Abstract
Descending projections from sensory areas of the cerebral cortex are among the largest pathways in the brain, suggesting that they are important for subcortical processing. Although corticofugal inputs have been shown to modulate neuronal responses in the thalamus and midbrain, the behavioral importance of these changes remains unknown. In the auditory system, one of the major descending pathways is from cortical layer V pyramidal cells to the inferior colliculus in the midbrain. We examined the role of these neurons in experience-dependent recalibration of sound localization in adult ferrets by selectively killing the neurons using chromophore-targeted laser photolysis. When provided with appropriate training, animals normally relearn to localize sound accurately after altering the spatial cues available by reversibly occluding one ear. However, this ability was lost after eliminating corticocollicular neurons, whereas normal sound-localization accuracy was unaffected. The integrity of this descending pathway is therefore critical for learning-induced localization plasticity.
Keywords: Auditory cortex, corticofugal projection, inferior colliculus, chromophore-targeted neuronal degeneration, apoptosis, adult plasticity, sound localization, binaural cues, ferret
Introduction
Plasticity of cortical processing is important for enabling humans and other species to interact effectively with their constantly changing environments and is believed to provide the basis by which learning can improve perceptual abilities1, 2. However, there is growing evidence, particularly for the auditory system, that learning is also associated with plasticity at lower levels of the pathway3, 4, 5. Electrical stimulation and inactivation studies have shown that cortical feedback can alter the representation of sensory information at almost every stage of subcortical processing5, 6, 7, 8, 9, 10, 11. Although these findings suggest that corticofugal modulation could contribute to learning-induced plasticity and subsequent changes in behavior, there is currently no direct evidence for this and the role of descending cortical projections in sensory processing remains unclear.
The most widely studied descending sensory pathway is the projection from the auditory cortex to the inferior colliculus in the midbrain12. The sensitivity of inferior colliculus neurons to sound frequency13, 14, intensity15, duration16 and location17, 18 has been shown to change after manipulating activity in the auditory cortex in various ways. However, the electrical stimulation and inactivation methods that have been used in these studies are also likely to affect other cells in the cortex in addition to those projecting to the inferior colliculus. We used a chromophore-targeted neuronal degeneration technique19, 20 to investigate the behavioral consequences of selectively eliminating layer V neurons in the primary auditory cortical areas that project to the inferior colliculus (Fig. 1).
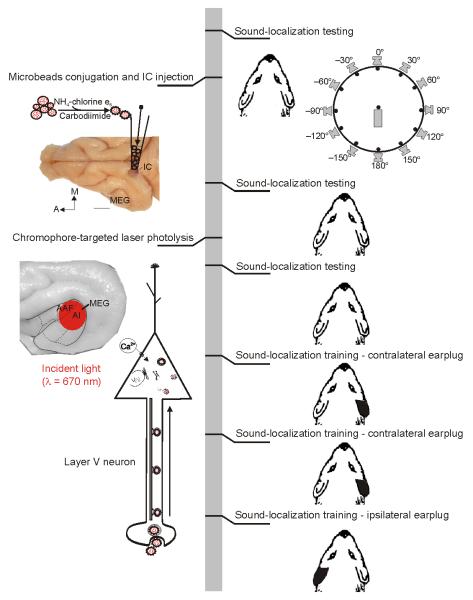
Figure 1. Experimental design.
The vertical gray bar represents the chronological order with behavioral measurements on the right and surgical procedures on the left. After obtaining baseline data from two blocks of testing on a 12-speaker sound-localization task, we gave the ferrets multiple injections of fluorescent microspheres conjugated with chlorine e6 monoethylene diamine disodium (chlorine e6) in the left inferior colliculus. Two more blocks of behavioral testing were followed by ablation of retrograde-labeled layer V corticocollicular neurons by illumination of the ipsilateral auditory cortex with near-infrared light. After re-testing the ability of the animals to localize sound, we examined their capacity to relearn to localize sound after altering the spatial cues available. This was done by providing sound localization training over a 2-week period while the ferrets wore a unilateral earplug, first in the right ear, contralateral to the corticocollicular pathway lesion, then again in the right ear, and finally in the left ear. A, anterior; AAF, anterior auditory field; A1, primary auditory cortex; IC, inferior colliculus; M, medial. Calibration bar represents 5 mm.
We examined the role of this pathway in auditory localization and its recalibration by experience. Differences in the level and time of arrival of the sound at the two ears, along with spectral cues provided by each external ear, provide the basis for determining the spatial location of a sound source21. The processing of these localization cues takes place in the brainstem and information from all three is combined in the inferior colliculus22. However, an intact auditory cortex is necessary for normal sound localization23, 24, 25, implying that further processing takes place at higher levels. Moreover, the cortex appears to be engaged during both training-induced improvements in auditory spatial discrimination26 and adaptation to changes in the balance of inputs between the two ears (F.R.N., O. Kacelnik, V.M.B., J.K. Bizley, D.R.M., A.J.K., unpublished observation).
We found that adaptation to altered sound-localization cues was prevented in the contralateral hemifield after eliminating the auditory corticocollicular projection on one side of the brain. Thus, one function of the auditory cortex in spatial hearing is to provide signals that are transmitted via descending cortical pathways to bring about experience-driven changes in localization.
Results
Corticollicular neuron loss does not impair localization
We determined the ability of ferrets with lesions of the left corticocollicular pathway to localize sound by measuring the accuracy with which they approached each of 12 loudspeakers located at 30° intervals in the horizontal plane. Auditory localization behavior was measured using broadband noise bursts presented at a range of sound levels and durations before and after each stage of the procedure to ablate the left corticocollicular pathway (Fig. 1). The performance of all ferrets at each of these stages overlapped with the normal range of values27 (Fig. 2). Briefly, all of the ferrets showed a reduction in localization accuracy for lateral and posterior targets as the stimulus was shortened in duration (Fig. 2a-c). However, localization precision remained consistent, with >80% of all incorrect responses being made to an adjacent loudspeaker location. Consequently, the overall incidence of both left-right errors (0.18 ± 0.23%) and front-back errors (1.35 ± 1.75%) was very low.
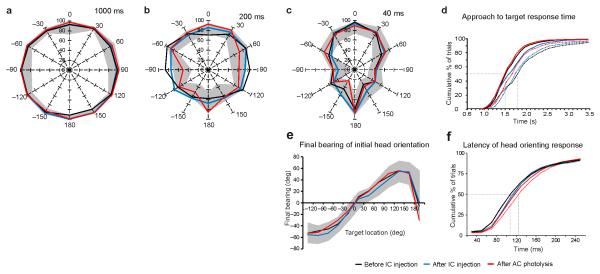
Figure 2. Effect of unilateral auditory corticocollicular lesions on sound-localization accuracy.
(a,b,c) Percentage correct scores at each of the 12 loudspeakers positioned at equal intervals in the horizontal plane. 0° is directly in front of the ferret and negative speaker angles denote stimulus locations on the ferret’s left. Accuracy is shown at three different stimulus sound durations, 1,000 ms (a), 200 ms (b) and 40 ms (c). (d) Cumulative response time in the auditory-localization approach-to-target task, subdivided by whether the ferrets approached the correct reward spout (solid lines) or not (dotted lines). (e,f) The initial sound-evoked head-orienting responses were recorded in the same trials, from which we derived the mean final head bearing for each stimulus location (e) and the latency of those movements (f). The gray bands correspond to 1 s.d. on either side of the mean values achieved by control ferrets and the different colors represent the mean values for the ferrets with corticocollicular lesions before and after each stage of the chromophore-targeted laser photolysis procedure. AC, auditory cortex.
We used univariate logistic regression to cumulatively model the percentage of correct responses as a function of different factors (stimulus duration, target location, stimulus level, individual animal and surgical procedure). We measured the percentage of responses explained by the cumulative model after each factor was introduced, and used the Wald χ2 statistic to determine the significance of the contribution of each factor. The Wald χ2 is given as
and can be used to estimate the probability of obtaining the model parameters () by chance, under the null hypothesis that the mean of is θ0. We found that stimulus duration was the most important predictor of response accuracy, accounting for 79.8% of responses (χ2 = 1757, P < 0.001, degrees of freedom = 5). Successive inclusion of the other factors contributed much less to the percentage of responses explained by the cumulative model: target location improved the fits by only 1.3% (so that the cumulative model explained 81.1% of responses, χ2 = 863, P < 0.001, degrees of freedom = 11), animal by 0.4% (cumulative prediction 81.5%, χ2 = 67, P < 0.001, degrees of freedom = 2) and stimulus level by just 0.2% (81.7%, χ2 = 35, P < 0.001, degrees of freedom = 4). In contrast, the factor surgical procedure was not significant (χ2 = 5, P = 0.091, degrees of freedom = 2), indicating that injection of microbeads into the midbrain and subsequent exposure of the primary auditory cortex to near-infrared light did not alter the localization ability of these ferrets.
The time taken by the ferrets to respond in the approach-to-target task (Fig. 2d) did not differ either between ferrets (F2,7247 = 2.858, P = 0.121) or with surgical procedure (F2,9447 = 0.691, P = 0.525), indicating that their mobility was unimpaired. As in normal ferrets27, correct responses were made more quickly than incorrect ones (χ22 = 903.229, P < 0.001) and this relationship was preserved at each stage of the procedure that we used to kill corticocollicular neurons (Fig. 2d).
Because our neuronal degeneration technique involved making multiple tracer injections into the inferior colliculus, we also measured the accuracy and latency of the ferrets’ sound-evoked orienting movements, as these are likely to rely on midbrain circuitry28, 29. Final bearing increased with target eccentricity in the frontal hemifield (F11,22037 = 85.441, P < 0.0001) (Fig. 2e) but did not vary between animals (F2,12782 = 0.622, P = 0.553), following the corticocollicular lesions (F2,4024 = 0.779, P = 0.518) or with stimulus duration (F5,10355 = 0.706, P = 0.631). Similarly, we found no differences in head movement latency (Fig. 2f) between animals (F2,9015 = 1.420, P = 0.291), after surgical manipulations (F2,4027 = 0.047, P = 0.955) or with stimulus duration (F5,10221 = 2.530, P = 0.098). In summary, these data indicate that ablation of the left auditory corticocollicular pathway did not affect sound localization, as measured by either the initial orienting response or the subsequent selection of sound-source location.
Plasticity is impaired after corticocollicular neuron loss
Localization in the horizontal plane relies principally on binaural cues21. Consequently, if the relationship between these cues and sound direction is altered by occluding one ear, localization accuracy will be impaired. We have shown previously, however, that adult ferrets can adapt to a substantial degree to the altered cues if auditory localization training is provided30. We examined whether this was also the case in ferrets with corticocollicular pathway lesions. This was initially done by inserting an earplug in the right ear, contralateral to the corticocollicular lesion (Fig. 1). Their performance was compared with that of two other groups of ferrets, one that followed the same sequence of behavioral testing, but with a different procedure of tracer injections and/or laser illumination (see Online Methods), and a second that received an earplug in one ear only without any manipulation of the corticocollicular pathway. Because of the similarity between the data (data not shown), all of these ferrets were included in a single control group.
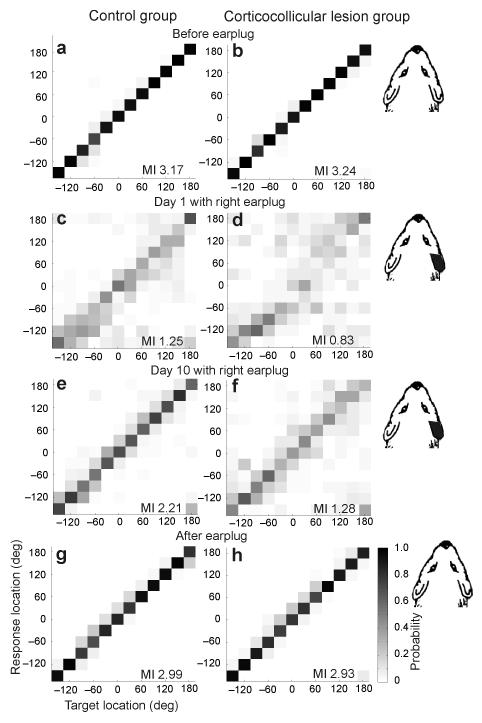
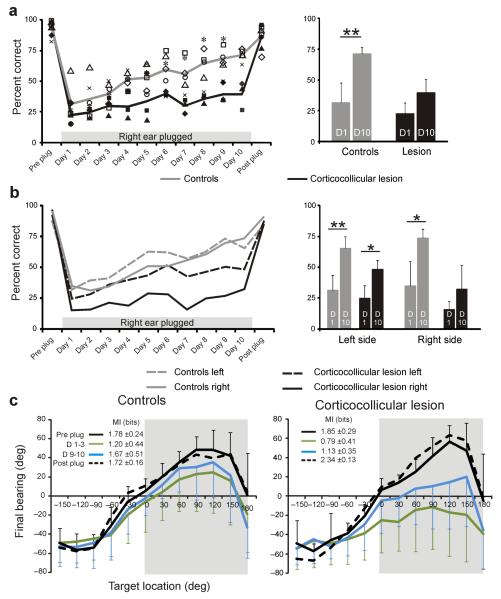
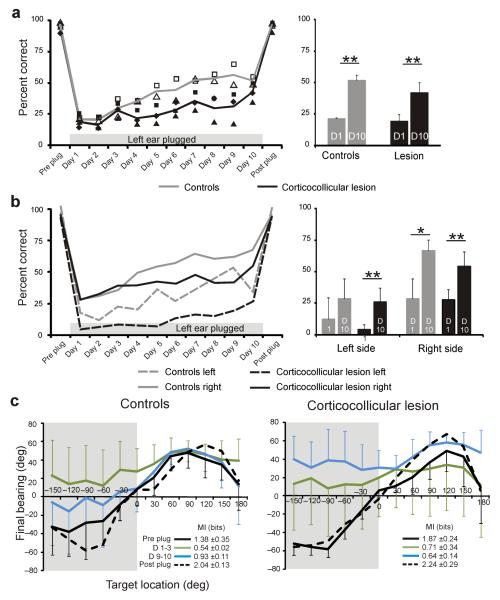
In both control animals and those with corticocollicular lesions, plugging the right ear resulted in an increase in the number and magnitude of localization errors throughout the horizontal plane (shown for 1,000-ms noise bursts in Figs. 3 and 4). Consistent with our previous study30, most ferrets had slightly larger deficits in the hemifield ipsilateral to the earplug, and we found no difference between the control and lesion groups in the scores achieved on the first day of monaural occlusion (t test, P = 0.096). With daily training, the performance of the control group steadily improved and approached pre-plug levels by the tenth day of monaural occlusion (Figs. 3a,c,e and 4a,b). We quantified this improvement by measuring the mutual information between target and response locations. The mutual information values fell from 3.17 bits (close to the theoretical maximum of 3.58 bits) before earplug insertion to 1.25 bits on the day the ear was plugged, but rose to 2.21 bits by the tenth day. The percentage correct scores increased significantly between these sessions (t test, P < 0.01), and this improvement was seen for both the left (P < 0.01) and right (P < 0.01) sides of space (Fig. 4a,b).
Figure 3. Effect of monaural occlusion on auditory-localization accuracy by the control ferrets (left column) and the ferrets with left auditory corticocollicular lesions (right column).
Each plot shows the distribution of the conditional probabilities of the response. (a,b) Data from the last session before inserting an earplug. (c,d) Data from the first day with an earplug in the right ear. (e,f) Data from the last (tenth) day with an earplug in the right ear. (g,h) Data from the first session after the plug was removed. Stimulus (target) location is plotted along the abscissa and response location along the ordinate of each panel, with negative numbers representing the left hemifield. Gray scale represents the conditional probability of the response location selected by the ferrets for each target location. The mutual information (MI) in bits between response and stimulus locations is given in each panel.
Figure 4. Effect of occluding the right ear on sound-localization accuracy.
(a) Percentage correct scores (averaged across all speaker locations) in the session before the right ear was plugged (Preplug), on each of the 10 d over which the plug was worn (day 1–10) and in the session following its removal (Postplug). Data from control animals are shown in gray and from the ferrets with corticocollicular lesions in black; the symbols represent different animals and the lines show the mean scores. The mean (± s.d.) scores on the first (D1) and tenth (D10) day of monaural occlusion are shown for each group on the right. (b) Percentage correct scores for the left and right speaker locations are plotted separately, as indicated. Prior to insertion of the earplug, all of the ferrets scored ≥90% correct, but performed poorly when the right ear was first plugged. Daily training with the earplug in place led to a recovery in localization accuracy, except on the right side of space for the ferrets with corticocollicular lesions. *P < 0.05, **P < 0.01. (c) Accuracy of the head-orienting responses made by the same ferrets at the start of each trial, shown by plotting final head bearing against target location, with negative numbers indicating locations on the ferrets’ left. Mean (± s.d.) preplug and postplug data are shown in black, whereas data averaged over the first three (D1–3) and last two (D9–10) days are shown by the green and blue lines, respectively. The gray background indicates the side on which the earplug was worn. The corresponding mutual information value between target location and final head bearing is shown in each panel. Note the lack of recovery in the accuracy of acoustic orientation behavior in the ferrets with corticocollicular lesions.
The ferrets with corticocollicular lesions showed a more modest increase in mutual information values during the period of monaural occlusion (Fig. 3d,f) and the differences between the percentage correct scores on the first and tenth days of monaural occlusion were not significant, as a result of a lack of improvement in the right hemifield (t tests, P > 0.05; Fig. 4a,b). The regression line fitted to the scores averaged across animal and speaker location over the period of earplug wearing were steeper in the controls (y = 4.43x + 28.58, R2 = 0.96) than in the lesion group (y = 1.76x + 22.7, R2 = 0.74, F3,32 = 7.76, P < 0.001). This was a result of a lack of plasticity in the right hemifield for the ferrets with left corticocollicular lesions, where the regression line slope was lower than that on the left side or on either side for the control group (post hoc t tests, P < 0.05). We obtained the same findings when the ferrets were tested with shorter noise bursts of either 200 ms or 40 ms in duration. Again, no learning occurred in the contralateral hemifield (Supplementary Fig. 1), although normal ferrets do exhibit adaptive plasticity at these shorter sound durations30.
Training-induced plasticity of sound-localization accuracy after occluding one ear in normal ferrets is also seen in the head-orienting responses30. In both the control and lesion groups, acoustic-orienting behavior was disrupted when the right ear was occluded. This was particularly the case for stimuli presented on the side of the earplug, in response to which ferrets with corticocollicular lesions often turned toward the other side (Fig. 4c and Supplementary Fig. 2). Head-orienting accuracy largely recovered in the control ferrets, as indicated by the change in mutual information between final head bearing and target location. Indeed, by the end of the period of monaural occlusion, these ferrets were consistently turning toward the appropriate side and a clear correlation was evident between the location of targets presented within ±120° of the anterior midline and final head bearing. In contrast, less adaptation occurred in the ferrets with corticocollicular pathway lesions, whose head movements toward targets contralateral to the lesion remained extremely variable and inaccurate. These results therefore suggest that the integrity of the auditory corticocollicular pathways is necessary for learning-induced plasticity of both measures of sound localization.
Removal of the earplug restored both head-orienting and approach-to-target response accuracy to near pre-plug levels (Figs. 3 and 4 and Supplementary Fig. 2) in all of the ferrets. This indicates that the ferrets with corticocollicular lesions, which performed poorly while one ear was occluded, could still localize sounds accurately but only if a balanced binaural input was restored. A small after-effect was apparent in the approach data, as shown by a tendency to respond toward the side of the previously plugged ear for sounds presented in the frontal region of space (Fig. 3g,h). After an interval of at least 1 week, we plugged the right ear again (Fig. 1). In the control group, the initial deficits were smaller than when the earplug was first occluded (data not shown), consistent with the idea that the neural changes associated with a previous period of learning partially persist30. These ferrets then showed a significant improvement in performance with training (t tests for comparison of day 1 and day 10 scores, P < 0.05). Again, no adaptation took place in the right hemifield when the right ears of the ferrets with corticocollicular lesions were plugged for the second time (P > 0.05), although a significant improvement in scores was observed in the left hemifield (P < 0.05)
To determine whether this asymmetry in adaptive plasticity reflected the ear that was occluded or the side of space from which the stimuli were presented, we removed the earplug from the right ear at the end of the training period and plugged the left ear instead (Fig. 1). Comparison with data from naive animals in which the left ear was occluded30 revealed that there was no difference in the initial deficits produced by plugging the left or right ears (ANOVA, F2,11 = 0.838, P = 0.45). In contrast, ‘reverse occlusion’ of the ears had a particularly disruptive effect on the performance of all of the ferrets in the left hemifield (Fig. 5), most likely because they had learned to make greater use of spectral cues provided by the left ear when the plug was in the other ear. Both of the groups showed an overall improvement in approach-to-target response accuracy and, as before, this occurred at a slower rate in the ferrets with corticocollicular lesions than in the controls (F3,32 = 4.18, P = 0.013; Fig. 5a). Similarly, head-orienting accuracy partially improved with training in the controls but remained severely impaired in the lesion group (Fig. 5c). A small and comparable increase in the percentage of correct scores occurred on the side of the earplug (comparison of slopes, F3,32 = 2.49, P = 0.310), whereas, once again, the rate of recovery in the right hemifield was lower in the ferrets with left corticocollicular pathway lesions (F3,32 = 4.23, P = 0.026) (Fig. 5b).
Figure 5. Effect of occluding the left ear on sound-localization accuracy.
(a) Percentage correct scores (averaged across all speaker locations) in the session before the left ear was plugged (Preplug), on each of the 10 d over which the plug was worn (day 1-10) and in the session following its removal (Postplug). Data from control ferrets are shown in gray and from the ferrets with corticocollicular lesions in black. (b) Percentage correct scores for the left and right speaker locations are plotted separately, as indicated. (c) Accuracy of the head-orienting responses made by the same ferrets, shown by plotting final head bearing against target location. Data are otherwise presented as described in Figure 4. The previous experience of wearing an earplug in the right ear resulted in a larger deficit when the left earplug was first inserted. As with occlusion of the right ear, more complete recovery was seen in the control ferrets than in the ferrets with corticocollicular lesions. *P < 0.05, **P < 0.01.
Cortical cell loss following laser photolysis
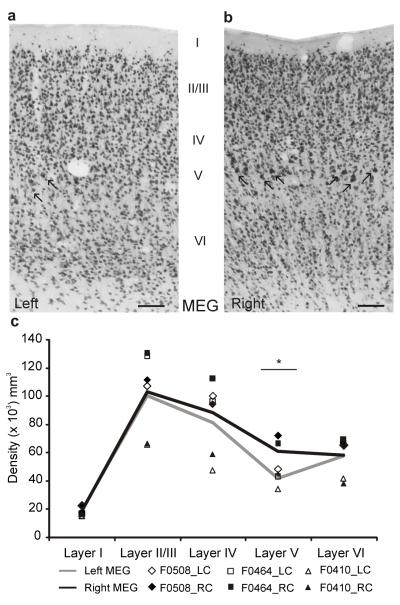
Following behavioral testing, we assessed the effectiveness of the lesions histologically. The density of NeuN-positive neurons in the primary auditory areas in the middle ectosylvian gyrus (MEG) of the ferrets with corticocollicular lesions differed both between the left and right hemispheres (multivariate ANOVA (MANOVA), F1,4 = 10.596, P = 0.009) and across cortical layers (F4,10 = 7.009, P = 0.006) (Supplementary Table 1). Significant left-right differences were restricted to layer V (paired t tests, P = 0.023; Fig. 6).
Figure 6. Reduction in density of layer V neurons in the primary auditory cortex in the corticocollicular lesion group.
(a,b) We used antibody to NeuN as a neuronal marker and found that fewer large pyramidal cells (arrows) were present in layer V of the MEG in the left (lesioned) hemisphere (a) than in the right hemisphere (b). Calibration bars represent 0.1 mm. (c) Using the optical fractionator as a stereological estimator of the number of neurons in each layer of the MEG, we calculated and plotted the neuronal density across cortical layers in each of the ferrets that received corticocollicular lesions. The symbols indicate different ferrets and the lines are the mean values, plotted in black for the right MEG and in gray for the left (lesioned) side. The density of neurons in layer V was lower in the left MEG than in the right (t2 = 4.483, *P < 0.05), with no significant differences between the two hemispheres in other layers. LC, left cortex; RC, right cortex.
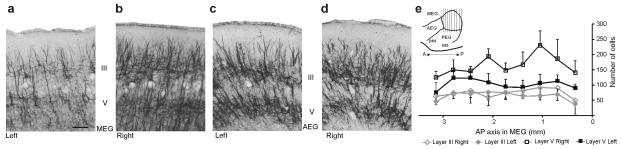
To confirm that this was the result of a loss of layer V projection neurons rather than of nonspecific damage, we used a monoclonal antibody to nonphosphorylated neurofilament H (SMI32) to label pyramidal cells (Fig. 7). Layers III and V were more sparsely stained in the left MEG, which had been illuminated with near-infrared light, than in the right control MEG (Fig. 7a,b), whereas no left-right differences were found in other auditory areas (Fig. 7c,d). This was because of a reduction in the number of SMI32-positive neurons in layer V in the left MEG (Fig.7e and Supplementary Table 2), resulting in a left-right ratio of 0.58 ± 0.11, compared with a ratio of 1.07 ± 0.17 in control ferrets. Having confirmed that the samples were normally distributed (Kolmogorov-Smirnov two-tailed tests, P > 0.6 in each case), we found no difference in the number of SMI32-positive neurons in layer III between the left and right sides of the MEG (MANOVA, F1,8 = 3.252, P = 0.088) or along its anteroposterior extent (F8,18 = 1.726, P = 0.160). However, the number of pyramidal cells in layer V was significantly lower on the left side than on the right (F1,8 = 60.628, P < 0.001), without any anteroposterior variation (F8,18 = 2.083, P = 0.093). These results therefore indicate that chromophore-targeted laser photolysis produced a selective loss of ~40% of pyramidal cells in layer V of the primary auditory cortical areas in the MEG.
Figure 7. Reduction in the number of layer V pyramidal neurons in the primary auditory cortex following corticocollicular lesions.
(a-d) Staining with the SMI32 antibody, a marker of layer III and layer V pyramidal cortical neurons, was sparser on the left (lesioned) side of the MEG, resulting in a less distinct bilaminar appearance (a) than on the right side (b), whereas no differences between the hemispheres were found in other parts of the auditory cortex, such as the adjacent anterior ectosylvian gyrus (AEG) (c,d). Calibration bar represents 0.1 mm. (e) The mean (± s.d.) number of SMI32-positive cells was plotted at different antero-posterior levels of the MEG, counting from the posterior corner of the suprasylvian sulcus (antero-posterior = 0). The number of SMI32-positive cells in layer V was significantly lower (P < 0.01) in the left MEG than on the right side, whereas no differences were found for layer III. The inset indicates the antero-posterior location of the sections used for quantifying SMI32-positive staining. The sparser staining in layer III of the left MEG in a was a result of there being fewer apical dendrites extending from layer V neurons. PEG, posterior ectosylvian gyrus.
Selective loss of corticocollicular neurons
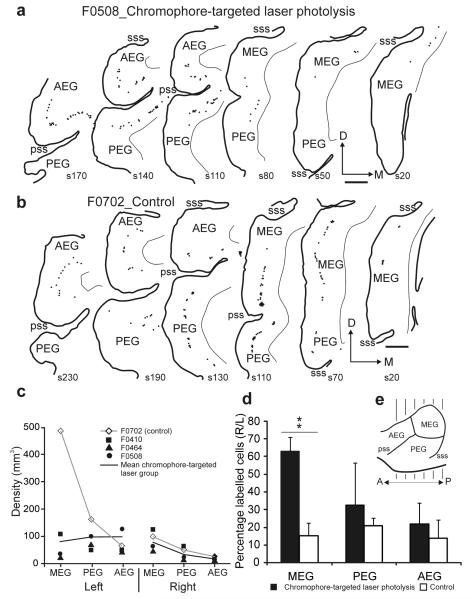
In each case, injections of fluorescent microbeads were similar in volume and location and included all subdivisions of the left inferior colliculus, extending medially into the periaqueductal gray (Supplementary Fig. 3). Labeled cells were found bilaterally in the inferior colliculus, in other subcortical nuclei and in cortical layer V of all three subdivisions of the ectosylvian gyrus (Fig. 8 and Supplementary Table 3). In normal ferrets31 and in control ferrets in which inferior colliculus injections were not followed by laser illumination (case F0702; Fig. 8b,c), the highest density of retrograde labeling was in the ipsilateral left MEG. However, following corticocollicular lesions, the density of labeling was reduced in the ipsilateral MEG, but not in other auditory cortical areas (Fig. 8c,d), including the contralateral MEG, indicating that the lesion was restricted to the region illuminated by the laser.
Figure 8. (a,b) Two examples of the distribution of retrograde-labeled fluorescent cells in the left auditory cortex for a ferret from the corticocollicular lesion group (a) and one from the control group (b).
Coronal sections of the ectosylvian gyrus were arranged from anterior to posterior and numbered starting from its posterior extent (e). Calibration bar represents 1 mm. (c) Density of retrograde-labeled fluorescent cells plotted for the different regions of the auditory cortex (MEG, PEG and AEG) in each hemisphere. Three ferrets with left corticocollicular lesions (filled symbols and black line showing the average density) were compared with the control case F0702 (open symbols and gray line) in which beads were injected in the left inferior colliculus, but the cortex was not exposed to the laser. (d) Mean (± s.d.) percentage of retrograde-labeled cells in the right hemisphere, contralateral to the inferior colliculus injection sites, for the different regions of the right auditory cortex. The significant increase (**P < 0.01) in this percentage in the MEG of the animals in the lesion group (black bars) indicates a substantial loss of left corticocollicular neurons compared with the controls (open bars). D, dorsal; P, posterior; pss, pseudosylvian sulcus; sss, suprasylvian sulcus.
Because no differences in labeling density were found in the right hemisphere between controls and ferrets with left corticocollicular lesions, we used the proportion of fluorescent cells in the right auditory cortex as an index of cell loss (Fig. 8d). In control ferrets, 16.37 ± 6.73% of labeled cells were in the right hemisphere and this proportion was consistent across different auditory cortical areas (ANOVA, F1,8 = 35.598, P = 1.973). This indicates that >80% of the corticocollicular input is normally uncrossed. In contrast, following chromophore-targeted laser photolysis, the proportion rose significantly to >60% in the MEG (F1,5 = 11.740, P = 0.006), suggesting that at least two-thirds of the cells potentially labeled in the left primary auditory cortex could have been lost (Fig. 8d).
Discussion
Using a technique for inducing targeted neuronal apoptosis, we were able to selectively eliminate most of the descending projection from the primary auditory cortical areas on one side of the brain to the ipsilateral inferior colliculus in the midbrain. This manipulation had no effect on the ferrets’ sound-localization abilities but severely disrupted their capacity to learn to localize using markedly altered spatial cue values, even under closed-loop conditions, where the stimulus was long enough for the ferrets to have potentially benefited from dynamic spatial cues or made corrective adjustments in their responses.
Learning was disrupted when we examined the effects of corticocollicular lesions on both the initial orienting response of the ferrets and their perceived sound direction, as assessed by measuring which loudspeaker was approached in order to receive a fluid reward. Although it is well established that the integrity of several cortical fields, including the primary auditory cortex, is required for normal sound localization when animals have to select or discriminate between different targets23, 24, 25, the accuracy of orienting responses is unaffected by lesions of the primary auditory cortex alone28 (F.R.N., O. Kacelnik, V.M.B., J.K. Bizley, D.R.M., A.J.K., unpublished observation). Nevertheless, our results indicate that descending projections from the primary auditory areas are an essential component of the pathway responsible for the adaptive plasticity of head-orienting responses that is required when different spatial cues provide conflicting information. The circuitry involved has yet to be determined, but the auditory cortex projects to different regions of the inferior colliculus, including its external cortex31, 32. This part of the inferior colliculus is a major source of auditory input to the superior colliculus33, which has a well-established role in sensory-guided orienting behavior29.
Dynamic processing of spatial information is necessary if humans and other species are to maintain accurate sound localization in different acoustic environments. Over a longer timescale, adaptation to altered spatial cues is possible, both during development, when the correspondence between cue values and directions in space changes naturally as a result of head and ear growth34, 35, and in adulthood30, 36, 37, 38. The training-dependent recovery of accurate sound localization by adult ferrets when inputs to one ear were reduced with a unilateral earplug seems to involve a reweighting of different cues; the ferrets appear to learn to ignore the altered cues and to rely more on other information, particularly the spectral cues provided by the contralateral external ear, that are less affected by the earplug30. Our results indicate that the corticocollicular pathway is critical for this process.
Compared with the control ferrets, plasticity was particularly impaired on the side of space contralateral to the ablated corticocollicular pathway. This implies that the predominantly ipsilateral corticocollicular pathway mediates plasticity in the opposite hemifield, presumably because each auditory cortex mainly represents the contralateral side of space39, 40. Similarly, unilateral cooling of cat cortical association areas disrupts the multisensory enhancement of orientation behavior for targets in the contralateral hemifield without affecting responses to modality-specific targets41.
In humans, vertical localization and front-back discrimination rely primarily on the spectral cues provided by the near ear42, 43. Moreover, humans can adapt to novel spectral cues independently for each ear38. This supports the possibility that plasticity in the neural processing of the spectral cues provided by the contralateral open ear contributes to the adaptation seen when the other ear is plugged. But in the horizontal plane, adaptation must also involve either a shift or a reduction in sensitivity to the unnatural binaural localization cues produced by the earplug. The minimal aftereffect observed following earplug removal indicates that sensitivity to interaural level differences (ILDs) is suppressed, whereas interaural time differences do seem to be used following adaptation30. As long as the corticocollicular pathway is intact, the auditory system continues to reweight the cues according to their relative salience, thereby enabling accurate sound localization to be recovered when an earplug is inserted into the same ear for a second time or even into the opposite ear.
The laser photolysis technique19 can be used to target specific neuronal populations very effectively without damaging nearby nontargeted neurons, glia or axons of passage20. Our anatomical data suggest that by focusing the beam of near-infrared light at the level of layer V, neurons in other cortical layers were preserved, as were those labeled in layer V outside of the beam spot. Because lesions of the primary auditory cortex impair the localization of brief sounds23, 24, 25, the lack of any such deficits also suggests that there was no nonspecific damage.
We estimated that at least two-thirds of the ipsilateral auditory corticocollicular neurons were killed. This is consistent with the value reported when the same technique was used to target layer VI visual corticogeniculate cells, which resulted in changes in the response properties of both cortical and thalamic neurons44. It was not possible to reach layer V projection neurons lying deep in the sulci surrounding the MEG. Nevertheless, elimination of most of this pathway led to a clear behavioral change, as the ferrets exhibited greatly reduced auditory-localization plasticity. It seems likely that the surviving neurons in this pathway, potentially including the minority that project to the contralateral inferior colliculus, together with nonprimary corticocollicular neurons, were responsible for the limited adaptive changes that were observed.
Electrical stimulation and inactivation studies have shown that the auditory cortex can modulate various response properties of inferior colliculus neurons, including their selectivity to sound frequency13, 14 and location17, 18. Although sensitivity to ILDs is inherited from the brainstem45 or created in inferior colliculus itself46, it is strongly influenced by descending inputs18. The presence of corticofugal terminals in the part of the inferior colliculus31 that receives inputs from brainstem areas that process ILDs and spectral cues47 suggests that sensitivity to those cues is also likely to be under cortical control. In fact, individual inferior colliculus neurons can carry information about all three cues in different aspects of their spike discharge patterns22, raising the possibility that selective corticofugal modulation of particular coding strategies could alter the contribution of each cue to the output of the neurons and therefore to the location percept.
On the basis of the results of electrical stimulation studies, it was previously proposed12 that cortical neurons can facilitate or inhibit the responses of inferior colliculus neurons according to how well matched their response properties are. According to this model, activity in the corticocollicular pathway should reinforce spatial response properties shared between primary auditory cortex and inferior colliculus neurons, shift the spatial selectivity of unmatched inferior colliculus neurons toward that of the cortical neurons, and inhibit the responses of inferior colliculus neurons to sound locations that differ from those to which the cortical neurons are tuned. If corticofugal modulation does contribute to training-induced localization plasticity, activity in the cortex and midbrain would have to be influenced by the earplug in different ways for the primary auditory cortex to trigger a reorganization of subcortical processing. This could be achieved by top-down mechanisms48 and reinforcement-based neuromodulator release49, 50, which enable learning-induced plasticity to take place in the auditory cortex. Our results indicate that descending corticofugal pathways are also part of the circuitry responsible for learning, suggesting that the physiological changes that bring about adaptive changes in behavior may actually occur subcortically.
Methods
Animals
The experiments were approved by the Committee on Animal Care and Ethical Review of the University of Oxford and licensed by the UK Home Office. We used 24 ferrets (Mustela putorius furo) in this study.
Five ferrets received fluorescent microbead injections (retrobeads, Lumafluor) in the inferior colliculus to anatomically determine the optimal number and location of injections in the inferior colliculus and the survival time for maximizing the retrograde labeling of corticocollicular neurons. Three ferrets were used to examine the behavioral consequences of chromophore-targeted laser photolysis of the descending pathway from the auditory cortex to the inferior colliculus. The photolysis protocol included two steps, injection of chlorine e6-conjugated fluorescent microbeads in the left inferior colliculus followed by illumination of the left auditory cortex with near-infrared light. Before and after each of these steps, the ferrets were tested for their ability to localize sound in the horizontal plane (Fig. 1). Their capacity to compensate for the altered spatial cues produced by occluding each ear was then examined; this was always tested first for the ear contralateral to the corticocollicular lesion. Another 16 ferrets provided control data for the behavioral experiments. Three of these followed the same sequence of behavioral testing as the ferrets with corticollicular lesions (that is, both ears were plugged sequentially). In one case (F0538), no surgical manipulations were carried out; in a second case (F0702), fluorescent microbeads were injected in the inferior colliculus, but no laser photolysis of the cortex was carried out, and a third ferret (F0543) received fluorescent microbead injections in the left superior colliculus followed by laser illumination of the left MEG. The remaining 13 control ferrets either provided normal sound-localization data (n = 10), or were used to examine the effects of plugging one ear only (n = 3).
Behavioral testing
The ferrets were maintained on a water-regulation procedure and trained by positive reinforcement to carry out a sound-localization task27, 30. They were tested twice daily in blocks of 14 d in a circular arena (radius, 75 cm) housed in a double-walled sound-attenuated room with 12 loudspeakers (Audax TW025MO) and reward spouts located at 30° intervals around the perimeter. Each ferret was trained to initiate a trial by mounting a central raised platform and licking a spout, at which point its head was at the center of the arena. This triggered the presentation of a single burst of broadband noise with a low-pass cut-off frequency of 30 kHz from one of the speakers. The ferret was rewarded with water if it approached the speaker from which the stimulus had been played and licked the spout beneath. After an incorrect response, the stimulus was presented from the same location up to two more times (correction trials). If the ferret still made an error, a continuous burst of noise was presented (easy trials) until the ferret made a correct response. Correction trials and easy trials were not included in the data analysis.
Stimuli were generated by TDT System II hardware (Tucker-Davis Technologies) and the output of each speaker was digitally flattened and matched for sound level. The test sessions were controlled by a custom-built program that registered the position of the ferret at the different spout locations, presented the stimuli and delivered the rewards accordingly. The sound durations were 2,000, 1,000, 500, 200,100 and 40 ms, presented at five different levels that ranged from 84 to 56 dB sound pressure level in 7-dB steps. In each session, the sound duration was kept constant while the sound levels were randomly varied.
The initial head movement was measured by tracking the position of a reflective strip attached to the ferret’s head with a vertically mounted infrared-sensitive camera and a video contrast–detection device (HVS Image). Our software calculated the angles relative to the initial head position at a 50-Hz frame rate during the first second after the onset of sound. The latency of the head movement was taken as the time when the head first moved in the same angular direction for at least three successive frames. Trials in which the initial head angle deviated by ≥7° or in which the head movement latency was ≥500 ms were excluded from the analysis. The final head bearing was automatically assigned by the software on the basis of the mean of the last three data points after the peak angular acceleration was reached.
Each 14-d testing block started with the longest sound duration, which was sequentially reduced after at least 300 trials were completed per sound duration. Two blocks of testing were provided before and after injecting microbeads in the inferior colliculus and after laser illumination of the auditory cortex. The effects of occluding one ear were examined at three different sound durations, 1,000, 200 and 40 ms.
Chromophore-targeted laser photolysis of the corticocollicular pathway
The photolysis technique was developed previously19, 20. Chlorine e6 (Porphirine Products) was activated with N-cyclohexyl-N′-(2-morpholinoethyl) carboiimide methyl-p-toluenesulfonate (Sigma-Aldrich) and attached to the latex surface of fluorescent microbeads (red retrobeads IX) using gentle agitation on a rocker table (70 rpm) at 0 °C. The reaction was stopped after 65 min with 0.1 M glycine buffer (pH = 8) and a pellet was produced by a series of high-speed centrifugations (169,537 g).
Conjugated microbeads were injected in the inferior colliculus (or superior colliculus) of anesthetized ferrets. After sedation with Domitor (medetomidine hydrochloride, 0.1 mg per kg intramuscular, Pfizer), anesthesia was induced with Saffan (0.3% alphadolone acetate (wt/vol), 0.9% alphaxolone (wt/vol), 2 ml per kg body weight, intramuscular, Schering-Plough Animal Health) and maintained with an intravenous infusion of Domitor (0.22 mg per kg per h) and Ketaset (5 mg per kg per h, ketamine hydrochloride, Fort Dodge Animal Health) in saline. Atrocare (0.06 mg per kg per h of atropine sulfate, Animalcare) and Dexadreson (0.5 mg per kg per h of dexamethasone, Intervet UK) were administered to minimize pulmonary secretions and prevent cerebral edema, respectively. Perioperative analgesia was provided with Vetergesic (buprenorphine hydrochloride, 0.03 mg per kg, subcutaneous, Alstoe Animal Health) and Metacam (meloxicam, 0.2 mg per kg, subcutaneous, Boehringer Ingelheim). The electrocardiogram was monitored and body temperature maintained at 38 °C.
The ferret was mounted in a sterotaxic frame and its eyes were protected with Viscotears (Novartis Pharmaceuticals). After exposing the skull, a craniotomy revealed the most posterior corner of the occipital cortex, which was aspirated to expose the inferior colliculus. Pressure injections of conjugated microbeads were made with a microinjector (Nanojet II, Drummond Scientific) in the left inferior colliculus using a glass micropipette with a 15–30-μm tip diameter. Three to four individual injections of 18.4 nl were made in each of the penetrations (Supplementary Fig. 3). In one of the control cases (F0543), the same number of injections were centered in the superior colliculus.
After 4–6 weeks, during which the ferrets recommenced behavioral testing, the three ferrets in the corticocollicular lesion group and two of the three control ferrets (cases F0702 with beads in the inferior colliculus and F0543 with beads in the superior colliculus) were anesthetized as before and the left MEG was exposed. Nothing further was done with F0702, which therefore represented a sham-operated control. In the three ferrets in the cortico-collicular lesion group and in the control ferret with superior colliculus injections (F0543), the MEG was illuminated with a 670-nm wavelength near-infrared light from a 300-mW laser diode (Flatbeam-Laser 670, Schäfter + Kirchhoff). The laser light was adjusted with beam-shaping optics to create a 1.35-mm spot focused at the level of layer V, ~1 mm from the pial surface. The center of the spot was always placed in the center of the MEG, where primary auditory cortex is located. The laser intensity was increased to 247 mW and maintained for 10 min.
Earplugging
Earplug insertion and removal were carried out under sedation (Domitor, 0.1 mg per kg, intramuscular). The ear was checked before earplug insertion and following its removal by otoscopic examination and tympanometry (Kamplex KLT25 Audiometer, P.C. Werth). The ear was occluded by inserting a customized foam plug (Earfit, Aearo) into the auditory meatus and by filling the concha of the external ear with Otoform-K2 silicone impression material (Dreve Otoplastik). Auditory brainstem response audiometry showed that these plugs produced ~40 dB of attenuation across a broad range of frequencies, and acoustical measurements showed that this attenuation gradually rolls off for frequencies of <3.5 kHz.
Histological processing
After the behavioral experiments or after a survival period of at least 4 weeks following microbead injections in the inferior colliculus in the ferrets used only for anatomy, the ferrets were killed by overdose (Euthatal, 2 ml of 200 mg ml−1 of pentobarbital sodium, Merial Animal Health) and perfused intracardially with 0.9% saline (wt/vol) followed by 4% paraformaldehyde (wt/vol) in 0.1 M phosphate buffer, pH 7.4. Ten series of 35-μm-thick frozen brain sections were cut.
Sections from the even-numbered serials were washed in phosphate buffer, mounted in gelatin-coated slides, dried and coverslipped using Krystalon as mounting media (HARLECO Krystalon). These sections were used to analyze the number and distribution of retrograde-labeled cells in different auditory brain regions under fluorescence microscopy. Sections from odd series were stained to visualize Nissl substance (using 0.2% cresyl violet (wt/vol)), cytochrome oxidase activity (using 0.025% cytochrome C (wt/vol, Sigma-Aldrich) and 0.05% DAB (wt/vol) in 0.1 M phosphate buffer containing 4% sucrose (wt/vol) at 37 °C), SMI32 neurofilament (monoclonal mouse antibody to SMI32, 1:2,000, Covance), neuronal nuclei (NeuN, monoclonal mouse antibody to NeuN, 1:500, Chemicon Europe) or Doublecortin immunoreactivity (guinea pig polyclonal antibody to Doublecortin, 1:500, Chemicon). Appropriate secondary biotinylated antibodies were used (biotinylated antibodies to mouse or guinea pig IgG, Vector Labs) and the reaction product was visualized by incubating the sections in the avidin biotin complex (Vectastain Elite ABC Kit, Vector Labs) and using 3,3′-diaminobenzidine (0.4 mM of DAB, Sigma-Aldrich) as chromogen in the presence of 9.14 mM H2O2.
Data analysis
Behavioral data were analyzed using MATLAB (MathWorks) and Microsoft Office Excel 2007 software (Microsoft). Statistical comparisons were carried out using SPSS software (version 16 for Windows, SPSS).
The approach-to-target data were considered to be independent events and to follow a binomial distribution (correct or incorrect response). Therefore, we fitted a univariate logistic regression to our data to model the influence of different factors (stimulus parameters, surgical manipulations) on the percentage of correct responses. Both forward and backward methods were used to test the validity of the analysis.
The mutual information between the approach-to-target response location or final head bearing and target location was calculated as
where r is the response location or final head bearing, s is the target location, MI(r;s) is the mutual information between r and s, p(r,s) is the joint probability of r and s (obtained from the conditional probability values and equivalent to p(r|s)p(s)), p(s) equals 1/12, as there were 12 equiprobable speakers, and p(r) is obtained from the overall distribution of the response (either approach-to-target or head bearing) locations or, equivalently, from summing p(r,s) across all s. ANOVAs and t tests were also used, as indicated.
Histological analysis and photography were carried out using a Leica DMR microscope (Leica Microsystems), fitted with filters for fluorescence (530-nm light emission) and a digital camera (Microfire, Olympus America). Histological reconstructions were performed using Neurolucida 7 software (MBF Bioscience, MicroBrightField). Unbiased stereological estimates of neuronal numbers and cortical layer volumes were made using the optical fractionator probe with StereoInvestigator software (version 7, MBF Bioscience). The parameters were set to obtain a coefficient of error, which represents the precision of the population size estimate, of <0.05). Kolmogorov-Smirnov two-tailed tests were performed to test whether the samples were normally distributed, and differences between hemispheres and cortical layers were explored by repeated-measures MANOVA and Scheffé post hoc tests, or by paired, two-tailed Student’s t tests.
Supplementary Material
Acknowledgments
We are grateful to J.D. Macklis and R. Fricker-Gates for helping us to set up the chromophore-targeted laser photolysis technique and to B. Willmore for statistical advice. K. Allen and A. Fieger assisted with the early stages of the project, and J. Bizley, R. Campbell, D. Kumpik and S. Spires contributed to the behavioral testing and provided valuable discussion. This work was supported by the Wellcome Trust through a Principal Research Fellowship to A.J.K. (WT076508AIA) and a project grant to A.J.K. and D.R.M. (WT069600/Z/02/Z).
REFERENCES
- 1.Gilbert CD, Li W, Piech V. Perceptual learning and adult cortical plasticity. J. Physiol. 2009;587:2743–2751. doi: 10.1113/jphysiol.2009.171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahmen JC, King AJ. Learning to hear: plasticity of auditory cortical processing. Curr. Opin. Neurobiol. 2007;17:456–464. doi: 10.1016/j.conb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Edeline JM, Weinberger NM. Thalamic short-term plasticity in the auditory system: associative returning of receptive fields in the ventral medial geniculate body. Behav. Neurosci. 1991;105:618–639. doi: 10.1037//0735-7044.105.5.618. [DOI] [PubMed] [Google Scholar]
- 4.Tzounopoulos T, Kraus N. Learning to encode timing: mechanisms of plasticity in the auditory brainstem. Neuron. 2009;62:463–469. doi: 10.1016/j.neuron.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suga N, Ma X. Multiparametric corticofugal modulation and plasticity in the auditory system. Nat. Rev. Neurosci. 2003;4:783–794. doi: 10.1038/nrn1222. [DOI] [PubMed] [Google Scholar]
- 6.Sillito AM, Jones HE, Gerstein GL, West DC. Feature-linked synchronization of thalamic relay cell firing induced by feedback from the visual cortex. Nature. 1994;369:479–482. doi: 10.1038/369479a0. [DOI] [PubMed] [Google Scholar]
- 7.Krupa DJ, Ghazanfar AA, Nicolelis MA. Immediate thalamic sensory plasticity depends on corticothalamic feedback. Proc. Natl. Acad. Sci. USA. 1999;96:8200–8205. doi: 10.1073/pnas.96.14.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao Z, Suga N. Modulation of cochlear hair cells by the auditory cortex in the mustached bat. Nat. Neurosci. 2002;5:57–63. doi: 10.1038/nn786. [DOI] [PubMed] [Google Scholar]
- 9.Perrot X, Ryvlin P, Isnard J, Guénot M, Catenoix H, Fischer C, Mauguière F, Collet L. Evidence for corticofugal modulation of peripheral auditory activity in humans. Cereb. Cortex. 2006;16:941–948. doi: 10.1093/cercor/bhj035. [DOI] [PubMed] [Google Scholar]
- 10.Alvarado JC, Stanford TR, Vaughan JW, Stein BE. Cortex mediates multisensory but not unisensory integration in superior colliculus. J. Neurosci. 2007;27:12775–12786. doi: 10.1523/JNEUROSCI.3524-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo F, Wang Q, Kashani A, Yan J. Corticofugal modulation of initial sound processing in the brain. J. Neurosci. 2008;28:11615–11621. doi: 10.1523/JNEUROSCI.3972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suga N. Role of corticofugal feedback in hearing. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2008;194:169–183. doi: 10.1007/s00359-007-0274-2. [DOI] [PubMed] [Google Scholar]
- 13.Ma X, Suga N. Plasticity of bat’s central auditory system evoked by focal electric stimulation of auditory and/or somatosensory cortices. J. Neurophysiol. 2001;85:1078–1087. doi: 10.1152/jn.2001.85.3.1078. [DOI] [PubMed] [Google Scholar]
- 14.Yan J, Zhang Y, Ehret G. Corticofugal shaping of frequency tuning curves in the central nucleus of the inferior colliculus of mice. J. Neurophysiol. 2005;93:71–83. doi: 10.1152/jn.00348.2004. [DOI] [PubMed] [Google Scholar]
- 15.Yan J, Ehret G. Corticofugal modulation of midbrain sound processing in the house mouse. Eur. J. Neurosci. 2002;16:119–128. doi: 10.1046/j.1460-9568.2002.02046.x. [DOI] [PubMed] [Google Scholar]
- 16.Ma X, Suga N. Corticofugal modulation of duration-tuned neurons in the midbrain auditory nucleus in bats. Proc. Natl. Acad. Sci. USA. 2001;98:14060–14065. doi: 10.1073/pnas.241517098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X, Jen PH. Corticofugal modulation of directional sensitivity in the midbrain of the big brown bat, Eptesicus fuscus. Hear. Res. 2005;203:201–215. doi: 10.1016/j.heares.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Nakamoto KT, Jones SJ, Palmer AR. Descending projections from auditory cortex modulate sensitivity in the midbrain to cues for spatial position. J. Neurophysiol. 2008;99:2347–2356. doi: 10.1152/jn.01326.2007. [DOI] [PubMed] [Google Scholar]
- 19.Macklis JD. Transplanted neocortical neurons migrate selectively into regions of neuronal degeneration produced by chromophore-targeted laser photolysis. J. Neurosci. 1993;13:3848–3863. doi: 10.1523/JNEUROSCI.13-09-03848.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 21.King AJ, Doubell TP, Schnupp JWH. The shape of ears to come. Trends Cog. Neurosci. 2001;5:261–270. doi: 10.1016/s1364-6613(00)01660-0. [DOI] [PubMed] [Google Scholar]
- 22.Chase SM, Young ED. Cues for sound localization are encoded in multiple aspects of spike trains in the inferior colliculus. J. Neurophysiol. 2008;99:1672–1682. doi: 10.1152/jn.00644.2007. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins WM, Merzenich MM. Role of cat primary auditory cortex for sound-localization behavior. J. Neurophysiol. 1984;52:819–847. doi: 10.1152/jn.1984.52.5.819. [DOI] [PubMed] [Google Scholar]
- 24.Kavanagh GL, Kelly JB. Contributions of auditory cortex to sound localization in the ferret (Mustela putorius) J. Neurophysiol. 1987;57:1746–1766. doi: 10.1152/jn.1987.57.6.1746. [DOI] [PubMed] [Google Scholar]
- 25.Heffner HE, Heffner RS. Effect of bilateral auditory cortex lesions on sound localization in Japanese macaques. J. Neurophysiol. 1990;64:915–931. doi: 10.1152/jn.1990.64.3.915. [DOI] [PubMed] [Google Scholar]
- 26.Spierer L, Tardif E, Sperdin H, Murray MM, Clarke S. Learning-induced plasticity in auditory spatial representations revealed by electrical neuroimaging. J. Neurosci. 2007;27:5474–5483. doi: 10.1523/JNEUROSCI.0764-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nodal FR, Bajo VM, Parsons CH, Schnupp JWH, King AJ. Sound localization behavior in ferrets: comparison of acoustic orientation and approach-to-target responses. Neuroscience. 2008;2008;154:397–408. doi: 10.1016/j.neuroscience.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson GC, Masterton RB. Brain stem auditory pathways involved in reflexive head orientation to sound. J. Neurophysiol. 1978;41:1183–1202. doi: 10.1152/jn.1978.41.5.1183. [DOI] [PubMed] [Google Scholar]
- 29.Lomber SG, Payne BR, Cornwell P. Role of the superior colliculus in analyses of space: superficial and intermediate layer contributions to visual orienting, auditory orienting, and visuospatial discriminations during unilateral and bilateral deactivations. J. Comp. Neurol. 2001;2001;441:44–57. doi: 10.1002/cne.1396. [DOI] [PubMed] [Google Scholar]
- 30.Kacelnik O, Nodal FR, Parsons CH, King AJ. Training-induced plasticity of auditory localization in adult mammals. PLoS Biol. 2006;4:e71. doi: 10.1371/journal.pbio.0040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bajo VM, Nodal FR, Bizley JK, Moore DR, King AJ. The ferret auditory cortex: descending projections to the inferior colliculus. Cereb. Cortex. 2007;17:475–491. doi: 10.1093/cercor/bhj164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winer JA, Larue DT, Diehl JJ, Hefti BJ. Auditory cortical projections to the cat inferior colliculus. J. Comp. Neurol. 1998;400:147–174. [PubMed] [Google Scholar]
- 33.King AJ, Jiang ZD, Moore DR. Auditory brainstem projections to the ferret superior colliculus: anatomical contribution to the neural coding of sound azimuth. J. Comp. Neurol. 1998;390:342–365. [PubMed] [Google Scholar]
- 34.Knudsen EI, Esterly SD, Knudsen PF. Monaural occlusion alters sound localization during a sensitive period in the barn owl. J. Neurosci. 1984;4:1001–1011. doi: 10.1523/JNEUROSCI.04-04-01001.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King AJ, Parsons CH, Moore DR. Plasticity in the neural coding of auditory space in the mammalian brain. Proc. Natl. Acad. Sci. USA. 2000;97:11821–11828. doi: 10.1073/pnas.97.22.11821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bauer RW, Matuzsa JL, Blackmer F, Glucksberg S. Noise localization after unilateral attenuation. J. Acoust. Soc. Am. 1966;40:441–444. [Google Scholar]
- 37.Florentine M. Relation between lateralization and loudness in asymmetrical hearing losses. J. Am. Audiol. Soc. 1976;1:243–251. [PubMed] [Google Scholar]
- 38.Van Wanrooij MM, Van Opstal AJ. Sound localization under perturbed binaural hearing. J. Neurophysiol. 2007;97:715–726. doi: 10.1152/jn.00260.2006. [DOI] [PubMed] [Google Scholar]
- 39.Mrsic-Flogel TD, King AJ, Schnupp JWH. Encoding of virtual acoustic space stimuli by neurons in ferret primary auditory cortex. J. Neurophysiol. 2005;93:3489–3503. doi: 10.1152/jn.00748.2004. [DOI] [PubMed] [Google Scholar]
- 40.Woods TM, Lopez SE, Long JH, Rahman JE, Recanzone GH. Effects of stimulus azimuth and intensity on the single-neuron activity in the auditory cortex of the alert macaque monkey. J. Neurophysiol. 2006;96:3323–3337. doi: 10.1152/jn.00392.2006. [DOI] [PubMed] [Google Scholar]
- 41.Jiang W, Jiang H, Stein BE. Two corticotectal areas facilitate multisensory orientation behavior. J. Cogn. Neurosci. 2002;14:1240–1255. doi: 10.1162/089892902760807230. [DOI] [PubMed] [Google Scholar]
- 42.Hofman PM, van Opstal AJ. Binaural weighting of pinna cues in human sound localization. Exp. Brain Res. 2003;148:458–470. doi: 10.1007/s00221-002-1320-5. [DOI] [PubMed] [Google Scholar]
- 43.Jin C, Corderoy A, Carlile S, van Schaik A. Contrasting monaural and interaural spectral cues for human sound localization. J. Acoust. Soc. Am. 2004;115:3124–3141. doi: 10.1121/1.1736649. [DOI] [PubMed] [Google Scholar]
- 44.Eyding D, Macklis JD, Neubacher U, Funke K, Wörgötter F. Selective elimination of corticogeniculate feedback abolishes the electroencephalogram dependence of primary visual cortical receptive fields and reduces their spatial specificity. J. Neurosci. 2003;23:7021–7033. doi: 10.1523/JNEUROSCI.23-18-07021.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis KA, Ramachandran R, May BJ. Single-unit responses in the inferior colliculus of decerebrate cats. II. Sensitivity to interaural level differences. J. Neurophysiol. 1999;82:164–175. doi: 10.1152/jn.1999.82.1.164. [DOI] [PubMed] [Google Scholar]
- 46.Pollak GD, Burger RM, Park TJ, Klug A, Bauer EE. Roles of inhibition for transforming binaural properties in the brainstem auditory system. Hear. Res. 2002;168:60–78. doi: 10.1016/s0378-5955(02)00362-3. [DOI] [PubMed] [Google Scholar]
- 47.Oliver DL. Neuronal organization of the inferior colliculus. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. Springer; New York: 2005. pp. 69–114. [Google Scholar]
- 48.Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J. Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 50.Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nat. Rev. Neurosci. 2004;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.