Abstract
Objectives: Ventricular septal defect (VSD) is the most common congenital heart disease (CHD). Genome-wide linkage analysis revealed a potential CHD susceptibility locus in the homeodomain leucine zipper-encoding (HOMEZ) gene in a South Indian population. The present study aimed to identify potential pathogenic mutations for HOMEZ and to provide insights into the etiology of isolated VSD in the Chinese population. Methods: Case–control mutational analysis was performed in 400 patients with isolated VSD and 400 healthy controls. Protein-coding exton of HOMEZ and their flanking sequences were amplified by polymerase chain reaction and sequenced on an ABI3730 Automated Sequencer. CLC workbench software was used to compare the conservatism of the HOMEZ protein with other multiple species. The ExPASy-ProtScale online tool was used to predicate the alignment of the hydrophobic features. Results: Two novel heterozygous missense mutations (c.116 C>T; c. 630T>A) were identified in HOMEZ gene exon-2. The two mutations lead to alanine to valine substitution at position 39 and serine to arginine at position 210, which are highly conserved among many species. The hydropathicity of the valine and arginine residue at the position 39 and 210 were significantly different from the wild type. Conclusions: We have identified two novel heterozygous missense mutations in HOMEZ gene exon-2 in isolated VSD patients in the Chinese population and have found that these two mutations resulted in alteration of the hydropathicity of the HOMEZ protein. Therefore, the two missense mutations of the HOMEZ gene are directly linked with the etiology of isolated VSD in the Chinese population.
Ventricular septal defect (VSD) is the most common congenital heart disease (CHD) and is present in 33% of all affected infants (Correa-Villasenor et al., 1993). Reports of prevalence of VSD in population-based studies have shown large variations: 0.9–6.0 per 1000 live-births (Ferencz et al., 1985). Recent studies have suggested that up to 4% of asymptomatic neonates, at birth, had VSD (Sands et al., 1999). The causes of VSD are largely unknown. However, epidemiologic studies revealed a significant environmental contribution to the pathogenesis of VSD (Burki and Babar, 2001; Burdn et al., 2006). Familial aggregation and twin studies indicated the presence of genetic factors for susceptibility to this condition (Maestri et al., 1988; Oyen et al., 2010).
The homeodomain leucine zipper-encoding (HOMEZ) gene is a vertebrate homeobox gene. Bayarsaihan and coworkers first identified HOMEZ through database analysis by using the mouse sequence as query and mapped it to chromosome 14q11.2 by genomic sequence analysis (Nagase et al., 2000; Bayarsaihan et al., 2003). The gene contains two exons, the second of which contains most of the coding sequence (Bayarsaihan et al., 2003). The deduced 549-amino acid protein, which is translated with the gene has a calculated molecular mass of about 61 KD (Bayarsaihan et al., 2003). The HOMEZ gene encodes a protein with an unusual structural organization, which contains three atypical homeodomains, two leucine zipper-like motifs, proline- and serine-rich motifs, and an acidic domain. It also has a putative nuclear localization signal within homeodomain 2 (Bayarsaihan et al., 2003).
Studies demonstrated that a first-cousin marriage might be a significant risk factor for specific types of CHD in a consanguineous population (Becker et al., 2001; Tadmouri et al., 2009). Inbreeding studies suggest an autosomal recessive component in the cause of some congenital heart defects (Rose et al., 1985; Badaruddoza et al., 1994). Recently, a genome-wide linkage analysis implicated linkage of CHD with the polymorphism (rs1055061; c.1505G>A, p.Arg502Gln) of HOMEZ in a family-based South Indian population study (McGregor et al., 2010). Moreover, mutational analysis revealed three novel heterozygous sequence variations of HOMEZ in 54 Indian probands with CHD (McGregor et al., 2010).
Based on the above studies, we hypothesized that HOMEZ may play an important role in the development of VSD. The aims of our study were to identify potential pathogenic mutations for HOMEZ and to provide insights into the etiology of isolated VSD in the Chinese population.
Materials and Methods
Study population
A total of 400 nonsyndromic VSD patients and 400 control subjects with no reported cardiac phenotype were recruited. All participates were matched by ethnicity, gender, and age. An informed consent form was obtained from their parents or guardians. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee (Internal Review Board) of TEDA-international Cardiovascular Hospital, Tianjin, China.
Clinical assessment of the patients included anthropometric measurement and physical examination for dysmorphism and malformation. The patients also underwent chest X-ray examination, electrocardiogram, and ultrasonic echocardiogram. All patients underwent open heart surgery for repair of VSD and were confirmed as isolated VSD without other major congenital malformations.
DNA extraction and mutational analysis
Genomic DNA was extracted from peripheral blood leukocytes by the QIAamp blood kit (Qiagen, Hilden, Germany) according to the manufacturer's instruction. Then, the genomic DNA was tested on a 1% agarose gel and NanoDrop 2000 instrument. The DNA was stored at −20°C before use.
The protein-coding exon (exon-2) of the HOMEZ gene and the partial flanking sequences were amplified by polymerase chain reaction (PCR) with a pair of HOMEZ gene-specific primers (Exon-2 F: 5-AGTTGGGACGACAGGCACGAAC-3, Exon-2 R: 5-GCGGGTGAAACATAGTCAAGT-3; 2673 bp). The PCR primers were designed by using GeneTool Software. PCR cycling conditions were as follows: 94°C for 5 min once, 35 cycles of 94°C for 1 min, annealing temperature 60°C for 1 min, 72°C for 3 min, and 72°C for 10 min once.
The PCR products were sequenced on an ABI3730 Automated Sequencer (PE Biosystems, Foster City, CA). The data were compared with sequences from the NCBI GenBank (HOMEZ: NM_020834.2).
CLC workbench software was used to compare the conservatism of the HOMEZ protein with other multiple species. The alignment of the hydrophobic features between the wild type and mutant type was predicted by the ExPASy-ProtScale tool (www.expasy.org/cgi-bin/pro- tscale.pl). The online tool of Polyphen (genetics.bwh.harvard.edu/pph/) was used to predict possible impact of an amino acid substitution on the structure and function of a human protein.
Results
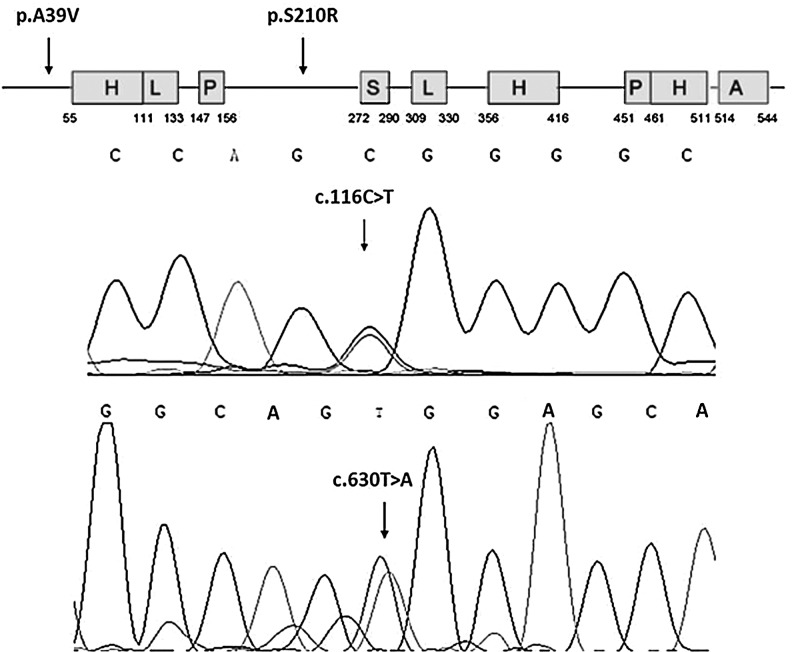
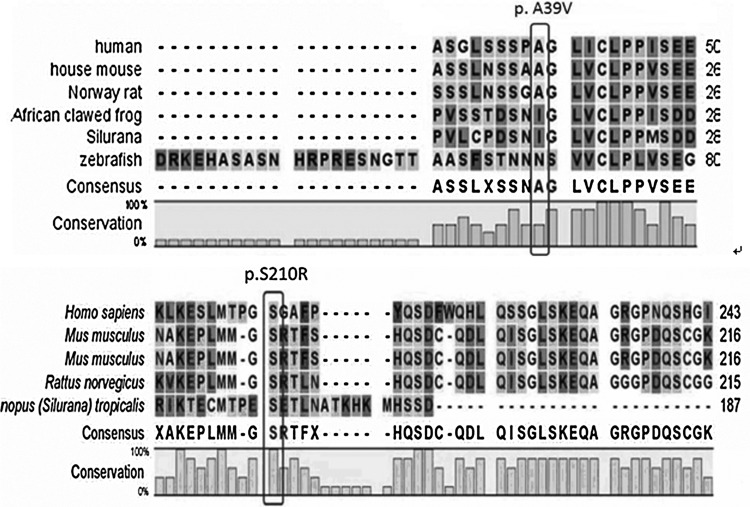
Two novel heterozygous missense mutations (c. 116C>T; c. 630T>A) were identified in HOMEZ gene exon-2 (Fig. 1) when mutational analysis was performed in 400 isolated VSD patients. By comparing with the GenBank data, the two mutations lead to an alanine to valine substitution at the position 39 (p.A39V) and serine to arginine at position 210 (p.S210R) in the HOMEZ protein. The position 39 and position 210 of the HOMEZ protein were known to be highly conserved among many species (Fig. 2), suggesting that this position might play an important role in maintaining the protein function.
FIG. 1.
Two missense mutations (p.A39V; p.S210R) in the protein of HOMEZ (indicated by an arrow; H, homeodomain; L, leucine zipper motif; P, proline-rich motif; S, serine-rich sequence; A, acidic domain.) (upper). Corresponding single transitions are observed at position 116 (C>T) and position 630 (T>A) of the HOMEZ gene as C/T and T/A double peak (indicated by an arrow) (lower). HOMEZ, homeodomain leucine zipper-encoding.
FIG. 2.
Multiple-sequence alignment of partial amino acid sequence of the HOMEZ protein in different species is shown. The alignment data indicate that alanine at position 39 and serine at position 210 (indicated by pane) are conserved in different species in the HOMEZ protein.
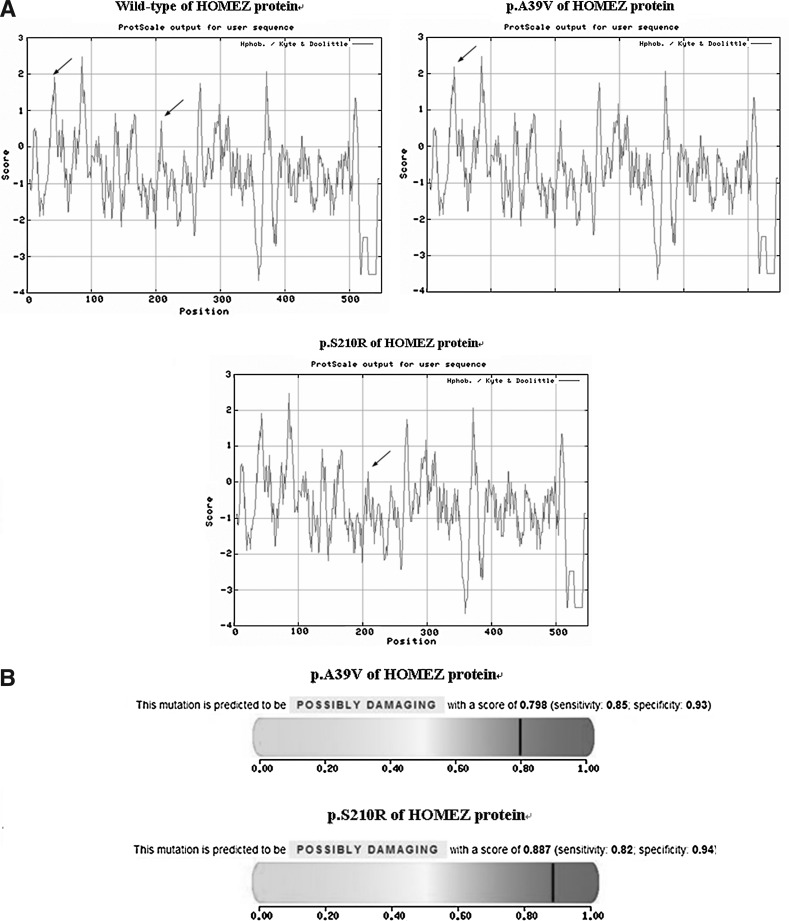
To further explore the role of this amino acid, an online tool ExPASy-ProtScale was used. This approach was defined by a numerical value assigned to predict the hydrophobicity or hydrophilicity scales, the secondary structure conformational parameters, and other scales, which are based on different chemical and physical properties of the amino acids. The hydropathicity of the valine residue at the position 39 and the arginine residue at the position 210 were significantly different from the wild type and the substitution may, in turn, result in modification of the protein structure (Fig. 3, A). Furthermore, when predicted by Polyphen, the changes of these two amino acids were shown to probably damage the protein structure (Fig. 3, B).
FIG. 3.
(A) Hydropathy plot of the wild type, p.A39V, and p.S21P0R of the HOMEZ protein prepared in the Expasy ProtScale Web site according to the Kyte and Doolittle algorithm. Hydrophobic segments have values above zero in the Y-axis, amino acid position is plotted on the x-axis beginning with the N-terminus. The hydrophobicity scores of p.A39V of the HOMEZ protein are higher than the wild type, and p.S210R of the HOMEZ protein is lower than the wild type (the hydrophilicity of corresponding amino acids in HOMEZ protein is indicated by arrows). (B) The results of Polyphen indicate that the changes of these two amino acids were shown to probably damage the protein structure (possibly damaging score: p.A39V=0.798, p.S210R=0.887).
A total of 400 unrelated healthy control subjects were also recruited for mutational screen, and the alteration was not detected in any of them.
We also identified some nonpathogenic variants, as follows: rs117273314, rs117434701, rs1055061, rs79723196, rs76331664, rs10131813, and rs10131813. All of those SNPs were performed association analysis between isolated VSD case patients and 400 healthy controls, but none of them showed statistical differences.
Discussion
Congenital heart malformations occur in more than 0.5% to 1% of live births and are the most common birth defects in newborns (D'Alton et al., 1993). The atrial septal defect and VSD account for the greatest proportion of CHD (Hoffman et al., 1995). In recent years, our knowledge of the etiology of certain CHD has increased significantly. Great strides have been made in the identification of genes responsible for both syndromic and nonsyndromic forms of CHD, and their role in cardiogenesis (Pierpont et al., 2000; Bentham and Bhattacharya, 2008). Since the genome-wide association study (GWAS) technology was used for complex disease genetics, CHD has been at the forefront of a rapidly moving field (Perreault et al., 2010; Wooten et al., 2010). Studies with GWAS methods have identified many genetic variants and loci that are associated with CHD.
Familial aggregation studies have indicated that parental consanguinity is a risk factor for CHD (Maestri et al., 1988; Oyen et al., 2010). This implies that recessive inheritance contributes to the susceptibility of this condition. For example, unlike other Mendelian forms of CHD, the Ellis van-Creveld syndrome is inherited in an autosomal-recessive manner (Ellis and van Creveld, 1940). The hallmark features of the Ellis van-Creveld syndrome are congenital heart defects, which occur in at least 50% of affected individuals, as well as chondroectodermal dysplasia and bilateral postaxial polydactyly (McKusick et al., 1964; da Silva et al., 1980).
To explore the recessive model of CHD, McGregor and coworkers conducted a genome-wide linkage analysis utilizing high-density oligonucleotide microarrays and enrolling 83 Indian CHD probands (phenotypic distribution: conotruncal lesions, right-sided obstructive lesions, septal defects, single-ventricle lesions, valvular defects, and other complex defects) born to unaffected consanguineous parents. Then, mutational analysis in the coding regions of potential predisposing genes and genetic association study were performed in 54 Indian probands and a United States population (McGregor et al., 2010). In the results, two-point linkage analysis resulted in two SNPs (rs1055061 and rs12433225) with log-of-odds scores above the standard threshold of 3.3. Of the two markers, rs1055061 lies within an exon of HOMEZ, causing a nonsynonymous amino acid substitution (Arg>Glu). Three heterozygous variations were found in the 5′ UTR region (c.116G>A), in exon 1(Gly10Arg), and in the 3′UTR (c.+193delT) of the HOMEZ gene when resequencing, and mutational analysis were performed in 54 Indian probands. In addition, no significant differences were detected in the genotype or allele distribution between 325 patients with CHD and 605 population-based controls in an American population of mixed European ancestry (McGregor et al., 2010).
Our patients were sporadic and were from many provinces of China, with no specific parental consanguinity among the patients. In the results, our study for the first time has identified two heterozygous missense mutations (c.116 C>T, p.A39V; c. 630T>A, p.S210R) of the HOMEZ gene in the isolated VSD patients. The two mutations, respectively, lead to an alanine to valine substitution at the position 39 (p.A39V) and serine to arginine at position 210 (p.S210R) in the HOMEZ protein. Although the substitutions are not laid within the unusual structural organization, these positions are highly conserved among many species (human, rats, mice, etc.), which suggest that they might play an important role in maintaining the protein function. Additionally, the hydropathicity of the valine and arginine residue at the position 39 and 210 of the HOMEZ protein were significantly different from the wild type and the substitutions may, in turn, result in modification of the protein structure. Owing to the discussion above, we believe that these two novel heterozygous missense mutations of HOMEZ are directly linked with the etiology of isolated VSD in the Chinese population.
Limitation of study
There are limitations of this study that should be discussed. First, modern genetics demonstrated that the introns and noncoding exons might play an important role in the gene expression regulation (Mattick, 1994; Ren, 2010). In our study, only the coding exons and the partial flanking sequences of HOMEZ were amplified and sequenced. Therefore, our study does not rule out the role of introns and noncoding exons of HOMEZ in the development of isolated VSD that will be one of the future directions for genetic studies in CHD. In fact, there are other genetic factors involved in VSD in Chinese patients as we recently reported (Xuan et al., 2012). Second, the findings from the present study that only involves the Chinese population should be carefully interpreted in other populations. The relationship between HOMEZ and isolated VSD in other populations warrants further study. In addition, the function of these two novel heterozygous missense mutations needs further experimental investigation to confirm.
In summary, the present study by using mutational analysis of HOMEZ in 400 Chinese patients with isolated VSD revealed two novel missense mutations (c.116 C>T, p.A39V; c. 630T>A, p.S210R) in exon-2. These two mutations have not been previously reported in CHD. It is possible that the etiology of isolated VSD might be directly linked with the two mutations. The effect of the mutations on the expression levels and the concrete functional role of these mutations in the development of the septum and the formation of isolated VSD should be addressed in further studies.
Acknowledgments
The work described in this article was fully supported by the National Natural Science Foundation of China (No. 81170148), National Basic Research Program of China 2010CB529500, International Science & Technology Cooperation Program of China 2009DFB30560, and Tianjin Municipal Science and Technology Commission 09ZCZDSF04200 & 10JCYBJC26400, Tianjin Municipal Research Grant for Applied Basic and Frontier Technology & Natural Science Funds (12JCYBJC17400), China, Binhai Key Platform for Creative Research Program (2012-BH110004), Tianjin Binhai New Area Health Bureau (2011BHKZ001 & 2012BWKZ008) & (2011BHKY002 & 2012BWKY024), and Hong Kong Research Grants Council Grants (GRF CUHK4789/09M & 4774/12M).
Author Disclosure Statement
No competing financial interests exist.
References
- Badaruddoza Afzal M. Akhtaruzzaman Inbreeding and congenital heart diseases in a north Indian population. Clin Genet. 1994;45:288–291. doi: 10.1111/j.1399-0004.1994.tb04032.x. [DOI] [PubMed] [Google Scholar]
- Bayarsaihan D. Enkhmandakh B. Makeyev A, et al. Homez, a homeobox leucine zipper gene specific to the vertebrate lineage. Proc Natl Acad Sci. 2003;100:10358–10363. doi: 10.1073/pnas.1834010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker SM. Al Halees Z. Molina C. Paterson RM. Consanguinity and congenital heart disease in Saudi Arabia. Am J Med Genet. 2001;99:8–13. doi: 10.1002/1096-8628(20010215)99:1<8::aid-ajmg1116>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Bentham J. Bhattacharya S. Genetic mechanisms controlling cardiovascular development. Ann N Y Acad Sci. 2008;1123:10–19. doi: 10.1196/annals.1420.003. [DOI] [PubMed] [Google Scholar]
- Burdn F. Szumilo J. Dudka J, et al. Congenital ventricular septal defects and prenatal exposure to cyclooxygenase inhibitors. Braz J Med Biol Res. 2006;39:925–934. doi: 10.1590/s0100-879x2006000700011. [DOI] [PubMed] [Google Scholar]
- Burki MK. Babar GS. Prevalence and pattern of congenital heart disease in Hazara. J Ayub Med Coll Abbottabad. 2001;13:16–18. [PubMed] [Google Scholar]
- Correa-Villasenor A. Ferencz C. Loffredo C. Magee C. Paternal exposures and cardiovascular malformations. The Baltimore-Washington Infant Study Group. J Expo Anal Environ Epidemiol. 1993;3(Suppl 1):173–185. [PubMed] [Google Scholar]
- D'Alton ME. DeCherney AH. Prenatal diagnosis. N Engl J Med. 1993;328:114–120. doi: 10.1056/NEJM199301143280208. [DOI] [PubMed] [Google Scholar]
- da Silva EO. Janovitz D. de Albuquerque SC. Ellis-van Creveld syndrome: report of 15 cases in an inbred kindred. J Med Genet. 1980;17:349–356. doi: 10.1136/jmg.17.5.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RWB. van Creveld SA. Syndrome characterized by ectodermal dysplasia, polydactyly, chondrodysplasia, and congenital morbus cordis: report of three cases. Arch Dis Child. 1940;15:65. doi: 10.1136/adc.15.82.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferencz C. Rubin JD. McCarter RJ, et al. Congenital heart disease: prevalence at livebirth. The Baltimore-Washington Infant Study. Am J Epidemiol. 1985;121:31–36. doi: 10.1093/oxfordjournals.aje.a113979. [DOI] [PubMed] [Google Scholar]
- Hoffman JI. Incidence of congenital heart disease: I. postnatal incidence. Pediatr Cardiol. 1995;16:103–113. doi: 10.1007/BF00801907. [DOI] [PubMed] [Google Scholar]
- Maestri NE. Beaty TH. Liang KY, et al. Assessing familial aggregation of congenital cardiovascular malformations in case-control studies. Genet Epidemiol. 1988;5:343–354. doi: 10.1002/gepi.1370050505. [DOI] [PubMed] [Google Scholar]
- Mattick JS. Introns: evolution and function. Curr Opin Genet Dev. 1994;4:823–831. doi: 10.1016/0959-437x(94)90066-3. [DOI] [PubMed] [Google Scholar]
- McGregor TL. Misri A. Bartlett J, et al. Consanguinity mapping of congenital heart disease in a South Indian population. PLoS One. 2010;5:e10286. doi: 10.1371/journal.pone.0010286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKusick VA. Egeland JA. Eldridge R, et al. The Ellis-van Creveld syndrome. Bull Johns Hopkins Hosp. 1964;115:306–336. [PubMed] [Google Scholar]
- Nagase T. Kikuno R. Ishikawa KI, et al. Prediction of the coding sequences of unidentified human genes. XVI. The complete sequences of 150 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 2000;7:65–73. doi: 10.1093/dnares/7.1.65. [DOI] [PubMed] [Google Scholar]
- Oyen N. Poulsen G. Wohlfahrt J, et al. Recurrence of discordant congenital heart defects in families. Circ Cardiovasc Genet. 2010;3:122–128. doi: 10.1161/CIRCGENETICS.109.890103. [DOI] [PubMed] [Google Scholar]
- Perreault LP. Andelfinger GU. Asselin G. Dube MP. Partitioning of copy-number genotypes in pedigrees. BMC Bioinform. 2010;11:226. doi: 10.1186/1471-2105-11-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpont ME. Markwald RR. Lin AE. Genetic aspects of atrio-ventricular septal defects. Am J Med Genet. 2000;97:289–296. [PubMed] [Google Scholar]
- Ren B. Transcription: Enhancers make non-coding RNA. Nature. 2010;465:173–174. doi: 10.1038/465173a. [DOI] [PubMed] [Google Scholar]
- Rose V. Gold RJ. Lindsay G. Allen M. A possible increase in the incidence of congenital heart defects among the offspring of affected parents. J Am Coll Cardiol. 1985;6:376–382. doi: 10.1016/s0735-1097(85)80175-3. [DOI] [PubMed] [Google Scholar]
- Sands AJ. Casey FA. Craig BG, et al. Incidence and risk factors for ventricular septal defect in “low risk” neonates. Arch Dis Child Fetal Neonatal Ed. 1999;81:F61–F63. doi: 10.1136/fn.81.1.f61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadmouri GO. Nair P. Obeid T, et al. Consanguinity and reproductive health among Arabs. Reprod Health. 2009;6:17. doi: 10.1186/1742-4755-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten EC. Iyer LK. Montefusco MC, et al. Application of gene network analysis techniques identifies AXIN1/PDIA2 and endoglin haplotypes associated with bicuspid aortic valve. PLoS One. 2010;5:e8830. doi: 10.1371/journal.pone.0008830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan C. Wang BB. Gao G, et al. A novel variation of PLAGL1 in Chinese patients with isolated ventricular septal defect. Genet Test Mol Biomarkers. 2012;16:984–987. doi: 10.1089/gtmb.2012.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]