Abstract
Background:
Gentamicin (GM) nephrotoxicity has been related to oxidative stress. Garlic and metformin (MF) have anti-oxadant activity and therefore, this study was aimed to evaluate the preventive and curative effects of garlic, MF and their combination on GM indeced tubular toxicity in Wistar rats.
Methods:
In a pre-clinical study, 70 male Wistar rats were randomly designated into 7 groups of 10 and treated as follows: Group 1: Received saline for 20 days. Group 2: Were injected 100 mg/kg/d of GM intraperitoneally (ip), for 10 days and saline for 10 more days. Group 3: Received GM for 10 days then 20 mg/kg garlic ip for the next 10 days. Group 4: Received GM for 10 days and MF (100 mg/kg) orally for the next 10 days. Group 5: Received GM for 10 days and a combination of MF and garlic for the next 10 days (100 and 20 mg/kg, respectively). Group 6: The same as group 5but with half-doses of MF and Garlic. Group 7: Received GM for 10 days together with a combination ofMF and garlic. On 20th day of the experiment the serum blood urea nitrogen (BUN) and creatinine (Cr) were measured and compared in different groups.
Results:
GM injection significantly increased the serum BUN and Cr (P < 0.05). Administration of MF, garlic or their combination with or after injection of GM (high doses) could atenuate BUN and Cr.
Conclusions:
The results indicate that MF and garlic or their combination have curative and protective activity against GM nephrotoxicity.
Keywords: Garlic, gentamicin, metformin, nephrotoxicity
INTRODUCTION
In contrast to its nephrotoxicity, the aminoglycoside antibiotic gentamicin (GM) is still considered to be an important agent against life-threatening infections.[1,2] During the last decade, the goal of reducing or protecting against its nephrotoxicity has attracted much effort and attention.[1–3] The pathogenesis of GM-nephrotoxicity has shown the involvement of oxygen free radicals, and someof free radical scavengers have been shown to ameliorate the nephrotoxicity.[1–5] Herbal therapies are used by a substantial proportion of people in the world.[6] Herbs are often administered in combination with therapeutic drugs, which may raise the potential of herb-drug antioxidant activity. Indeed recent trends in controlling and treating diseases tend to favor natural antioxidant compounds rather than synthetic ones.[6,7] Garlic, is a commonly worldwide used food, and its medical properties have been well-recognized since the ancient times.[8] Garlic is known for its properties, as an antioxidant against free radicals.[8,9] Apart from superiorities of Metformin (MF) to other anti-diabetic drugs,[10,11] various investigations strongly suggects that this antidiabetic agent prevents oxidative stress-induced death in several cell types through a mechanism dependent on the mitochondrial permeability transition pore (PTP) opening.[10–13] Thus, MFmayafford protection against GM-induced tubular injury by affecting the mitochondria through a mechanism dependent on the mitochondrial PTP opening.[12–21] Due tothe relative safety and effectiveness of antioxidant agents, they seem to be good candidates for testing in human. Furthermore, co-administration of herbal medicines with synthetic drugs is very common, and may potentiate their antioxidant properties. However, their combination effectsneed testing. Therefore, we investigated the effect of garlic juice and MF co-administration in attenuation of GM indeced tubular toxicity in Wistar rats.
METHODS
Drugs and chemicals
MF Hexal, Germany was supplied as white-powder, dosolved in distilled water freshly, as an aqueous solution to be given as a single daily oral dose of 100 mg/kg/day.[17] GM treatment protocol used in the present study has been reported in a previous study.[22]
Garlic extract preparation
Fresh garlic was purchased at the peak of their maturity from a local grower in Hamadan, Iran, in May 2011. The garlics were cleaned, separately chopped, crushed and then macerated with 96% ethanol for 48 h. The debris was removed by centrifugation at 200 g for 5 min. The supernatant was then filtered and rotary-evaporated at 40°C. The extract was frozen and stored at –20°C. The frozen extract was reconstituted with normal saline to prepare final concentration when needed.[23]
Determination of total flavonoids
The amount of total flavonoids in the garlic extract was determined colorimetrically using the method of Shirzad and coworkers[23,24] with minor modification. In this method, 0.5mLof garlic extract or rutin (standard flavonoid compound) was mixed with 1.5 mL of methanol, 0.1 mL of 10% aluminum chloride, 0.1 mL of 1mpotassium acetate, and 2.8 mL of distilled water. Then it was left at room temperature for 30 min. The absorbance of the reaction mixture was measured at 415 nm prepared using rutin solutions at concentrations of 25-500 ppm in methanol. The experiment was repeated in triplicate. Total flavonoids were expressed in terms of rutin equivalents (in mg/g).
Determination of total phenolic compounds
The amount of total phenolic compounds in the garlic extract was determined calorimetrically using the Folin–Ciocalteu reagent described by Bahmani et al.,[25] with minor modification. In brief, 5 mL of garlic extract or gallic acid (standard phenolic compound) was mixed with Folin-Ciocalteu reagent (1:10 diluted with distilled water) and aqueous Na2CO3 (4 mL, 1m). The mixture was allowed to stand for 15 min, and the total phenols were determined by colorimetry at 765 nm. A standard curve was prepared using 0, 50, 100, 150, 200, and 250mg/L solutions of gallic acid in methanol: water (50:50, vol/vol). Total phenol values were expressed in terms of gallic acid equivalent (in mg/g). The experiment was repeated in triplicate.
Determination of antioxidant activity
The ferric thiocyanate method was employed to evaluate antioxidant activity of the extract.[26] In a suitable vial, 500 μg of the extract was dissolved in ethanol and added to a reaction mixture containing 2.88 mL of 2.5% linoleic acid and 9 mL of 40mm phosphate buffer. The vial was incubated at 40°C for 96 h. Every 12 h (during incubation), 0.1 mL of the vial content was diluted with 9.7 mL of 75% ethanol, 0.1 mL of ammonium thiocyanate, and 0.1 mL of FeCl2. The absorbance of sample was measured at 500 nm, and the percentage inhibition (the capacity to inhibit the peroxide formation in linoleic acid) was determined using the following equation;
Percentage of inhibition = (1-[absorbance of sample/absorbance of control])×100
A high inhibition percentage indicates a high antioxidant activity. Ethanol within the sample and without reagents was used as the negative control.
Allicin determination
Allicin content was measured in garlic extract using the method of Miron et al.[25] In brief, 200 mg of extract was added to 1.0 mL (final volume) of 2-nitro-5-thiobenzoate (1.2 × 10–4m) in 50 mm sodium phosphate and 1 mm ethylenediamine tetraacetic acid (pH 7.2). The decrease in optical density at 412 nm was determined after a 30 min incubation at room temperature.[23] The concentration (C) of allicin was calculated according to the following equation:
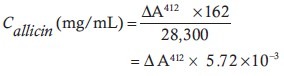
ΔA412 in the above formula is the decrease in optical density compared with the initial absorption at 412 nm.
Animals
Study samples included 70 male Wistar rats with a weight range of 200-250 g. The rats were purchased from Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. All animals were similarly handled in the animal house of the research center, had free access to food and water. They were housed at a controlled temperature (25°C ± 3°C) and humidity (50-60%) environment with a 12 h dark-light cycle (lights on at 7 AM) and allowed free access to pelleted diet and tap water. Their general health state and activity were monitored closely during the experiment. The animal experimentation was conducted in accordance with the National Institute of Health guide for the careful use of laboratory animals.
Experimental design
The animals were divided into seven groups (10 rats each) as follows:
Group 1: Received saline for 20 days. Group 2: Were injected 100 mg/kg/d of GM intraperitoneally (ip), for 10 days and saline for 10 more days. Group 3: Received GM for 10 days then 20 mg/kg garlic ip for the next 10 days. Group 4: Received GM for 10 days and MF (100 mg/kg) orally for the next 10 days. Group 5: Received GM for 10 days and a combination of MF and garlic for the next 10 days (100 and 20 mg/kg, respectively). Group 6: The same as group 5 but with half-doses of MF and Garlic. Group 7: Received GM for 10 days together with a combination of MF and garlic.
In the first day (before experiment) and on the final day (20th day), serum samples were obtained to measure blood urea nitrogen (BUN), serum creatinine (Cr) for all rats. Rats were sacrificed by injecting ketamine (ip) under general anesthesia.
Determination of serum blood urea nitrogen and Cr levels
BUN and Cr levels were measured using colorimetric method employing commercial kits by an auto analyzer (BT 300, Japan).
Statistical analysis
Data were expressed as mean ± SEM. One way ANOVA was applied to compare the serum BUN and Cr levels between the groups. Values of P < 0.05 were considered statistically significant.
RESULTS
The effect of metformin on BUN and Cr levels
The data for serum levels of BUN and Cr are demonstrated in Figure 1. No significant diffrences were observed before the experiment.
Figure 1.
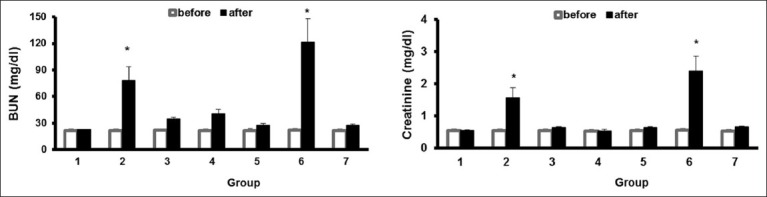
The serum levels of BUN and creatinine before and after the experiment in seven groups of animals. Group 1, sham group; group 2, positive control group treated with gentamicin (GM); group 3 treated with GM for 10 days and post-treatment with garlic extract intraperitoneally (ip) for next 10 days; group 4 treated with GM for 10 days and post-treatment with MF (oral) for next 10 days; group 5, treated with GM for 10 days and post-treatment with combination of MF (oral) and garlic extract (ip) for the next 10 day (100 and 20 mg/kg, respectively); group 6, treated with GM and combination of low dose (50 and 10 mg/kg, respectively); of MF (oral) and garlic extract (ip) for 10 days, and group 7, treated with GM and combination of high dose of MF (oral) and garlic extract (ip) for 10 days. The symbol (*) stands for significant difference from other groups (P< 0.05)
The levels of BUN and Cr in group II which received GM were significantly higher than the ones in group 1, after the experiment (P < 0.05). The results indicate that GM injection induced nephrotoxicity in animals. The levels of BUN and Cr. in goups which received garlic or MF were significantly lesser than group 2 which receive GM. Co-administration of high-doses of MF and garlic vanished the induced nephrotoxicity. However, their low-doses had no effect on GM nephrotoxicity.
DISCUSSION
The main objective of this reasearch was to determine the protective and curative role of garlic, MF and combination of both on GM induced nepgrotoxicity. The findings indicate that 10 days post-treatment with garlic, MF, or combination of both after 10 days of GM administration, the serum levels of BUN and Cr reduced significantly (P < 0.05), when compared with GM treated group. Similar result was observed when co-administration of GM, MF and garlic was applid for 10 days. In this group of animals, the serum levels of BUN and Cr were also reduced statistically (P < 0.05) when compared with the positive control group. When we administered concorently MF and garlic juice with GM, they could prevent GM induced nephrotoxicity. These results indicate that MF and garlic have curative effect other than protective activity. The aminoglycoside antibiotic GM is still widely used against infections by Gram-positive and Gram-negative aerobic bacteria. However, its use has been limited due to renal impairment that occurs in up to 30% of treated patients.[1,2] Moreover, GM has been widely used as a model to study renal failure in experimental animals.[3] The drug may accumulate in epithelial tubular cells causing a range of effects starting with loss of the brush border in epithelial cells and ending in overt tubular necrosis, activation of apoptosis, and massive proteolysis. GM also causes cell death by generation of free radicals, phospholipidosis, extracellular calcium-sensing receptor stimulation and energetic catastrophe, reducedrenalblood flow, and inflammation.[1–5] Various drugs or antioxidents have been shown to ameliorate GM-nephrotoxicity. Because of their relative safety and effectiveness, antioxidant agents seem to be good candidates for testing in human. To the best of our knowledge, this is the first study in which the combination of MF and Garlic extract was applied for the treatment of GM-tubular toxicity. Garlic is known for its antioxidant properties against free radicals.[27] The protective effect of thegarlic-derived antioxidant S-allylcysteine onrenalinjury and oxidative stress induced by ischemia and reperfusion was shown by Segoviano-Murillo et al.[28] The results of this study showed that garlic possess high level of antioxidant activity. In another study, Pedraza-Chaverrí et al. showed that S-allylmercaptocysteine (one of the water soluble organo-sulfur compounds found in garlic extract scavenges hydroxyl radical in vitro and attenuates GM-induced oxidative and nitrosative stress and renal damage in vivo.[29] MF is used for the treatment of diabetes as a sugar-lowering agent.[18,21] MF exerts its metabolic activity through the induction of the adenosine monophosphate-activated protein kinase (AMPK) pathway, which acts as a sensor detecting variations of intracellular energy levels.[21] Alterations in epithelial cell polarity and in the subcellular distributions of epithelial ion transport proteins are key molecular consequences of acute kidney injury and intracellular energy depletion.[30] AMPK, a cellular energy sensor, is rapidly activated in response to renal ischemia, and AMPK activity may influence the maintenance or recovery of epithelial cell organization in mammalian renal epithelial cells subjected to energy depletion.[31–34] At a molecular level, energy deprivation causes key energy-dependent membrane proteins to become displaced and dysfunctional.[30,31] Specially, in the proximal tubule, the Na, K, ATPase (Adenosin triphosphatase) is internalized from the basolateral membrane, disrupting the cell's capacity to maintain normal transepithelial sodium transport.[12,18,34–36] Preservation of a polarized plasma membrane distribution of Na, K, ATPase in renal epithelia is essential for the maintenance of both solute reabsorption and volume homeostasis. It was shown that Na, K, ATPase becomes mislocalized after energy deprivation.[18,37–43] ATP depletion also perturbs the distribution of tight junction proteins, further disrupting epithelial cell polarity and organization[12,18,36] and leading to back leak of extracellular fluid into the urinary space. Such molecular insults result in accumulation of potentially harmful toxins.[1] MF activates AMPK in rat kidney lysates.[41,42,43] MF treatment increases detectable p-AMPK in a dose-dependent manner and MF-induced AMPK activation occurs in proximal tubules as well as in distal segments.[41,42,43] Mitochondria represents one of the major cellular sources of reactive oxygen species (ROS) generation,[10,43–50] and mitochondrial toxicity can also be mediated by ROS. ROS are normally produced at low levels by mitochondria themselves. However, under pathological conditions, the intracellular and intramitochondrial ROS content may be amplified.[10,43–50] In accordance with our laboratory results, prevention of histologic changes due to GM toxicity by MF was shown by Morales et al.[18] They found that control and MF-treated rats showed no structural alterations in renal tissues, while massive and diffuse cell necrosis was observed in the proximal tubules of kidneys from rats injected with GM.[18] Similar results were obtained in our study.
CONCLUSIONS
The results of our study showed that Garlic extract could safely be used together with MF to incease the antioxident potency to ameliorate GM-tubular toxicity.
ACKNOWLEDGMENTS
This paper was derived from MD thesis and the study was granted by research deputy of Shahrekord University of Medical Sciences (Grant #994).
Footnotes
Source of Support: Shahrekord University of Medical Sciences
Conflict of Interest: None declared
REFERENCES
- 1.Ali BH. Agents ameliorating or augmenting experimental gentamicin nephrotoxicity: Some recent research. Food ChemToxicol. 2003;41:1447–52. doi: 10.1016/s0278-6915(03)00186-8. [DOI] [PubMed] [Google Scholar]
- 2.Rafieian-Kopaei M, Nasri H, Nematbakhsh M, Baradaran A, Gheissari A, Rouhi H, et al. Erythropoietin ameliorates genetamicin-induced renal toxicity: A biochemical and histopathological study. J Nephropathology. 2012;1:109–16. doi: 10.5812/nephropathol.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tavafi M. Inhibition of gentamicin–induced renal tubular cell necrosis. J Nephropathology. 2012;1:83–6. doi: 10.5812/nephropathol.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nematbakhsh M, Ashrafi F, Pezeshki Z, Fatahi Z, Kianpoor F, Sanei MH, et al. A histopathological study of nephrotoxicity, hepatoxicity or testicular toxicity: Which one is the first observation as side effect of Cisplatin-induced toxicity in animal model. J Nephropathology. 2012;1:190–3. doi: 10.5812/nephropathol.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali BH. The effect of Nigella sativa oil on gentamicin nephrotoxicity in rats. Am J Chin Med. 2004;32:49–55. doi: 10.1142/S0192415X04001710. [DOI] [PubMed] [Google Scholar]
- 6.Craig W, Beck L. Phytochemicals: Health protective effects. Can J Diet Pract Res. 1999;60:78–84. [PubMed] [Google Scholar]
- 7.Khajehdehi P. Turmeric: Reemerging of a neglected Asian traditional remedy. J Nephropathology. 2012;1:17–22. doi: 10.5812/jnp.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morihara N, Sumioka I, Ide N, Moriguchi T, Uda N, Kyo E. Aged garlic extract maintains cardiovascular homeostasis in mice and rats. J Nutr. 2006;136:777S–81S. doi: 10.1093/jn/136.3.777S. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee SK, Mukherjee PK, Maulik SK. Garlic as an antioxidant: The good, the bad and the ugly. Phytother Res. 2003;17:97–106. doi: 10.1002/ptr.1281. [DOI] [PubMed] [Google Scholar]
- 10.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: An update. Ann Intern Med. 2002;137:25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 11.El-Kaissi S, Sherbeeni S. Pharmacological management of type 2 diabetes mellitus: An update. Curr Diabetes Rev. 2011;7:392–405. doi: 10.2174/157339911797579160. [DOI] [PubMed] [Google Scholar]
- 12.Zorov DB. Amelioration of aminoglycoside nephrotoxicity requires protection of renal mitochondria. Kidney Int. 2010;77:841–3. doi: 10.1038/ki.2010.20. [DOI] [PubMed] [Google Scholar]
- 13.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348:607–14. [PMC free article] [PubMed] [Google Scholar]
- 14.Rouhi H, Ganji F. Effect of N-acetyl cysteine on serum lipoprotein (a) and proteinuria in type 2 diabetic patients. J Nephropathology. 2013;2:6166. doi: 10.5812/nephropathol.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tolouian R, Hernandez GT. Prediction of diabetic nephropathy: The need for a sweet biomarker. J Nephropathology. 2013;2:4–5. doi: 10.5812/nephropathol.8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tavafi M. Diabetic nephropathy and antioxidants. J Nephropathology. 2013;2:20–7. doi: 10.5812/nephropathol.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahimi Z. ACE insertion/deletion (I/D) polymorphism and diabetic nephropathy. J Nephropathology. 2012;1:143–51. doi: 10.5812/nephropathol.8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morales AI, Detaille D, Prieto M, Puente A, Briones E, Arévalo M, et al. Metformin prevents experimental gentamicin-induced nephropathy by a mitochondria-dependent pathway. Kidney Int. 2010;77:861–9. doi: 10.1038/ki.2010.11. [DOI] [PubMed] [Google Scholar]
- 19.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: A new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–14. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadkhodaee M. Erythropoietin; bright future and new hopes for an old drug. J Nephropathology. 2012;1:81–2. doi: 10.5812/nephropathol.7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fryer LG, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–32. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 22.Amini FG, Rafieian-Kopaei M, Nematbakhsh M, Baradaran A, Nasri H. Ameliorative effects of metformin on renal histologic and biochemical alterations of gentamicin-induced renal toxicity in Wistar rats. J Res Med Sci. 2012;17:621–5. [PMC free article] [PubMed] [Google Scholar]
- 23.Shirzad H, Taji F, Rafieian-Kopaei M. Correlation between antioxidant activity of garlic extracts and WEHI-164fibrosarcoma tumor growth in BALB/c mice. J Med Food. 2011;14:969–74. doi: 10.1089/jmf.2011.1594. [DOI] [PubMed] [Google Scholar]
- 24.Bahmani M, Rafieian-kopaei M, Parsaei P, Mohsenzadegan A. The anti-leech effect of peganumharmala L.extract and some anti-parasite drugs on Limnatis Nilotica. Afr J Microbiol Res. 2012;6:2586–90. [Google Scholar]
- 25.Rafieian-Kopaei M, Asgary S, Adelnia A, Setorki M, Khazaei M, Kazemi S, et al. The effects of cornelian cherry on atherosclerosis and atherogenic factors in hypercholesterolemic rabbits. J Med Plants Res. 2011;5:2670–6. [Google Scholar]
- 26.Miron T, Rabinkov A, Mirelman D, Weiner L, Wilchek M. A spectrophotometric assay for allicin and alliinase (Alliinlyase) activity: Reaction of 2-nitro-5-thiobenzoate with thiosulfinates. Anal Biochem. 1998;265:317–25. doi: 10.1006/abio.1998.2924. [DOI] [PubMed] [Google Scholar]
- 27.Hassan HA, El-Agmy SM, Gaur RL, Fernando A, Raj MH, Ouhtit A. In vivo evidence of hepato-and reno-protective effect of garlic oil against sodium nitrite-induced oxidative stress. Int J Biol Sci. 2009;5:249–55. doi: 10.7150/ijbs.5.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segoviano-Murillo S, Sánchez-González DJ, Martínez-Martínez CM, Cruz C, Maldonado PD, Pedraza-Chaverrí J. S-allylcysteine ameliorates ischemia and reperfusion induced renal damage. Phytother Res. 2008;22:836–40. doi: 10.1002/ptr.2396. [DOI] [PubMed] [Google Scholar]
- 29.Pedraza-Chaverrí J, Barrera D, Maldonado PD, Chirino YI, Macías-Ruvalcaba NA, Medina-Campos ON, et al. S-allylmercaptocysteine scavenges hydroxyl radical and singlet oxygen in vitro and attenuates gentamicin-induced oxidative and nitrosative stress and renal damage in vivo. BMC Clin Pharmacol. 2004;4:5. doi: 10.1186/1472-6904-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seo-Mayer PW, Thulin G, Zhang L, Alves DS, Ardito T, Kashgarian M, et al. Preactivation of AMPK by metformin may ameliorate the epithelial cell damage caused by renal ischemia. Am J Physiol Renal Physiol. 2011;301:F1346–57. doi: 10.1152/ajprenal.00420.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takiar V, Nishio S, Seo-Mayer P, King JD Jr, Li H, Zhang L, et al. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci U S A. 2011;108:2462–7. doi: 10.1073/pnas.1011498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tolou-Ghamari Z. Nephro and neurotoxicity, mechanisms of rejection: A review on tacrolimus and cyclosporin in organ transplantation. J Nephropathology. 2012;1:23–30. doi: 10.5812/jnp.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tayebi Khosroshahi H. Short history about renal transplantation program in Iran and the world: Special focus on world kidney day 2012. J Nephropathology. 2012;1:5–10. doi: 10.5812/jnp.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiritoshi S, Nishikawa T, Sonoda K, Kukidome D, Senokuchi T, Matsuo T, et al. Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: Potential role in diabetic nephropathy. Diabetes. 2003;52:2570–7. doi: 10.2337/diabetes.52.10.2570. [DOI] [PubMed] [Google Scholar]
- 35.Einollahi B. Are acquired cystic kidney disease and autosomaldominantpolycystic kidney disease risk factors for renal cell carcinoma in kidney transplant patients? J Nephropathology. 2012;1:65–8. doi: 10.5812/nephropathol.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ardalan MR, Samadifar Z, Vahedi A. Creatine monohydrate supplement induced interstitial nephritis. J Nephropathology. 2012;1:117–20. doi: 10.5812/nephropathol.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Assadi F. The epidemic of pediatric chronic kidney disease: The danger of skepticism. J Nephropathology. 2012;1:61–4. doi: 10.5812/nephropathol.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kari J. Epidemiology of chronic kidney disease in children. J Nephropathology. 2012;1:162–3. doi: 10.5812/nephropathol.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahni N, Gupta KL. Dietary antioxidents and oxidative stress in predialysis chronic kidney patients. J Nephropathology. 2012;1:134–42. doi: 10.5812/nephropathol.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nasri H. Sudden onset of acute renal failure requiring dialysis associated with large B-cell lymphoma of colon. J Nephropathology. 2012;1:202–6. doi: 10.5812/nephropathol.8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plotnikov EY, Chupyrkina AA, Jankauskas SS, Pevzner IB, Silachev DN, Skulachev VP, et al. Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochim Biophys Acta. 2011;1812:77–86. doi: 10.1016/j.bbadis.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Takiyama Y, Harumi T, Watanabe J, Fujita Y, Honjo J, Shimizu N, et al. Tubular injury in a rat model of type 2 diabetes is prevented by metformin: A possible role of HIF-1ޛ expression and oxygen metabolism. Diabetes Res Clin Pract. 2011;60:981–92. doi: 10.2337/db10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyauchi K, Takiyama Y, Honjyo J, Tateno M, Haneda M. Upregulated IL-18 expression in type 2 diabetic subjects with nephropathy: TGF-beta1 enhanced IL-18 expression in human renal proximal tubular epithelial cells. Diabetes Res Clin Pract. 2009;83:190–9. doi: 10.1016/j.diabres.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 44.Maghsoudi AR, Baradaran-Ghahfarokhi M, Ghaed-Amini F, Nasri H, Dehghani Mobarakeh M, Rafieian-Kopaei M. Renal failure and sub mental lymphadenopathy in a 68 years old woman. J Nephropathology. 2012;1:198–201. doi: 10.5812/nephropathol.8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gheissari A, Mehrasa P, Merrikhi A, Madihi Y. Acute kidney injury: A pediatric experience over 10 years at a tertiary care center. J Nephropathology. 2012;1:101–8. doi: 10.5812/nephropathol.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gheissari A, Hemmatzadeh S, Merrikhi A, Fadaei Tehrani S, Madihi Y. Chronic kidney disease in children: A report from a tertiary care center over 11 years. J Nephropathology. 2012;1:177–82. doi: 10.5812/nephropathol.8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gheissari A, Javanmard SH, Shirzadi R, Amini M, Khalili N. The effects of blocking Angiotensin receptors on early stages of diabetic nephropathy. Int J Prev Med. 2012;3:477–82. [PMC free article] [PubMed] [Google Scholar]
- 48.Kam-Tao Li PK, Burdmann EA, Mehta RL. Acute kidney injury: global health alert. J Nephropathology. 2013;2:90–7. doi: 10.12860/JNP.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solati M, Mahboobi HR. Paraoxonase enzyme activity and dyslipidemia in chronic renal failure patients. J Nephropathology. 2012;1:123–5. doi: 10.5812/nephropathol.8106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ashrafi F, Haghshenas S, Nematbakhsh M, Nasri H, Talebi A, Eshraghi-Jazi F, et al. The Role of Magnesium Supplementation in Cisplatin-induced Nephrotoxicity in a Rat Model: No Nephroprotectant Effect. Int J Prev Med. 2012;3:637–43. [PMC free article] [PubMed] [Google Scholar]


