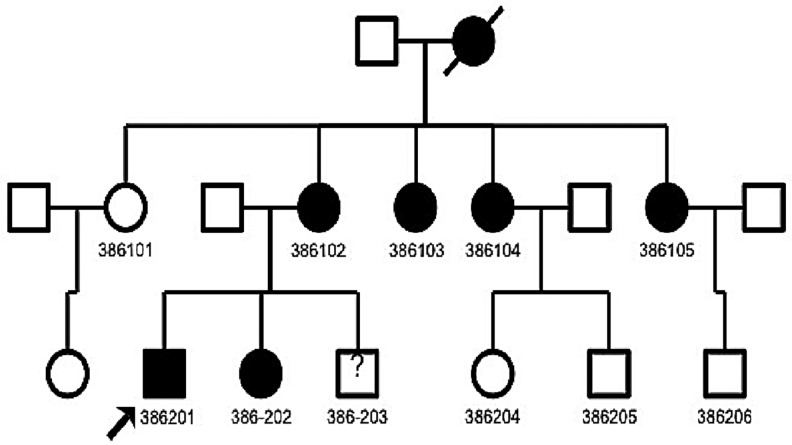
There were a total of 11 subjects (5 women and 6 men) identified. Nine of them were interviewed, and blood samples were collected from six members. The individuals ranged in age from 14 to 55 years with a mean of 32 years. Unfortunately, the matriarch of the family was deceased and history of diabetes was obtained from familial interviews. No genetic testing was performed on the matriarch. The first progeny found harboring the A3243G mutation were four sisters. The next progeny included four men and two women representing the offspring of the previous affected generation (Fig. 1). Clinical features of the subjects are summarized in Table 1. Relevant associated illnesses are listed, including malignant brain tumor, stroke, and Crohn's disease requiring liver transplant.
FIG. 1.
Three-generation pedigree of a single family affected with the A3243G mitochondrial DNA (mtDNA) mutation. The affected individuals are represented by filled symbols. The proband (386201) is indicated by an arrow. No data were available for individual 386–203.
Table 1.
Summary of Clinical and Molecular Data of Several Members in the Pedigree with Diabetes Mellitus
| Subjects | Gender | Age at test (years) | Age at onset (years) | Degree of hearing loss | Audiogram shape | Diabetes mellitus | Associated illnesses | Heteroplasmy of A3243G (%) |
|---|---|---|---|---|---|---|---|---|
| 386101 | F | - | No | Normal hearing | No | No | No | 0 |
| 386102 | F | 55 | Unknown | Severe | Flat | No | Malignant brain tumor | 5.51 |
| 386104 | F | 46 | 40+ | Moderate to severe | Gently sloping | Yes | No | 14.33 |
| 386105 | F | 46 | 35+ | Severe | Gently sloping | Yes | No | 10.70 |
| 386201 | M | 29 | Unknown | Moderate to severe | Gently sloping | Yes | MELAS; stroke | 27.74 |
| 386202 | F | 31 | Unknown | Mild | High frequency | Yes | Liver transplant secondary to Crohn's disease | 25.89 |
| 386204 | F | 19 | Normal hearing | |||||
| 386205 | M | 15 | Normal hearing | |||||
| 386206 | M | 14 | Normal hearing |
MELAS, mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes.
Genetic testing
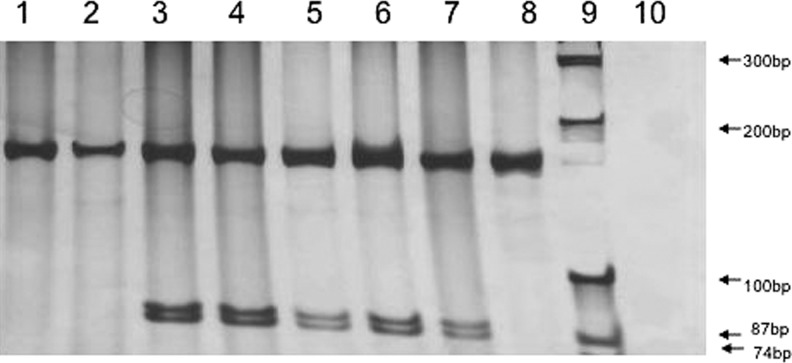
Genomic DNA was extracted from peripheral blood. Polymerase chain reaction amplification of the mtDNA portion coding for tRNAleu(UUR) was performed. The amplified 161-bp fragment was digested with ApaI. Digested products were analyzed on a 7% polyacrylamide gel. Since the A3243G mutation created an ApaI site, the restriction enzyme cleaves the amplicon into 87- and 74-bp fragments only when the A3243G mutation is present (Fig. 2; lanes 3 to 7). Direct DNA sequence analysis confirmed the presence of the A3243G mutation (data not shown). Quantification from the proportion of digested and undigested PCR product revealed that heteroplasmy levels ranged from 0% to 27.74% in our study group (Fig. 2 and Table 1). Older patients tended to have lower levels of heteroplasmy in peripheral blood. Patient 386101 had 0% heteroplasmy and no clinically significant hearing loss or other illnesses. Patient 386102 (age 55) had 5.51% heteroplasmy and presented with a malignant brain tumor. The patients with 14.33% (386104) and 10.70% (386105) heteroplasmy did not present with any associated illnesses other than hearing loss. However, patients with heteroplasmy levels greater than 20% had incidental history of stroke or Crohn's disease requiring liver transplant (386201 and 386202, respectively).
FIG. 2.
Identification of the A3243G mutation in mtDNA using the ApaI restriction enzyme analysis of polymerase chain reaction (PCR) products. The primer pair generates a PCR product of 161 bp. PCR products amplified from mutant A3243G allele(s) are digested into 87 and 74 bp, whereas normal control DNA products are not digested. Lane 1 for normal control DNA; lanes 2 and 8 for DNAs for individuals without A3243G mutations; lanes 3–7 for individuals with A3243G mutations; lane 9 for 100 bp DNA ladder; lane 10 for negative control; lane 3: 386202; lane 4: 386201; lane 5: 386105; lane 6: 386104; lane 7: 386102; lane 8: 386101.
Audiometry
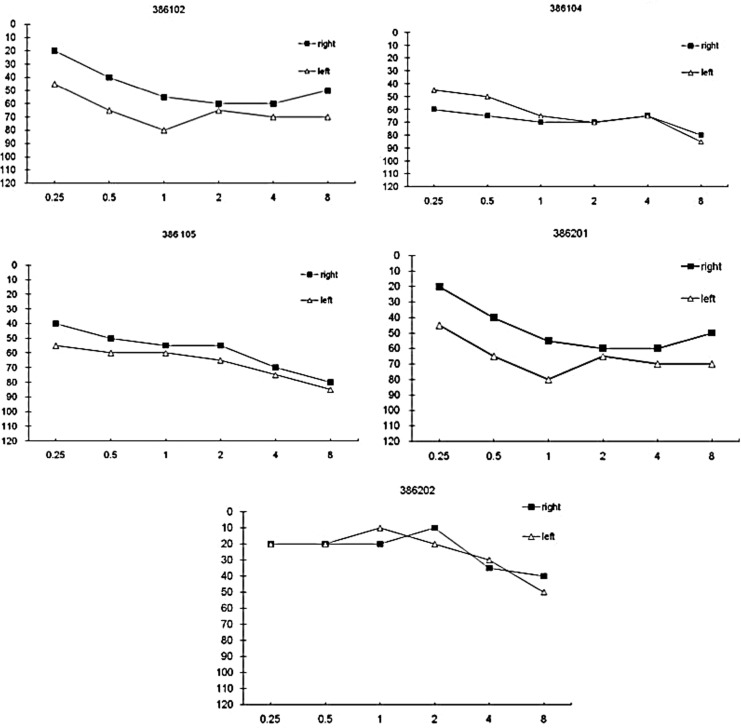
Hearing loss was noted in five out of nine interviewed patients (Fig. 3 and Table 1). All affected patients were noted to be aged 20 or older. Two patients reported hearing loss onset in approximately the mid-third decade of life. The remaining three patients with hearing loss did not recall the age at onset. The severity of hearing loss ranged from mild to severe with no clear correlation with age. However, older patients tended to have more severe hearing loss. The audiogram results display a symmetrical and gently sloping pattern with more severe loss in higher frequencies. One patient (386202) had a mild hearing loss only at high frequency levels. All patients with diabetes had some level of hearing loss, but hearing loss did not necessarily present with diabetes mellitus in our patient cohort. There was no association between degree of hearing loss and other associated illnesses in this family. Two patients (386104 and 386105) with moderate to severe hearing loss did not have any other clinical findings. However, two subjects (386201 and 386202) with mild to moderate hearing loss presented with malignant brain tumor, stroke, or Crohn's disease. All patients under 20 years of age presented with normal hearing and no other clinical symptoms.
FIG. 3.
Pure-tone audiometry demonstrating mild to severe hearing loss of five individuals with the A3243G mtDNA mutation.
Discussion
The mutations in the mitochondrial genome cause a dysfunction in the oxidative phosphorylation of the cell (Karkos et al., 2004). Therefore, metabolically active tissues, such as skeletal muscle, heart, brain, and cochlea, are especially sensitive to mitochondrial mutation and dysfunction (Karkos et al., 2004). In the ear, the stria vascularis, which is critical to maintenance of the ionic potential, is highly metabolically active. Consequently, impaired oxidative phosphorylation is believed to result in free oxygen production with resulting loss of function and hearing loss (Sue et al., 1998). In the A3243G mutation, multiple biochemical aspects of the tRNAleu(URR) gene are affected, which include structure stabilization, methylation, amino-acylation, and codon recognition (Finsterer, 2007). As a result, the A3243G mutation causes markedly reduced adenosine triphosphate production, increased lactate production, impaired cell calcium homeostasis, increased reactive oxygen species, and reduced insulin secretion. The loss of these critical biochemical functions results in systemic dysfunction and may manifest as MELAS (Finsterer, 2007).
Most pathological mtDNA mutations harbor a mixture of mutated and wild-type mtDNA, described as heteroplasmy (Chinnery et al., 2000a). Interestingly, the phenotypic expression of mitochondrial defects only occurs when the heteroplasmic levels of the corresponding tissue exceed a critical threshold level (Larsson and Clayton, 1995). It has been hypothesized that pathologic conditions of mitochondrial diseases may occur due to the organ-specific vulnerability to energy needs (Shoffner and Wallace, 1990; Larsson and Clayton, 1995). However, understanding this critical threshold has not been a simple quantitative task.
In our study, we measured the percent heteroplasmy levels in peripheral blood. However, the use of heteroplasmy to determine disease severity has not been clearly elucidated, and severity of symptoms does not always demonstrate a linear correlation (Chinnery et al., 1997; Xing et al., 2007). The use of peripheral blood has previously been shown to have an inverse relationship with the onset of hearing loss and diabetes; for example, increased heteroplasmy results in an earlier age of symptom onset (Olsson et al., 1998). Conversely, Chinnery et al. (2000a) found no statistically significant correlation between degree of heteroplasmy and severity of hearing loss. Our data suggest that patients with a higher rate of mutation demonstrate a tendency toward more severe clinical phenotypes. Patient 386201 was noted to have MELAS and 27.74% heteroplasmy at age 29. Interestingly, patient 386202 with the next highest degree of heteroplasmy (25.89%) was noted to have a mild to moderate degree of hearing loss and diabetes at age 31. Our oldest patient, 386102, was noted to have 5.51% heteroplasmy with flat hearing loss and malignant brain tumor but no evidence of diabetes or other clinical findings.
One possible mechanism for clinical variability may involve the heteroplasmic state of A3243G and other mtDNA mutations. The contribution of genetic background is well recognized in Leber's hereditary optic neuropathy (Howell, 1999). The A12308G polymorphism has also been shown to increase the risk of strokes in MELAS (Pulkes et al., 2000). While high levels of the A3243G mutation or heteroplasmy levels in muscle or blood are associated with hearing loss or stroke-like episodes, there may be other unknown confounders contributing to the clinical presentation (Van de Ouweland et al., 1992; Chinnery et al., 2000a; Deschauer et al., 2004). Therefore, some have hypothesized that there may be unaccounted mtDNA or DNA background attributing for the presentation of the MELAS syndrome. Intrafamilial clustering of phenotype would indirectly point to mtDNA background. However, our patients presented with variable phenotypes, for example, 386201 and 386202, despite similar levels of heteroplasmy and age suggesting possible genetic or environmental confounders.
Additionally, no studies to date have been able to fully account for the effect of age on blood heteroplasmy levels. Olsson et al. (1998) attempted to calculate the decline of heteroplasmy with age in peripheral blood in their study. They were able to derive from their patients (n=23) the annual decrease in the proportion of mutant mitochondria in blood as roughly 0.44%. However, this was estimated by looking at patients at one time point. In contrast, t'Hart et al. (1996) examined blood levels at two time points. They calculated the decline to average 0.69% per year in their patient population (n=17). However, this study also assumes a linear decline, which is uncertain. Our analysis does not allow us to comment on the rate of decline, but it does bring into question the degree of heteroplasmy of our older patients and its relation to the phenotype presentation. For example, patient 386102 was noted to have 5.51% heteroplasmy with severe, flat hearing loss but no evidence of diabetes. This may be accounted for by a higher degree of heteroplasmy at a younger age, which triggered the critical threshold for loss of function. Nevertheless, the absence of diabetes in the context of severe hearing loss suggests the possibility of confounding contributors to the phenotype.
In terms of age of onset, two patients with diabetes but no other associated illness in this study began to recognize hearing impairment in their mid-third decade of life. Previous reports suggest that patients with sensorineural hearing loss due to the A3243G mutation have onset hearing impairment from the 20s to the 50s (Chinnery et al., 2000a; Nagata et al., 2001). The youngest patients (386–204, 386–205, 386–206) in the present study had a normal hearing test at 19, 15, and 14 years of age, respectively, and were not analyzed for the A3243G mutation. The clinical manifestations associated with mtDNA mutations are very variable and relate in part to the severity and proportion of the mutant mtDNA. It is recognized that both genetic and environmental may act synergistically and cause hearing loss in individuals with a lower mutation load. For example, aminoglycoside antibiotics may precipitate deafness in persons with a particular mtDNA genotype (Prezant et al., 1993). Thus, asymptomatic individuals who have family history of maternal inheritance of hearing loss should avoid ototoxic agents, such as aminoglycoside, which may further compromise cochlear function (Estivill et al., 1998). For now even diagnosed, there is no curative treatment for patients who have mtDNA disease. Current therapy is limited to symptomatic relief and early detection of treatable symptoms such as cardiac disease and diabetes.
Many studies have investigated mtDNA-associated hearing loss (Table 2). Yamasoba et al. (1996) reported symmetric, bilateral SNHL initially affecting higher frequencies followed by deterioration of hearing from 1.5 to 7.9 dB per year. Liu et al. (2008) noted NSHL associated with mitochondrial mutations was often postlingual in onset with great variability in severity and a sloping audiogram. The A3243G mutation is also generally characterized by sloping hearing loss. Tamagawa et al. (1997) studied the audiological findings of nine patients with A3243G, and Sue et al. (1998) reviewed the audiometric findings of 18 patients with the MELAS syndrome. Their findings supported the notion of a progressive disease process with higher frequencies affected initially and a sloping hearing loss pattern, which is often seen with mtDNA hearing loss. In advanced stages of hearing loss, defined by a pure tone average more than 60 dB and serial audiometry, a flat shape suggesting progressive cochlear involvement was appreciated. Sue et al. (1998) further noted the presence of stepwise progression in at least five patients, partial reversibility in two patients, and asymmetry in four patients, suggesting that variable presentations are possible. The audiometry of our patient cohort is consistent with sloping hearing loss noted and more severe hearing loss associated with flat morphology of the audiogram.
Table 2.
Audiologic Features of the A3243G Mitochondrial DNA Mutation Described in the Literature
| Case | Sex | Onset of hearing loss | Age at audiogram | Shape of audiogram | Asymmetric hearing loss | Source |
|---|---|---|---|---|---|---|
| 1 | M | 12 | 17 | Sharp slope | No | Tamagawa et al. (1997) |
| 2 | F | 14 | 14 | Sloping | No | Tamagawa et al. (1997) |
| 3 | F | 17 | 20 | Sloping | No | Tamagawa et al. (1997) |
| 4 | M | 30 | 44 | Sloping | No | Tamagawa et al. (1997) |
| 5 | M | 24 | 35 | Flat | Yes | Tamagawa et al. (1997) |
| 6 | F | 39 | 42 | Sharp slope | No | Tamagawa et al. (1997) |
| 7 | F | 45 | 52 | Sloping | No | Tamagawa et al. (1997) |
| 8 | F | 40 | 50 | Sloping | No | Tamagawa et al. (1997) |
| 9 | F | 18 | 45 | Flat | No | Tamagawa et al. (1997) |
| 10 | F | 29 | 33 | Sloping | No | Yamasoba et al. (1996) |
| 11 | F | 26 | 38 | Sloping | Yes | Yamasoba et al. (1996) |
| 12 | F | 33 | 42 | Sloping | No | Yamasoba et al. (1996) |
| 13 | F | 39 | 54 | Flat | No | Yamasoba et al. (1996) |
| 14 | M | 55 | 61 | Sloping | Yes | Yamasoba et al. (1996) |
| 15 | F | - | 72 | Sloping | No | Sue et al. (1998) |
| 16 | M | 65 | 63 | Sloping | Yes | Sue et al. (1998) |
| 17 | F | 61 | 61 | High frequency | No | Sue et al. (1998) |
| 18 | M | 50 | 63 | Sloping | Yes | Sue et al. (1998) |
| 19 | F | 37 | 57 | Sloping | No | Sue et al. (1998) |
| 20 | F | 16 | 22 | High frequency | No | Sue et al. (1998) |
| 21 | M | - | 20 | Normal | No | Sue et al. (1998) |
| 22 | F | <14 | 27 | Flat | No | Sue et al. (1998) |
| 23 | F | 30 | 47 | Flat | Yes | Sue et al. (1998) |
| 24 | F | 40 | 35 | Sloping | Yes | Sue et al. (1998) |
| 25 | F | 28 | 40 | Sloping | No | Sue et al. (1998) |
| 26 | M | 15 | 44 | Sloping | No | Sue et al. (1998) |
| 27 | F | <12 | 40 | Flat | Yes | Sue et al. (1998) |
| 28 | F | - | 19 | Normal | No | Sue et al. (1998) |
| 29 | M | - | 12 | Normal | No | Sue et al. (1998) |
| 30 | F | 35 | 33 | High frequency | Yes | Sue et al. (1998) |
| 31 | M | - | 15 | Normal | No | Sue et al. (1998) |
| 32 | M | 35 | 38 | Flat | No | Sue et al. (1998) |
Our study is limited by the number of subjects, details of exposure history, and lack of multiple testing time points to assess heteroplasmy stability. However, the relationship between heteroplasmy in blood and severity of hearing loss is not simply dismissible as seen with our patients. Future studies will need to address the stability of heteroplasmy over time and whether decay is exponential or linear. It remains uncertain if peak heteroplasmy levels at a young age may be adequate to breach the threshold level for loss of function alone or does an environmental insult, such as medication or nutrition, upset a critical balance. In conclusion, level of heteroplasmy may further help us to determine the severity of mtDNA mutations; however, a clear association continues to require further investigation.
Conclusions
Heteroplasmy levels are an important consideration in determining the severity of phenotype. However, additional studies are necessary to clarify the relationship between degree of heteroplasmy and phenotypic presentation of mtDNA mutations.
Acknowledgments
The authors thank the family for their kind participation in this study. This work was supported by NIH Grants DCR01 05575, DC012546 and DC012115.
Author Disclosure Statement
No competing financial interests exist.
References
- Chinnery PF. Elliott C. Green GR, et al. The spectrum of hearing loss due to mitochondria DNA defects. Brain. 2000a;123:82–92. doi: 10.1093/brain/123.1.82. [DOI] [PubMed] [Google Scholar]
- Chinnery PF. Howell N. Lightowlers RN. Turnbull DM. Molecular pathology of MELAS and MERRF. The relationship between mutation load and clinical phenotypes. Brain. 1997;120:1713–1721. doi: 10.1093/brain/120.10.1713. [DOI] [PubMed] [Google Scholar]
- Chinnery PF. Johnson MA. Wardell TM, et al. The epidemiology of pathogenic mitochondrial DNA mutations. Ann Neurol. 2000b;48:188–193. [PubMed] [Google Scholar]
- Deschauer M. Chinnery PF. Schaefer AM, et al. No association of the mitochondrial DNA A12308G polymorphism with increased risk of stroke in patients with the A3243G mutation. J Neurol Neurosurg Psychiatry. 2004;75:1200–1207. doi: 10.1136/jnnp.2003.026278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estivill X. Govea N. Barcelo E, et al. Familial progressive sensorineural deafness is mainly due o the mtDNA A1555G mutation and is enhanced by treatment of aminoglycosides. Am J Hum Genet. 1998;62:27–35. doi: 10.1086/301676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterer J. Genetic, pathogenetic, and phenotypic implications of the mitochondrial A3243G tRNALeu(UUR) mutation. Acta Neurol Scand. 2007;116:1–14. doi: 10.1111/j.1600-0404.2007.00836.x. [DOI] [PubMed] [Google Scholar]
- Gold M. Rapin I. Non-Mendelian mitochdonrial inheritance as a cause of progressive genetic sensorineural hearing loss. Int J Pediatr Otorhinolaryngol. 1994;30:91–104. doi: 10.1016/0165-5876(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Goto Y. Horai S. Matsuoka T, et al. Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS): a correlative study of the clinical features and mitochondrial DNA mutation. Neurology. 1992;42:545–550. doi: 10.1212/wnl.42.3.545. [DOI] [PubMed] [Google Scholar]
- Guan MX. Molecular pathogenetic mechanism of maternally inherited deafness. Ann N Y Acad Sci. 2004;1011:259–271. doi: 10.1007/978-3-662-41088-2_25. [DOI] [PubMed] [Google Scholar]
- Harrison TJ. Boles RG. Johnson DR, et al. Macular pattern retinal dystrophy, adult-onset diabetes, and deafness: a family study of A3243G mitochondrial heteroplasmy. Am J Ophthalmol. 1997;124:217–221. doi: 10.1016/s0002-9394(14)70787-1. [DOI] [PubMed] [Google Scholar]
- Hirano M. Pavlakis S. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (MELAS): Current concepts. J Child Neurol. 1994;9:4–13. doi: 10.1177/088307389400900102. [DOI] [PubMed] [Google Scholar]
- Hsu CH. Kwon H. Perng CL, et al. Hearing loss in mitochondrial disorders. Ann N Y Acad Sci. 2005;1042:36–47. doi: 10.1196/annals.1338.004. [DOI] [PubMed] [Google Scholar]
- Hutchin TP. Cortopassi GA. Mitochondrial defects and hearing loss. Cell Mol Life Sci. 2000;57:1927–1937. doi: 10.1007/PL00000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchin TP. Thompson KR. Parker M, et al. Prevalence of mitochondrial DNA mutations in childhood/congenital onset of non-syndromal sensorineural hearing impairment. J Med Genet. 2001;38:229–231. doi: 10.1136/jmg.38.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell N. Human mitochondrial diseases: answering questions and questioning answers. Int Rev Cytol. 1999;186:49–116. doi: 10.1016/s0074-7696(08)61051-7. [DOI] [PubMed] [Google Scholar]
- Karkos PD. Waldron M. Johnson IJ. The MELAS syndrome. Review of the literature: the role of the otologist. Clin Otolaryngol. 2004;29:1–4. doi: 10.1111/j.1365-2273.2004.00769.x. [DOI] [PubMed] [Google Scholar]
- Larsson NG. Clayton DA. Molecular genetic aspects of human mitochondrial disorders. Annu Rev Genet. 1995;29:151–178. doi: 10.1146/annurev.ge.29.120195.001055. [DOI] [PubMed] [Google Scholar]
- Liu XZ. Angeli S. Ouyang XM, et al. Audiological and genetic features of the mtDNA mutations. Acta Otolaryngol. 2008;128:732–738. doi: 10.1080/00016480701719011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majamaa K. Moilanen JS. Uimonen S, et al. Epidemiology of A3243G, the mutation for mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes: Prevalence of the mutation in an adult population. Am J Hum Genet. 1998;63:447–454. doi: 10.1086/301959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwaring N. Jones MM. Wang JJ, et al. Population prevalence of the MELAS A3243G mutation. Mitochondrion. 2007;7:230–233. doi: 10.1016/j.mito.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Montazer Zohour M. Tabatabaiefar MA. Dehkordi FA, et al. Large-scale screening of mitochondrial DNA mutations among Iranian patients with prelingual nonsyndromic hearing impairment. Genet Test Mol Biomarkers. 2012;16:271–278. doi: 10.1089/gtmb.2011.0176. [DOI] [PubMed] [Google Scholar]
- Nagata H. Kumahara K. Tomemori T, et al. Frequency and clinical features of patients with sensorineural hearing loss associated with the A3243G mutation of the mitochondrial DNA in otorhinolaryngic clinics. J Hum Genet. 2001;46:595–599. doi: 10.1007/s100380170027. [DOI] [PubMed] [Google Scholar]
- Olsson C. Zethelius B. Lagerstrom-Fermer M, et al. Level of heteroplasmy for the mitochondrial mutation A3243G correlates with age at onset of diabetes and deafness. Hum Mutat. 1998;12:52–58. doi: 10.1002/(SICI)1098-1004(1998)12:1<52::AID-HUMU8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Prezant TR. Agapian JV. Bohlman MC, et al. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat Genet. 1993;4:289–294. doi: 10.1038/ng0793-289. [DOI] [PubMed] [Google Scholar]
- Pulkes T. Sweeney MG. Hanna MG. Increased risk of stroke in patients with the A12308G polymorphism in mitochondria. Lancet. 2000;356:2068–2069. doi: 10.1016/s0140-6736(00)03408-5. [DOI] [PubMed] [Google Scholar]
- Shoffner JM., 4th Wallace DC. Oxidative phosphorylation diseases. Disorders of two genomes. Adv Hum Genet. 1990;19:267–330. [PubMed] [Google Scholar]
- Sue CM. Lipset LJ. Crimmins DS, et al. Cochlear origin of hearing loss in MELAS syndrome. Ann Neurol. 1998;43:350–359. doi: 10.1002/ana.410430313. [DOI] [PubMed] [Google Scholar]
- Tamagawa Y. Kitamura K. Hagiwara H, et al. Audiologic findings in patients with a point mutation at nucleotide 3,243 of mitochondrial DNA. Ann Otol Rhinol Laryngol. 1997;1997;106:338–342. doi: 10.1177/000348949710600414. [DOI] [PubMed] [Google Scholar]
- t'Hart LM. Jansen JJ. Lemkes HH, et al. Heteroplasmy levels of a mitochondrial gene mutation associated with diabetes mellitus decrease in leucocyte DNA upon aging. Hum Mutat. 1996;7:193–197. doi: 10.1002/(SICI)1098-1004(1996)7:3<193::AID-HUMU2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- van de Ouweland JM. Lemkes HH. Ruitenbeek K, et al. Mutation in mitochondrial tRNA Leu(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat Genet. 1992;1:368–371. doi: 10.1038/ng0892-368. [DOI] [PubMed] [Google Scholar]
- Xing G. Chen Z. Xin C. Mitochondrial rRNA and tRNA and hearing function. Cell Res. 2007;17:227–239. doi: 10.1038/sj.cr.7310124. [DOI] [PubMed] [Google Scholar]
- Yamasoba T. Oka Y. Tsukuda K, et al. Auditory findings in patients with maternally inherited diabetes and deafness harboring a point mutation in the mitochondrial transfer RNALEU(UUR) gene. Laryngoscope. 1996;106:49–53. doi: 10.1097/00005537-199601000-00010. [DOI] [PubMed] [Google Scholar]