Abstract
Aims
Prolonged sedentary time is ubiquitous in developed economies and is associated with an adverse cardio-metabolic risk profile and premature mortality. This study examined the associations of objectively assessed sedentary time and breaks (interruptions) in sedentary time with continuous cardio-metabolic and inflammatory risk biomarkers, and whether these associations varied by sex, age, and/or race/ethnicity.
Methods and results
Cross-sectional analyses with 4757 participants (≥20 years) from the 2003/04 and 2005/06 US National Health and Nutrition Examination Survey (NHANES). An Actigraph accelerometer was used to derive sedentary time [<100 counts per minute (cpm)] and breaks in sedentary time. Independent of potential confounders, including moderate-to-vigorous exercise, detrimental linear associations (P for trends <0.05) of sedentary time with waist circumference, HDL-cholesterol, C-reactive protein, triglycerides, insulin, HOMA-%B, and HOMA-%S were observed. Independent of potential confounders and sedentary time, breaks were beneficially associated with waist circumference and C-reactive protein (P for trends <0.05). There was limited evidence of meaningful differences in associations with biomarkers by age, sex, or race/ethnicity. Notable exceptions were sex-differences in the associations of sedentary time and breaks with HDL-cholesterol, and race/ethnicity differences in the association of sedentary time with waist circumference with associations detrimental in non-Hispanic whites, null in Mexican Americans, and beneficial in non-Hispanic blacks.
Conclusion
These are the first population-representative findings on the deleterious associations of prolonged sedentary time with cardio-metabolic and inflammatory biomarkers. The findings suggest that clinical communications and preventive health messages on reducing and breaking up sedentary time may be beneficial for cardiovascular disease risk.
Keywords: Epidemiology, Cardiovascular risk factors, Prevention, Population, Sedentary, Accelerometry
Introduction
Cardiovascular disease (CVD) is the leading cause of premature death in the USA and Europe.1,2 Elevated levels of cardio-metabolic and inflammatory biomarkers have been associated with increased risk of CVD and premature mortality, even at levels below traditional cutpoints of categorically defined risk.3,4 Lack of regular exercise is an established modifiable risk factor for these biomarkers as well as incident CVD and premature mortality.5 More recently, time spent in self-reported sedentary behaviour has been recognized as a unique risk factor, with detrimental associations observed with several of these outcomes.6 Both of these behavioural risk factors are highly prevalent, with objective measures revealing remarkably low levels of moderate–vigorous exercise7 and high levels of sedentary time8 in adult populations. Furthermore, they are independent of each other, with the adverse influence of prolonged sedentary time being seen independent of exercise,9,10 and in those who are physically active at or above recommended levels.9 These findings are consistent with the idea that sedentary behaviours have the potential to influence risk of disease, independent of exercise participation.
To date, the majority of evidence on associations of sedentary time with cardio-metabolic biomarkers has been derived from self-report measures. Only three studies, from Europe and Australia, have examined these associations using an objectively derived (via accelerometers) measure of sedentary time.11–15 These studies, less prone to the biases and errors of self-report, showed that total sedentary time was detrimentally associated with waist circumference, triglycerides, 2 h plasma glucose,11,12 and insulin.13,14 However, these findings are limited by small sample size11,12,14,15 a focus on high-risk individuals,14 and have only evaluated white adults of European descent.11–15 Given that variations in the existence or strength of the associations of self-reported sedentary time with measures of cardio-metabolic health have been noted (mostly by sex6 but also by race/ethnicity16), it is important to establish (particularly in large, representative samples) whether such differences exist for objectively derived sedentary time.
Furthermore, just as focusing exclusively on exercise time ignores the substantial daily contribution of both light-intensity incidental activity and sedentary time,12 focusing only on the total time spent sedentary might overlook important issues, such as how sedentary time is accumulated. An accelerometer-measurement study of 168 Australian adults showed accruing sedentary time in shorter periods (with more interruptions) to be less detrimental for cardio-metabolic health than accruing sedentary time in prolonged periods (with fewer interruptions).15 Here, breaks (interruptions) in sedentary time were beneficially associated with adiposity, triglycerides, and 2 h plasma glucose, independent of total sedentary and exercise time.15 No other studies have explored this ‘breaks’ hypothesis in relation to cardio-metabolic biomarkers.
Given the comparative lack of information about the relationships of sedentary time with cardio-metabolic and inflammatory risk in non-white populations, and the need to confirm and extend on earlier findings regarding breaks in sedentary time, our aim was to examine the associations of accelerometer-derived total sedentary time and breaks in sedentary time with cardio-metabolic and inflammatory risk biomarkers in a large, ethnically diverse sample of US adults.
Methods
The National Health and Nutrition Examination Survey (NHANES) is a cross-sectional study that uses a complex, multistage probability design to obtain a representative sample of the USA civilian non-institutionalized population. The methods are described in detail at: http://www.cdc.gov/nchs/nhanes.htm.17 The survey consists of a household interview and an examination conducted in a mobile examination centre (MEC). For this study, data were drawn from the 2003/04 and 2005/06 NHANES cycles. For adults, response rates for the examination component were 69% in 2003/04 and 71% in 2005/06.17 The study complies with the Declaration of Helsinki, the National Center for Health Statistics Ethics Review Board approved the protocols, and written informed consent was obtained.
Study sample
From an initial sample size of 20 472, adult participants (≥20 years; excluding those who were pregnant or taking insulin) who wore an accelerometer were included in the present study (eligible sample: 7797). Those with missing values for outcome variables or covariates or with insufficient valid accelerometer data were also excluded. Data from 4757 adults were available for full analyses (full sample), with sub-samples of 2118 available for fasting analyses (fasting sub-sample), and 910 for 2 h plasma glucose analysis (oral glucose tolerance test: OGTT sub-sample). Further details are provided in the Supplementary material oline, Figure S1.
Accelerometer data collection and analysis
All ambulatory participants attending the MEC were eligible for the accelerometer component (Actigraph 7164; Actigraph, LLC, Fort Walton Beach, FLA).17 The Actigraph accelerometer is a small (5.1 × 4.1 × 1.5 cm), lightweight (0.4 kg) instrument that records integrated acceleration information as an activity count, which provides an objective estimate of the intensity of bodily movement (particularly ambulatory movement).8 As these activity counts are time and date stamped, detailed data on the time, volume, and intensity of movement can be derived. This differs from a pedometer, which only measures the volume of movement. Lack of, or minimal movement recorded by the accelerometer can be used to derive the time spent sedentary. The accelerometer was worn on the right hip during waking hours (except for water-based activities) for 7 days. Initial screening excluded data from monitors not in calibration and data identified as questionable at the download phase.17 An automated program (SAS 9.1: http://riskfactor.cancer.gov/tools/nhanes_pam/)8 was adapted and used to implement quality control procedures, derive wear time, and summarize minute-by-minute data. Non-wear time was defined as intervals of at least 60 consecutive minutes of 0 cpm, with allowance for up to 2min of observations of some limited movement (<50 cpm) within these periods. Days with at least 10 h of wear time that did not contain excessively high counts (>20 000 cpm) were considered valid. To estimate a longer-term pattern of sedentary time, only participants with at least four valid days (10+ h of wear), including at least 1 weekend day, were included in the analyses.
Accelerometer counts were used to classify all worn time as either sedentary (<100 cpm),8,11–14 light-intensity activity (100–1951 cpm), or moderate–vigorous intensity physical activity (exercise, ≥1952 cpm):18 the intensity level recommended for health in current physical activity guidelines.5 Interruptions in sedentary time, or a transition from a sedentary (<100 cpm) to an active state (≥100 cpm), was considered a break.15 The number of breaks was summed over valid days15 and the mean duration of breaks was calculated. Correction for the influence of variation in wear time on both the sedentary (total and breaks) and exercise variables was achieved by standardizing these values using the residuals obtained when regressing the variables on wear time.19 To facilitate interpretation of the clinical significance of the findings, sedentary time, breaks, and exercise (corrected for wear time) were examined as quartiles. Light-intensity time was not examined as it had an almost perfect inverse correlation (Spearman's ρ = −0.98) with sedentary time (corrected for wear) and would have caused collinearity.
Cardio-metabolic outcomes
Waist circumference was measured to the nearest 0.1 cm at the level of the iliac crest. Resting systolic and diastolic blood pressures were measured three to four times with a mercury sphygmomanometer by trained staff and are reported as averages (excluding the first reading and questionable values).17 Non-fasting serum measures of HDL-cholesterol were collected and analysed using the Roche/Boehringer-Mannheim Diagnostics direct HDL method. Non-fasting C-reactive protein concentrations were measured by latex-enhanced nephelometry on a Behring Nephelometer.
Additionally, in a sub-sample of participants who attended the morning examination (half of all sampled), fasting measures were obtained for triglycerides, plasma glucose, and insulin. There was no exclusion for the fasting sample based on drug treatment, and no specific instructions were given with respect to a drug washout period for those in this portion of the study. Triglycerides were measured enzymatically at both time points, using the Beckman Synchron LX20 analyser in 2003/04 and the Hitachi 717/912 analysers in 2005/06. Glucose was analysed using the hexokinase method, using the Roche Cobas Mira analyser in 2003/04 and the Roche/Hitachi 911 analyser in 2005/06. Insulin was analysed via the Tosoh AIA-PACK IRI immunoenzymometric assay in 2003/04 and the Merocodia Insulin ELISA immunoassay in 2005/06. To account for differences in the methods, we applied correction equations to the fasting plasma glucose and insulin values, after truncating values to assay-specific detectable ranges (to avoid negative estimates). These corrected values were used in the Homeostatic Model Assessment to provide measures of β-cell function (HOMA-%B) and insulin sensitivity (HOMA-%S). Furthermore, all fasting participants in the 2005/06 survey underwent an OGTT from which 2 h plasma glucose values were obtained. All outcomes were treated continuously, with natural log transformation applied to non-normal distributions.
Covariates
Socio-demographics
Interviewer-administered questionnaires obtained socio-demographic information. Age (years) at the time of the survey was treated continuously, allowing for curvilinear relationships by also modelling age-squared (where statistically significant). Categories of educational attainment, marital status and poverty–income ratio, and self-reported race/ethnicity were used, as listed in the Supplementary material online, Table S1.
Behaviours
Smoking status was categorized according to serum-cotinine levels. A single 24 h diet-recall coupled with US Department of Agriculture food composition data measured intakes of total energy and saturated fat (as a percentage of total energy) and alcohol. Additionally, potassium, fibre, caffeine, and calcium intakes were considered with respect to blood pressure. Dietary variables were examined as quintiles, except for alcohol, which was collapsed into sex-specific categories based on US Dietary guidelines.20
Medical history
Dichotomous variables were generated from self-reported medical history for diabetes, CVD, and cancer and (for females only) use of oral contraceptives, hormone replacement and post-menopausal status. Current medication use was recorded, and coded according to the Lexicon Plus (Cerner Multum Inc.) database.17
Statistical analysis
To obtain population-representative findings, linearized estimates with weightings were computed in Stata v.11 (College Station, TX, Stata Corporation). Two year sample weights for each NHANES cycle were combined to provide 4 year weights for the 2003–06 survey periods. Sub-sample weights were used for the fasting and OGTT outcomes; all other analyses used examination weights that were reweighted to account for the non-random absence of accelerometer data.7 Statistical significance was set at P < 0.05 for main effects, and P < 0.1 for interactions; tests were two-sided.
Linear regression analyses examined the associations of total sedentary time and breaks in sedentary time with cardio-metabolic outcomes. Model 1 adjusted only for age, sex, and race/ethnicity. Model 2 additionally adjusted for socio-demographic, behavioural, and medical confounding factors that a backward elimination process had previously identified as specifically relevant for each outcome (P < 0.2 for retention: see Supplementary material online, Table S2) as well as quartiles of exercise time. To examine the extent to which central obesity may mediate the associations observed with other cardio-metabolic outcomes, Model 3 further adjusted for waist circumference. The associations for breaks were examined having adjusted for sedentary time (quartiles). The findings from Model 2 are presented as the main results; the results from Model 1 and Model 3 are reported in Supplementary material online, Table S4. Results are reported as marginal means for each quartile of exposure, back-transformed from the log scale for the non-normal outcomes.
Interactions examined whether associations varied by sex, race/ethnicity, or age (adjusting as per Model 2, but without weighting, to avoid the problem of inflated standard errors). Race/ethnicity comparisons excluded the ‘Other’ race/ethnicity group, where the small sample size precluded meaningful interpretation. Stratified analyses differed from the main analyses in that they adjusted for reproductive variables (in female only models, if P < 0.2); routinely adjusted for poverty–income ratio (all race/ethnicity models); used collapsed sampling strata and response categories for smoking and alcohol when required due to insufficient numbers; and used sex-specific, race/ethnicity specific, or sex and race/ethnicity specific quartiles as appropriate. Age interactions were tested using continuous age, centred on the mean (unweighted mean = 53.0 years).
Results
The population-weighted socio-demographic and behavioural characteristics of the sub-samples (fasting, OGTT) were similar to the full sample and to all potentially eligible participants (see Supplementary material online, Table S1). Furthermore, there were minimal differences between those included in the samples and the eligible (but excluded) participants in terms of their demographic characteristics and study outcomes. The average age of the full sample was 46.5 years (SD 14.2), with 50% males. Accelerometer wear time was 14.6 (SD 1.60) h/day (91.2 h total, SD 17.1), of which an average 8.44 (1.45) h/day was spent sedentary, and a median 0.34 h/day (interquartile range 0.15, 0.61) was spent in exercise. Sedentary time was broken up an average of 556 (SD 108) times across the entire wear time (92.5 times, SD 15.6 per valid day), with a mean break duration of 4.12 (SD 1.26) min.
Clear differences by sex and race/ethnicity in accelerometer and cardio-metabolic characteristics were observed (see Supplementary material online, Table S3). Females were more sedentary, but had more breaks and a more-favourable cardio-metabolic profile than did males. Mexican Americans were the least sedentary, but had significantly higher triglycerides than did non-Hispanic whites and non-Hispanic blacks. In the full sample, exercise had a moderate inverse correlation with sedentary time (Spearman's ρ = −0.57, P < 0.001) and a very weak correlation with breaks (Spearman's ρ = 0.03, P = 0.04). Sedentary time and breaks were weakly correlated overall (Pearson's r = −0.25, P < 0.001).
Overall associations of total sedentary time and breaks with cardio-metabolic risk biomarkers
Following adjustment for covariates, including exercise (Table 1), there were significant detrimental, linear associations of total sedentary time with waist circumference, HDL-cholesterol, C-reactive protein, triglycerides, insulin, HOMA-%B, and HOMA-%S. The magnitude of the average difference between the top and bottom quartiles of sedentary time (a difference of 2.3 h), was clinically meaningful for triglycerides (0.26 mmol/L), insulin (11.6 pmol/L), HOMA-%B (11.6%), and HOMA-%S (36%).3
Table 1.
Adjusted means (95% CI) for continuous cardio-metabolic biomarkers across quartiles of total sedentary time and breaks in sedentary time in US adults ≥20 years (NHANES 2003–06)
| Cardio-metabolic biomarker | Mean (SD) or Median (IQR) | Sedentary variable | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for trend |
|---|---|---|---|---|---|---|---|
| Full sample (n= 4757) | |||||||
| Waist circumference, cm | 96.8 (12.7) | Total | 96.3 (95.3, 97.4) | 96.6 (95.4, 97.8) | 96.5 (95.4, 97.6) | 97.9 (97.0, 98.8)* | 0.0495 |
| Breaks | 99.2 (97.9, 100.6) | 96.8 (95.5, 98.1)* | 96.3 (95.4, 97.2)*** | 95.1 (94.0, 96.1)*** | <0.001 | ||
| Systolic blood pressure, mmHGa | 119 (110, 132) | Total | 121 (121, 122) | 121 (120, 123) | 121 (119, 122) | 120 (119, 122) | 0.056 |
| Breaks | 121 (119, 122) | 121 (120, 122) | 121 (120, 122) | 121 (120, 123) | 0.664 | ||
| Diastolic blood pressure, mmHG | 91.2 (15.4) | Total | 91.4 (89.6, 93.2) | 91.5 (89.5, 93.5) | 91.4 (89.9, 92.8) | 90.7 (89.1, 92.4) | 0.503 |
| Breaks | 91.8 (90.5, 93.2) | 91.5 (89.6, 93.5) | 91.3 (90.1, 92.4) | 90.3 (88.7, 92.0) | 0.095 | ||
| HDL-cholesterol, mmol/La | 1.34 (1.09, 1.63) | Total | 1.38 (1.34, 1.41) | 1.36 (1.34, 1.38) | 1.35 (1.32, 1.37) | 1.32 (1.30, 1.35)* | 0.014 |
| Breaks | 1.34 (1.32, 1.37) | 1.35 (1.32, 1.38) | 1.34 (1.32, 1.36) | 1.37 (1.35, 1.40) | 0.150 | ||
| C-reactive protein, mg/dLa | 0.18 (0.07, 0.42) | Total | 0.17 (0.15, 0.19) | 0.17 (0.16, 0.19) | 0.18 (0.17, 0.20) | 0.19 (0.17, 0.21) | 0.032 |
| Breaks | 0.20 (0.18, 0.21) | 0.17 (0.16, 0.19)* | 0.18 (0.16, 0.19)* | 0.16 (0.15, 0.18)*** | 0.001 | ||
| Fasting sub-sample (n = 2118) | |||||||
| Fasting triglycerides, mmol/La | 1.30 (0.90, 1.96) | Total | 1.22 (1.16, 1.28) | 1.34 (1.27, 1.42)* | 1.41 (1.35, 1.48)*** | 1.48 (1.38, 1.58)*** | <0.001 |
| Breaks | 1.35 (1.28, 1.43) | 1.41 (1.35, 1.48) | 1.33 (1.25, 1.42) | 1.35 (1.28, 1.43) | 0.700 | ||
| Fasting plasma glucose, mmol/La | 5.35 (5.01, 5.79) | Total | 5.49 (5.39, 5.60) | 5.51 (5.43, 5.59) | 5.47 (5.40, 5.54) | 5.52 (5.41, 5.63) | 0.872 |
| Breaks | 5.55 (5.46, 5.64) | 5.51 (5.43, 5.60) | 5.41 (5.33, 5.49)* | 5.51 (5.40, 5.62) | 0.267 | ||
| Insulin, pmol/La | 42.1 (24.6, 73.3) | Total | 36.2 (33.6, 39.1) | 40.1 (36.7, 43.8)* | 42.3 (39.5, 45.3)** | 47.8 (44.5, 51.4)*** | <0.001 |
| Breaks | 43.6 (40.7, 46.6) | 42.2 (38.7, 46.0) | 39.8 (37.2, 42.7) | 41.0 (37.0, 45.4) | 0.233 | ||
| HOMA-%Ba | 67.9 (96.1, 49.4) | Total | 61.2 (58.2, 64.4) | 65.1 (61.9, 68.5) | 68.7 (65.7, 71.8)** | 72.8 (68.2, 77.7)*** | <0.001 |
| Breaks | 67.5 (63.9, 71.3) | 67.3 (63.6, 71.1) | 67.4 (63.9, 71.0) | 66.1 (61.3, 71.3) | 0.707 | ||
| HOMA-%Sa | 124 (70.1, 211) | Total | 145 (134, 158) | 130 (120, 142)* | 123 (115, 132)** | 109 (101, 118)*** | <0.001 |
| Breaks | 120 (113, 129) | 124 (113, 135) | 131 (122, 140) | 127 (115, 141) | 0.259 | ||
| OGTT sub-sample (n = 910) | |||||||
| 2 h plasma glucose, mmol/La | 5.94 (4.83, 7.55) | Total | 5.89 (5.55, 6.24) | 6.09 (5.76, 6.44) | 6.28 (6.02, 6.55) | 6.32 (5.92, 6.75) | 0.119 |
| Breaks | 6.29 (6.03, 6.57) | 6.19 (5.83, 6.56) | 6.35 (5.95, 6.79) | 6.32 (5.78, 6.91) | 0.831 | ||
Data are adjusted means (95% CI), weighted to the USA population. Adjusted for age, sex, race/ethnicity, and quartiles of exercise (≥1952 cpm) h/day, sedentary time (breaks only) plus significant socio-demographic, behavioural, and medical history covariates identified through outcome-specific backward elimination (retained at P < 0.2: see Supplementary material online, Table S2). Quartile cutpoints, corrected for wear time, are 7.24, 8.51, 9.57 h/day for total sedentary time; 470, 559, 645 for breaks in sedentary time. *P < 0.05; **P < 0.01; ***P < 0.001 from Quartile 1 (reference).
aBack-transformed from the log scale.
Bold indicates P < 0.05.
Independent of total sedentary time (as well as exercise and potential confounders), breaks in sedentary time were significantly detrimentally associated with waist circumference, C-reactive protein, and fasting plasma glucose (Table 1), although only the first two were consistent, linear associations. The associations with waist circumference were particularly strong and clinically relevant. Here, Quartiles 2, 3, and 4 were all significantly different from Quartile 1 (reference) and corresponded to, on average, a 2.4, 2.9, and 4.1 cm lower waist circumference, respectively.
Sex differences
Significant interactions (P < 0.1) between sedentary time and sex were seen with blood pressure, HDL-cholesterol, triglycerides, insulin, HOMA-%S, and HOMA-%B. For breaks, significant interactions were seen with these same outcomes, except for HOMA-%B, and also with 2 h plasma glucose. However, HDL-cholesterol was the only outcome for which there was any evidence that an association with sedentary time or breaks was present for one sex but not the other (sedentary time in males; breaks in females). Detailed results are available in Supplementary material online, Table S5.
Racial/ethnic differences
Associations of total sedentary time with waist circumference, systolic blood pressure, insulin, HOMA-%B, and HOMA-%S differed significantly by race/ethnicity (Figure 1A–E). The most marked difference between the racial/ethnic groups was for waist circumference, where sedentary time was detrimentally associated in non-Hispanic whites only, with the association null for Mexican Americans, and beneficial in non-Hispanic blacks. In contrast, sedentary time had significant detrimental associations with insulin, HOMA-%B, and HOMA-%S within all racial/ethnic groups, with only the shape of the relationship differing. No association between sedentary time and systolic blood pressure was evident within any racial/ethnic group (despite the interaction). With one exception, HDL-cholesterol (Figure 1F), associations of breaks did not differ by race/ethnicity (P > 0.1). Breaks had a linear, significant, beneficial association with HDL-cholesterol in non-Hispanic whites but no association within both Mexican Americans and non-Hispanic blacks. Detailed stratified results are available in Supplementary material online, Table S6.
Figure 1.
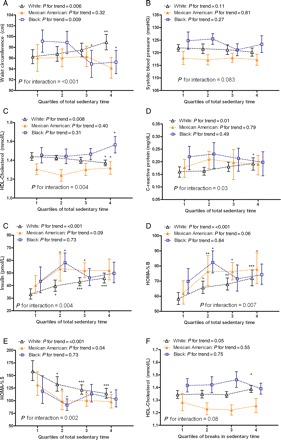
Associations of total sedentary time (A–E) and breaks in sedentary time (F) with cardio-metabolic biomarkers by race/ethnicity (Model 2) in US adults ≥20 years (NHANES 2003–06). Only associations where a significant interaction (P < 0.1) was observed are shown. *P < 0.05; **P < 0.01; ***P < 0.001 from Quartile 1 (reference). Quartile cutpoints for total sedentary time (hours/day) are: 7.39, 8.59, 9.72 for non-Hispanic whites (clear triangle), 6.20, 7.48, 8.85 for Mexican Americans (filled orange triangle), and 7.19, 8.49, 9.51 for non-Hispanic blacks (clear blue square). Quartile cutpoints for breaks are: 472, 558, 645 for non-Hispanic whites, 478, 575, 649 for Mexican Americans, and 449, 545, 638 for non-Hispanic blacks. Sedentary variables standardized for wear time prior to categorization.
Age differences
We examined whether the differences in cardio-metabolic outcomes between quartiles of sedentary time or breaks (relative to the bottom quartile) varied with each additional year of age. Significant interactions were observed for waist circumference (P = 0.002), HDL-cholesterol (P = 0.037), and C-reactive protein (P = 0.005) for sedentary time, and systolic blood pressure (P = 0.005) for breaks. For each of these outcomes, the effect of sedentary time or breaks was the same in direction and significance as was seen previously in the overall analysis (Table 1). Detailed results are presented in the Supplementary material online, Table S7.
Discussion
Prolonged sitting time is a common feature of contemporary society.21 However, compared with other modifiable health behaviours, such as diet, smoking, and lack of exercise, the potential health risks of this ubiquitous behaviour are relatively unknown. This is the first study in a large, representative, multi-ethnic, population-based sample to examine the associations of objectively derived total sedentary time and breaks in sedentary time with cardio-metabolic and inflammatory risk biomarkers. Independent of exercise time and other potential confounders, total sedentary time was detrimentally associated with several biomarkers, whereas breaks, independent of sedentary time, were beneficially associated with waist circumference, C-reactive protein, and fasting plasma glucose. These findings complement and build upon previous results from smaller and/or less-diverse populations and highlight the importance of considering prolonged sedentary time as a distinct health risk behaviour that warrants explicit advice in future public health guidelines.5 In particular, the findings are likely to have implications for settings where prolonged sitting is widespread, such as office workplaces.
Our findings overall, and among non-Hispanic white participants, were consistent with those observed among Australian and European adults;11–15 namely, the strongest associations with sedentary time were observed for triglycerides and markers of insulin resistance, rather than for blood pressure. This is consistent with plausible physiological mechanisms: fewer skeletal muscle contractions may result in reduced lipoprotein lipase activity and clearance of plasma triglycerides, reduced clearance of an oral glucose load from plasma, and less glucose-stimulated insulin secretion.21 The magnitude of the differences between the top and bottom quartiles were clinically meaningful for triglycerides and insulin resistance suggesting that, in theory, population wide reductions in sedentary time (of a magnitude of 1–2 h per day) could have a substantial impact on CVD prevention.22
This study also showed, for the first time, that sedentary time was detrimentally associated with C-reactive protein, while breaks were beneficially associated. Given that C-reactive protein is an inflammatory marker associated with increased risk of several major disease, including coronary heart disease and vascular mortality,4 inflammation may be an adjunct pathway (along with reduced muscular contractions) though which prolonged sedentary time may impact on CVD risk. Furthermore, as the correlation between sedentary time and light-intensity time was almost perfectly inverse, findings could also reflect the benefits of light-intensity activity.11,12
A key contribution of this study is the confirmation and extension of our previous findings15 indicating that patterns of sedentary time accumulation are important in addition to amount of sedentary time. This was particularly pertinent for waist circumference where those in the top quartile of breaks had, on average, a 4.1 cm lower waist circumference than those in the lowest quartile. Of importance to note is that a break could be as short as 1min and not necessarily entail ‘exercise’, suggesting that regular breaks from sedentary time are probably feasible in many contexts. However, the measure of breaks is relatively unsophisticated: it does not differentiate between breaks of a long and/or high intensity, and those of a short, low intensity. More detailed examination of sedentary time patterns, as well as laboratory experimental studies and real-world intervention trials examining the effects of reducing and/or breaking up sedentary time, are needed.
A unique element of this study was the examination of the relationship of objectively derived sedentary time with cardio-metabolic biomarkers by race/ethnicity. The patterning of findings by race/ethnicity has some coherence with studies showing racial/ethnic differences in the relationship of waist circumference with visceral adiposity,23 the compensatory responses to insulin resistance,24 lipoprotein responsiveness to exercise,25 and the relationship between triglycerides and insulin resistance.26 For waist circumference in particular, the racial/ethnic differences were quite pronounced—with no evidence of a detrimental association of sedentary time at all with Mexican Americans and non-Hispanic blacks. Regardless of whether the racial/ethnic differences are biological, or due to unmeasured confounding factors, it is clear that this field of research needs to expand beyond predominantly white populations, to explore this heterogeneity and avoid potentially inappropriate generalization of findings.
A strength of our study is the objective measurement of exposure variables. Compared with self-report, objective measures are more precise, less biased, and reduce the potential for differential measurement errors. However, some error could still be present. First, estimates depend heavily on wear time, which was estimated rather than directly measured. Second, the uniaxial accelerometer predominantly captures ambulatory activities and cannot distinguish between different postures or variations in walking conditions. Thus, though the sedentary cutpoint (<100 cpm) provides a useful estimate of sitting time,8 some standing still time may also be included as sedentary time.
Although we controlled for confounding using several well-measured relevant variables, including measures of health status, residual confounding is possible. For example, adjustments were not made for occupational characteristics (unavailable for the 2005/06 survey). This is unlikely to be an important issue, as a sensitivity analysis of the 2003/04 data (not reported) adjusted for employment status and work type, did not attenuate any of the associations statistically or in terms of effect size; most interactions were also unaffected. There may have been some selection bias as we excluded a large proportion of participants, predominantly for lacking sufficient accelerometer data to acquire habitual estimates of sedentary time; however, this bias is likely to be minimal, particularly in view of the reweighting for accelerometer non-response. Of importance to note is that these associations were cross-sectional. Thus, reverse causation is a possibility, and causality cannot be determined.
In summary, these population-based findings provide further evidence on the deleterious associations of sedentary time with cardio-metabolic health in adults, and provide novel evidence on the relationship of sedentary time with the inflammatory biomarker C-reactive protein. Furthermore, we found significant beneficial associations of breaking up sedentary time with cardio-metabolic health—particularly waist circumference—independent of overall sedentary time. In general, these associations were consistent across sex, age, and race/ethnicity subgroups. Prolonged sedentary time is likely to increase with future technological and social innovations,21 and it is important to consider a whole of day approach to physical activity promotion. Reducing and regularly breaking up sedentary time may be an important adjunct health message, alongside the well-established recommendation for regular participation in exercise. While further evidence of a causal nature is required from longitudinal and intervention studies, less sitting time would be unlikely to do harm, and would, at the very least, contribute to increase overall levels of energy expenditure.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported a National Health and Medical Research Council (NHMRC #569861)/National Heart Foundation of Australia (PH 08B 3905) Postdoctoral Fellowship to G.N.H.; a Victorian Health Promotion Foundation Public Health Research Fellowship to D.W.D.; and Queensland Health Core Research Infrastructure grant and NHMRC Program Grant funding (#301200) to E.A.H.W. and N.O. All data used in this study were collected by the National Center for Health Statistics, Centers for Disease Control and Prevention.
Conflict of interest: none declared.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. doi:10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Allender S, Scarborough P, Peto V, Raynor M, Leal J, Luengo-Fernandez R, Gray A European Heart Network. European Cardiovascular Disease Statistics 2008. http://www.ehnheart.org/cdv-statistics.html. 24 June 2010.
- 3.Hu G, Qiao Q, Tuomilehto J, Eliasson M, Feskens EJ, Pyorala K. Plasma insulin and cardiovascular mortality in non-diabetic European men and women: a meta-analysis of data from eleven prospective studies. Diabetologia. 2004;47:1245–1256. doi: 10.1007/s00125-004-1433-4. [DOI] [PubMed] [Google Scholar]
- 4.Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. doi:10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. doi:10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 6.Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38:105–113. doi: 10.1097/JES.0b013e3181e373a2. doi:10.1097/JES.0b013e3181e373a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 8.Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167:875–881. doi: 10.1093/aje/kwm390. doi:10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc. 2009;41:998–1005. doi: 10.1249/MSS.0b013e3181930355. doi:10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- 10.Dunstan DW, Barr ELM, Healy GN, Salmon J, Shaw JE, Balkau B, Magliano DJ, Cameron AJ, Zimmet PZ, Owen N. Television viewing time and mortality: the AusDiab study. Circulation. 2010;121:384–391. doi: 10.1161/CIRCULATIONAHA.109.894824. doi:10.1161/CIRCULATIONAHA.109.894824. [DOI] [PubMed] [Google Scholar]
- 11.Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ, Owen N. Objectively measured light-intensity physical activity is independently associated with 2-h plasma glucose. Diabetes Care. 2007;30:1384–1389. doi: 10.2337/dc07-0114. doi:10.2337/dc07-0114. [DOI] [PubMed] [Google Scholar]
- 12.Healy GN, Wijndaele K, Dunstan DW, Shaw JE, Salmon J, Zimmet PZ, Owen N. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Diabetes Care. 2008;31:369–371. doi: 10.2337/dc07-1795. doi:10.2337/dc07-1795. [DOI] [PubMed] [Google Scholar]
- 13.Balkau B, Mhamdi L, Oppert JM, Nolan J, Golay A, Porcellati F, Laakso M, Ferrannini E. Physical activity and insulin sensitivity: the RISC study. Diabetes. 2008;57:2613–2618. doi: 10.2337/db07-1605. doi:10.2337/db07-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekelund U, Griffin SJ, Wareham NJ. Physical activity and metabolic risk in individuals with a family history of type 2 diabetes. Diabetes Care. 2007;30:337–342. doi: 10.2337/dc06-1883. doi:10.2337/dc06-1883. [DOI] [PubMed] [Google Scholar]
- 15.Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ, Owen N. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008;31:661–666. doi: 10.2337/dc07-2046. doi:10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- 16.Richmond TK, Walls CE, Gooding HC, Field AE. Television viewing is not predictive of BMI in Black and Hispanic young adult females. Obesity (Silver Spring) 2010;18:1015–1020. doi: 10.1038/oby.2009.391. doi:10.1038/oby.2009.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Survey Data. National Center for Health Statistics (NCHS) http://www.cdc.gov/nchs/nhanes.htm. 30th May 2009.
- 18.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–781. doi: 10.1097/00005768-199805000-00021. doi:10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Willett W, Stampfer M. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Department of Health and Human Services. U.S. Department of Agriculture. Dietary Guidelines for Americans. Washington, DC: U.S. Government Printing Office; 2005. [Google Scholar]
- 21.Hamilton MT, Hamilton DG, Zderic TW. The role of low energy expenditure and sitting on obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56:2655–2667. doi: 10.2337/db07-0882. doi:10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 22.Emberson J, Whincup P, Morris R, Walker M, Ebrahim S. Evaluating the impact of population and high-risk strategies for the primary prevention of cardiovascular disease. Eur Heart J. 2004;25:484–491. doi: 10.1016/j.ehj.2003.11.012. doi:10.1016/j.ehj.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Carroll JF, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, Bae S, Cardarelli R. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity (Silver Spring) 2008;16:600–607. doi: 10.1038/oby.2007.92. doi:10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 24.Goran MI, Bergman RN, Cruz ML, Watanabe R. Insulin resistance and associated compensatory responses in african-american and Hispanic children. Diabetes Care. 2002;25:2184–2190. doi: 10.2337/diacare.25.12.2184. doi:10.2337/diacare.25.12.2184. [DOI] [PubMed] [Google Scholar]
- 25.Bergeron J, Couillard C, Despres JP, Gagnon J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C. Race differences in the response of postheparin plasma lipoprotein lipase and hepatic lipase activities to endurance exercise training in men: results from the HERITAGE Family Study. Atherosclerosis. 2001;159:399–406. doi: 10.1016/s0021-9150(01)00515-9. doi:10.1016/S0021-9150(01)00515-9. [DOI] [PubMed] [Google Scholar]
- 26.Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008;196:696–703. doi: 10.1016/j.atherosclerosis.2006.12.018. doi:10.1016/j.atherosclerosis.2006.12.018. [DOI] [PubMed] [Google Scholar]


